Profiling of O-acetylated Gangliosides Expressed in Neuroectoderm Derived Cells
Abstract
1. Introduction
2. Results
2.1. O-acetylated Sialic Acid Species Are Highly Expressed on Glycolipids in Cancer Cells
2.2. Profiling of O-acetylated Gangliosides in Breast Cancer, Melanoma, and Neuroblastoma Cell Lines
2.3. O-acetylated Ganglioside Species Expression Increases in GD3 Synthase Overexpressing Clones
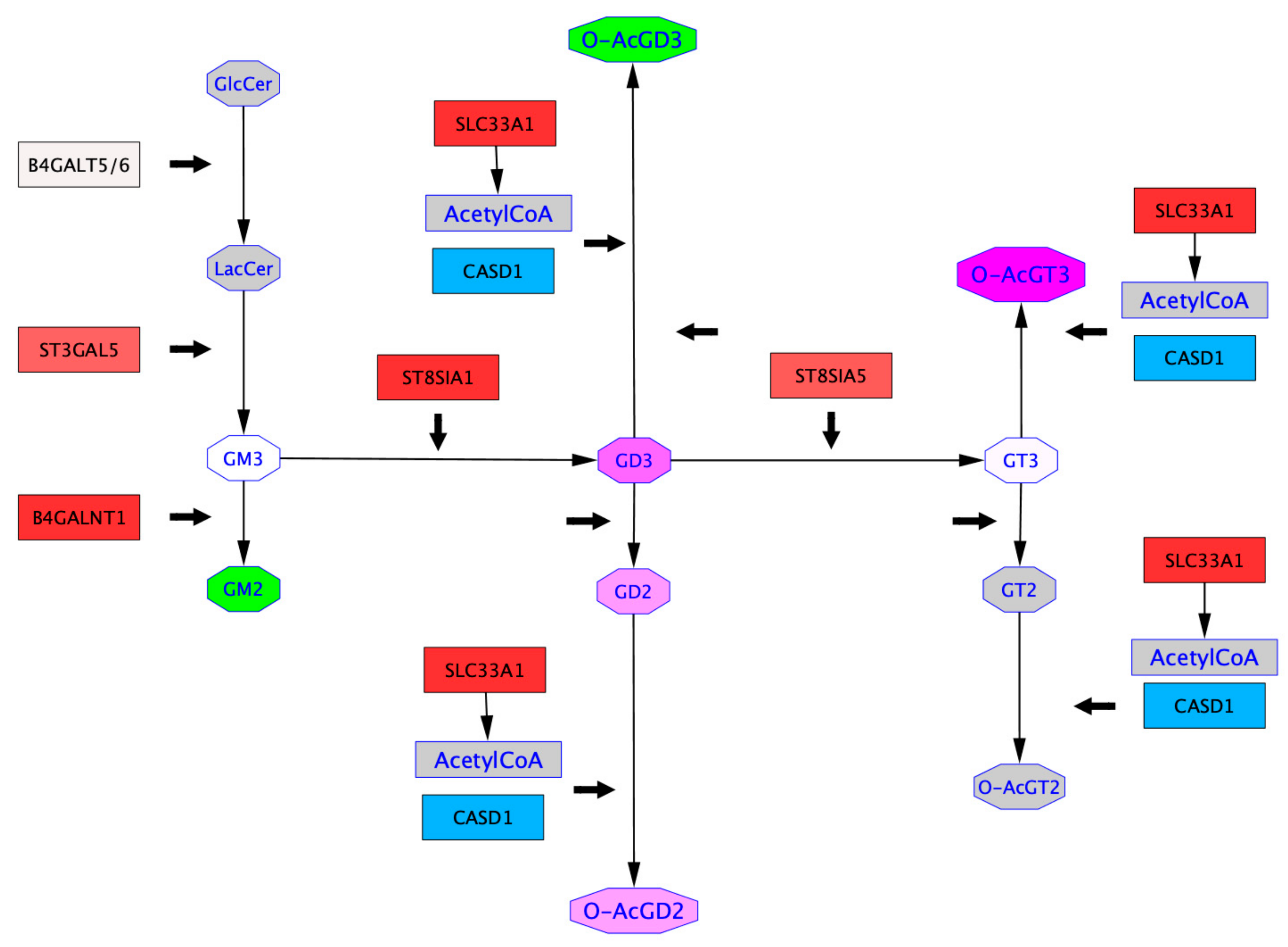
2.4. Mapping of GTs Gene Expression and Gangliosides Content on a Ganglioside Metabolism Patway
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. RNA Extraction and Quantitative Real Time Polymerase Chain Reaction
4.3. DMB Derivatization of Sialic Acids
4.4. Quantitation Analysis of DMB-Sia on Micro-LC/ESI-MS3
4.5. Extraction of Native Gangliosides
4.6. Mass Spectrometry Analysis
4.7. In Silico Mapping of Glycosyltransferase Gene Expression and Ganglioside Species Quantification
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Breast Cancer |
| GlcCer | Glucosylceramide |
| LacCer | Lactosylceramide |
| LC-ESI/MS | Liquid Chromatography Electrospray Ionisation/Mass Spectrometry |
| Neu5,9Ac2 | 9-O-acetyl-N-acetylneuraminic acid |
| OAcGD2 | O-acetylated GD2 |
| OAcGD3 | O-acetylated GD3 |
| RPLC-MS | Reverse-Phase Liquid Chromatography Mass Spectrometry |
| SCLC | Small Cell Lung Carcinoma |
| SOAT | Sialyl-O-acetyltransferase |
| SIAE | Sialyl-O-acetylesterase |
| TACA | Tumor Associated Carbohydrate Antigen |
| TLC | Thin Layer Chromatography |
References
- Hakomori, S. Aberrant glycosylation in cancer cell membranes as focused on glycolipids: Overview and perspectives. Cancer Res. 1985, 45, 2405–2414. [Google Scholar] [PubMed]
- Julien, S.; Bobowski, M.; Steenackers, A.; Le Bourhis, X.; Delannoy, P. How Do Gangliosides Regulate RTKs Signaling? Cells 2013, 2, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Cazet, A.; Bobowski, M.; Rombouts, Y.; Lefebvre, J.; Steenackers, A.; Popa, I.; Guérardel, Y.; Le Bourhis, X.; Tulasne, D.; Delannoy, P. The ganglioside G(D2) induces the constitutive activation of c-Met in MDA-MB-231 breast cancer cells expressing the G(D3) synthase. Glycobiology 2012, 22, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Fukumoto, S.; Kawaguchi, H.; Sato, S.; Ueda, R.; Furukawa, K. Ganglioside G(D2) in small cell lung cancer cell lines: Enhancement of cell proliferation and mediation of apoptosis. Cancer Res. 2001, 61, 4244–4252. [Google Scholar] [PubMed]
- Furukawa, K.; Hamamura, K.; Aixinjueluo, W.; Furukawa, K. Biosignals modulated by tumor-associated carbohydrate antigens: Novel targets for cancer therapy. Ann. N. Y. Acad. Sci. 2006, 1086, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Dinutuximab: First global approval. Drugs 2015, 75, 923–927. [Google Scholar] [CrossRef]
