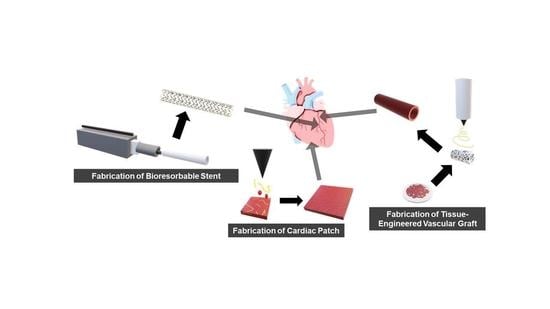
Bioresorbable Polymeric Scaffold in Cardiovascular Applications
Abstract
:1. Introduction
Use of Biomaterials in the Treatment of CHD
2. Bioresorbable Stents
2.1. Development of Coronary Stents
2.2. Polymers in BRS Application
2.3. Degradation Profile of BRS
2.4. Processing Methods
2.4.1. Extrusion
2.4.2. Dip Coating
2.4.3. Spinning and Braiding
2.4.4. 3D Printing
2.5. Current BRS
2.5.1. BRS Clinical Experience
2.5.2. Looking Forward: Polymeric BRS
3. Vascular Grafts
3.1. Polymers for Bioresorbable Vascular Graft Scaffold Application
3.2. Strategies and Approaches for a Bioresorbable Vascular Graft Scaffold
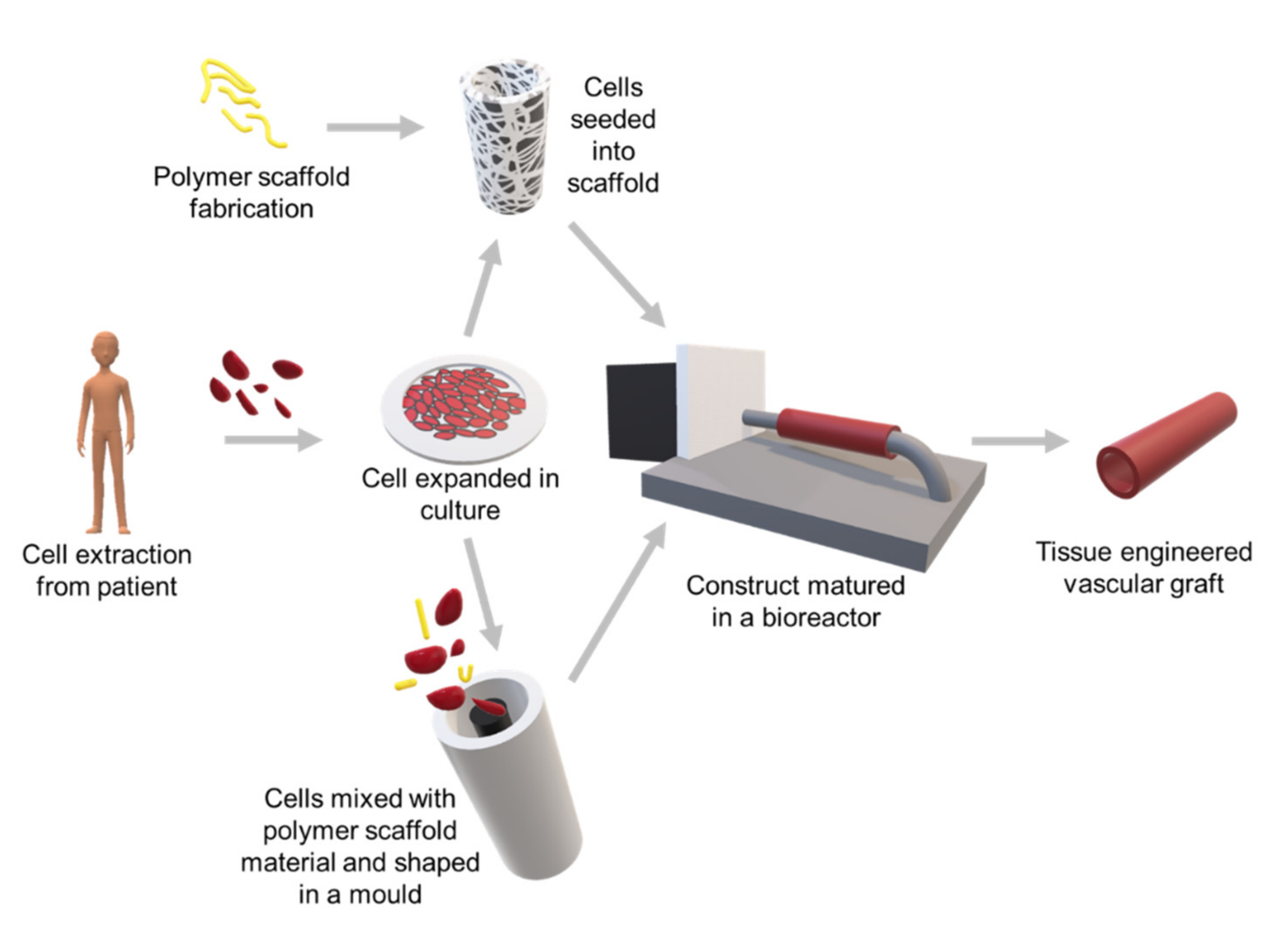
3.3. Fabrication of Bioresorbable Vascular Graft Scaffolds
3.3.1. Electrospinning
3.3.2. Gas Foaming
3.3.3. Solvent Casting/Particulate Leaching
3.3.4. Emulsion Freeze Drying
3.3.5. Thermal-Induced Phase Separation (TIPS)
3.4. Preclinical Studies of Bioresorbable Vascular Graft
3.5. Challenges Ahead: Bioresorbable Vascular Graft
4. Cardiac Patches
4.1. Commercially Available Cardiac Patches
4.2. Material Choice for Cardiac Patches
4.2.1. Natural Biopolymers
4.2.2. Synthetic Materials
4.3. Material Fabrication—3D Bioprinting
4.4. Future Challenges—The Ideal Cardiac Patch (From a Materials Perspective)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMS | Bare Metal Stent |
| BRS | Bioresorbable Scaffolds |
| BTI | Bioabsorbable Therapeutics Inc |
| CABG | Coronary Artery Bypass Grafting |
| CAD | Computer-Aided Design |
| CHD | Coronary Heart Disease |
| CPCs | Cardiac Progenitor Cells |
| CT | Computed Tomography |
| CVD | Cardiovascular Disease |
| DES | Drug Eluting Stent |
| ECs | Endothelial Cell |
| ECFCs | Endothelial Colony Forming Cells |
| ECM | Extracellular Matrix |
| FDM | Fuse Deposition Modelling |
| FFF | Fusion Filament Fabrication |
| GAG | Glycosaminoglycans |
| GelMA | Gelatin Methacrylate |
| hMSCs | Human Mesenchymal Stem Cells |
| ISR | In-Stent Restenosis |
| LDLP | Low Density Lipoproteins |
| LL | Lumen Loss |
| LLL | Late Lumen Loss |
| LVEF | Left Ventricular Ejection Fraction |
| MI | Myocardial Infarction |
| MSCs | Mesenchymal Stem Cells |
| PC | Polycarbonate |
| PCL | Polycaprolactone |
| PDLGA | Poly [(D, L-lactic-co-Glycolic Acid)] |
| PEG | Polyethylene Glycol |
| PEUU | Poly(ester Urethane) Urea |
| PGA | Polyglycolic Acid |
| PGS | Poly (glycerol sebacate) |
| PLA | Polylactic Acid |
| PLLA | Poly L-lactic Acid |
| POBA | Plain Old Balloon Angioplasty |
| PPC | Propylene Carbonate |
| PTFE | Polytetrafluoroethylene |
| ScT | Scaffold Thrombosis |
| ST | Stent Thrombosis |
| TEVG | Tissue Engineered Vascular Grafts |
| TIPS | Thermal-Induced Phase Separation |
| TLF | Target Lesion Failure |
| TPU | Thermoplastic Polyurethane |
| UTS | Ultimate Tensile Strength |
References
- Sanchis-Gomar, F.; Perez-Quilis, C.; Leischik, R.; Lucia, A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann. Transl. Med. 2016, 4, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansilal, S.; Castellano, J.M.; Fuster, V. Global burden of CVD: Focus on secondary prevention of cardiovascular disease. Int. J. Cardiol. 2015, 201, S1–S7. [Google Scholar] [CrossRef] [Green Version]
- WHO. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 9 March 2020).
