Adipokines Expression and Effects in Oocyte Maturation, Fertilization and Early Embryo Development: Lessons from Mammals and Birds
Abstract
1. Introduction
2. Oocyte Maturation, Fertilization, and Early Embryo Development in Mammals
2.1. Oocyte Maturation and Fertilization
2.2. Cleavage and Blastocyst Formation
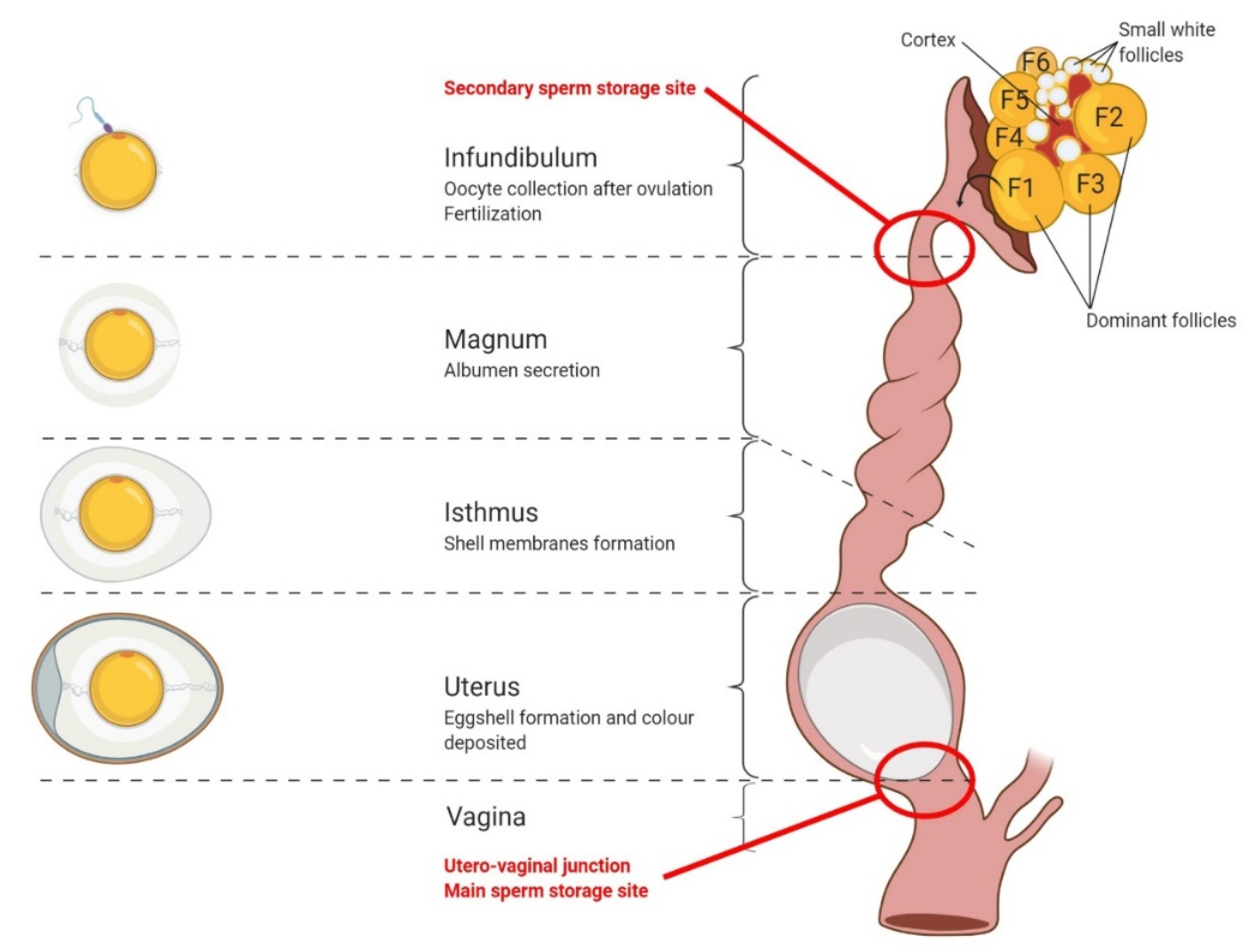
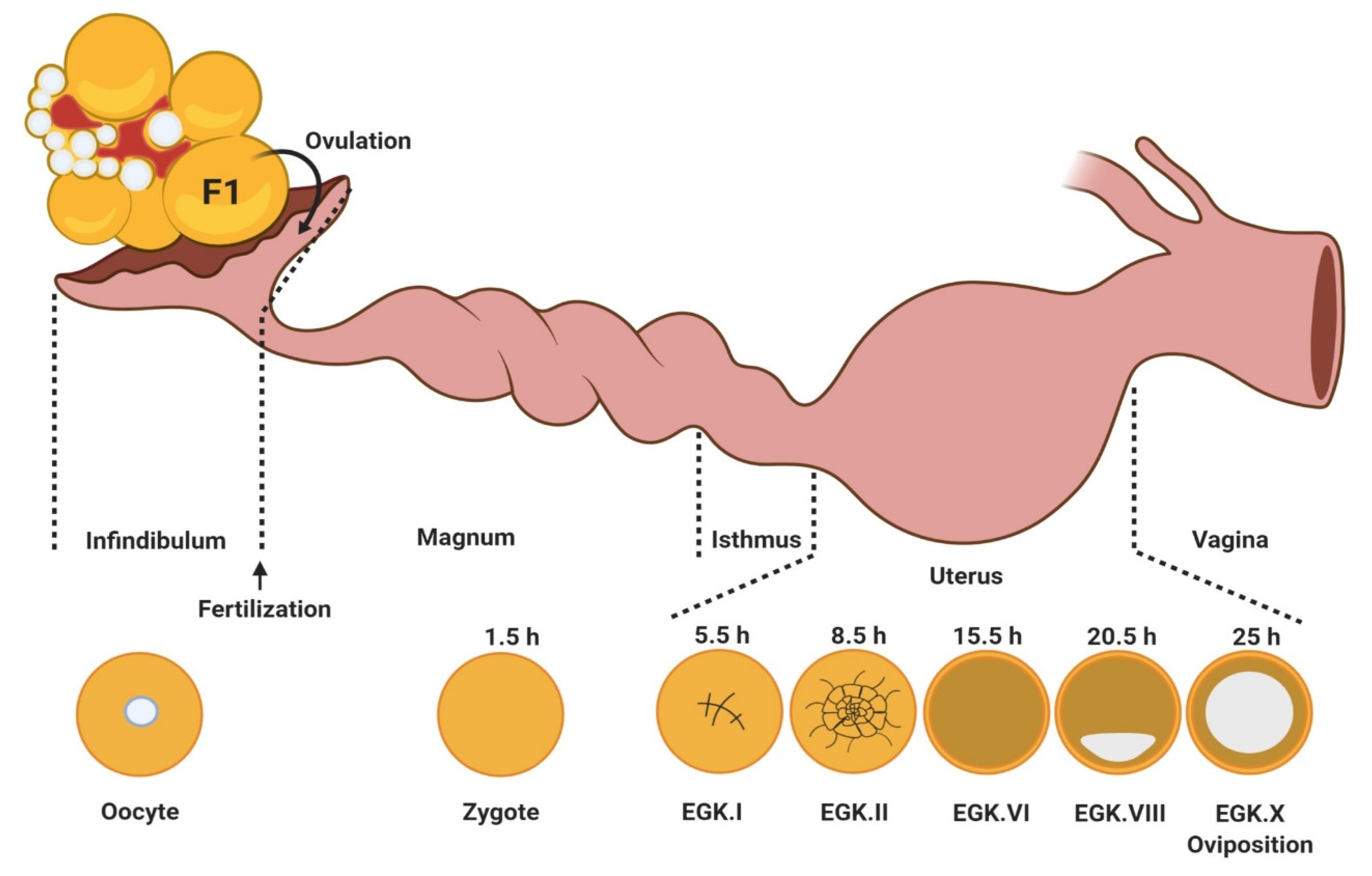
3. Oocyte Maturation, Fertilization, and Early Embryo Development in Birds
3.1. Oocyte Maturation
3.2. Fertilization
- Penetration of the spermatozoon in the ovular cytoplasm;
- Activation of the oocyte;
- Fusion of the haploid nuclei of the two gametes and the reconstitution of a new diploid cell: the zygote.