- Alvarez-Rueda, N.; Desselle, A.; Cochonneau, D.; Chaumette, T.; Clemenceau, B.; Leprieur, S.; Bougras, G.; Supiot, S.; Mussini, J.-M.; Barbet, J.; et al. A Monoclonal Antibody to O-Acetyl-GD2 Ganglioside and Not to GD2 Shows Potent Anti-Tumor Activity without Peripheral Nervous System Cross-Reactivity. PLoS ONE 2011, 6, e25220. [Google Scholar] [CrossRef]
- Terme, M.; Dorvillius, M.; Cochonneau, D.; Chaumette, T.; Xiao, W.; Diccianni, M.B.; Barbet, J.; Yu, A.L.; Paris, F.; Sorkin, L.S.; et al. Chimeric antibody c.8B6 to O-acetyl-GD2 mediates the same efficient anti-neuroblastoma effects as therapeutic ch14.18 antibody to GD2 without antibody induced allodynia. PLoS ONE 2014, 9, e87210. [Google Scholar] [CrossRef]
- Cochonneau, D.; Terme, M.; Michaud, A.; Dorvillius, M.; Gautier, N.; Frikeche, J.; Alvarez-Rueda, N.; Bougras, G.; Aubry, J.; Paris, F.; et al. Cell cycle arrest and apoptosis induced by O-acetyl-GD2-specific monoclonal antibody 8B6 inhibits tumor growth in vitro and in vivo. Cancer Lett. 2013, 333, 194–204. [Google Scholar] [CrossRef]
- Kniep, B.; Kniep, E.; Ozkucur, N.; Barz, S.; Bachmann, M.; Malisan, F.; Testi, R.; Rieber, E.P. 9-o-acetyl GD3 protects tumor cells from apoptosis. Int. J. Cancer 2006, 119, 67–73. [Google Scholar] [CrossRef]
- Malisan, F.; Franchi, L.; Tomassini, B.; Ventura, N.; Condò, I.; Rippo, M.R.; Rufini, A.; Liberati, L.; Nachtigall, C.; Kniep, B.; et al. Acetylation suppresses the proapoptotic activity of GD3 ganglioside. J. Exp. Med. 2002, 196, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Cavdarli, S.; Dewald, J.H.; Yamakawa, N.; Guérardel, Y.; Terme, M.; Le Doussal, J.-M.; Delannoy, P.; Groux-Degroote, S. Identification of 9-o-acetyl-N-acetylneuraminic acid (Neu5,9Ac2) as main O-acetylated sialic acid species of GD2 in breast cancer cells. Glycoconj. J. 2019, 36, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Baumann, A.-M.T.; Bakkers, M.J.G.; Buettner, F.F.R.; Hartmann, M.; Grove, M.; Langereis, M.A.; de Groot, R.J.; Mühlenhoff, M. 9-o-Acetylation of sialic acids is catalysed by CASD1 via a covalent acetyl-enzyme intermediate. Nat. Commun. 2015, 6, 7673. [Google Scholar] [CrossRef] [PubMed]
- Mandal, C.; Schwartz-Albiez, R.; Vlasak, R. Functions and Biosynthesis of O-Acetylated Sialic Acids. Top. Curr. Chem. 2015, 366, 1–30. [Google Scholar]
- Ravindranath, M.H.; Higa, H.H.; Cooper, E.L.; Paulson, J.C. Purification and characterization of an O-acetylsialic acid-specific lectin from a marine crab Cancer antennarius. J. Biol. Chem. 1985, 260, 8850–8856. [Google Scholar]