- Karabulut, A.; Cakmak, M. Treatment strategies in the left main coronary artery disease associated with acute coronary syndromes. J. Saudi Heart Assoc. 2015, 27, 272–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramadan, R.; Boden, W.E.; Kinlay, S. Management of Left Main Coronary Artery Disease. J. Am. Heart Assoc. 2018, 7, e008151. [Google Scholar] [CrossRef]
- Knight, D.K.; Gillies, E.R.; Mequanint, K. Vascular Grafting Strategies in Coronary Intervention. Front. Mater. 2014, 1. [Google Scholar] [CrossRef] [Green Version]
- Streeter, B.W.; Davis, M.E. Therapeutic Cardiac Patches for Repairing the Myocardium. In Cell Biology and Translational Medicine, Volume 5: Stem Cells: Translational Science to Therapy; Turksen, K., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–24. [Google Scholar]
- Williams, D.F. Challenges With the Development of Biomaterials for Sustainable Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 127. [Google Scholar] [CrossRef] [Green Version]
- Bose, S.; Bandyopadhyay, A. Chapter 1—Introduction to Biomaterials. In Characterization of Biomaterials; Bandyopadhyay, A., Bose, S., Eds.; Academic Press: Oxford, UK, 2013; pp. 1–9. [Google Scholar]
- Tibbitt, M.W.; Rodell, C.B.; Burdick, J.A.; Anseth, K.S. Progress in material design for biomedical applications. Proc. Natl. Acad. Sci. USA 2015, 112, 14444. [Google Scholar] [CrossRef] [Green Version]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [Green Version]
- Smit, N.W.; ten Sande, J.N.; Parvizi, M.; van Amersfoorth, S.C.M.; Plantinga, J.A.; van Spreuwel-Goossens, C.A.F.M.; van Dongen, E.M.W.M.; van Dessel, P.F.H.M.; Kluijtmans, S.G.J.M.; Meijborg, V.M.F.; et al. Recombinant human collagen-based microspheres mitigate cardiac conduction slowing induced by adipose tissue-derived stromal cells. PLoS ONE 2017, 12, e0183481. [Google Scholar] [CrossRef] [Green Version]
- Piskin, E. Biodegradable polymers as biomaterials. J. Biomater. Sci. Polym. Ed. 1995, 6, 775–795. [Google Scholar] [CrossRef]
- Tomberli, B.; Mattesini, A.; Baldereschi, G.I.; Di Mario, C. Breve historia de los stents coronarios. Rev. Española Cardiol. 2018, 71, 312–319. [Google Scholar] [CrossRef]
- Cassar, A.; Holmes, D.R., Jr.; Rihal, C.S.; Gersh, B.J. Chronic coronary artery disease: Diagnosis and management. Mayo Clin. Proc. 2009, 84, 1130–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, J.; Gunn, J.; Serruys, P.W. Coronary stents: Historical development, current status and future directions. Br. Med Bull. 2013, 106, 193–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, S.; Serruys, P.W. Coronary stents: Current status. J. Am. Coll. Cardiol. 2010, 56, S1–S42. [Google Scholar] [CrossRef] [Green Version]
- Serruys, P.W.; De Jaegere, P.; Kiemeneij, F.; Macaya, C.; Rutsch, W.; Heyndrickx, G.; Emanuelsson, H.; Marco, J.; Legrand, V.; Materne, P. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. N. Engl. J. Med. 1994, 331, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Fischman, D.L.; Leon, M.B.; Baim, D.S.; Schatz, R.A.; Savage, M.P.; Penn, I.; Detre, K.; Veltri, L.; Ricci, D.; Nobuyoshi, M. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. N. Engl. J. Med. 1994, 331, 496–501. [Google Scholar] [CrossRef]
- Hoffmann, R.; Mintz, G.S.; Dussaillant, G.R.; Popma, J.J.; Pichard, A.D.; Satler, L.F.; Kent, K.M.; Griffin, J.; Leon, M.B. Patterns and mechanisms of in-stent restenosis: A serial intravascular ultrasound study. Circulation 1996, 94, 1247–1254. [Google Scholar] [CrossRef]
- Chitkara, K.; Gershlick, A. Second versus first-generation drug-eluting stents. J. Interv. Cardiol. 2010, 5, 23–26. [Google Scholar] [CrossRef]
- Tada, T.; Byrne, R.A.; Simunovic, I.; King, L.A.; Cassese, S.; Joner, M.; Fusaro, M.; Schneider, S.; Schulz, S.; Ibrahim, T.; et al. Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: Results from a registry of 18,334 patients. JACC Cardiovasc. Interv. 2013, 6, 1267–1274. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, A.; Jinnouchi, H.; Torii, S.; Virmani, R.; Finn, A.V. Understanding the Impact of Stent and Scaffold Material and Strut Design on Coronary Artery Thrombosis from the Basic and Clinical Points of View. Bioengineering 2018, 5, 71. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S. Very very late stent thrombosis: 9.5 years after DES implantation. Indian Heart J. 2016, 68 (Suppl. 2), S39–S43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ang, H.Y.; Huang, Y.Y.; Lim, S.T.; Wong, P.; Joner, M.; Foin, N. Mechanical behavior of polymer-based vs. metallic-based bioresorbable stents. J. Thorac. Dis. 2017, 9, S923–S934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ormiston, J.A.; Serruys, P.W. Bioabsorbable coronary stents. Circ. Cardiovasc. Interv. 2009, 2, 255–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berglund, J.; Guo, Y.; Wilcox, J.N. Challenges related to development of bioabsorbable vascular stents. EuroIntervention 2009, 5 (Suppl. F), F72–F79. [Google Scholar] [CrossRef]
- Neamtu, I.; Chiriac, A.; Ghilan, A.; Nita, L.; Balan, V.; Nistor, M. Current Concepts on Cardiovascular Stent Devices. Mini Rev. Med. Chem. 2014, 14. [Google Scholar] [CrossRef]
- Akinapelli, A.; Chen, J.P.; Roy, K.; Donnelly, J.; Dawkins, K.; Huibregtse, B.; Hou, D. Current State of Bioabsorbable Polymer-Coated Drug-Eluting Stents. Curr. Cardiol. Rev. 2017, 13, 139–154. [Google Scholar] [CrossRef] [Green Version]
- Soares, J.; Moore, J. Biomechanical Challenges to Polymeric Biodegradable Stents. Ann. Biomed. Eng. 2015, 44. [Google Scholar] [CrossRef]
- Leibundgut, G. A Novel, Radiopaque, Bioresorbable Tyrosine-Derived Polymer for Cardiovascular Scaffolds, Cardiac Interventions Today, vol., no 2018, p. EUROPEAN FEATURED TECHNOLOGY. 2018. Available online: https://citoday.com/articles/2018-july-aug/a-novel-radiopaque-bioresorbable-tyrosine-derived-polymer-for-cardiovascular-scaffolds?c4src=archive:feed. (accessed on 29 February 2020).