3.3. Early Embryo Development
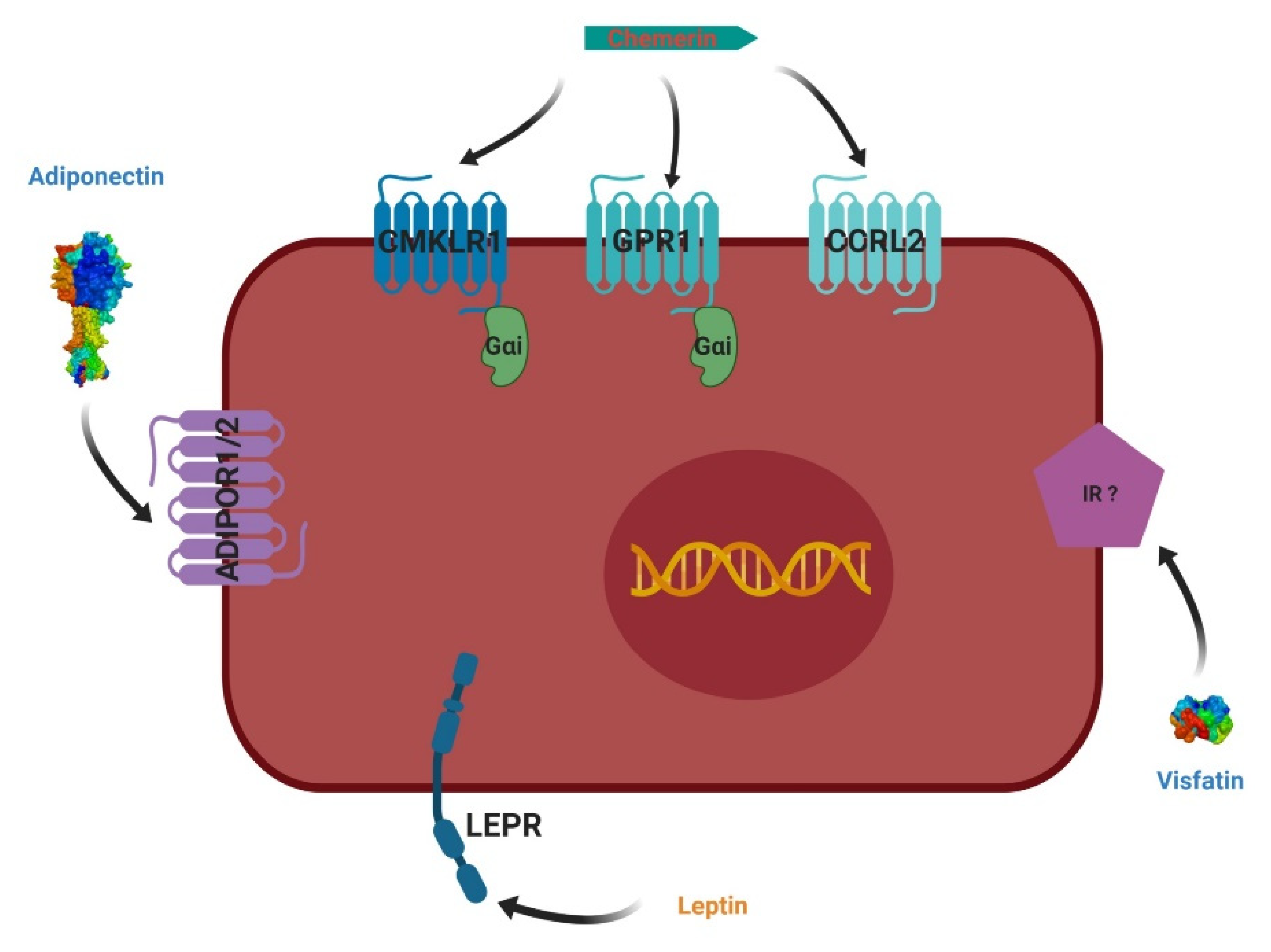
4. Adipokines and Their Receptors
4.1. Leptin
4.2. Adiponectin
4.3. Visfatin
4.4. Chemerin
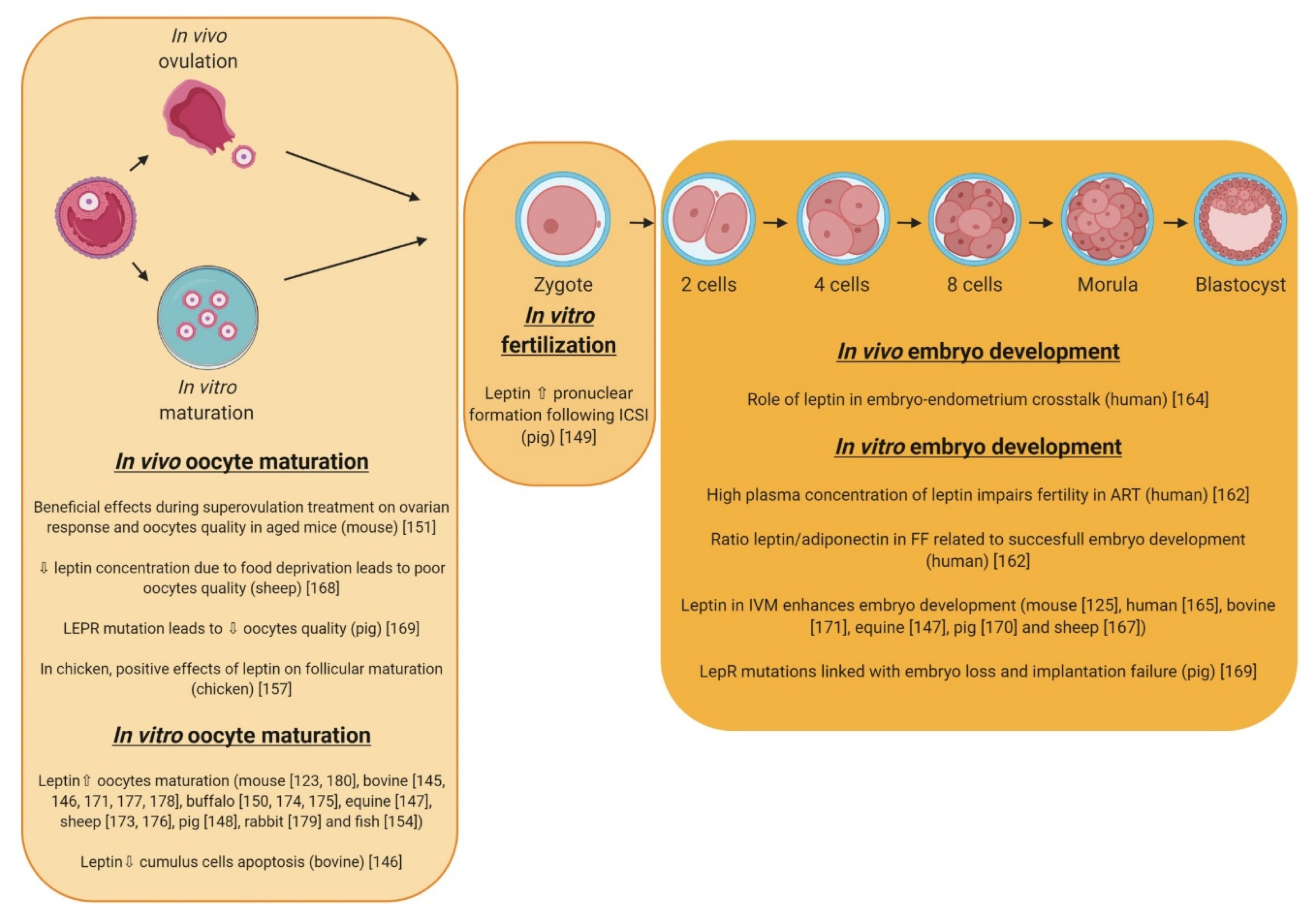
5. Involvement of Adipokines in the Oocyte Maturation, Fertilization, and Early Embryo Development in Mammals and Birds
5.1. Leptin
5.1.1. Oocyte Maturation
5.1.2. Fertilization and Embryo Development
5.2. Adiponectin
5.3. Visfatin
5.4. Chemerin
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADIPOQ | Adiponectin |
| ADIPOR1 | Adiponectin receptor 1 |
| ADIPOR2 | Adiponectin receptor 2 |
| apM1 | Adipose most abundant gene transcript 1 protein |
| BMI | Body Mass Index |
| CCRL2 | CC motif chemokine receptor like 2 |
| cDNA | Complementary deoxyribonucleic acid |
| CMKLR1 | Chemokine like receptor 1 |
| COCs | Cumulus-Oocyte-Complexe |
| COX2 | Cytochrome c oxidase subunit II |
| EDD | Early embryo development |
| EGK | Eyal-Giladi and Kochav |
| E2 | Estradiol |
| FSH | Follicule stimulating hormone |
| GPR1 | G protein coupled receptor 1 |
| GV | Germinal vesicule |
| hCG | human Chorionic Gonadotropin |
| HH | Hamburger and Hamilton |
| HMW | High molecular weight |
| HPG | Hypothalamus pituitary gonadal |
| ICM | Inner cell mass |
| IGF-1 | Insulin like Growth Factor alpha |
| IVF | In Vitro Fertilization |
| IVM | In Vitro Maturation |
| KO | Knockout |
| LEPR | Leptin receptor |
| LH | Luteinizing Hormone |
| MAPK | Mitogen-Activated Protein Kinases |
| mRNA | Messenger Ribonucleic acid |
| NAMPT | Nicotinamide phosphoribosyltransferase |
| NMN | Mononucleotide nicotinamide |
| P4 | Progesterone |
| P450ssc | P450 side-chain cleavage enzyme |
| PBEF | Pre-B-cell colony-enhancing factor |
| PCOS | Polycystic ovary syndrome |
| PGES | Prostaglandine E synthase |
| PI3K | Phosphoinositide 3-kinase |
| PM | Perivitellin membrane |
| RARRES 2 | Retinoic Acid Receptor Responder protein 2 |
| STAT3 | Signal transducer and activator of transcription 3 |
| SNPs | Single nucleotide polymorphisme |
| TE | Trophectoderm |
| TIG2 | Tazarotene-induced gene 2 |
| VEGF | Vascular endothelial growth factor |
| WAT | White adipose tissue |
| ZP | Zona pellucida |
References
- Stein, A.D.; Zybert, P.A.; van de Bor, M.; Lumey, L.H. Intrauterine famine exposure and body proportions at birth: The Dutch Hunger Winter. Int. J. Epidemiol. 2004, 33, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.W.; Robinson, F.E.; Charles, R.G.; Weingardt, R. Effect of feed allowance during rearing and breeding on female broiler breeders. 2. Ovarian morphology and production. Poult. Sci. 1992, 71, 1750–1761. [Google Scholar] [CrossRef] [PubMed]
- Renema, R.A.; Robinson, F.E.; Proudman, J.A.; Newcombe, M.; McKay, R.I. Effects of body weight and feed allocation during sexual maturation in broiler breeder hens. 2. Ovarian morphology and plasma hormone profiles. Poult. Sci. 1999, 78, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Estienne, A.; Bongrani, A.; Reverchon, M.; Rame, C.; Ducluzeau, P.H.; Froment, P.; Dupont, J. Involvement of Novel Adipokines, Chemerin, Visfatin, Resistin and Apelin in Reproductive Functions in Normal and Pathological Conditions in Humans and Animal Models. Int. J. Mol. Sci. 2019, 20, 4431. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, S.E.; Gee, L.L.; Wachtel, M.S.; Frezza, E.E. Adipose tissue: The new endocrine organ? A review article. Dig. Dis. Sci. 2009, 54, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Barbe, A.; Bongrani, A.; Mellouk, N.; Estienne, A.; Kurowska, P.; Grandhaye, J.; Elfassy, Y.; Levy, R.; Rak, A.; Froment, P.; et al. Mechanisms of Adiponectin Action in Fertility: An Overview from Gametogenesis to Gestation in Humans and Animal Models in Normal and Pathological Conditions. Int. J. Mol. Sci. 2019, 20, 1526. [Google Scholar] [CrossRef]
- Dupont, J.; Pollet-Villard, X.; Reverchon, M.; Mellouk, N.; Levy, R. Adipokines in human reproduction. Horm. Mol. Biol. Clin. Investig. 2015, 24, 11–24. [Google Scholar] [CrossRef]
- Dupont, J.; Reverchon, M.; Cloix, L.; Froment, P.; Rame, C. Involvement of adipokines, AMPK, PI3K and the PPAR signaling pathways in ovarian follicle development and cancer. Int. J. Dev. Biol. 2012, 56, 959–967. [Google Scholar] [CrossRef]
- Reverchon, M.; Rame, C.; Bertoldo, M.; Dupont, J. Adipokines and the female reproductive tract. Int. J. Endocrinol. 2014, 2014, 232454. [Google Scholar] [CrossRef]
- Reverchon, M.; Rame, C.; Dupont, J. [Chemerin: A pro-inflammatory adipokine involved in the reproduction function?]. Med. Sci. (Paris) 2015, 31, 493–498. [Google Scholar] [CrossRef]
- Elfassy, Y.; Bastard, J.P.; McAvoy, C.; Fellahi, S.; Dupont, J.; Levy, R. Adipokines in Semen: Physiopathology and Effects on Spermatozoas. Int. J. Endocrinol. 2018, 2018, 3906490. [Google Scholar] [CrossRef]
- Elfassy, Y.; McAvoy, C.; Fellahi, S.; Dupont, J.; Feve, B.; Levy, R.; Bastard, J.-P. Seminal plasma adipokines: Involvement in human reproductive functions. Eur. Cytokine Netw. 2017, 28, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K. Role of zona pellucida glycoproteins during fertilization in humans. J. Reprod. Immunol. 2015, 108, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.V.; Skinner, S.M.; Carino, C.; Wang, N.; Cartwright, J.; Dunbar, B.S. Structure and function of the proteins of the mammalian Zona pellucida. Cells Tissues Organs. 2000, 166, 148–164. [Google Scholar] [CrossRef] [PubMed]
- Payne, D.; Flaherty, S.P.; Barry, M.F.; Matthews, C.D. Preliminary observations on polar body extrusion and pronuclear formation in human oocytes using time-lapse video cinematography. Hum. Reprod. 1997, 12, 532–541. [Google Scholar] [CrossRef]
- Bell, C.E.; Calder, M.D.; Watson, A.J. Genomic RNA profiling and the programme controlling preimplantation mammalian development. Mol. Hum. Reprod. 2008, 14, 691–701. [Google Scholar] [CrossRef]
- Edwards, R.G.; Purdy, J.M.; Steptoe, P.C.; Walters, D.E. The growth of human preimplantation embryos in vitro. Am. J. Obs. Gynecol. 1981, 141, 408–416. [Google Scholar] [CrossRef]
- Trounson, A.O.; Mohr, L.R.; Wood, C.; Leeton, J.F. Effect of delayed insemination on in-vitro fertilization, culture and transfer of human embryos. J. Reprod. Fertil. 1982, 64, 285–294. [Google Scholar] [CrossRef]
- Harlow, G.M.; Quinn, P. Development of preimplantation mouse embryos in vivo and in vitro. Aust. J. Biol. Sci. 1982, 35, 187–193. [Google Scholar] [CrossRef]
- Kidder, G.M.; McLachlin, J.R. Timing of transcription and protein synthesis underlying morphogenesis in preimplantation mouse embryos. Dev. Biol. 1985, 112, 265–275. [Google Scholar] [CrossRef]
- Levy, J.B.; Johnson, M.H.; Goodall, H.; Maro, B. The timing of compaction: Control of a major developmental transition in mouse early embryogenesis. J. Embryol. Exp. Morphol. 1986, 95, 213–237. [Google Scholar] [PubMed]
- Enders, A.C.; Lantz, K.C.; Schlafke, S. The morula-blastocyst transition in two Old World primates: The baboon and rhesus monkey. J. Med. Primatol. 1990, 19, 725–747. [Google Scholar] [PubMed]
- Reima, I.; Lehtonen, E.; Virtanen, I.; Flechon, J.E. The cytoskeleton and associated proteins during cleavage, compaction and blastocyst differentiation in the pig. Differentiation 1993, 54, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Van Soom, A.; Boerjan, M.L.; Bols, P.E.; Vanroose, G.; Lein, A.; Coryn, M.; De Kruif, A. Timing of compaction and inner cell allocation in bovine embryos produced in vivo after superovulation. Biol. Reprod. 1997, 57, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.K.; Lane, M.; Spitzer, A.; Batt, P.A. Enhanced rates of cleavage and development for sheep zygotes cultured to the blastocyst stage in vitro in the absence of serum and somatic cells: Amino acids, vitamins, and culturing embryos in groups stimulate development. Biol. Reprod. 1994, 50, 390–400. [Google Scholar] [CrossRef]