- Sharma, V.; Chatterjee, M.; Mandal, C.; Sen, S.; Basu, D. Rapid diagnosis of Indian visceral leishmaniasis using achatininH, a 9-O-acetylated sialic acid binding lectin. Am. J. Trop. Med. Hyg. 1998, 58, 551–554. [Google Scholar] [CrossRef]
- Sugiyama, E.; Hara, A.; Uemura, K.; Taketomi, T. Application of matrix-assisted laser desorption ionization time-of-flight mass spectrometry with delayed ion extraction to ganglioside analyses. Glycobiology 1997, 7, 719–724. [Google Scholar] [CrossRef]
- Slenter, D.N.; Kutmon, M.; Hanspers, K.; Riutta, A.; Windsor, J.; Nunes, N.; Mélius, J.; Cirillo, E.; Coort, S.L.; Digles, D.; et al. WikiPathways: A multifaceted pathway database bridging metabolomics to other omics research. Nucleic Acids Res. 2018, 46, D661–D667. [Google Scholar] [CrossRef]
- Kutmon, M.; Lotia, S.; Evelo, C.T.; Pico, A.R. WikiPathways App for Cytoscape: Making biological pathways amenable to network analysis and visualization. F1000Research 2014, 3, 152. [Google Scholar] [CrossRef]
- Krengel, U.; Bousquet, P.A. Molecular recognition of gangliosides and their potential for cancer immunotherapies. Front. Immunol. 2014, 5, 325. [Google Scholar] [CrossRef]
- Rabu, C.; McIntosh, R.; Jurasova, Z.; Durrant, L. Glycans as targets for therapeutic antitumor antibodies. Future Oncol. 2012, 8, 943–960. [Google Scholar] [CrossRef]
- Modak, S.; Cheung, N.-K.V. Disialoganglioside directed immunotherapy of neuroblastoma. Cancer Investig. 2007, 25, 67–77. [Google Scholar] [CrossRef]
- Fuller, M.; Duplock, S.; Hein, L.K.; Rigat, B.A.; Mahuran, D.J. Liquid chromatography/electrospray ionisation-tandem mass spectrometry quantification of GM2 gangliosides in human peripheral cells and plasma. Anal. Biochem. 2014, 458, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Busch, C.M.; Desai, A.V.; Moorthy, G.S.; Fox, E.; Balis, F.M. A validated HPLC-MS/MS method for estimating the concentration of the ganglioside, GD2, in human plasma or serum. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1102–1103, 60–65. [Google Scholar] [CrossRef]
- Zhang, G.; Ji, L.; Kurono, S.; Fujita, S.C.; Furuya, S.; Hirabayashi, Y. Developmentally regulated O-acetylated sialoglycans in the central nervous system revealed by a new monoclonal antibody 493D4 recognizing a wide range of O-acetylated glycoconjugates. Glycoconj. J. 1997, 14, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Rossdam, C.; Konze, S.A.; Oberbeck, A.; Rapp, E.; Gerardy-Schahn, R.; von Itzstein, M.; Buettner, F.F.R. Approach for Profiling of Glycosphingolipid Glycosylation by Multiplexed Capillary Gel Electrophoresis Coupled to Laser-Induced Fluorescence Detection to Identify Cell-Surface Markers of Human Pluripotent Stem Cells and Derived Cardiomyocytes. Anal. Chem. 2019, 91, 6413–6418. [Google Scholar] [CrossRef]