- Ang, H.Y.; Bulluck, H.; Wong, P.; Venkatraman, S.S.; Huang, Y.; Foin, N. Bioresorbable stents: Current and upcoming bioresorbable technologies. Int. J. Cardiol. 2017, 228, 931–939. [Google Scholar] [CrossRef]
- Onuma, Y.; Ormiston, J.; Serruys, P.W. Bioresorbable scaffold technologies. Circ. J. 2011, 75, 509–520. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Garcia, H.M.; Serruys, P.W.; Campos, C.M.; Muramatsu, T.; Nakatani, S.; Zhang, Y.J.; Onuma, Y.; Stone, G.W. Assessing bioresorbable coronary devices: Methods and parameters. JACC. Cardiovasc. Imaging 2014, 7, 1130–1148. [Google Scholar] [CrossRef] [Green Version]
- Onuma, Y.; Serruys, P.W. Bioresorbable scaffold: The advent of a new era in percutaneous coronary and peripheral revascularization? Circulation 2011, 123, 779–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajjar, C.R.; King, M.W. Resorbable Fiber-Forming Polymers for Biotextile Applications; Springer: Cham, Switzerland, 2014. [Google Scholar]
- Kang, E.Y.; Lih, E.; Kim, I.H.; Joung, Y.K.; Han, D.K. Effects of poly(L-lactide-ε-caprolactone) and magnesium hydroxide additives on physico-mechanical properties and degradation of poly(L-lactic acid). Biomater. Res. 2016, 20, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra, A.J.; Ciurana, J. Stent’s Manufacturing Field: Past, Present, and Future Prospects. In Angiography; IntechOpen: London, UK, 2018. [Google Scholar]
- Bink, N.; Mohan, V.B.; Fakirov, S. Recent advances in plastic stents: A comprehensive review. Int. J. Polym. Mater. Polym. Biomater. 2019, 1–22. [Google Scholar] [CrossRef]
- Chaturvedi, E.; Rajput, N.; Upadhyaya, S.; Pandey, P. Experimental Study and Mathematical Modeling for Extrusion using High Density Polyethylene. Mater. Today: Proc. 2017, 4, 1670–1676. [Google Scholar] [CrossRef]
- Rawal, A.; Mukhopadhyay, S. 4 - Melt spinning of synthetic polymeric filaments. In Advances in Filament Yarn Spinning of Textiles and Polymers; Zhang, D., Ed.; Woodhead Publishing: Oxford, UK; Witney: Oxford, UK, 2014; pp. 75–99. [Google Scholar]
- Ramzipoor, K.; Alfred, N.; Wang, L.; Lee, C.Y. Stent Fabrication via Tubular Casting Processes. Google Patents US 2017/0157806 A1, 8 June 2017. [Google Scholar]
- Esposito, G. From fortitude 150 to aptitude 115: Clinical update. Presented at European Association for Percutaneous Cardiovascular Interventions, Paris, France, 17 May 2017. [Google Scholar]
- Regazzoli, D.; Leone, P.P.; Colombo, A.; Latib, A. New generation bioresorbable scaffold technologies: An update on novel devices and clinical results. J. Thorac. Dis. 2017, 9, S979–S985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMahon, S.; Bertollo, N.; Cearbhaill, E.D.O.; Salber, J.; Pierucci, L.; Duffy, P.; Dürig, T.; Bi, V.; Wang, W. Bio-resorbable polymer stents: A review of material progress and prospects. Prog. Polym. Sci. 2018, 83, 79–96. [Google Scholar] [CrossRef]
- Onuma, Y.; Serruys, P.W. Bioresorbable Scaffolds: From Basic Concept to Clinical Applications; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Martinez, A.W.; Chaikof, E.L. Microfabrication and nanotechnology in stent design. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2011, 3, 256–268. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-H.; Lee, S.-Y.; Horng, S.; Guy, L.-G.; Yu, T.-B. In vitro and in vivo degradation of microfiber bioresorbable coronary scaffold. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2018, 106, 1842–1850. [Google Scholar] [CrossRef] [Green Version]
- Zilberman, M.; Schwade, N.D.; Eberhart, R.C. Protein-loaded bioresorbable fibers and expandable stents: Mechanical properties and protein release. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2004, 69, 1–10. [Google Scholar] [CrossRef]
- Cabrera, M.S.; Sanders, B.; Goor, O.J.; Driessen-Mol, A.; Oomens, C.W.; Baaijens, F.P. Computationally designed 3D printed self-expandable polymer stents with biodegradation capacity for minimally invasive heart valve implantation: A proof-of-concept study. 3D Print. Addit. Manuf. 2017, 4, 19–29. [Google Scholar] [CrossRef]
- Ware, H.O.T.; Farsheed, A.C.; Akar, B.; Duan, C.; Chen, X.; Ameer, G.; Sun, C. High-speed on-demand 3D printed bioresorbable vascular scaffolds. Mater. Today Chem. 2018, 7, 25–34. [Google Scholar] [CrossRef]
- Guerra, A.J.; Cano, P.; Rabionet, M.; Puig, T.; Ciurana, J. 3D-printed PCL/PLA composite stents: Towards a new solution to cardiovascular problems. Materials 2018, 11, 1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, J.; Onuma, Y.; Ormiston, J.; Abizaid, A.; Waksman, R.; Serruys, P. Bioresorbable scaffolds: Rationale, current status, challenges, and future. Eur. Heart J. 2014, 35, 765–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Yang, K.; Cheng, R.; Xiang, Y.; Yuan, T.; Cheng, Y.; Sarmento, B.; Cui, W. The current status of biodegradable stent to treat benign luminal disease. Mater. Today 2017, 20, 516–529. [Google Scholar] [CrossRef]
- Kereiakes, D.J.; Ellis, S.G.; Metzger, C.; Caputo, R.P.; Rizik, D.G.; Teirstein, P.S.; Litt, M.R.; Kini, A.; Kabour, A.; Marx, S.O.; et al. 3-Year Clinical Outcomes With Everolimus-Eluting Bioresorbable Coronary Scaffolds. J. Am. Coll. Cardiol. 2017, 70, 2852. [Google Scholar] [CrossRef]
- The Next Step in Bioresorbable Stent Technologies. Available online: https://www.dicardiology.com/article/next-step-bioresorbable-stent-technologies (accessed on 9 December 2019).