- Surani, M.A. Zona pellucida denudation, blastocyst proliferation and attachment in the rat. J. Embryol. Exp. Morphol. 1975, 33, 343–353. [Google Scholar]
- Dobrinsky, J.R.; Johnson, L.A.; Rath, D. Development of a culture medium (BECM-3) for porcine embryos: Effects of bovine serum albumin and fetal bovine serum on embryo development. Biol. Reprod. 1996, 55, 1069–1074. [Google Scholar] [CrossRef]
- Seshagiri, P.B.; Hearn, J.P. In-vitro development of in-vivo produced rhesus monkey morulae and blastocysts to hatched, attached, and post-attached blastocyst stages: Morphology and early secretion of chorionic gonadotrophin. Hum. Reprod. 1993, 8, 279–287. [Google Scholar] [CrossRef]
- Keskintepe, L.; Burnley, C.A.; Brackett, B.G. Production of viable bovine blastocysts in defined in vitro conditions. Biol. Reprod. 1995, 52, 1410–1417. [Google Scholar] [CrossRef]
- King, W.A.; Linares, T.; Gustavsson, I. Cytogenetics of Pre-Implantation Embryos Sired by Bulls Heterozygous for the 1-29 Translocation. Hereditas 1981, 94, 219–224. [Google Scholar] [CrossRef]
- Lovell, T.M.; Gladwell, R.T.; Groome, N.P.; Knight, P.G. Ovarian follicle development in the laying hen is accompanied by divergent changes in inhibin A, inhibin B, activin A and follistatin production in granulosa and theca layers. J. Endocrinol. 2003, 177, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L. Reproduction in the female. In Sturkie’s Avian Physiology, 6th ed.; Scanes, C.G., Ed.; Elsevier: London, UK, 2015; pp. 635–665. [Google Scholar]
- Sturkie’s, C.S. Avian Physiology 2014, 6th ed.; Springer: New York, NY, USA, 2014. [Google Scholar]
- Johnson, J.R.; Santos, S.D.; Johnson, T.; Pieper, U.; Strumillo, M.; Wagih, O.; Sali, A.; Krogan, N.J.; Beltrao, P. Prediction of Functionally Important Phospho-Regulatory Events in Xenopus laevis Oocytes. PLoS Comput. Biol. 2015, 11, e1004362. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharp, S.B.; Pierce, J.G. Binding and Degradation of Doubly Radioiodinated Luteinizing-Hormone by Mouse Leydig-Cells. Endocrinology 1983, 113, 1784–1790. [Google Scholar] [CrossRef] [PubMed]
- Doi, O.; Takai, T.; Nakamura, T.; Tanabe, Y. Changes in the pituitary and plasma LH, plasma and follicular progesterone and estradiol, and plasma testosterone and estrone concentrations during the ovulatory cycle of the quail (Coturnix coturnix japonica). Gen. Comp. Endocrinol. 1980, 41, 156–163. [Google Scholar] [CrossRef]
- Furr, B.J.; Bonney, R.C.; England, R.J.; Cunningham, F.J. Luteinizing hormone and progesterone in peripheral blood during the ovulatory cycle of the hen Gallus domesticus. J. Endocrinol. 1973, 57, 159–169. [Google Scholar] [CrossRef]
- Tanabe, Y.; Nakamura, T.; Omiya, Y.; Yano, T. Changes in the plasma LH, progesterone, and estradiol during the ovulatory cycle of the duck (Anas platyrhynchos domestica) exposed to different photoperiods. Gen. Comp. Endocrinol. 1980, 41, 378–383. [Google Scholar] [CrossRef]
- Bakst, M.R. Fate of fluorescent stained sperm following insemination: New light on oviducal sperm transport and storage in the turkey. Biol. Reprod. 1994, 50, 987–992. [Google Scholar] [CrossRef][Green Version]
- Thibault, C.; Gerard, M.; Menezo, Y. Preovulatory and ovulatory mechanisms in oocyte maturation. J. Reprod. Fertil. 1975, 45, 605–610. [Google Scholar] [CrossRef]
- Brillard, J.P. Sperm storage and transport following natural mating and artificial insemination. Poult. Sci. 1993, 72, 923–928. [Google Scholar] [CrossRef]
- Sasanami, T.; Matsuzaki, M.; Mizushima, S.; Hiyama, G. Sperm storage in the female reproductive tract in birds. J. Reprod. Dev. 2013, 59, 334–338. [Google Scholar] [CrossRef]
- Sauveur, B.; Zybko, A.; Colas, B. Dietary Proteins and Egg Quality. 1. Effects of Some Protein-Sources on Egg Quality and Functional-Properties. Ann. De Zootech. 1979, 28, 271–295. [Google Scholar] [CrossRef]
- Eyal-Giladi, H.; Kochav, S. From cleavage to primitive streak formation: A complementary normal table and a new look at the first stages of the development of the chick. I. General morphology. Dev. Biol. 1976, 49, 321–337. [Google Scholar] [CrossRef]
- Kochav, S.; Ginsburg, M.; Eyal-Giladi, H. From cleavage to primitive streak formation: A complementary normal table and a new look at the first stages of the development of the chick. II. Microscopic anatomy and cell population dynamics. Dev. Biol. 1980, 79, 296–308. [Google Scholar] [CrossRef]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. Dev. Dyn. 1992, 195, 231–272. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Baumann, H.; Morella, K.K.; White, D.W.; Dembski, M.; Bailon, P.S.; Kim, H.; Lai, C.F.; Tartaglia, L.A. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc. Natl. Acad. Sci. USA 1996, 93, 8374–8378. [Google Scholar] [CrossRef]
- Bjorbaek, C.; Uotani, S.; da Silva, B.; Flier, J.S. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J. Biol. Chem. 1997, 272, 32686–32695. [Google Scholar] [CrossRef]
- Dixit, V.D.; Mielenz, M.; Taub, D.D.; Parvizi, N. Leptin induces growth hormone secretion from peripheral blood mononuclear cells via a protein kinase C- and nitric oxide-dependent mechanism. Endocrinology 2003, 144, 5595–5603. [Google Scholar] [CrossRef]
- O’Rourke, L.; Yeaman, S.J.; Shepherd, P.R. Insulin and leptin acutely regulate cholesterol ester metabolism in macrophages by novel signaling pathways. Diabetes 2001, 50, 955–961. [Google Scholar] [CrossRef]
- Bates, S.H.; Stearns, W.H.; Dundon, T.A.; Schubert, M.; Tso, A.W.K.; Wang, Y.; Banks, A.S.; Lavery, H.J.; Haq, A.K.; Maratos-Flier, E.; et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 2003, 421, 856–859. [Google Scholar] [CrossRef]
- Saladin, R.; Staels, B.; Auwerx, J.; Briggs, M. Regulation of ob gene expression in rodents and humans. Horm. Metab. Res. 1996, 28, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, G. Leptin signalling. Cell Signal. 2002, 14, 655–663. [Google Scholar] [CrossRef]
- Munzberg, H.; Morrison, C.D. Structure, production and signaling of leptin. Metabolism 2015, 64, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Terauchi, Y.; Froguel, P.; Tobe, K.; Nagai, R.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of receptors for adiponectin that mediates anti-diabetic and anti-atherogenic effeccts. Circulation 2003, 108, 113. [Google Scholar]
- Sommer, G.; Garten, A.; Petzold, S.; Beck-Sickinger, A.G.; Blüher, M.; Stumvoll, M.; Fasshauer, M. Visfatin/PBEF/Nampt: Structure, regulation and potential function of a novel adipokine. Clin. Sci. (Lond.) 2008, 115, 13–23. [Google Scholar] [CrossRef]
- Buechler, C.; Feder, S.; Haberl, E.M.; Aslanidis, C. Chemerin Isoforms and Activity in Obesity. Int. J. Mol. Sci. 2019, 20, 1128. [Google Scholar] [CrossRef]
- Mattern, A.; Zellmann, T.; Beck-Sickinger, A.G. Processing, signaling, and physiological function of chemerin. Iubmb Life 2014, 66, 19–26. [Google Scholar] [CrossRef]
- Bondue, B.; Wittamer, V.; Parmentier, M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev. 2011, 22, 331–338. [Google Scholar] [CrossRef]
- Yoshimura, T.; Oppenheim, J.J. Chemokine-like receptor 1 (CMKLR1) and chemokine (C-C motif) receptor-like 2 (CCRL2); two multifunctional receptors with unusual properties. Exp. Cell Res. 2011, 317, 674–684. [Google Scholar] [CrossRef]
