3-Hydroxytyrosol Promotes Angiogenesis In Vitro by Stimulating Endothelial Cell Migration
Abstract
:1. Introduction
2. Results
2.1. Evaluation of HT Effect on Human Umbilical Vascular Endothelial Cells
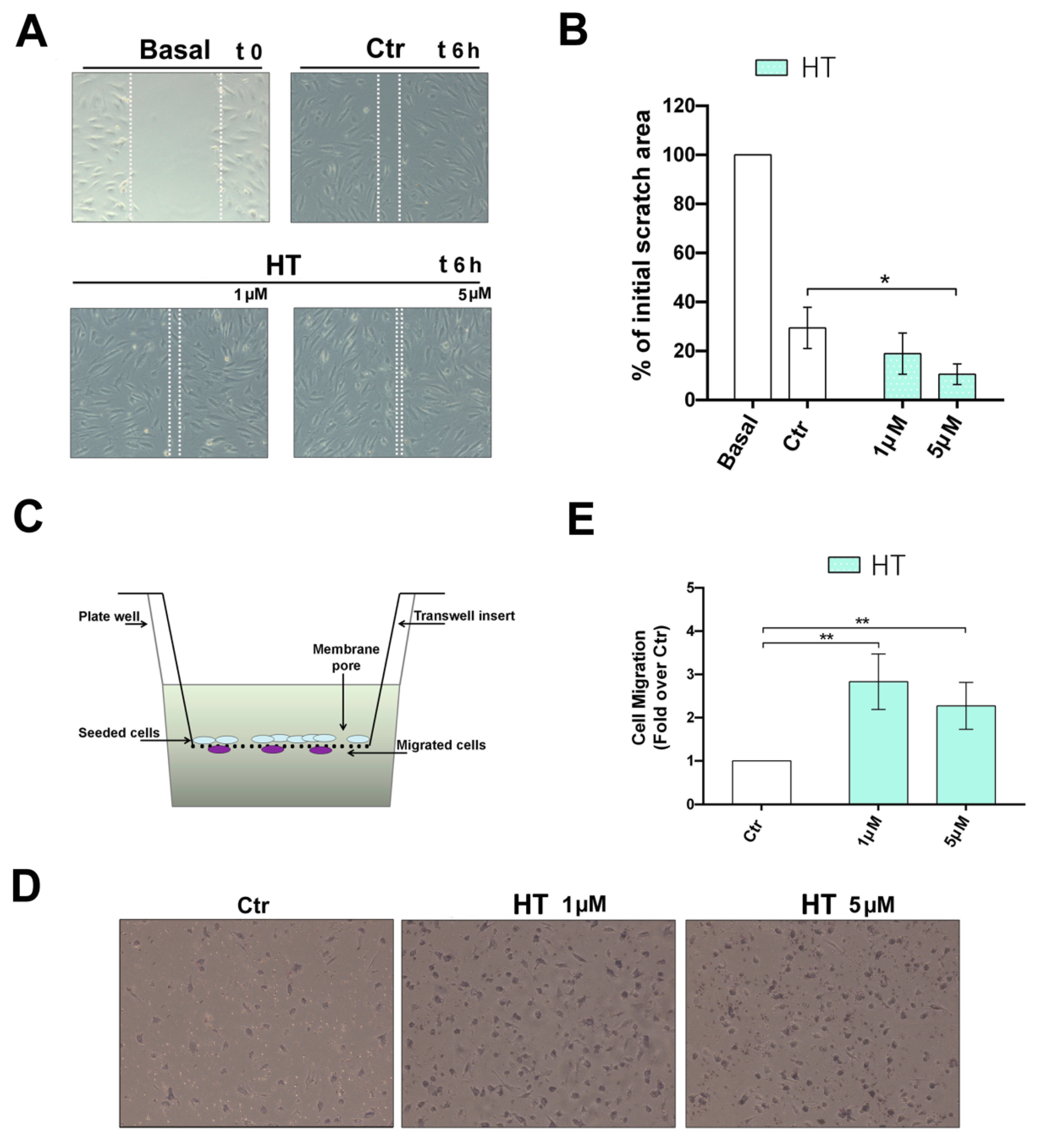
2.2. Improvement of the Migratory Capacity of Endothelial Cells Exposed to HT
2.3. HT Induces the Expression of Migration-Linked Proteins
2.4. HT Induces Capillary Network Formation
2.5. HT Induces the Expression of Angiogenesis Key Proteins
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Cells
4.3. Determination of Cell Viability by MTT Assay
4.4. Determination of Cell Proliferation by BrdU Assay
4.5. Scratch Wound Healing Assay
4.6. Boyden Chamber Cell Migration Assay
4.7. Capillary-Like Tube Formation on Gel
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hong, X.Y.; Lin, J.; Gu, W.W. Risk factors and therapies in vascular diseases: An umbrella review of updated systematic reviews and meta-analyses. J. Cell Physiol. 2019, 234, 8221–8232. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; De Pergola, G. The Mediterranean Diet: Its definition and evaluation of a priori dietary indexes in primary cardiovascular prevention. Int. J. Food Sci. Nutr. 2018, 69, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Barak, Y.; Fridman, D. Impact of Mediterranean Diet on Cancer: Focused Literature Review. Cancer Genom. Proteom. 2017, 14, 403–408. [Google Scholar]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [Green Version]
- Hu, T.; He, X.W.; Jiang, J.G.; Xu, X.L. Hydroxytyrosol and its potential therapeutic effects. J. Agric. Food Chem. 2014, 62, 1449–1455. [Google Scholar] [CrossRef]
- Tejada, S.; Pinya, S.; Del Mar Bibiloni, M.; Tur, J.A.; Pons, A.; Sureda, A. Cardioprotective Effects of the Polyphenol Hydroxytyrosol from Olive Oil. Curr. Drug Targets 2017, 18, 1477–1486. [Google Scholar] [CrossRef]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, E2001. [Google Scholar] [CrossRef] [Green Version]
- Peyrol, J.; Riva, C.; Amiot, M.J. Hydroxytyrosol in the Prevention of the Metabolic Syndrome and Related Disorders. Nutrients 2017, 9, E306. [Google Scholar] [CrossRef]