- Seth, A.; Onuma, Y.; Chandra, P.; Bahl, V.K.; Manjunath, C.N.; Mahajan, A.U.; Kumar, V.; Goel, P.K.; Wander, G.S.; Kaul, U.; et al. Three-year clinical and two-year multimodality imaging outcomes of a thin-strut sirolimus-eluting bioresorbable vascular scaffold: MeRes-1 trial. EuroIntervention 2019, 15, 607–614. [Google Scholar] [CrossRef]
- Granada, J.F. Fortitude, Aptitude, and Magnitude: Progressively Thin-Strut BRS Based on Ultra-High MW Amorphous PLLA. Presented at Transcatheter Cardiovascular Therapeutics 2017, Denver, CO, USA, 31 October 2017. [Google Scholar]
- Alaide, C.; Saud Ahmed, K.; Azeem, L.; Boris, V.; Miguel, M.; Juan, A.D.; Jaime, F.; Luca, T.; Giovanni, E.; Marco, F.; et al. RENASCENT II: First in Human Evaluation of a Novel Sirolimus-Eluting Ultra-High Molecular Weight APTITUDE® Bioresorbable Scaffold: 9-and 24-Months Imaging and Clinical Results. EuroIntervention 2020. [Google Scholar] [CrossRef]
- Antonio, C. Renascent III: Nine Month Clinical and Imaging Outcomes with a Thin Strut 98 Micrometer Bioresorbable Coronary Scaffold. Presentated at Transcatheter Cardiovascular Therapeutics 2018, San Diego, CA, USA, 23 September 2018. [Google Scholar]
- Han, Y.; Xu, B.; Fu, G.; Wang, X.; Xu, K.; Jin, C.; Tao, L.; Li, L.; Hou, Y.; Su, X.; et al. A Randomized Trial Comparing the NeoVas Sirolimus-Eluting Bioresorbable Scaffold and Metallic Everolimus-Eluting Stents. JACC: Cardiovasc. Interv. 2018, 11, 260–272. [Google Scholar] [CrossRef]
- Trafton, A. Study Reveals Why Polymer Stents Failed. Available online: http://news.mit.edu/2018/study-reveals-why-polymer-stents-failed-0226 (accessed on 25 March 2020).
- Adriaenssens, T. Very Late Stent Thrombosis. In Cardiovascular OCT Imaging; Jang, I.-K., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 125–137. [Google Scholar]
- Chen, D.; Su, Z.; Weng, L.; Cao, L.; Chen, C.; Zeng, S.; Zhang, S.; Wu, T.; Hu, Q.; Xiao, J. Effect of inflammation on endothelial cells induced by poly-L-lactic acid degradation in vitro and in vivo. J. Biomater. Sci. Polym. Ed. 2018, 29, 1909–1919. [Google Scholar] [CrossRef]
- Adriaenssens, T. Exploring evidence and experience with magnesium scaffold Magmaris. Presented at European Association for Percutaneous Cardiovascular Interventions, Paris, France, 23 May 2019. [Google Scholar]
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The Tissue-Engineered Vascular Graft-Past, Present, and Future. Tissue Eng. Part B Rev. 2016, 22, 68–100. [Google Scholar] [CrossRef]
- Antoniou, G.A.; Chalmers, N.; Georgiadis, G.S.; Lazarides, M.K.; Antoniou, S.A.; Serracino-Inglott, F.; Smyth, J.V.; Murray, D. A meta-analysis of endovascular versus surgical reconstruction of femoropopliteal arterial disease. J. Vasc. Surg. 2013, 57, 242–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohr, F.W.; Morice, M.C.; Kappetein, A.P.; Feldman, T.E.; Stahle, E.; Colombo, A.; Mack, M.J.; Holmes, D.R., Jr.; Morel, M.A.; Van Dyck, N.; et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013, 381, 629–638. [Google Scholar] [CrossRef]
- Kindi, H.A.; Samaan, A.; Hosny, H. NOBLE and EXCEL: The debate for excellence in dealing with left main stenosis. Glob. Cardiol. Sci. Pract. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Harskamp, R.E.; Lopes, R.D.; Baisden, C.E.; de Winter, R.J.; Alexander, J.H. Saphenous vein graft failure after coronary artery bypass surgery: Pathophysiology, management, and future directions. Ann. Surg. 2013, 257, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Ballotta, E.; Renon, L.; Toffano, M.; Da Giau, G. Prospective randomized study on bilateral above-knee femoropopliteal revascularization: Polytetrafluoroethylene graft versus reversed saphenous vein. J. Vasc. Surg. 2003, 38, 1051–1055. [Google Scholar] [CrossRef] [Green Version]
- Brewster, D.C.; Hospital, F.M.G.; School, H.M. Current controversies in the management of aortoiliac occlusive disease. J. Vasc. Surg. 1997, 25, 365–379. [Google Scholar] [CrossRef] [Green Version]
- Hadinata, I.E.; Hayward, P.A.; Hare, D.L.; Matalanis, G.S.; Seevanayagam, S.; Rosalion, A.; Buxton, B.F. Choice of conduit for the right coronary system: 8-year analysis of Radial Artery Patency and Clinical Outcomes trial. Ann. Thorac. Surg. 2009, 88, 1404–1409. [Google Scholar] [CrossRef] [PubMed]
- Hehrlein, F.W.; Schlepper, M.; Loskot, F.; Scheld, H.H.; Walter, P.; Mulch, J. The use of expanded polytetrafluoroethylene (PTFE) grafts for myocardial revascularization. J. Cardiovasc. Surg. 1984, 25, 549–553. [Google Scholar]
- Greenwald, S.; Berry, C. Improving vascular grafts: The importance of mechanical and haemodynamic properties. J. Pathol. 2000, 190, 292–299. [Google Scholar] [CrossRef]
- Davies, M.G.; Hagen, P.-O. Pathophysiology of vein graft failure: A review. Eur. J. Vasc. Endovasc. Surg. 1995, 9, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Deutsch, M.; Meinhart, J.; Zilla, P.; Howanietz, N.; Gorlitzer, M.; Froeschl, A.; Stuempflen, A.; Bezuidenhout, D.; Grabenwoeger, M. Long-term experience in autologous in vitro endothelialization of infrainguinal ePTFE grafts. J. Vasc. Surg. 2009, 49, 352–362, discussion 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chlupac, J.; Filova, E.; Bacakova, L. Blood vessel replacement: 50 years of development and tissue engineering paradigms in vascular surgery. Physiol. Res. 2009, 58 (Suppl. 2), S119–S139. [Google Scholar] [PubMed]
- Stowell, C.E.T.; Wang, Y. Quickening: Translational design of resorbable synthetic vascular grafts. Biomaterials 2018, 173, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.; Azevedo, H.; Malafaya, P.; Silva, S.; Oliveira, J.; Silva, G.; João Mano, R.S.; Reis, R. 16—Natural Polymers in Tissue Engineering Applications. In Handbook of Biopolymers and Biodegradable Plastics; Ebnesajjad, S., Ed.; William Andrew Publishing: Boston, MA, USA, 2013; pp. 385–425. [Google Scholar]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Loh, X.J. Polyhydroxyalkanoates: Opening doors for a sustainable future. NPG Asia Mater. 2016, 8, e265. [Google Scholar] [CrossRef]