- Kristal, B.S.; Brown, A.M. Apoptogenic ganglioside GD3 directly induces the mitochondrial permeability transition. J. Biol. Chem. 1999, 274, 23169–23175. [Google Scholar] [CrossRef]
- Sa, G.; Das, T.; Moon, C.; Hilston, C.M.; Rayman, P.A.; Rini, B.I.; Tannenbaum, C.S.; Finke, J.H. GD3, an overexpressed tumor-derived ganglioside, mediates the apoptosis of activated but not resting T cells. Cancer Res. 2009, 69, 3095–3104. [Google Scholar] [CrossRef] [PubMed]
- Constantine-Paton, M.; Blum, A.S.; Mendez-Otero, R.; Barnstable, C.J. A cell surface molecule distributed in a dorsoventral gradient in the perinatal rat retina. Nature 1986, 324, 459–462. [Google Scholar] [CrossRef]
- Farrer, R.G.; Quarles, R.H. GT3 and its O-acetylated derivative are the principal A2B5-reactive gangliosides in cultured O2A lineage cells and are down-regulated along with O-acetyl GD3 during differentiation to oligodendrocytes. J. Neurosci. Res. 1999, 57, 371–380. [Google Scholar] [CrossRef]
- Waki, H.; Masuzawa, A.; Kon, K.; Ando, S. A new O-acetylated trisialoganglioside, 9-o-acetyl GT2, in cod brain. J. Biochem. 1993, 114, 459–462. [Google Scholar] [CrossRef]
- Ravindranaths, M.H.; Paulson, J.C.; Irie, R.F. Human melanoma antigen O-acetylated ganglioside GD3 is recognized by Cancer antennarius lectin. J. Biol. Chem. 1988, 263, 2079–2086. [Google Scholar]
- Fahr, C.; Schauer, R. Detection of sialic acids and gangliosides with special reference to 9-O-acetylated species in basaliomas and normal human skin. J. Investig. Dermtol. 2001, 116, 254–260. [Google Scholar] [CrossRef]
- Mukherjee, K.; Chava, A.K.; Mandal, C.; Dey, S.N.; Kniep, B.; Chandra, S.; Mandal, C. O-acetylation of GD3 prevents its apoptotic effect and promotes survival of lymphoblasts in childhood acute lymphoblastic leukaemia. J. Cell. Biochem. 2008, 105, 724–734. [Google Scholar] [CrossRef]
- Birks, S.M.; Danquah, J.O.; King, L.; Vlasak, R.; Gorecki, D.C.; Pilkington, G.J. Targeting the GD3 acetylation pathway selectively induces apoptosis in glioblastoma. Neuro Oncol. 2011, 13, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Marquina, G.; Waki, H.; Fernandez, L.E.; Kon, K.; Carr, A.; Valiente, O.; Perez, R.; Ando, S. Gangliosides expressed in human breast cancer. Cancer Res. 1996, 56, 5165–5171. [Google Scholar]