- Covas, M.I.; Nyyssönen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.J.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Bäumler, H.; et al. EUROLIVE Study Group. The effect of polyphenols in olive oil on heart disease risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef]
- Dell’Agli, M.; Maschi, O.; Galli, G.V.; Fagnani, R.; Dal Cero, E.; Caruso, D.; Bosisio, E. Inhibition of platelet aggregation by olive oil phenols via cAMP-phosphodiesterase. Br. J. Nutr. 2008, 99, 945–951. [Google Scholar] [CrossRef] [Green Version]
- Petroni, A.; Blasevich, M.; Salami, M.; Papini, N.; Montedoro, G.F.; Galli, C. Inhibition of platelet aggregation and eicosanoid production by phenolic components of olive oil. Thromb. Res. 1995, 78, 151–160. [Google Scholar] [CrossRef]
- González-Santiago, M.; Martín-Bautista, E.; Carrero, J.J.; Fonollá, J.; Baró, L.; Bartolomé, M.V.; Gil-Loyzaga, P.; López-Huertas, E. One-month administration of hydroxytyrosol, a phenolic antioxidant present in olive oil, to hyperlipemic rabbits improves blood lipid profile, antioxidant status and reduces atherosclerosis development. Atherosclerosis 2006, 188, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Manna, C.; Napoli, D.; Cacciapuoti, G.; Porcelli, M.; Zappia, V. Olive oil phenolic compounds inhibit homocysteine-induced endothelial cell adhesion regardless of their different antioxidant activity. J. Agric. Food Chem. 2009, 57, 3478–3482. [Google Scholar] [CrossRef] [PubMed]
- De Pablos, R.M.; Espinosa-Oliva, A.M.; Hornedo-Ortega, R.; Cano, M.; Arguelles, S. Hydroxytyrosol protects from aging process via AMPK and autophagy; a review of its effects on cancer, metabolic syndrome, osteoporosis, immune-mediated and neurodegenerative diseases. Pharmacol. Res. 2019, 143, 58–72. [Google Scholar] [CrossRef]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- D’Angelo, S.; Manna, C.; Migliardi, V.; Mazzoni, O.; Morrica, P.; Capasso, G.; Pontoni, G.; Galletti, P.; Zappia, V. Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil. Drug Metab. Dispos. 2001, 29, 1492–1498. [Google Scholar]
- European Commission Regulation EC No. 1924/2006 on Nutrition and Health Claims Made on Foods. Off. J. Eur. Union 2012, L310, 36. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02006R1924-20121129&from=EN (accessed on 7 May 2020).