- Kakisis, J.D.; Liapis, C.D.; Breuer, C.; Sumpio, B.E. Artificial blood vessel: The Holy Grail of peripheral vascular surgery. J. Vasc. Surg. 2005, 41, 349–354. [Google Scholar] [CrossRef] [Green Version]
- Nicolas, F.L.; Gagnieu, C.H. Denatured thiolated collagen. II. Cross-linking by oxidation. Biomaterials 1997, 18, 815–821. [Google Scholar] [CrossRef]
- Habermehl, J.; Skopinska, J.; Boccafoschi, F.; Sionkowska, A.; Kaczmarek, H.; Laroche, G.; Mantovani, D. Preparation of ready-to-use, stockable and reconstituted collagen. Macromol. Biosci. 2005, 5, 821–828. [Google Scholar] [CrossRef]
- Silver, F.H.; Horvath, I.; Foran, D.J. Viscoelasticity of the vessel wall: The role of collagen and elastic fibers. Crit. Rev. Biomed. Eng. 2001, 29, 279–301. [Google Scholar] [CrossRef]
- Ibrahim, H.; El-Zairy, E. Chitosan as a biomaterial—Structure, properties, and electrospun nanofibers. Concepts Compd. Altern. Antibact. 2015, 81–101. [Google Scholar] [CrossRef] [Green Version]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Conklin, B.S.; Richter, E.R.; Kreutziger, K.L.; Zhong, D.S.; Chen, C. Development and evaluation of a novel decellularized vascular xenograft. Med. Eng. Phys. 2002, 24, 173–183. [Google Scholar] [CrossRef]
- Peck, M.; Gebhart, D.; Dusserre, N.; McAllister, T.N.; L’Heureux, N. The evolution of vascular tissue engineering and current state of the art. Cells Tissues Organs 2012, 195, 144–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seifu, D.G.; Purnama, A.; Mequanint, K.; Mantovani, D. Small-diameter vascular tissue engineering. Nat. Rev. Cardiol. 2013, 10, 410–421. [Google Scholar] [CrossRef]
- Zhu, Y.; Cao, Y.; Pan, J.; Liu, Y. Macro-alignment of electrospun fibers for vascular tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92, 508–516. [Google Scholar] [CrossRef]
- Zhu, Y.; Leong, M.F.; Ong, W.F.; Chan-Park, M.B.; Chian, K.S. Esophageal epithelium regeneration on fibronectin grafted poly(L-lactide-co-caprolactone) (PLLC) nanofiber scaffold. Biomaterials 2007, 28, 861–868. [Google Scholar] [CrossRef]
- Ju, Y.M.; Choi, J.S.; Atala, A.; Yoo, J.J.; Lee, S.J. Bilayered scaffold for engineering cellularized blood vessels. Biomaterials 2010, 31, 4313–4321. [Google Scholar] [CrossRef]
- Fiqrianti, I.A.; Widiyanti, P.; Manaf, M.A.; Savira, C.Y.; Cahyani, N.R.; Bella, F.R. Poly-L-lactic Acid (PLLA)-Chitosan-Collagen Electrospun Tube for Vascular Graft Application. J. Funct. Biomater. 2018, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- Marcolin, C.; Draghi, L.; Tanzi, M.; Fare, S. Electrospun silk fibroin-gelatin composite tubular matrices as scaffolds for small diameter blood vessel regeneration. J. Mater. Sci. Mater. Med. 2017, 28, 80. [Google Scholar] [CrossRef]
- Raeisdasteh Hokmabad, V.; Davaran, S.; Ramazani, A.; Salehi, R. Design and fabrication of porous biodegradable scaffolds: A strategy for tissue engineering. J. Biomater. Sci. Polym. Ed. 2017, 28, 1797–1825. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold techniques and designs in tissue engineering functions and purposes: A review. Adv. Mater. Sci. Eng. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Costantini, M.; Barbetta, A. 6—Gas foaming technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds; Deng, Y., Kuiper, J., Eds.; Woodhead Publishing: Oxford, UK; Witney: Oxford, UK, 2018; pp. 127–149. [Google Scholar]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikos, A.G.; Temenoff, J.S. Formation of highly porous biodegradable scaffolds for tissue engineering. Electron. J. Biotechnol. 2000, 3, 23–24. [Google Scholar] [CrossRef]
- Harris, L.D.; Kim, B.-S.; Mooney, D.J. Open pore biodegradable matrices formed with gas foaming. J. Biomed. Mater. Res. 1998, 42, 396–402. [Google Scholar] [CrossRef]
- Fernández-Colino, A.; Wolf, F.; Rütten, S.; Schmitz-Rode, T.; Rodríguez-Cabello, J.C.; Jockenhoevel, S.; Mela, P. Small Caliber Compliant Vascular Grafts Based on Elastin-Like Recombinamers for in situ Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behr, J.-M.; Irvine, S.A.; Thwin, C.-S.; Shah, A.H.; Bae, M.-C.K.; Zussman, E.; Venkatraman, S. Matching Static and Dynamic Compliance of Small-Diameter Arteries, with Poly(lactide-co-caprolactone) Copolymers: In Vitro and In Vivo Studies. Macromol. Biosci. 2020, 20, 1900234. [Google Scholar] [CrossRef] [PubMed]
- Badhe, R.V.; Bijukumar, D.; Chejara, D.R.; Mabrouk, M.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Kondiah, P.P.D.; Pillay, V. A composite chitosan-gelatin bi-layered, biomimetic macroporous scaffold for blood vessel tissue engineering. Carbohydr. Polym. 2017, 157, 1215–1225. [Google Scholar] [CrossRef]
- Clyne, A.M. Thermal processing of tissue engineering scaffolds. J. Heat Transf. 2011, 133, 034001. [Google Scholar] [CrossRef]
- Schugens, C.; Maquet, V.; Grandfils, C.; Jérôme, R.; Teyssie, P. Biodegradable and macroporous polylactide implants for cell transplantation: 1. Preparation of macroporous polylactide supports by solid-liquid phase separation. Polymer 1996, 37, 1027–1038. [Google Scholar] [CrossRef] [Green Version]
- Norouzi, S.K.; Shamloo, A. Bilayered heparinized vascular graft fabricated by combining electrospinning and freeze drying methods. Mater. Sci. Eng. C 2019, 94, 1067–1076. [Google Scholar] [CrossRef]
- Yuan, L.; Li, X.; Ge, L.; Jia, X.; Lei, J.; Mu, C.; Li, D. Emulsion Template Method for the Fabrication of Gelatin-Based Scaffold with a Controllable Pore Structure. ACS Appl. Mater. Interfaces 2019, 11, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Park, I.K.; Kim, J.M.; Lee, J.H. In vitro and in vivo characteristics of PCL scaffolds with pore size gradient fabricated by a centrifugation method. Biomaterials 2007, 28, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, J.; Zhao, Y.; Wen, L.; Yuan, X.; Fan, Y. Formation of porous PLGA scaffolds by a combining method of thermally induced phase separation and porogen leaching. J. Appl. Polym. Sci. 2008, 109, 1232–1241. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; McNulty, J.; Salick, M.R.; Peng, X.-F.; Turng, L.-S. Approaches to Fabricating Multiple-Layered Vascular Scaffolds Using Hybrid Electrospinning and Thermally Induced Phase Separation Methods. Ind. Eng. Chem. Res. 2016, 55, 882–892. [Google Scholar] [CrossRef]