- Mlinac, K.; Fabris, D.; Vukelić, Z.; Rožman, M.; Heffer, M.; Bognar, S.K. Structural analysis of brain ganglioside acetylation patterns in mice with altered ganglioside biosynthesis. Carbohydr. Res. 2013, 382, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Corfield, A.P.; Myerscough, N.; Warren, B.F.; Durdey, P.; Paraskeva, C.; Schauer, R. Reduction of sialic acid O-acetylation in human colonic mucins in the adenoma-carcinoma sequence. Glycoconj. J. 1999, 16, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Kohla, G.; Lrhorfi, A.L.; Sipos, B.; Kalthoff, H.; Gerwig, G.J.; Kamerling, J.P.; Schauer, R.; Tiralongo, J. O-acetylation and de-o-acetylation of sialic acids in human colorectal carcinoma. Eur. J. Biochem. 2004, 271, 281–290. [Google Scholar] [CrossRef]
- Arming, S.; Wipfler, D.; Mayr, J.; Merling, A.; Vilas, U.; Schauer, R.; Schwartz-Albiez, R.; Vlasak, R. The human Cas1 protein: A sialic acid-specific O-acetyltransferase? Glycobiology 2011, 21, 553–564. [Google Scholar] [CrossRef]
- Cazet, A.; Groux-Degroote, S.; Teylaert, B.; Kwon, K.-M.; Lehoux, S.; Slomianny, C.; Kim, C.-H.; Le Bourhis, X.; Delannoy, P. GD3 synthase overexpression enhances proliferation and migration of MDA-MB-231 breast cancer cells. Biol. Chem. 2009, 390, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Guérardel, Y.; Chang, L.-Y.; Fujita, A.; Coddeville, B.; Maes, E.; Sato, C.; Harduin-Lepers, A.; Kubokawa, K.; Kitajima, K. Sialome analysis of the cephalochordate Branchiostoma belcheri, a key organism for vertebrate evolution. Glycobiology 2012, 22, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Diaz, S.; Ferreira, I.; Lamblin, G.; Roussel, P.; Manzi, A.E. New sialic acids from biological sources identified by a comprehensive and sensitive approach: Liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS) of SIA quinoxalinones. Glycobiology 1997, 7, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Sommer, U.; Herscovitz, H.; Welty, F.K.; Costello, C.E. LC-MS-based method for the qualitative and quantitative analysis of complex lipid mixtures. J. Lipid Res. 2006, 47, 804–814. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]


 Ceramide;
Ceramide;  Galactose;
Galactose;  Glucose;
Glucose;  N-acetyl-galactosamine;
N-acetyl-galactosamine;  N-acetyl-neuraminic acid.
N-acetyl-neuraminic acid.
 Ceramide;
Ceramide;  Galactose;
Galactose;  Glucose;
Glucose;  N-acetyl-galactosamine;
N-acetyl-galactosamine;  N-acetyl-neuraminic acid.
N-acetyl-neuraminic acid.
 Ceramide;
Ceramide;  Galactose;
Galactose;  Glucose;
Glucose;  N-acetyl-galactosamine;
N-acetyl-galactosamine;  N-acetyl-neuraminic acid, ° dehydration Ac: O-acetyl group.
N-acetyl-neuraminic acid, ° dehydration Ac: O-acetyl group.
 Ceramide;
Ceramide;  Galactose;
Galactose;  Glucose;
Glucose;  N-acetyl-galactosamine;
N-acetyl-galactosamine;  N-acetyl-neuraminic acid, ° dehydration Ac: O-acetyl group.
N-acetyl-neuraminic acid, ° dehydration Ac: O-acetyl group.