- Friedman-Einat, M.; Boswell, T.; Horev, G.; Girishvarma, G.; Dunn, I.C.; Talbot, R.; Sharp, P. The chicken leptin gene: Has it been cloned? General Comp. Endocrinol. 1999, 115, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.J.; Dunn, I.C.; Waddington, D.; Boswell, T. Chicken leptin. Gen. Comp. Endocrinol. 2008, 158, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Millar, R.P. Identification of Genuine/Authentic Avian Leptin: Some Answers and More Questions. Endocrinology 2014, 155, 3203–3205. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seroussi, E.; Cinnamon, Y.; Yosefi, S.; Genin, O.; Smith, J.G.; Rafati, N.; Bornelöv, S.; Andersson, G.; Friedman-Einat, M. Identification of the Long-Sought Leptin in Chicken and Duck: Expression Pattern of the Highly GC-Rich Avian leptin Fits an Autocrine/Paracrine Rather Than Endocrine Function. Endocrinology 2016, 157, 737–751. [Google Scholar] [CrossRef]
- Seroussi, E.; Pitel, F.; Leroux, S.; Morisson, M.; Bornelöv, S.; Miyara, S.; Yosefi, S.; Cogburn, L.A.; Burt, D.W.; Anderson, L.; et al. Mapping of leptin and its syntenic genes to chicken chromosome 1p. BMC Genet. 2017, 18, 77. [Google Scholar]
- Bado, A.; Levasseur, S.; Attoub, S.; Kermorgant, S.; Laigneau, J.-P.; Bortoluzzi, M.-N.; Moizo, L.; Lehy, T.; Guerre-Millo, M.; Le Marchand-Brustel, Y.; et al. The stomach is a source of leptin. Nature 1998, 394, 790–793. [Google Scholar] [CrossRef]
- Sagawa, N.; Yura, S.; Itoh, H.; Kakui, K.; Takemura, M.; Nuamah, M.A.; Ogawa, Y.; Masuzaki, H.; Nakao, K.; Fujii, S. Possible role of placental leptin in pregnancy—A review. Endocrine 2002, 19, 65–71. [Google Scholar] [CrossRef]
- Friedman-Einat, M.; Seroussi, E. Avian Leptin: Bird’s-Eye View of the Evolution of Vertebrate Energy-Balance Control. Trends Endocrinol. Metab. 2019, 30, 819–832. [Google Scholar] [CrossRef]
- Lei, M.M.; Wu, S.Q.; Li, X.W.; Wang, C.L.; Chen, Z.; Shi, Z.D. Leptin receptor signaling inhibits ovarian follicle development and egg laying in chicken hens. Reprod. Biol. Endocrinol. 2014, 12, 25. [Google Scholar] [CrossRef]
- Lei, M.; Wu, S.; Shao, X.; Li, X.; Chen, Z.; Ying, S.; Shi, Z. Creating leptin-like biofunctions by active immunization against chicken leptin receptor in growing chickens. Domest. Anim. Endocrinol. 2015, 50, 55–64. [Google Scholar] [CrossRef]
- Seroussi, E.; Knytl, M.; Pitel, F.; Elleder, D.; Krylov, V.; Leroux, S.; Morisson, M.; Yosefi, S.; Miyara, S.; Ganesan, S.; et al. Avian Expression Patterns and Genomic Mapping Implicate Leptin in Digestion and TNF Signaling, Suggesting that Their Interacting Adipokine Role is Unique to Mammals. Int. J. Mol. Sci. 2019, 20, 4489. [Google Scholar] [CrossRef] [PubMed]
- Dunn, I.C.; Boswell, T.; Friedman-Einat, M.; Eshdat, Y.; Burt, D.W.; Paton, I.R. Mapping of the leptin receptor gene (LEPR) to chicken chromosome 8. Anim. Genet. 2000, 31, 290. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, W.; Liu, Z.L.; Li, N. cDNA cloning, genomic structure, chromosomal mapping and expression analysis of ADIPOQ (adiponectin) in chicken. Cytogenet. Genome Res. 2006, 112, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Maddineni, S.; Ocon-Grove, O.; Hendricks, C.; Vasilatos-Younken, R.; Hadley, J.A. Expression of adiponectin and its receptors in avian species. Gen. Comp. Endocrinol. 2013, 190, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Ocon-Grove, O.M.; Metzger, S.L. Molecular cloning and tissue expression of chicken AdipoR1 and AdipoR2 complementary deoxyribonucleic acids. Domest. Anim. Endocrinol. 2007, 33, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Diot, M.; Reverchon, M.; Rame, C.; Baumard, Y.; Dupont, J. Expression and effect of NAMPT (visfatin) on progesterone secretion in hen granulosa cells. Reproduction 2015, 150, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Diot, M.; Reverchon, M.; Rame, C.; Froment, P.; Brillard, J.-P.; Brière, S.; Leveque, G.; Guillaume, D.; Dupont, J. Expression of adiponectin, chemerin and visfatin in plasma and different tissues during a laying season in turkeys. Reprod. Biol. Endocrin. 2015, 13, 81. [Google Scholar] [CrossRef]
- Li, J.; Meng, F.; Song, C.; Wang, Y.; Leung, F.C. Characterization of chicken visfatin gene: cDNA cloning, tissue distribution, and promoter analysis. Poult. Sci. 2012, 91, 2885–2894. [Google Scholar] [CrossRef]
- Mellouk, N.; Rame, C.; Barbe, A.; Grandhaye, J.; Froment, P.; Dupont, J. Chicken Is a Useful Model to Investigate the Role of Adipokines in Metabolic and Reproductive Diseases. Int. J. Endocrinol. 2018, 2018, 4579734. [Google Scholar] [CrossRef]
- Maddineni, S.; Metzger, S.; Ocon, O.; Hendricks, G.; Ramachandran, R. Adiponectin gene is expressed in multiple tissues in the chicken: Food deprivation influences adiponectin messenger ribonucleic acid expression. Endocrinology 2005, 146, 4250–4256. [Google Scholar] [CrossRef]
- Maeda, K.; Okubo, K.; Shimomura, I.; Funahashi, T.; Matsuzawa, Y.; Matsubara, K. CDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (Adipose most abundant gene transcript 1). Biochem. Biophys. Res. Commun. 1996, 221, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Liang, P.; Spiegelman, B.M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 1996, 271, 10697–10703. [Google Scholar] [CrossRef] [PubMed]
- Scherer, P.E.; Williams, S.; Fogliano, M.; Baldini, G.; Lodish, H.F. A Novel Serum-Protein Similar to C1q, Produced Exclusively in Adipocytes. J. Biol. Chem. 1995, 270, 26746–26749. [Google Scholar] [CrossRef]
- Nakano, Y.; Tobe, T.; ChoiMiura, N.H.; Mazda, T.; Tomita, M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J. Biochem. 1996, 120, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Pajvani, U.B.; Du, X.L.; Combs, T.P.; Berg, A.H.; Rajala, M.W.; Schulthess, T.; Engel, J.; Brownlee, M.; Scherer, P.E. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin—Implications for metabolic regulation and bioactivity. J. Biol. Chem. 2003, 278, 9073–9085. [Google Scholar] [CrossRef]
- Holland, W.L.; Scherer, P.E. Ronning After the Adiponectin Receptors. Science 2013, 342, 1460–1461. [Google Scholar] [CrossRef]
- Tao, C.; Sifuentes, A.; Holland, W.L. Regulation of glucose and lipid homeostasis by adiponectin: Effects on hepatocytes, pancreatic beta cells and adipocytes. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 43–58. [Google Scholar] [CrossRef]
- Tiwari, A.; Ocon-Grove, O.M.; Hadley, J.A.; Giles, J.R.; Johnson, P.A.; Ramachandran, R. Expression of Adiponectin and Its Receptors Is Altered in Epithelial Ovarian Tumors and Ascites-Derived Ovarian Cancer Cell Lines. Int. J. Gynecol. Cancer 2015, 25, 399–406. [Google Scholar] [CrossRef]
- Zhang, R.; Lin, Y.; Zhi, L.; Liao, H.; Zuo, L.; Li, Z.; Xu, Y. Expression profiles and associations of adiponectin and adiponectin receptors with intramuscular fat in Tibetan chicken. Br. Poult. Sci. 2017, 58, 151–157. [Google Scholar] [CrossRef]
- Hendricks, G.L.; Hadley, J.A.; Krzysik-Walker, S.M.; Prabhu, K.S.; Vasilatos-Younken, R.; Ramachandran, R. Unique Profile of Chicken Adiponectin, a Predominantly Heavy Molecular Weight Multimer, and Relationship to Visceral Adiposity. Endocrinology 2009, 150, 3092–3100. [Google Scholar] [CrossRef]
- Tahmoorespur, M.; Ghazanfari, S.; Nobari, K. Evaluation of adiponectin gene expression in the abdominal adipose tissue of broiler chickens: Feed restriction, dietary energy, and protein influences adiponectin messenger ribonucleic acid expression. Poult. Sci. 2010, 89, 2092–2100. [Google Scholar] [CrossRef] [PubMed]
- Samal, B.; Sun, Y.H.; Stearns, G.; Xie, C.S.; Suggs, S.; Mcniece, I. Cloning and Characterization of the Cdna-Encoding a Novel Human Pre-B-Cell Colony-Enhancing Factor. Mol. Cell. Biol. 1994, 14, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Q.; Heruth, D.P.; Ye, S.Q. Nicotinamide Phosphoribosyltransferase in Human Diseases. J. Bioanal. Biomed. 2011, 3, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Mills, K.F.; Yoon, M.J.; Imai, S.I. Nicotinamide Mononucleotide, a Key NAD(+) Intermediate, Treats the Pathophysiology of Diet- and Age-Induced Diabetes in Mice. Cell Metab. 2011, 14, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Uddin, G.M.; Youngson, N.A.; Sinclair, D.A.; Morris, M.J. Head to Head Comparison of Short-Term Treatment with the NAD Precursor Nicotinamide Mononucleotide (NMN) and 6 Weeks of Exercise in Obese Female Mice. Front. Pharmacol. 2016, 7, 258. [Google Scholar] [CrossRef]