- Kannan, R.Y.; Salacinski, H.J.; Butler, P.E.; Hamilton, G.; Seifalian, A.M. Current status of prosthetic bypass grafts: A review. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2005, 74, 570–581. [Google Scholar] [CrossRef]
- Sugiura, T.; Tara, S.; Nakayama, H.; Kurobe, H.; Yi, T.; Lee, Y.U.; Lee, A.Y.; Breuer, C.K.; Shinoka, T. Novel Bioresorbable Vascular Graft With Sponge-Type Scaffold as a Small-Diameter Arterial Graft. Ann. Thorac. Surg. 2016, 102, 720–727. [Google Scholar] [CrossRef] [Green Version]
- Centola, M.; Rainer, A.; Spadaccio, C.; De Porcellinis, S.; Genovese, J.A.; Trombetta, M. Combining electrospinning and fused deposition modeling for the fabrication of a hybrid vascular graft. Biofabrication 2010, 2, 014102. [Google Scholar] [CrossRef] [Green Version]
- Alessandrino, A.; Chiarini, A.; Biagiotti, M.; Dal Prà, I.; Bassani, G.A.; Vincoli, V.; Settembrini, P.; Pierimarchi, P.; Freddi, G.; Armato, U. Three-Layered Silk Fibroin Tubular Scaffold for the Repair and Regeneration of Small Caliber Blood Vessels: From Design to in vivo Pilot Tests. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef]
- Zhang, L.; Ao, Q.; Wang, A.; Lu, G.; Kong, L.; Gong, Y.; Zhao, N.; Zhang, X. A sandwich tubular scaffold derived from chitosan for blood vessel tissue engineering. J. Biomed. Mater. Res. Part A 2006, 77, 277–284. [Google Scholar] [CrossRef]
- Zhao, J.; Qiu, H.; Chen, D.-l.; Zhang, W.-x.; Zhang, D.-c.; Li, M. Development of nanofibrous scaffolds for vascular tissue engineering. Int. J. Biol. Macromol. 2013, 56, 106–113. [Google Scholar] [CrossRef]
- Sharifpoor, S.; Simmons, C.A.; Labow, R.S.; Paul Santerre, J. Functional characterization of human coronary artery smooth muscle cells under cyclic mechanical strain in a degradable polyurethane scaffold. Biomaterials 2011, 32, 4816–4829. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Crapo, P.M.; Wang, Y. Macroporous Elastomeric Scaffolds with Extensive Micropores for Soft Tissue Engineering. Tissue Eng. 2006, 12, 917–925. [Google Scholar] [CrossRef]
- Der Lei, B.v.; Wildevuur, C.R.H.; Dijk, F.; Blaauw, E.H.; Molenaar, I.; Nieuwenhuis, P. Sequential studies of arterial wall regeneration in microporous, compliant, biodegradable small-caliber vascular grafts in rats. J. Thorac. Cardiovasc. Surg. 1987, 93, 695–707. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Salick, M.R.; Cordie, T.; Crone, W.C.; Peng, X.-F.; Turng, L.-S. Morphology, mechanical properties, and shape memory effects of poly (lactic acid)/thermoplastic polyurethane blend scaffolds prepared by thermally induced phase separation. J. Cell. Plast. 2014, 50, 361–379. [Google Scholar] [CrossRef]
- Buttafoco, L.; Engbers-Buijtenhuijs, P.; Poot, A.A.; Dijkstra, P.J.; Vermes, I.; Feijen, J. Physical characterization of vascular grafts cultured in a bioreactor. Biomaterials 2006, 27, 2380–2389. [Google Scholar] [CrossRef]
- Engbers-Buijtenhuijs, P.; Buttafoco, L.; Poot, A.A.; Dijkstra, P.J.; de Vos, R.A.; Sterk, L.M.; Geelkerken, R.H.; Vermes, I.; Feijen, J. Biological characterisation of vascular grafts cultured in a bioreactor. Biomaterials 2006, 27, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Lovett, M.; Eng, G.; Kluge, J.A.; Cannizzaro, C.; Vunjak-Novakovic, G.; Kaplan, D.L. Tubular silk scaffolds for small diameter vascular grafts. Organogenesis 2010, 6, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Barreto-Ortiz, S.F.; Fradkin, J.; Eoh, J.; Trivero, J.; Davenport, M.; Ginn, B.; Mao, H.Q.; Gerecht, S. Fabrication of 3-dimensional multicellular microvascular structures. FASEB J. 2015, 29, 3302–3314. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.-C.; Jiang, L.; Huang, A.; Wang, X.-F.; Li, Q.; Turng, L.-S. Electrospun polycaprolactone/gelatin composites with enhanced cell–matrix interactions as blood vessel endothelial layer scaffolds. Mater. Sci. Eng. C 2017, 71, 901–908. [Google Scholar] [CrossRef]
- Hasan, A.; Memic, A.; Annabi, N.; Hossain, M.; Paul, A.; Dokmeci, M.R.; Dehghani, F.; Khademhosseini, A. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014, 10, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Rocco, K.A.; Maxfield, M.W.; Best, C.A.; Dean, E.W.; Breuer, C.K. In vivo applications of electrospun tissue-engineered vascular grafts: A review. Tissue Eng. Part B Rev. 2014, 20, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, G.; Hibino, N.; Ikada, Y.; Kurosawa, H.; Shin’oka, T. Successful application of tissue engineered vascular autografts: Clinical experience. Biomaterials 2003, 24, 2303–2308. [Google Scholar] [CrossRef]
- Patterson, J.T.; Gilliland, T.; Maxfield, M.W.; Church, S.; Naito, Y.; Shinoka, T.; Breuer, C.K. Tissue-engineered vascular grafts for use in the treatment of congenital heart disease: From the bench to the clinic and back again. Regen. Med. 2012, 7, 409–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fioretta, E.S.; Lintas, V.; Mallone, A.; Motta, S.E.; von Boehmer, L.; Dijkman, P.E.; Cesarovic, N.; Caliskan, E.; Rodriguez Cetina Biefer, H.; Lipiski, M.; et al. Differential Leaflet Remodeling of Bone Marrow Cell Pre-Seeded Versus Nonseeded Bioresorbable Transcatheter Pulmonary Valve Replacements. JACC: Basic Transl. Sci. 2020, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Mela, P. Subject- and Leaflet-Specific Remodeling of Polymeric Heart Valves for In Situ Tissue Engineering: Challenges Towards Clinical Translation. JACC: Basic Transl. Sci. 2020, 5, 32–34. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, W.; Radisic, M.; Vunjak-Novakovic, G. Can We Engineer a Human Cardiac Patch for Therapy? Circ. Res. 2018, 123, 244–265. [Google Scholar] [CrossRef]