- European Food Safety Authority (EFSA). Safety of hydroxytyrosol as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, 4728. [Google Scholar]
- Serreli, G.; Deiana, M. Biological Relevance of Extra Virgin Olive Oil Polyphenols Metabolites. Antioidants (Basel) 2018, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Ergul, A.; Alhusban, A.; Fagan, S.C. Angiogenesis: A harmonized target for recovery after stroke. Stroke 2012, 43, 2270–2274. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Morató, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Pérez-Mañá, C.; Khymenets, O.; Fitó, M.; Farré, M.; et al. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug Metab. Rev. 2016, 48, 218–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamalice, L.; Le Boeuf, F.; Huot, J. Endothelial cell migration during angiogenesis. Circ. Res. 2007, 100, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, D.; Cui, Y.; Zhang, W.; Weng, J.; Yu, L.; Chen, L.; Chen, Z.; Su, H.; Yu, S.; et al. Src Plays an Important Role in AGE-Induced Endothelial Cell Proliferation, Migration, and Tubulogenesis. Front. Physiol. 2018, 9, 765. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Wang, Y.C.; Hu, Z.B.; Xu, H.Y.; Sun, J.; Gao, Y.; Li, X.T.; Yang, C.B.; Xie, C.; Li, C.F.; et al. Simulated Microgravity Promotes Angiogenesis through RhoA-Dependent Rearrangement of the Actin Cytoskeleton. Cell Physiol. Biochem. 2017, 41, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.; Zhou, Z.; Jiang, B.; Lou, Y.; Guo, X. Autocrine VEGF and IL-8 Promote Migration via Src/Vav2/Rac1/PAK1 Signaling in Human Umbilical Vein Endothelial Cells. Cell Physiol. Biochem. 2017, 41, 1346–1359. [Google Scholar] [CrossRef]
- Ho, P.Y.; Zhong, W.B.; Ho, Y.S.; Lee, W.S. Terbinafine inhibits endothelial cell migration through suppression of the Rho-mediated pathway. Mol. Cancer Ther. 2006, 5, 3130–3138. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Dang, Y.; Zhou, X.; Huang, B.; Huang, X.; Zhang, Z.; Kwan, Y.W.; Chan, S.W.; Leung, G.P.; Lee, S.M.; et al. Formononetin promotes angiogenesis through the estrogen receptor alpha-enhanced ROCK pathway. Sci. Rep. 2015, 5, 16815. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, H.; Yan, L.; Du, W.; Zhang, M.; Chen, H.; Zhang, L.; Li, G.; Li, J.; Dong, Y.; et al. MMP-2 and MMP-9 contribute to the angiogenic effect produced by hypoxia/15-HETE in pulmonary endothelial cells. J. Mol. Cell Cardiol. 2018, 121, 36–50. [Google Scholar] [CrossRef]
- Huang, J.J.; Shi, Y.Q.; Li, R.L.; Hu, A.; Lu, Z.Y.; Weng, L.; Hao, C.N. Angiogenesis effect of therapeutic ultrasound on HUVECs through activation of the PI3K-Akt-eNOS signal pathway. Am. J. Transl. Res. 2015, 7, 1106–1115. [Google Scholar]
- Cicero, A.F.; Nascetti, S.; López-Sabater, M.C.; Elosua, R.; Salonen, J.T.; Nyyssönen, K.; Poulsen, H.E.; Zunft, H.J.; Kiesewetter, H.; de la Torre, K.; et al. Changes in LDL fatty acid composition as a response to olive oil treatment are inversely related to lipid oxidative damage: The EUROLIVE study. J. Am. Coll. Nutr. 2008, 27, 314–320. [Google Scholar] [CrossRef]
- Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; Lamuela-Raventos, R.M.; Serra-Majem, L.; Pintó, X.; Basora, J.; Muñoz, M.A.; Sorlí, J.V.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar]
- Toledo, E.; Salas-Salvadó, J.; Donat-Vargas, C.; Buil-Cosiales, P.; Estruch, R.; Ros, E.; Corella, D.; Fitó, M.; Hu, F.B.; Arós, F.; et al. Mediterranean Diet and Invasive Breast Cancer Risk Among Women at High Cardiovascular Risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Medina-Remón, A.; Casas, R.; Tressserra-Rimbau, A.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventos, R.M.; Estruch, R.; et al. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: A substudy of the PREDIMED trial. Br. J. Clin. Pharmacol. 