- Uddin, G.M.; Youngson, N.A.; Doyle, B.M.; Sinclair, D.A.; Morris, M.J. Nicotinamide mononucleotide (NMN) supplementation ameliorates the impact of maternal obesity in mice: Comparison with exercise. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Tian, X.; Shang, P.; Chen, H.; Kang, X.; Tian, Y.; Han, R. Characterization of the visfatin gene and its expression pattern and effect on 3T3-L1 adipocyte differentiation in chickens. Gene 2017, 632, 16–24. [Google Scholar] [CrossRef]
- Fietta, P.; Delsante, G. Focus on Adipokines. Theor. Biol. Forum 2013, 106, 103–129. [Google Scholar]
- Krzysik-Walker, S.M.; Hadley, J.A.; Pesall, J.E.; McFarland, D.C.; Vasilatos-Younken, R.; Ramachandran, R. Nampt/visfatin/PBEF affects expression of myogenic regulatory factors and is regulated by interleukin-6 in chicken skeletal muscle cells. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 159, 413–421. [Google Scholar] [CrossRef]
- Krzysik-Walker, S.M.; Ocon-Grove, O.M.; Maddineni, S.R.; Hendricks, G.L.; Ramachandran, R. Is visfatin an adipokine or myokine? Evidence for greater visfatin expression in skeletal muscle than visceral fat in chickens. Endocrinology 2008, 149, 1543–1550. [Google Scholar] [CrossRef]
- Cline, M.A.; Nandar, W.; Prall, B.C.; Bowden, C.N.; Denbow, D.M. Central visfatin causes orexigenic effects in chicks. Behav. Brain Res. 2008, 186, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Ocon-Grove, O.M.; Krzysik-Walker, S.M.; Maddineni, S.R.; Hendricks, G.L.; Ramachandran, R. NAMPT (visfatin) in the chicken testis: Influence of sexual maturation on cellular localization, plasma levels and gene and protein expression. Reproduction 2010, 139, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Zabel, B.A.; Nakae, S.; Zúñiga, L.; Kim, J.-Y.; Ohyama, T.; Alt, C.; Pan, J.; Suto, H.; Soler, D.; Allen, S.J.; et al. Mast cell-expressed orphan receptor CCRL2 binds chemerin and is required for optimal induction of IgE-mediated passive cutaneous anaphylaxis. J. Exp. Med. 2008, 205, 2207–2220. [Google Scholar] [CrossRef] [PubMed]
- Bozaoglu, K.; Bolton, K.; McMillan, J.; Zimmet, P.; Jowett, J.; Collier, G.; Walder, K.R.; Segal, D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 2007, 148, 4687–4694. [Google Scholar] [CrossRef]
- Meder, W.; Wendland, M.; Busmann, A.; Kutzleb, C.; Spodsberg, N.; John, H.; Richter, R.; Schleuder, D.; Meyer, M.; Forssmann, W. Characterization of human circulating TIG2 as a ligand for the orphan receptor ChemR23. Febs Lett. 2003, 555, 495–499. [Google Scholar] [CrossRef]
- Wittamer, V.; Franssen, J.-D.; Vulcano, M.; Mirjolet, J.-F.; Le Poul, E.; Migeotte, I.; Brézillon, S.; Tyldesley, R.; Blanpain, C.; Detheux, M.; et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J. Exp. Med. 2003, 198, 977–985. [Google Scholar] [CrossRef]
- Reverchon, M.; Cornuau, M.; Rame, C.; Guerif, F.; Royere, D.; Dupont, J. Chemerin inhibits IGF-1-induced progesterone and estradiol secretion in human granulosa cells. Hum. Reprod. 2012, 27, 1790–1800. [Google Scholar] [CrossRef]
- Li, L.; Huang, C.; Zhang, X.; Wang, J.; Ma, P.; Liu, Y.; Xiao, T.; Zabel, B.A.; Zhang, J.V. Chemerin-Derived Peptide C-20 Suppressed Gonadal Steroidogenesis. Am. J. Reprod. Immunol. 2014, 71, 265–277. [Google Scholar] [CrossRef]
- Li, L.; Ma, P.; Huang, C.; Liu, Y.; Zhang, Y.; Gao, C.; Xiao, T.; Ren, P.-G.; Zabel, B.A.; Zhang, J.V. Expression of chemerin and its receptors in rat testes and its action on testosterone secretion. J. Endocrinol. 2014, 220, 155–163. [Google Scholar] [CrossRef]
- De Henau, O.; DeGroot, G.-N.; Imbault, V.; Robert, V.; De Poorter, C.; Mcheik, S.; Gales, C.; Parmentier, M.; Springael, J.-Y. Signaling Properties of Chemerin Receptors CMKLR1, GPR1 and CCRL2. PLoS ONE 2016, 11, e0164179. [Google Scholar] [CrossRef]
- Marchese, A.; Cheng, R.; Lee, M.; Porter, C.; Heiber, M.; Goodman, M.; George, S.R.; Odowd, B. Mapping Studies of 2 G-Protein-Coupled Receptor Genes—An Amino-Acid Difference May Confer a Functional Variation between a Human and Rodent Receptor. Biochem. Biophys. Res. Commun. 1994, 205, 1952–1958. [Google Scholar] [CrossRef]
- Ingalls, A.M.; Dickie, M.M.; Snell, G.D. Obese, a new mutation in the house mouse. J. Hered. 1950, 41, 317–318. [Google Scholar] [CrossRef]
- Chehab, F.E.; Lim, M.E.; Lu, R.H. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat. Genet. 1996, 12, 318–320. [Google Scholar] [CrossRef]
- Hummel, K.P. Transplantation of Ovaries of the Obese Mouse. Anat. Rec. 1957, 128, 569. [Google Scholar]
- Barash, I.A.; Cheung, C.C.; Weigle, D.S.; Ren, H.; Kabigting, E.B.; Kuijper, J.L.; Clifton, D.K.; Steiner, R.A. Leptin is a metabolic signal to the reproductive system. Endocrinology 1996, 137, 3144–3147. [Google Scholar] [CrossRef]
- Akhter, N.; Carllee, T.; Syed, M.M.; Odle, A.; Cozart, M.A.; Haney, A.C.; Allensworth-James, M.L.; Benes, H.; Childs, G.V. Selective Deletion of Leptin Receptors in Gonadotropes Reveals Activin and GnRH-Binding Sites as Leptin Targets in Support of Fertility. Endocrinology 2014, 155, 4027–4042. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, M.; Ma, H.; Qu, J.; Wang, Y.; Hou, L.; Liu, L.; Wu, X. The impairment of reproduction in db/db mice is not mediated by intraovarian defective leptin signaling. Fertil. Steril. 2012, 97, 1183–1191. [Google Scholar] [CrossRef]
- Cheng, L.; Shi, H.; Jin, Y.; Li, X.; Pan, J.; Lai, Y.; Lin, Y.; Jin, Y.; Roy, G.; Zhao, A.; et al. Adiponectin Deficiency Leads to Female Subfertility and Ovarian Dysfunctions in Mice. Endocrinology 2016, 157, 4875–4887. [Google Scholar] [CrossRef]
- Cioffi, J.A.; Van Blerkom, J.; Antczak, M.; Shafer, A.; Wittmer, S.; Snodgrass, H.R. The expression of leptin and its receptors in pre-ovulatory human follicles. Mol. Hum. Reprod. 1997, 3, 467–472. [Google Scholar] [CrossRef]
- Antczak, M.; Van Blerkom, J. Oocyte influences on early development: The regulatory proteins leptin and STAT3 are polarized in mouse and human oocytes and differentially distributed within the cells of the preimplantation stage embryo. Mol. Hum. Reprod. 1997, 3, 1067–1086. [Google Scholar] [CrossRef]
- Ryan, N.K.; Woodhouse, C.M.; Van der Hoek, K.H.; Gilchrist, R.B.; Armstrong, D.T.; Norman, R.J. Expression of leptin and its receptor in the murine ovary: Possible role in the regulation of oocyte maturation. Biol. Reprod. 2002, 66, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, T.; Tahara, M.; Yokoi, T.; Masumoto, N.; Takeda, T.; Yamaguchi, M.; Tasaka, K.; Kurachi, H.; Murata, Y. Tyrosine phosphorylation of STAT3 by leptin through leptin receptor in mouse metaphase 2 stage oocyte. Biochem. Biophys. Res. Commun. 1999, 256, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Sato, N.; Fukuda, J.; Kodama, H.; Kumagai, J.; Tanikawa, H.; Nakamura, A.; Tanaka, T. Leptin promotes the development of mouse preimplantation embryos in vitro. Endocrinology 2002, 143, 1922–1931. [Google Scholar] [CrossRef]
- Kawamura, T.; Yoshida, K.; Sugawara, A.; Nagasaka, M.; Mori, N.; Takeuchi, K.; Kohzuki, M. Impact of exercise and angiotensin converting enzyme inhibition on tumor necrosis factor-alpha and leptin in fructose-fed hypertensive rats. Hypertens. Res. 2002, 25, 919–926. [Google Scholar] [CrossRef]
- Batista, A.; Silva, D.; Rêgo, M.J.B.D.M.; Silva, F.; Silva, E.; Beltrão, E.; Filho, M.G.; Wischral, A.; Guerra, M. The expression and localization of leptin and its receptor in goat ovarian follicles. Anim. Reprod. Sci. 2013, 141, 142–147. [Google Scholar] [CrossRef]