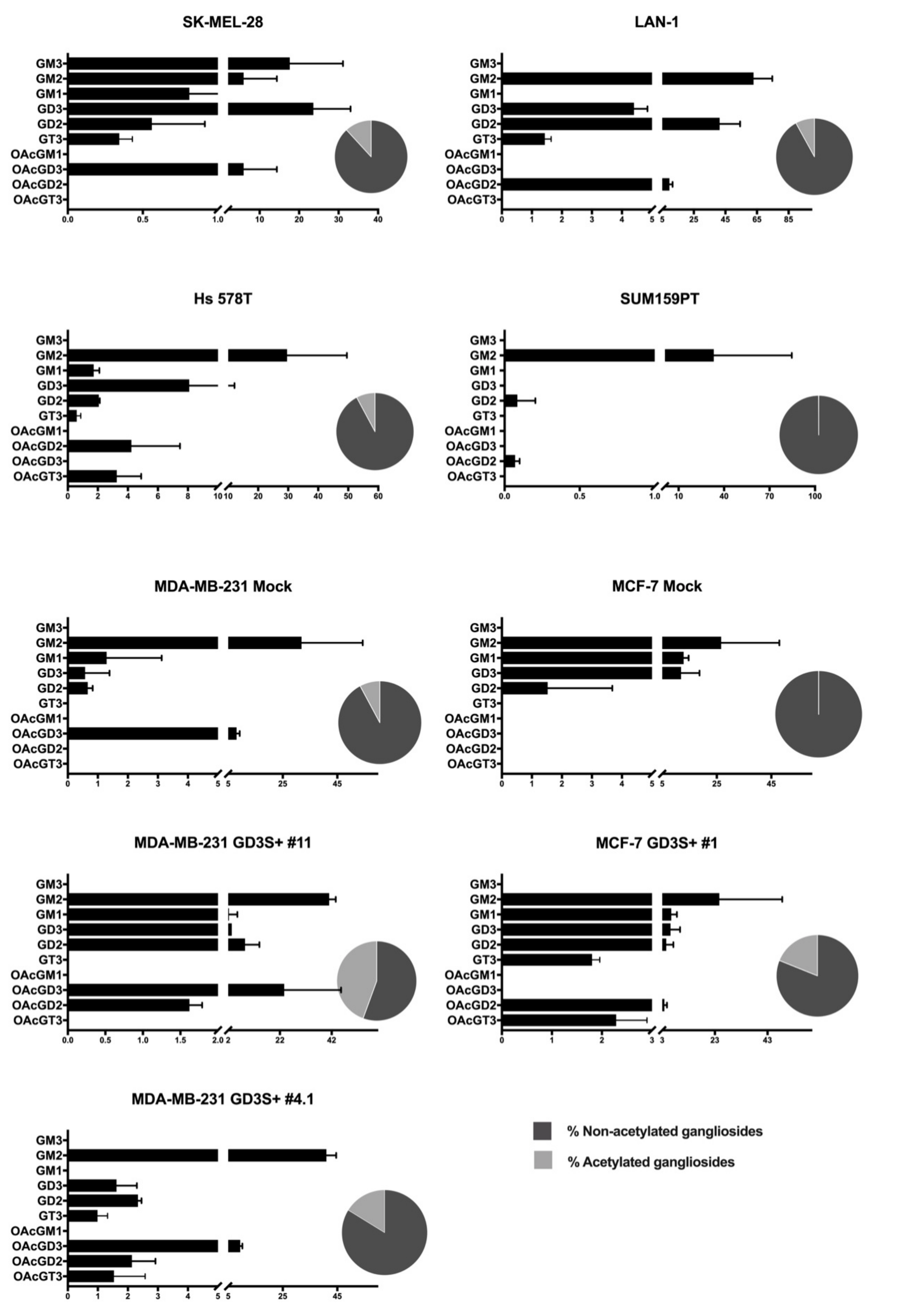

| GSL | Fatty Acids | Monoisotopic Mass [M-H]-m/z | Lactonization | SK-Mel-28 | LAN-1 | SUM 159 PT | Hs 578T | MDA-MB-231 | MDA-MB-231 GD3S+ Clone #4 | MDA-MB-231 GD3S+ Clone #11 | MCF-7 | MCF-7 GD3S+ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GM3 | C16 | 1151.7 | √ | √ | √ | √ | √ | |||||
| C18 | 1179.7 | √ | ||||||||||
| C22 | 1235.8 | √ | √ | √ | ||||||||
| C24 | 1263.8 | √ | √ | √ | √ | √ | ||||||
| GM2 | C16 | 1354.8 | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| C18 | 1382.8 | √ | ||||||||||
| C22 | 1438.9 | √ | √ | √ | √ | √ | √ | √ | √ | |||
| C24 | 1466.9 | √ | √ | √ | √ | √ | √ | |||||
| GM1 | C16 | 1516.8 | √ | √ | √ | √ | √ | √ | √ | |||
| C24 | 1629.0 | √ | √ | √ | √ | √ | √ | √ | √ | |||
| LacNAcGM1 | C16 | 1882.0 | √ | |||||||||
| GD3 | C16 | 1424.8 | -H2O | √ | √ | |||||||
| C16 | 1442.8 | √ | ||||||||||
| C18 | 1452.8 | -H2O | √ | |||||||||
| C22 | 1508.9 | -H2O | √ | √ | ||||||||
| C24 | 1536.9 | -H2O | √ | √ | √ | |||||||
| C24 | 1554.9 | √ | √ | √ | ||||||||
| GD2 | C16 | 1628.9 | -H2O | √ | √ | √ | √T | √ | √ | √T | ||
| C18 | 1671.9 | √ | √ | √ | √ | |||||||
| C24 | 1740.0 | -H2O | √ | √ | √ | √ | ||||||
| GD1b | C24 | 1902.1 | -H2O | √ | √ | |||||||
| GT3 | C24 | 1810.0 | -2H2O | √ | √ | |||||||
| GT2 | C24 | 2013.1 | -2H2O | √ | √ | |||||||
| OAcGM1 | C24 | 1671 | √ | √ | ||||||||
| OAcGD3 | C14 | 1438.8 | -H2O | √ | √T | √ | √ | |||||
| C16 | 1466.8 | -H2O | √ | √ | √T | |||||||
| C24 | 1578.9 | -H2O | √ | √ | ||||||||