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue Engineering Approaches in the Design of Healthy and Pathological In Vitro Tissue Models. Front. Bioeng. Biotechnol. 2017, 5. [Google Scholar] [CrossRef] [Green Version]
- Radisic, M.; Marsano, A.; Maidhof, R.; Wang, Y.; Vunjak-Novakovic, G. Cardiac tissue engineering using perfusion bioreactor systems. Nat. Protoc. 2008, 3, 719. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, W.-H.; Melnychenko, I.; Wasmeier, G.; Didié, M.; Naito, H.; Nixdorff, U.; Hess, A.; Budinsky, L.; Brune, K.; Michaelis, B. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat. Med. 2006, 12, 452–458. [Google Scholar] [CrossRef]
- Eschenhagen, T.; Fink, C.; Remmers, U.; Scholz, H.; Wattchow, J.; Weil, J.; Zimmermann, W.; Dohmen, H.H.; Schafer, H.; Bishopric, N.; et al. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: A new heart muscle model system. FASEB J. 1997, 11, 683–694. [Google Scholar] [CrossRef]
- Zimmermann, W.-H.; Eschenhagen, T. Cardiac Tissue Engineering for Replacement Therapy. Heart Fail. Rev. 2003, 8, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, W.-H.; Schneiderbanger, K.; Schubert, P.; Didié, M.; Münzel, F.; Heubach, J.F.; Kostin, S.; Neuhuber, W.L.; Eschenhagen, T. Tissue Engineering of a Differentiated Cardiac Muscle Construct. Circ. Res. 2002, 90, 223–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, L.; Gregorich, Z.R.; Zhu, W.; Mattapally, S.; Oduk, Y.; Lou, X.; Kannappan, R.; Borovjagin, A.V.; Walcott, G.P.; Pollard, A.E.; et al. Large Cardiac Muscle Patches Engineered From Human Induced-Pluripotent Stem Cell-Derived Cardiac Cells Improve Recovery From Myocardial Infarction in Swine. Circulation 2018, 137, 1712–1730. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The extracellular matrix in myocardial injury, repair, and remodeling. J. Clin. Invest. 2017, 127, 1600–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frangogiannis Nikolaos, G. The Extracellular Matrix in Ischemic and Nonischemic Heart Failure. Circ. Res. 2019, 125, 117–146. [Google Scholar] [CrossRef]
- LeGrice, I.; Pope, A.; Smaill, B. The Architecture of the Heart: Myocyte Organization and the Cardiac Extracellular Matrix. In Interstitial Fibrosis in Heart Failure; Villarreal, F.J., Ed.; Springer: New York, NY, USA, 2005; pp. 3–21. [Google Scholar]
- Cui, B.; Zheng, Y.; Sun, L.; Shi, T.; Shi, Z.; Wang, L.; Huang, G.; Sun, N. Heart Regeneration in Adult Mammals after Myocardial Damage. Acta Cardiol. Sin. 2018, 34, 115–123. [Google Scholar] [CrossRef]
- McCready, R.A.; Siderys, H.; Pittman, J.N.; Herod, G.T.; Halbrook, H.G.; Fehrenbacher, J.W.; Beckman, D.J.; Hormuth, D.A. Delayed postoperative bleeding from polytetrafluoroethylene carotid artery patches. J. Vasc. Surg. 1992, 15, 661–663. [Google Scholar] [CrossRef] [Green Version]
- Minale, C.; Nikol, S.; Hollweg, G.; Mittermayer, C.; Messmer, B.J. Clinical experience with expanded polytetrafluoroethylene Gore-Tex surgical membrane for pericardial closure: A study of 110 cases. J. Card. Surg. 1988, 3, 193–201. [Google Scholar] [CrossRef]
- Li, X.; Guo, Y.; Ziegler, K.R.; Model, L.S.; Eghbalieh, S.D.D.; Brenes, R.A.; Kim, S.T.; Shu, C.; Dardik, A. Current Usage and Future Directions for the Bovine Pericardial Patch. Ann. Vasc. Surg. 2011, 25, 561–568. [Google Scholar] [CrossRef] [Green Version]
- Umashankar, P.R.; Mohanan, P.V.; Kumari, T.V. Glutaraldehyde treatment elicits toxic response compared to decellularization in bovine pericardium. Toxicol. Int. 2012, 19, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Zimmermann, W.-H.; Garry, D.J.; Zhang, J. Patching the heart: Cardiac repair from within and outside. Circ. Res. 2013, 113, 922–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benzoni, P.; Ginestra, P.; Altomare, L.; Fiorentino, A.; De Nardo, L.; Ceretti, E.; Dell’Era, P. Biomanufacturing of a Chitosan/Collagen Scaffold to Drive Adhesion and Alignment of Human Cardiomyocyte Derived from Stem Cells. Procedia CIRP 2016, 49, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Denuziere, A.; Ferrier, D.; Domard, A. Interactions between chitosan and glycosaminoglycans (chondroitin sulfate and hyaluronic acid): Physicochemical and biological studies. Ann. Pharm. Fr. 2000, 58, 47–53. [Google Scholar]
- Rienks, M.; Papageorgiou, A.-P.; Frangogiannis, N.G.; Heymans, S. Myocardial Extracellular Matrix. Circ. Res. 2014, 114, 872–888. [Google Scholar] [CrossRef] [Green Version]
- Salick, M.R.; Napiwocki, B.N.; Sha, J.; Knight, G.T.; Chindhy, S.A.; Kamp, T.J.; Ashton, R.S.; Crone, W.C. Micropattern width dependent sarcomere development in human ESC-derived cardiomyocytes. Biomaterials 2014, 35, 4454–4464. [Google Scholar] [CrossRef] [Green Version]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the Development of Chitosan-Based Biomaterials for Tissue Engineering and Regenerative Medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Ghormade, V.; Pathan, E.K.; Deshpande, M.V. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017, 104, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Rashedi, I.; Talele, N.; Wang, X.-H.; Hinz, B.; Radisic, M.; Keating, A. Collagen scaffold enhances the regenerative properties of mesenchymal stromal cells. PLoS ONE 2017, 12, e0187348. [Google Scholar] [CrossRef] [Green Version]
- McMahan, S.; Taylor, A.; Copeland, K.M.; Pan, Z.; Liao, J.; Hong, Y. Current advances in biodegradable synthetic polymer based cardiac patches. J. Biomed. Mater. Res. Part A 2020, 108, 972–983. [Google Scholar] [CrossRef]