- Madeja, Z.E.; Warzych, E.; Peippo, J.; Lechniak, D.; Switonski, M. Gene expression and protein distribution of leptin and its receptor in bovine oocytes and preattachment embryos produced in vitro. Animals 2009, 3, 568–578. [Google Scholar] [CrossRef]
- Kirat, D.; Hamid, N.; Mohamed, W.; Shalaby, S. Leptin Gene Expression in Rabbits during Pregnancy and Fetal Life. Int. J. Biol. 2015, 7. [Google Scholar] [CrossRef][Green Version]
- Hu, Y.; Ni, Y.; Ren, L.; Dai, J.; Zhao, R. Leptin is involved in the effects of cysteamine on egg laying of hens, characteristics of eggs, and posthatch growth of broiler offspring. Poult. Sci. 2008, 87, 1810–1817. [Google Scholar] [CrossRef]
- Grzegorzewska, A.; Paczoska-Eliasiewicz, H. Expression of leptin receptors mRNA in hypothalamus, pituitary and gonads in chicken embryos. Acta Biol. Crac. Ser. Zool. 2006, 48, 67–73. [Google Scholar]
- Chabrolle, C.; Tosca, L.; Dupont, J. Regulation of adiponectin and its receptors in rat ovary by human chorionic gonadotrophin treatment and potential involvement of adiponectin in granulosa cell steroidogenesis. Reproduction 2007, 133, 719–731. [Google Scholar] [CrossRef]
- Schmidt, T.; Fischer, S.; Tsikolia, N.; Santos, A.N.; Rohrbach, S.; Ramin, N.; Thieme, R.; Fischer, B. Expression of adipokines in preimplantation rabbit and mice embryos. Histochem. Cell Biol. 2008, 129, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Palin, M.F.; Bordignon, V.V.; Murphy, B.D. Adiponectin and the Control of Female Reproductive Functions. Adiponectin 2012, 90, 239–287. [Google Scholar]
- Chabrolle, C.; Tosca, L.; Rame, C.; Lecomte, P.; Royere, D.; Dupont, J. Adiponectin increases insulin-like growth factor I-induced progesterone and estradiol secretion in human granulosa cells. Fertil. Steril. 2009, 92, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.L.; Li, L.L.; Yu, L.; Ge, C.Y.; Ma, H.T. Effects of (-)-hydroxycitric acid on lipid droplet accumulation in chicken embryos. Anim. Sci. J. 2018, 89, 237–249. [Google Scholar] [CrossRef]
- Choi, K.-H.; Joo, B.S.; Sun, S.-T.; Park, M.-J.; Son, J.-B.; Kil Joo, J.; Lee, K.-S. Administration of visfatin during superovulation improves developmental competency of oocytes and fertility potential in aged female mice. Fertil. Steril. 2012, 97, 1234. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, M.; Cornuau, M.; Cloix, L.; Ramé, C.; Guérif, F.; Royere, D.; Dupont, J. Visfatin is expressed in human granulosa cells: Regulation by metformin through AMPK/SIRT1 pathways and its role in steroidogenesis. Mol. Hum. Reprod. 2013, 19, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, M.; Rame, C.; Bunel, A.; Chen, W.; Froment, P.; Dupont, J. VISFATIN (NAMPT) Improves In Vitro IGF1-Induced Steroidogenesis and IGF1 Receptor Signaling Through SIRT1 in Bovine Granulosa Cells. Biol. Reprod. 2016, 94, 54. [Google Scholar] [CrossRef]
- Mellouk, N.; Rame, C.; Delaveau, J.; Rat, C.; Maurer, E.; Froment, P.; Dupont, J. Adipokines expression profile in liver, adipose tissue and muscle during chicken embryo development. Gen. Comp. Endocrinol. 2018, 267, 146–156. [Google Scholar] [CrossRef]
- Reverchon, M.; Bertoldo, M.J.; Rame, C.; Froment, P.; Dupont, J. CHEMERIN (RARRES2) Decreases In Vitro Granulosa Cell Steroidogenesis and Blocks Oocyte Meiotic Progression in Bovine Species. Biol. Reprod. 2014, 90, 102. [Google Scholar] [CrossRef]
- van Tol, H.T.A.; van Eerdenburg, F.J.C.M.; Colenbrander, B.; Roelen, B.A.J. Enhancement of bovine oocyte maturation by leptin is accompanied by an Upregulation in mRNA expression of leptin receptor isoforms in cumulus cells. Mol. Reprod. Dev. 2008, 75, 578–587. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, J.; Wu, Z.; Tian, J. Leptin enhances maturation and development of calf oocytes in vitro. Reprod. Domest. Anim. 2012, 47, 718–723. [Google Scholar] [CrossRef]
- Paula-Lopes, F.F.; Boelhauve, M.; Habermann, F.A.; Sinowatz, F.; Wolf, E. Leptin promotes meiotic progression and developmental capacity of bovine oocytes via cumulus cell-independent and -dependent mechanisms. Biol. Reprod. 2007, 76, 532–541. [Google Scholar] [CrossRef]
- Consiglio, A.L.; Dell’Aquila, M.E.; Fiandanese, N.; Ambruosi, B.; Cho, Y.S.; Bosi, G.; Arrighi, S.; Lacalandra, G.M.; Cremonesi, F. Effects of leptin on in vitro maturation, fertilization and embryonic cleavage after ICSI and early developmental expression of leptin (Ob) and leptin receptor (ObR) proteins in the horse. Reprod. Biol. Endocrin. 2009, 7, 113. [Google Scholar] [CrossRef]
- Craig, J.; Zhu, H.; Dyce, P.W.; Petrik, J.; Li, J.L. Leptin enhances oocyte nuclear and cytoplasmic maturation via the mitogen-activated protein kinase pathway. Endocrinology 2004, 145, 5355–5363. [Google Scholar] [CrossRef]
- Jin, Y.X.; Cui, X.S.; Han, Y.J.; Kim, N.H. Leptin accelerates pronuclear formation following intracytoplasmic sperm injection of porcine oocytes: Possible role for MAP kinase inactivation. Anim. Reprod. Sci. 2009, 115, 137–148. [Google Scholar] [CrossRef]
- Khaki, A.; Batavani, R.; Najafi, G.; Tahmasbian, H.; Belbasi, A.; Mokarizadeh, A. Effect of Leptin on In Vitro Nuclear Maturation and Apoptosis of Buffalo (Bubalus bubalis) Oocyte. Int. J. Fertil. Steril. 2014, 8, 43–50. [Google Scholar]
- Joo, J.K.; Joo, B.S.; Kim, S.C.; Choi, J.R.; Park, S.H.; Lee, K.S. Role of leptin in improvement of oocyte quality by regulation of ovarian angiogenesis. Anim. Reprod. Sci. 2010, 119, 329–334. [Google Scholar] [CrossRef]
- Danforth, D.R.; Arbogast, L.K.; Kaumaya, P.T.P.; Cohn, D.; Friedman, C.I. Endocrine gland vascular endothelial growth factor (EG-VEGF) regulates preantral follicle growth. Biol. Reprod. 2003, 68, 324–325. [Google Scholar] [CrossRef][Green Version]
- Shimizu, T.; Okamoto, H.; Chiba, S.; Matsui, Y.; Sugawara, T.; Akino, M.; Nan, J.; Kumamoto, H.; Onozuka, H.; Mikami, T. VEGF-mediated angiogenesis is impaired by angiotensin type 1 receptor blockade in cardiomyopathic hamster hearts. Cardiovasc. Res. 2003, 58, 203–212. [Google Scholar] [CrossRef]
- Song, Y.F.; Tan, X.Y.; Pan, Y.X.; Zhang, L.H.; Chen, Q.L. Fatty Acid beta-Oxidation Is Essential in Leptin-Mediated Oocytes Maturation of Yellow Catfish Pelteobagrus fulvidraco. Int. J. Mol. Sci. 2018, 19, 1457. [Google Scholar] [CrossRef]
- Dunning, K.R.; Russell, D.L.; Robker, R.L. Lipids and oocyte developmental competence: The role of fatty acids and beta-oxidation. Reproduction 2014, 148, R15–R27. [Google Scholar] [CrossRef]
- Paczoska-Eliasiewicz, H.E.; Gertler, A.; Proszkowiec, M.; Proudman, J.; Hrabia, A.; Sechman, A.; Mika, M.; Jacek, T.; Cassy, S.; Raver, N.; et al. Attenuation by leptin of the effects of fasting on ovarian function in hens (Gallus domesticus). Reproduction 2003, 126, 739–751. [Google Scholar] [CrossRef]
- Cassy, S.; Metayer, S.; Crochet, S.; Rideau, N.; Collin, A.; Tesseraud, S. Leptin receptor in the chicken ovary: Potential involvement in ovarian dysfunction of ad libitum-fed broiler breeder hens. Reprod. Biol. Endocrinol. 2004, 2, 72. [Google Scholar] [CrossRef][Green Version]
- Antczak, M.; Van Blerkom, J. Temporal and spatial aspects of fragmentation in early human embryos: Possible effects on developmental competence and association with the differential elimination of regulatory proteins from polarized domains. Hum. Reprod. 1999, 14, 429–447. [Google Scholar] [CrossRef]
- Schulz, L.C.; Roberts, R.M. Dynamic changes in leptin distribution in the progression from ovum to blastocyst of the pre-implantation mouse embryo. Reproduction 2011, 141, 767–777. [Google Scholar] [CrossRef][Green Version]
- Littwin, T.; Denker, H.W. Segregation during cleavage in the mammalian embryo? A critical comparison of whole-mount/CLSM and section immunohistochemistry casts doubts on segregation of axis-relevant leptin domains in the rabbit. Histochem. Cell Biol. 2011, 135, 553–570. [Google Scholar] [CrossRef]