| OAcGD2 | C16 | 1670 | -2H2O | √ | √ | √ | √ | √ | ||||
| C24 | 1782 | -H2O | √ | √ | ||||||||
| OAcGT3 | C16 | 1740.0 | -2H2O | √T | √ | √ | ||||||
| C24 | 1852.0 | -2H2O | √ | √ | ||||||||
| OAcGT2 | C24 | 2055.1 | √ | √ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavdarli, S.; Yamakawa, N.; Clarisse, C.; Aoki, K.; Brysbaert, G.; Le Doussal, J.-M.; Delannoy, P.; Guérardel, Y.; Groux-Degroote, S. Profiling of O-acetylated Gangliosides Expressed in Neuroectoderm Derived Cells. Int. J. Mol. Sci. 2020, 21, 370. https://doi.org/10.3390/ijms21010370
Cavdarli S, Yamakawa N, Clarisse C, Aoki K, Brysbaert G, Le Doussal J-M, Delannoy P, Guérardel Y, Groux-Degroote S. Profiling of O-acetylated Gangliosides Expressed in Neuroectoderm Derived Cells. International Journal of Molecular Sciences. 2020; 21(1):370. https://doi.org/10.3390/ijms21010370
Chicago/Turabian StyleCavdarli, Sumeyye, Nao Yamakawa, Charlotte Clarisse, Kazuhiro Aoki, Guillaume Brysbaert, Jean-Marc Le Doussal, Philippe Delannoy, Yann Guérardel, and Sophie Groux-Degroote. 2020. "Profiling of O-acetylated Gangliosides Expressed in Neuroectoderm Derived Cells" International Journal of Molecular Sciences 21, no. 1: 370. https://doi.org/10.3390/ijms21010370
APA StyleCavdarli, S., Yamakawa, N., Clarisse, C., Aoki, K., Brysbaert, G., Le Doussal, J.-M., Delannoy, P., Guérardel, Y., & Groux-Degroote, S. (2020). Profiling of O-acetylated Gangliosides Expressed in Neuroectoderm Derived Cells. International Journal of Molecular Sciences, 21(1), 370. https://doi.org/10.3390/ijms21010370