- Wang, Y.; Ameer, G.A.; Sheppard, B.J.; Langer, R. A tough biodegradable elastomer. Nat. Biotechnol. 2002, 20, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Tallawi, M.; Dippold, D.; Rai, R.; D’Atri, D.; Roether, J.A.; Schubert, D.W.; Rosellini, E.; Engel, F.B.; Boccaccini, A.R. Novel PGS/PCL electrospun fiber mats with patterned topographical features for cardiac patch applications. Mater. Sci. Eng. C 2016, 69, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bejleri, D.; Streeter, B.W.; Nachlas, A.L.Y.; Brown, M.E.; Gaetani, R.; Christman, K.L.; Davis, M.E. A Bioprinted Cardiac Patch Composed of Cardiac-Specific Extracellular Matrix and Progenitor Cells for Heart Repair. Adv. Heal. Mater. 2018, 7, 1800672. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Ha, D.-H.; Won Kim, S.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.; Park, H.-J.; Kim, S.-W.; Kim, H.; Park, J.Y.; Na, S.J.; Kim, H.J.; Park, M.N.; Choi, S.H.; Park, S.H.J.B. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Banerjee, M.N.; Bolli, R.; Hare, J.M. Clinical Studies of Cell Therapy in Cardiovascular Medicine. Circ. Res. 2018, 123, 266–287. [Google Scholar] [CrossRef]
- Zhang, J. Engineered Tissue Patch for Cardiac Cell Therapy. Curr. Treat. Options Cardiovasc. Med. 2015, 17, 399. [Google Scholar] [CrossRef] [Green Version]
- Wendel, J.S.; Ye, L.; Zhang, P.; Tranquillo, R.T.; Zhang, J.J. Functional consequences of a tissue-engineered myocardial patch for cardiac repair in a rat infarct model. Tissue Eng. Part A 2014, 20, 1325–1335. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.X.; Weng, Y.L.; Zhou, C.Q.; Wen, Z.Z.; Huang, H.; Wu, W.; Wang, J.F.; Wang, T. Comparison of cardiac stem cells and mesenchymal stem cells transplantation on the cardiac electrophysiology in rats with myocardial infarction. Stem Cell Rev. Rep. 2013, 9, 339–349. [Google Scholar] [CrossRef]
- Menasché, P.; Vanneaux, V.; Hagège, A.; Bel, A.; Cholley, B.; Parouchev, A.; Cacciapuoti, I.; Al-Daccak, R.; Benhamouda, N.; Blons, H.; et al. Transplantation of Human Embryonic Stem Cell–Derived Cardiovascular Progenitors for Severe Ischemic Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2018, 71, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Jammalamadaka, U.; Tappa, K.J.J.o.f.b. Recent advances in biomaterials for 3D printing and tissue engineering. J. Funct. Biomater. 2018, 9, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Material | Tg (°C) | Tm (°C) | Modulus (GPa) | Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|---|
| SS316L | NA | ~1400 | 193 | 668 | 40 |
| Co-Cr | NA | 1454 | 210 | 235 | 40 |
| PLA | 60 | 180–190 | 2–4 | 65 | 2–6 |
| PLLA | 60–65 | 175 | 2–4 | 60–70 | 2–6 |
| PDLLA | 55 | NA | 1–3.5 | 40 | 1–2 |
| PGA | 35–40 | 225–230 | 6–7 | 90–110 | 1–2 |
| PLGA (82/12) | 50 | 135–145 | 3.3–3.5 | 65 | 2–6 |
| PCL | 54 | 55–60 | 0.34–0.36 | 23 | 700–1000 |
| PC | 147 | 225 | 2–2.4 | 55–75 | 80–150 |
| Scaffold Materials | Scaffold Type | Technique of Fabrication | In Vitro/In Vivo Findings |
|---|---|---|---|
| PLCL scaffold with reinforced PLA nanofiber [114] | Synthetic | Freeze-drying, electrospinning |
|
| Degradable polar/hydrophobic/ionic (D-PHI) PU scaffold [119] | Synthetic | Particulate leaching |
|
| PGS scaffold [120] | Synthetic | Particulate leaching, freeze drying |
|
| PLLA/PCL scaffold [115] | Synthetic | Electrospinning, fused deposition modelling |
|
| PU/PLLA scaffold [121] | Synthetic | Multistep-dip coating |
|
| PLA/TPU scaffold [122] | Synthetic | TIPS |
|
| Crosslinked collagen/elastin scaffold [123,124] | Natural | Freeze drying |
|
| Silk scaffold [125] | Natural | Gel spinning, freeze drying |
|
| Fibrin hydrogel microfiber [126] | Natural | Electrospinning |
|
| Chitosan/gelatin scaffold [117] | Natural | TIPS, freeze drying |
|
| Elastin scaffold [103] | Natural | Gas foaming, particulate leaching |
|
| PCL/gelatin scaffold [127] | Hybrid | Electrospinning |
|
| Silk/PCL/Chitosan scaffold [118] | Hybrid | Electrospinning |
|
| TPU/PPC scaffold [112] | Hybrid | Electrospinning, TIPS |
|
| PCL/gelatin scaffold [108] | Hybrid | Electrospinning, Freeze-drying |
|
| Brand | Material | Purpose |
|---|---|---|
| CorMatrix Cor™ PATCH | Small Intestinal Submucosa Extra Cellular Matrix (SIS-ECM); Xenograft | Epicardial tissue support and repair |
| GORE-TEX® Cardiovascular Patch | Expanded Polytetrafluoroethylene (ePTFE) | To cover and support tissue following any injury or degenerative disease |
| Bard Cardiovascular Patch | Expanded Polytetrafluoroethylene (ePTFE) | Indicated for use in repair and closure of the cardiovascular system. |
| SteriGraft™—Pericardium | Pericardium Allograft Source | Pericardial defect, dura mater repair, and periodontal reconstruction |
| CardioCel® cardiovascular bio-scaffold | Acellular collagen sheet prepared from bovine pericardium; Xenograft | Repair of intracardiac defects; septal defects and annular repairs |
| Cryolife: Cardiac Tissue Matrix/Allograft | Allograft Source | Congenital reconstruction or as buttress material |
| PB—Bovine Pericardium Patch | Glutaraldehyde Bovine Pericardium; Xenograft | Cardiovascular repair and support |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toong, D.W.Y.; Toh, H.W.; Ng, J.C.K.; Wong, P.E.H.; Leo, H.L.; Venkatraman, S.; Tan, L.P.; Ang, H.Y.; Huang, Y. Bioresorbable Polymeric Scaffold in Cardiovascular Applications. Int. J. Mol. Sci. 2020, 21, 3444. https://doi.org/10.3390/ijms21103444
Toong DWY, Toh HW, Ng JCK, Wong PEH, Leo HL, Venkatraman S, Tan LP, Ang HY, Huang Y. Bioresorbable Polymeric Scaffold in Cardiovascular Applications. International Journal of Molecular Sciences. 2020; 21(10):3444. https://doi.org/10.3390/ijms21103444
Chicago/Turabian StyleToong, Daniel Wee Yee, Han Wei Toh, Jaryl Chen Koon Ng, Philip En Hou Wong, Hwa Liang Leo, Subramanian Venkatraman, Lay Poh Tan, Hui Ying Ang, and Yingying Huang. 2020. "Bioresorbable Polymeric Scaffold in Cardiovascular Applications" International Journal of Molecular Sciences 21, no. 10: 3444. https://doi.org/10.3390/ijms21103444
APA StyleToong, D. W. Y., Toh, H. W., Ng, J. C. K., Wong, P. E. H., Leo, H. L., Venkatraman, S., Tan, L. P., Ang, H. Y., & Huang, Y. (2020). Bioresorbable Polymeric Scaffold in Cardiovascular Applications. International Journal of Molecular Sciences, 21(10), 3444. https://doi.org/10.3390/ijms21103444