- Brannian, J.D.; Schmidt, S.M.; Kreger, D.O.; Hansen, K.A. Baseline non-fasting serum leptin concentration to body mass index ratio is predictive of IVF outcomes. Hum. Reprod. 2001, 16, 1819–1826. [Google Scholar] [CrossRef]
- Li, L.; Ferin, M.; Sauer, M.V.; Lobo, R.A. Ovarian adipocytokines are associated with early in vitro human embryo development independent of the action of ovarian insulin. J. Assist. Reprod. Genet. 2012, 29, 1397–1404. [Google Scholar] [CrossRef]
- Gonzalez, R.R.; Caballero-Campo, P.; Jasper, M.; Mercader, A.; Devoto, L.; Pellicer, A.; Simon, C. Leptin and leptin receptor are expressed in the human endometrium and endometrial leptin secretion is regulated by the human blastocyst. J. Clin. Endocrinol. Metab. 2000, 85, 4883–4888. [Google Scholar] [CrossRef]
- Cervero, A.; Horcajadas, J.A.; MartIn, J.; Pellicer, A.; Simon, C. The leptin system during human endometrial receptivity and preimplantation development. J. Clin. Endocrinol. Metab. 2004, 89, 2442–2451. [Google Scholar] [CrossRef]
- Kawamura, K.; Sato, N.; Fukuda, J.; Kodama, H.; Kumagai, J.; Tanikawa, H.; Murata, M.; Tanaka, T. The role of leptin during the development of mouse preimplantation embryos. Mol. Cell Endocrinol. 2003, 202, 185–189. [Google Scholar] [CrossRef]
- Fedorcsak, P.; Storeng, R. Effects of leptin and leukemia inhibitory factor on preimplantation development and STAT3 signaling of mouse embryos in vitro. Biol. Reprod. 2003, 69, 1531–1538. [Google Scholar] [CrossRef]
- Herrid, M.; Nguyen, V.L.; Hinch, G.; McFarlane, J.R. Leptin has concentration and stage-dependent effects on embryonic development in vitro. Reproduction 2006, 132, 247–256. [Google Scholar] [CrossRef][Green Version]
- Abecia, J.; Forcada, F.; Palacín, I.; Sánchez-Prieto, L.; Sosa, C.; Fernández-Foren, A.; Meikle, A. Undernutrition affects embryo quality of superovulated ewes. Zygote 2015, 23, 116–124. [Google Scholar] [CrossRef]
- Gonzalez-Añover, P.; Encinas, T.; Torres-Rovira, L.; Sanz, E.; Pallares, P.; Ros, J.; Gomez-Izquierdo, E.; Sanchez-Sanchez, R.; Gonzalez-Bulnes, A. Patterns of Corpora Lutea Growth and Progesterone Secretion in Sows with Thrifty Genotype and Leptin Resistance due to Leptin Receptor Gene Polymorphisms (Iberian Pig). Reprod. Domest. Anim. 2011, 46, 1011–1016. [Google Scholar] [CrossRef]
- Craig, J.A.; Zhu, H.; Dyce, P.W.; Wen, L.; Li, J. Leptin enhances porcine preimplantation embryo development in vitro. Mol. Cell Endocrinol. 2005, 229, 141–147. [Google Scholar] [CrossRef]
- Boelhauve, M.; Sinowatz, F.; Wolf, E.; Paula-Lopes, F.F. Maturation of bovine oocytes in the presence of leptin improves development and reduces apoptosis of in vitro-produced blastocysts. Biol. Reprod. 2005, 73, 737–744. [Google Scholar] [CrossRef]
- Ohkubo, T.; Tanaka, M.; Nakashima, K. Structure and tissue distribution of chicken leptin receptor (cOb-R) mRNA. Biochim. Biophys. Acta 2000, 1491, 303–308. [Google Scholar] [CrossRef]
- Macedo, T.J.S.; Santos, J.M.S.; Bezerra, M.; Éllida, S.; Menezes, V.G.; Gouveia, B.B.; Barbosa, L.M.R.; Lins, T.L.B.G.; Monte, A.P.O.; Barberino, R.S.; et al. Immunolocalization of leptin and its receptor in the sheep ovary and in vitro effect of leptin on follicular development and oocyte maturation. Mol. Cell Endocrinol. 2019, 495, 110506. [Google Scholar] [CrossRef]
- Panda, B.S.; Pandey, S.; Somal, A.; Parmar, M.S.; Bhat, I.A.; Baiju, I.; Bharti, M.K.; Kumar, G.S.; Chandra, V.; Sharma, G.T. Leptin supplementation in vitro improved developmental competence of buffalo oocytes and embryos. Theriogenology 2017, 98, 116–122. [Google Scholar] [CrossRef]
- Sheykhani, H.R.S.; Batavani, R.A.; Najafi, G.R. Protective effect of leptin on induced apoptosis with trichostatin A on buffalo oocytes. Vet. Res. Forum 2016, 7, 99–104. [Google Scholar]
- Kamalamma, P.; Kona, S.S.R.; Chakravarthi, V.P.; Kumar, A.V.N.S.; Punyakumari, B.; Rao, V.H. Effect of leptin on in vitro development of ovine preantral ovarian follicles. Theriogenology 2016, 85, 224–229. [Google Scholar] [CrossRef]
- Córdova, B.; Morato, R.; De Frutos, C.; Bermejo-Álvarez, P.; Paramio, T.; Gutierrez-Adan, A.; Mogas, T.; Paramio, M.-T. Effect of leptin during in vitro maturation of prepubertal calf oocytes: Embryonic development and relative mRNA abundances of genes involved in apoptosis and oocyte competence. Theriogenology 2011, 76, 1706–1715. [Google Scholar] [CrossRef]
- Arias-Alvarez, M.; Bermejo-Alvarez, P.; Gutierrez-Adan, A.; Rizos, D.; Lorenzo, P.L.; Lonergan, P. Effect of leptin supplementation during in vitro oocyte maturation and embryo culture on bovine embryo development and gene expression patterns. Theriogenology 2011, 75, 887–896. [Google Scholar] [CrossRef]
- Arias-Alvarez, M.; Garcia-Garcia, R.M.; Torres-Rovira, L.; Gonzalez-Bulnes, A.; Rebollar, P.G.; Lorenzo, P.L. Influence of leptin on in vitro maturation and steroidogenic secretion of cumulus-oocyte complexes through JAK2/STAT3 and MEK 1/2 pathways in the rabbit model. Reproduction 2010, 139, 523–532. [Google Scholar] [CrossRef]
- Ye, Y.; Kawamura, K.; Sasaki, M.; Kawamura, N.; Groenen, P.; Gelpke, M.D.S.; Kumagai, J.; Fukuda, J.; Tanaka, T. Leptin and ObRa/MEK signalling in mouse oocyte maturation and preimplantation embryo development. Reprod. Biomed. Online 2009, 19, 181–190. [Google Scholar] [CrossRef]
- Richards, J.S.; Liu, Z.; Kawai, T.; Tabata, K.; Watanabe, H.; Suresh, D.; Kuo, F.-T.; Pisarska, M.D.; Shimada, M. Adiponectin and its receptors modulate granulosa cell and cumulus cell functions, fertility, and early embryo development in the mouse and human. Fertil. Steril. 2012, 98, 471. [Google Scholar] [CrossRef]
- Zhang, N.; Hao, C.; Liu, X.; Zhang, S.; Zhang, F.; Zhuang, L.; Zhao, D. A potential determinant role of adiponectin and receptors for the early embryo development in PCOS patients with obesity hinted by quantitative profiling. Gynecol. Endocrinol. 2017, 33, 113–118. [Google Scholar] [CrossRef]
- Cikos, S.; Burkus, J.; Bukovska, A.; Fabian, D.; Rehak, P.; Koppel, J. Expression of adiponectin receptors and effects of adiponectin isoforms in mouse preimplantation embryos. Hum. Reprod. 2010, 25, 2247–2255. [Google Scholar] [CrossRef]
- Chappaz, E.; Albornoz, M.S.; Campos, D.; Che, L.; Palin, M.-F.; Murphy, B.D.; Bordignon, V. Adiponectin enhances in vitro development of swine embryos. Domest. Anim. Endocrinol. 2008, 35, 198–207. [Google Scholar] [CrossRef]
- Houde, A.A.; Murphy, B.D.; Mathieu, O.; Bordignon, V.; Palin, M.F. Characterization of swine adiponectin and adiponectin receptor polymorphisms and their association with reproductive traits. Anim. Genet. 2008, 39, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Zglejc-Waszak, K.; Waszkiewicz, E.M.; Franczak, A. Periconceptional undernutrition affects the levels of DNA methylation in the peri-implantation pig endometrium and in embryos. Theriogenology 2019, 123, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Maillard, V.; Uzbekova, S.; Guignot, F.; Perreau, C.; Rame, C.; Coyral-Castel, S.; Dupont, J. Effect of adiponectin on bovine granulosa cell steroidogenesis, oocyte maturation and embryo development. Reprod. Biol. Endocrin. 2010, 8. [Google Scholar] [CrossRef]
- Chabrolle, C.; Tosca, L.; Crochet, S.; Tesseraud, S.; Dupont, J. Expression of adiponectin and its receptors (AdipoR1 and AdipoR2) in chicken ovary: Potential role in ovarian steroidogenesis. Domest. Anim. Endocrinol. 2007, 33, 480–487. [Google Scholar] [CrossRef]
- Revollo, J.R.; Körner, A.; Mills, K.F.; Satoh, A.; Wang, T.; Garten, A.; Dasgupta, B.; Sasaki, Y.; Wolberger, C.; Townsend, R.R.; et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007, 6, 363–375. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, R.; Li, X.; Zhu, Q.; Liao, Y.; Tao, T.; Kang, X.; Liu, W.; Li, S.; Sun, Y. High concentration of chemerin caused by ovarian hyperandrogenism may lead to poor IVF outcome in polycystic ovary syndrome: A pilot study. Gynecol. Endocrinol. 2019, 35, 1072–1077. [Google Scholar] [CrossRef]
- Mellouk, N.; Bongrani, A.; Christelle, R.; Fabrice, G.; Joelle, D. Exploration of chemerin system in human granulosa cells: A new insight for polycystic ovarian syndrome. Hum. Reprod. 2018, 33, 34–35. [Google Scholar]




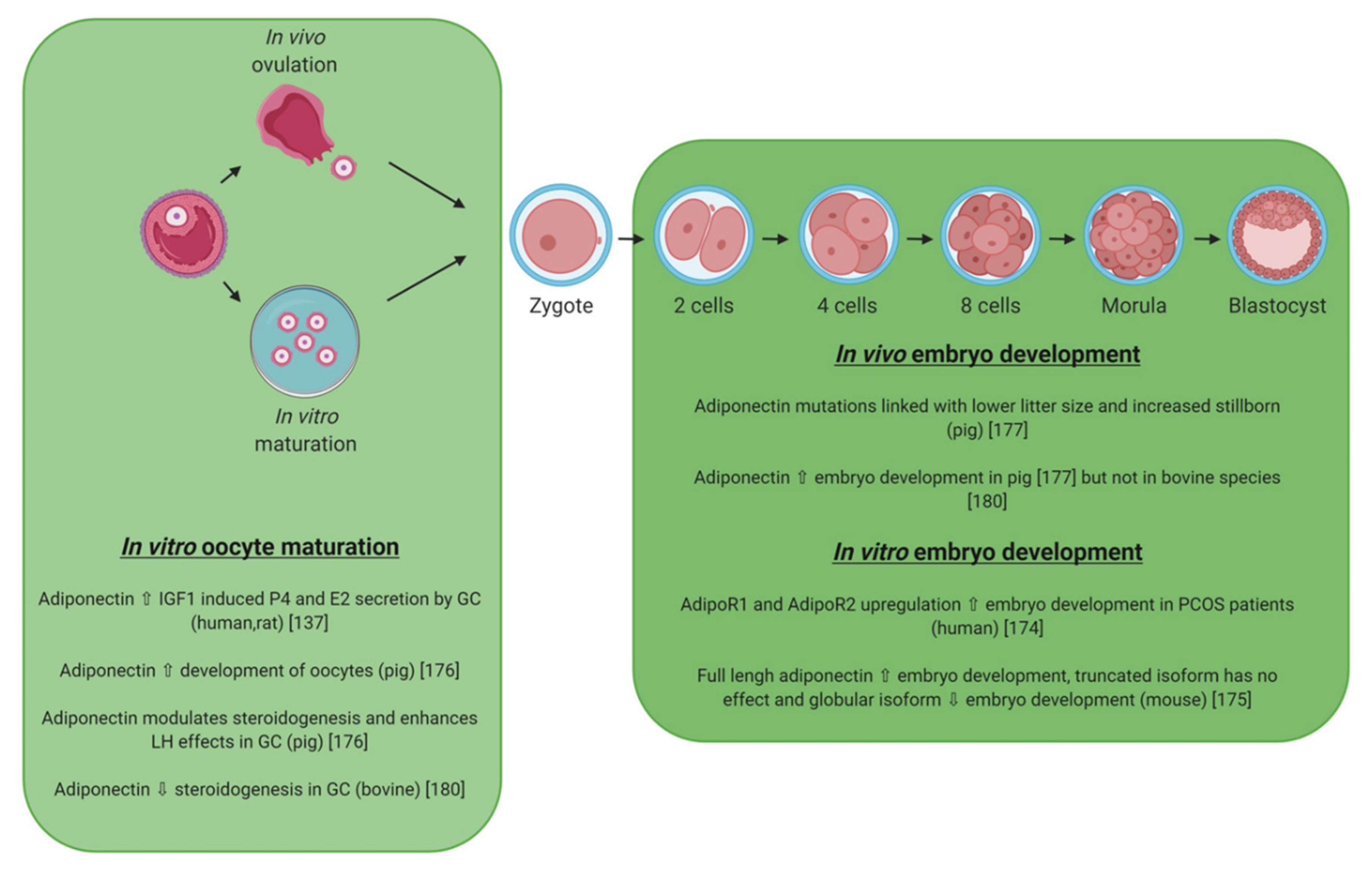
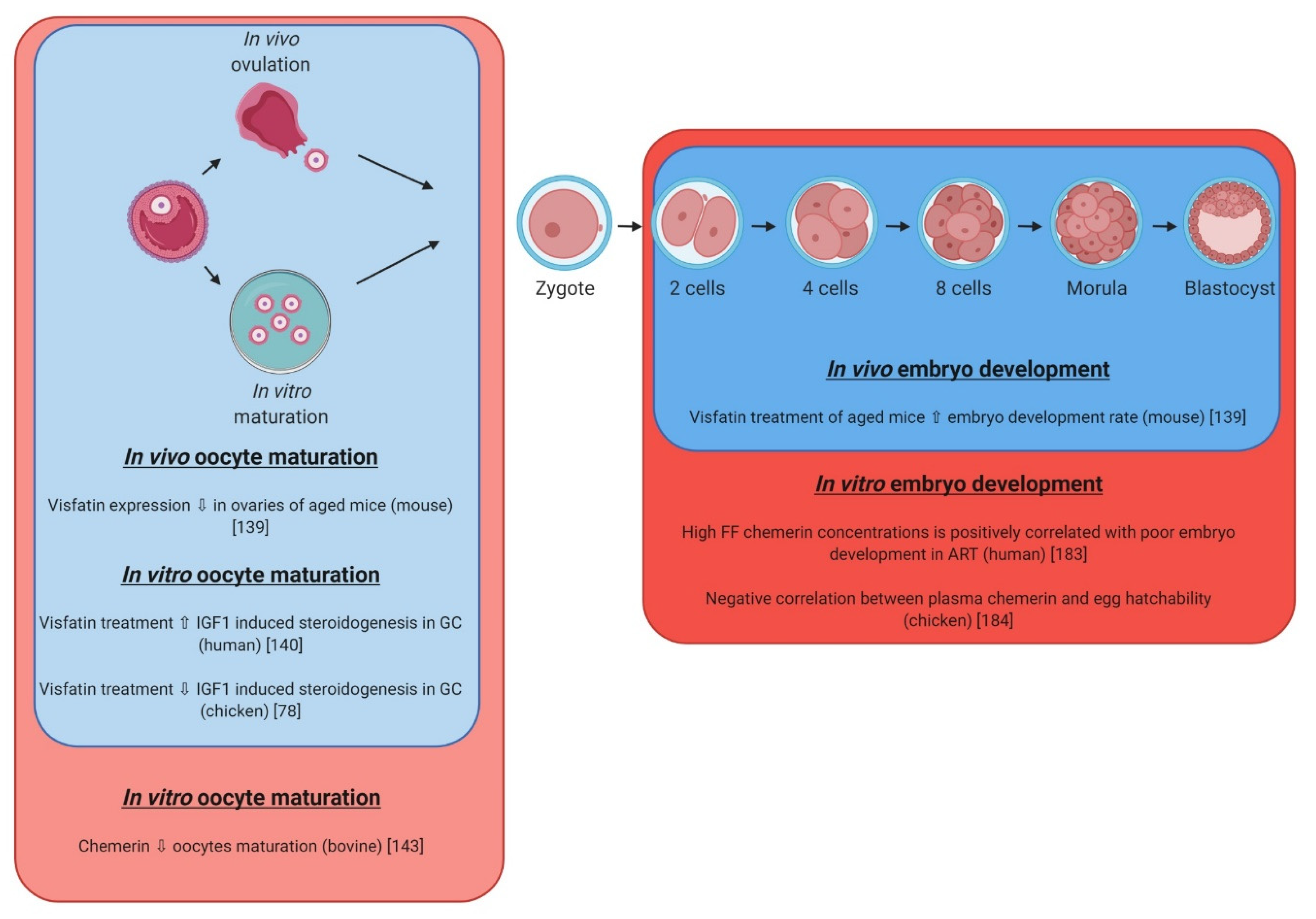
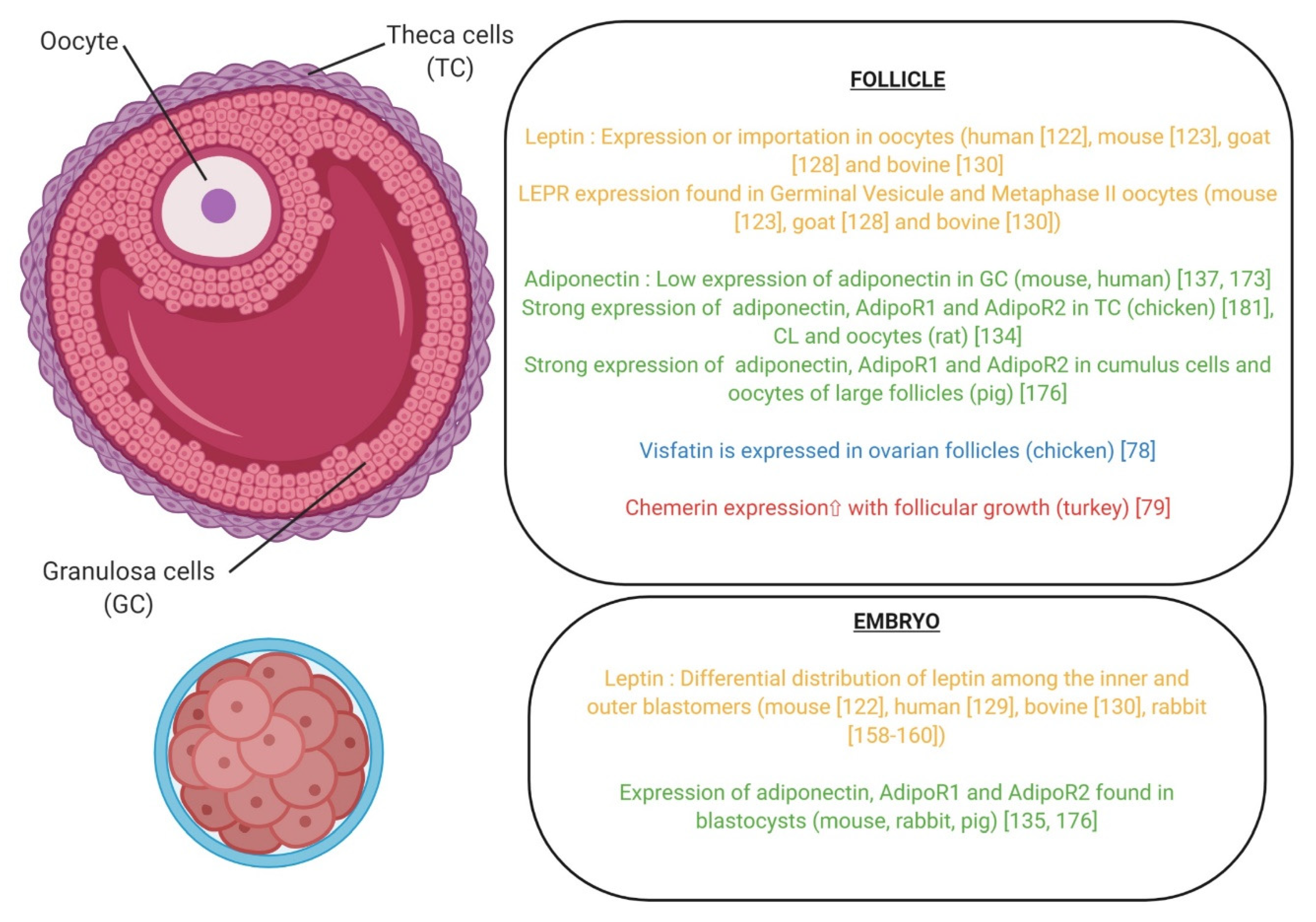
| Protein (Name) | Gene | Gene Location | Protein (Description) | Synthesis | References |
|---|---|---|---|---|---|
| Leptin | LEP/OB | 7q32.1 | 167 aa | WAT but also placenta | [53] |
| Leptin Receptor | LEPR | 1p31.3. | 1.165 aa (6 isoforms, a to f). LepR-b has intracellular signaling. LepR-e is a soluble receptor and binds plasma leptin. | LepR-b: strongly expressed in hypothalamus, but also in skeletal muscle. Ubiquitous | [54,55] |
| Adiponectin | ADIPOQ | 3q27.3. | 244 aa Full-length and Globular adiponectin (f and gADN) Three types of complex:Low molecular weight (LMW, 67 kDa), Middle molecular weight (MMW, 136 kDa), High molecular weight (HMW, >300 kDa). | Adipocytes | [56] |
| ADIPOR1 | ADIPO R1 | 1q32.1 | 375 aa | Skeletal muscles and ubiquitously | [56,57] |
| ADIPOR2 | ADIPO R2 | 12p13.33 | 386 aa | Liver and ubiquitously | [56,57] |
| Visfatin | NAMPT | 7q22.3 | 491 aa | ubiquitously | [58] |
| Chemerin | RARRES2 | 7q36.1 | 163 aa | White adipose tissue and liver | [59,60] |
| CMKLR1 | CMKLR1 | 12q23.3 | 373 aa | ubiquitously | [60,61,62] |
| GPR1 | GPR1 | 2q33.3 | 355 aa | ubiquitously | [60,61,62] |
| CCRL2 | CCRL2 | 3p21.31 | 344 aa | ubiquitously | [60,61,62] |
| Protein (Name) | Gene | Gene Location | Protein (Description) | Synthesis | References |
|---|---|---|---|---|---|
| Leptin | LEP | Chr 1 (1p) | 198 aa | mainly in brain and pituitary | [67,70,73] |
| Leptin Receptor | LEPR | Chr8 | 1146 aa | ubiquitously | [74] |
| Adiponectin | ADIPOQ | Chr9 | 244 aa | mainly fat tissue, heart, stomach and skin and ubiquitously | [75] |
| ADIPOR1 | ADIPOR1 | Chr32 | 376 aa | ubiquitously | [76,77] |
| ADIPOR2 | ADIPOR2 | Chr1 | 387 aa | ubiquitously | [76,77] |
| Visfatin | NAMPT | Chr1 | 493 aa | ubiquitously | [78,79,80] |
| Chemerin | RARRES2 | Chr2 | 162 aa | mainly liver (turkey) | [79] |
| CMKLR1 | CMKLR1 | Chr15 | 360 aa | ubiquitously | [79] |
| GPR1 | GPR1 | Chr7 | 420 aa | ubiquitously | [79] |
| CCRL2 | CCRL2 | Nd | nd | Pectoralis muscle and ubiquitously | [79] |
| Protein | Leptin | Leptin Receptor | Adiponectin | ADIPOR1 | ADIPOR2 | Visfatin | Chemerin | CMKLR1 | GPR1 | CCRL2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Identity % | 30 | 47 | 57 | 91 | 82 | 94 | 36 | 56 | 64 | nd |
| Accession number or ref. | [81] | P48357 and Q9I8V6 | [82] | [77] | [77] | [80] | Q99969 and A0A0K0PUH6 | Q99788 and A0A1D5P7P2 | F1NYB0 and P46091 |
| Adipokine Component Modified | Genetic Transformation | Ovarian Consequences | References |
|---|---|---|---|
| Leptin | Total Knockout (KO) | no mature follicles or corpora lutea were detected suppression of ovarian folliculogenesis and increase in ovarian granulosa cell apoptosis | [117] |
| Leptin-R | -Total KO (db mice) -Conditional deletion (cells expressing LH-β) | -Reduction of ovarian functions that are not due to Leptin-R expression in ovary -Reduction of litter size | [118,119] |
| Adiponectin | -Total KO | -Reduction of oocytes retrieval, disruption of estrous cycle, elevation of atretic follicles number, and decrease in late folliculogenesis | [120] |
| Protein | Oocytes | Embryos |
|---|---|---|
| Leptin | Mice (protein, [123]), Goat (protein, [127]), Human ([121,122]; Bovine [128] | Mouse and Human [122], Bovine [128], Rabbit [129], Chicken [130] |
| Leptin receptor | Rodent (protein, [123]), Goat (protein [127]), Bovine [128] | Chicken [131], Bovine [128] |
| Adiponectin | Rat [132], Bovine [9] | Rabbit [133], Chicken [81] |
| Adiponectin Receptors | Rat [132], Bovine [9], Pig [134], and Human [135] | Rabbit [133], Chicken [81,136], Pig [134] |
| Visfatin | Rodent [137], Human [138], Bovine [139] | Chicken [140] |
| Chemerin | Bovine [141] | Chicken [140] |
| Chemerin receptors | Bovine [141] | Chicken [140] |
| Cell Type | Species | Time of Incubation | Dose ng/mL | Origin of Leptin | Medium | Effects on Oocyte and/or Embryo | References |
|---|---|---|---|---|---|---|---|
| -Secondary follicle | sheep | 18 days | 25 | human | α-MEM+ |  MII (%) MII (%) | [171] |
| COCs | buffalo | 24 h (IVM), 48 h (cleavage rate) and day 8 post IVF (blastocyst rate) | 10 | nd | TCM-199 (IVM) FerTALP (IVC) |  cleavage and blastocyst rate cleavage and blastocyst rate | [172] |
| COCs | buffalo | 24 h (IVM) | 10 and 50 | mouse | TCM-199 |  oocyte nuclear maturation oocyte nuclear maturation | [173] |
| Preantral follicles and COCs | sheep | 6 days and 24 h for IVM | 10 | human | TCM-199 |  MII (%) MII (%) | [174] |
| COCs | buffalo | 24 h (IVM) | 10 | mouse | TCM-199 |  MII (%) MII (%) | [148] |
| COCs | calf | 24 h (IVM) | 1 or 10 | nd | TCM-199 |  MII (%) MII (%) | [143] |
| COCs | Prepubertal calf | 24 h (IVM) 48 h (cleavage) 8 days (blastocyst) | 10, 100 or 1000 | human | TCM-199 (IVM)FerTALP (IVC) | No effect on cleavage and blastocyst levels | [175] |
| COCs | bovine | 24 h (IVM) 7 days (blastocyst) | 10, 100 | human | TCM-199 FerTALP (IVC) |  cleavage rate and blastocyst yield with leptin 100 ng/mL cleavage rate and blastocyst yield with leptin 100 ng/mL | [176] |
| COCs | rabbit | 16 h (IVM) | 1, 10, 100 | nd | TCM-199 |  MII (%) MII (%)(nuclear oocyte maturation) | [177] |
| COCs | horse | 28 to 30 h (IVM) | 100 | human | TCM-199 |  in vitro oocyte maturation in vitro oocyte maturation | [145] |
| COCs | mouse | 24 h (IVM) | 10 | mouse | M16 |  oocyte nuclear maturation oocyte nuclear maturation | [178] |
| COCs | bovine | 20–22 h (IVM) | 1, 10 | human | TCM-199 |  MII (%) MII (%) | [144] |
| COCs | bovine | 22–24 h (IVM) 7 days (blastocyst) | 1, 10, 100 | human | TCM-199FerTALP (IVC) |  No effect on the cleavage rate but number of cells in blastocysts No effect on the cleavage rate but number of cells in blastocysts | [169] |
| COCs | pig | 24–48 h (IVM) | 10, 100, 1000 | human | TCM-199 |  MII (%) MII (%)with 10 and 100 ng/mL | [146] |
| Preovulatory follicle-enclosed oocytes | mouse | 24 h (IVM) | 10, 100, 1000 | human | TCM-199 |  GVBD (%) GVBD (%) | [123] |
 : increase;
: increase;  : decrease.
: decrease.© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estienne, A.; Brossaud, A.; Reverchon, M.; Ramé, C.; Froment, P.; Dupont, J. Adipokines Expression and Effects in Oocyte Maturation, Fertilization and Early Embryo Development: Lessons from Mammals and Birds. Int. J. Mol. Sci. 2020, 21, 3581. https://doi.org/10.3390/ijms21103581
Estienne A, Brossaud A, Reverchon M, Ramé C, Froment P, Dupont J. Adipokines Expression and Effects in Oocyte Maturation, Fertilization and Early Embryo Development: Lessons from Mammals and Birds. International Journal of Molecular Sciences. 2020; 21(10):3581. https://doi.org/10.3390/ijms21103581
Chicago/Turabian StyleEstienne, Anthony, Adeline Brossaud, Maxime Reverchon, Christelle Ramé, Pascal Froment, and Joëlle Dupont. 2020. "Adipokines Expression and Effects in Oocyte Maturation, Fertilization and Early Embryo Development: Lessons from Mammals and Birds" International Journal of Molecular Sciences 21, no. 10: 3581. https://doi.org/10.3390/ijms21103581
APA StyleEstienne, A., Brossaud, A., Reverchon, M., Ramé, C., Froment, P., & Dupont, J. (2020). Adipokines Expression and Effects in Oocyte Maturation, Fertilization and Early Embryo Development: Lessons from Mammals and Birds. International Journal of Molecular Sciences, 21(10), 3581. https://doi.org/10.3390/ijms21103581