2017, 83, 114–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Santiago, M.; Fonollá, J.; Lopez-Huertas, E. Human absorption of a supplement containing purified hydroxytyrosol, a natural antioxidant from olive oil, and evidence for its transient association with low-density lipoproteins. Pharmacol. Res. 2010, 61, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Huertas, E.; Fonolla, J. Hydroxytyrosol supplementation increases vitamin C levels in vivo. A human volunteer trial. Redox Biol. 2017, 11, 384–389. [Google Scholar] [CrossRef]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; de la Torre, R.; Martínez-González, M.Á.; Martínez-Lapiscina, E.H.; Fitó, M.; Pérez-Heras, A.; Salas-Salvadó, J.; et al. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Lapiscina, E.H.; Clavero, P.; Toledo, E.; San Julián, B.; Sanchez-Tainta, A.; Corella, D.; Lamuela-Raventós, R.M.; Martínez, J.A.; Martínez-Gonzalez, M.Á. Virgin olive oil supplementation and long-term cognition: The PREDIMED-NAVARRA randomized, trial. J. Nutr. Health Aging 2013, 17, 544–552. [Google Scholar] [CrossRef]
- Deveza, L.; Choi, J.; Yang, F. Therapeutic angiogenesis for treating cardiovascular diseases. Theranostics 2012, 2, 801–814. [Google Scholar] [CrossRef]
- Liao, Y.Y.; Chen, Z.Y.; Wang, Y.X.; Lin, Y.; Yang, F.; Zhou, Q.L. New progress in angiogenesis therapy of cardiovascular disease by ultrasound targeted microbubble destruction. BioMed Res. Int. 2014, 2014, 872984. [Google Scholar] [CrossRef]
- Martínez-Lara, E.; Peña, A.; Calahorra, J.; Cañuelo, A.; Siles, E. Hydroxytyrosol decreases the oxidative and nitrosative stress levels and promotes angiogenesis through HIF-1 independent mechanisms in renal hypoxic cells. Food Funct. 2016, 7, 540–548. [Google Scholar] [CrossRef]
- Lamy, S.; Ouanouki, A.; Béliveau, R.; Desrosiers, R.R. Olive oil compounds inhibit vascular endothelial growth factor receptor-2 phosphorylation. Exp. Cell Res. 2014, 322, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Calabriso, N.; Massaro, M.; Scoditti, E.; D’Amore, S.; Gnoni, A.; Pellegrino, M.; Storelli, C.; De Caterina, R.; Palasciano, G.; Carluccio, M.A. Extra virgin olive oil rich in polyphenols modulates VEGF-induced angiogenic responses by preventing NADPH oxidase activity and expression. J. Nutr. Biochem. 2016, 28, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Calabriso, N.; Massaro, M.; Pellegrino, M.; Storelli, C.; Martines, G.; De Caterina, R.; Carluccio, M.A. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: A potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch. Biochem. Biophys. 2012, 527, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Krkl, C.; Yiğit, M.V.; Özercan, İ.H.; Aygen, E.; Gültürk, B.; Artaş, G. The Effect of Ozonated Olive Oil on Neovascularizatıon in an Experimental Skin Flap Model. Adv. Skin Wound Care 2016, 29, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Fortes, C.; García-Vilas, J.A.; Quesada, A.R.; Medina, M.A. Evaluation of the anti-angiogenic potential of hydroxytyrosol and tyrosol, two bio-active phenolic compounds of extra virgin olive oil, in endothelial cell cultures. Food Chem. 2012, 134, 134–140. [Google Scholar] [CrossRef]
- Silva, S.; Sepodes, B.; Rocha, J.; Direito, R.; Fernandes, A.; Brites, D.; Freitas, M.; Fernandes, E.; Bronze, M.R.; Figueira, M.E. Protective effects of hydroxytyrosol-supplemented refined olive oil in animal models of acute inflammation and rheumatoid arthritis. J. Nutr. Biochem. 2015, 26, 360–368. [Google Scholar] [CrossRef]
- García-Vilas, J.A.; Quesada, A.R.; Medina, M.Á. Hydroxytyrosol targets extracellular matrix remodeling by endothelial cells and inhibits both ex vivo and in vivo angiogenesis. Food Chem. 2017, 221, 1741–1746. [Google Scholar] [CrossRef]
- Pastor, A.; Rodríguez-Morató, J.; Olesti, E.; Pujadas, M.; Pérez-Mañá, C.; Khymenets, O.; Fitó, M.; Covas, M.I.; Solá, R.; Motilva, M.J.; et al. Analysis of free hydroxytyrosol in human plasma following the administration of olive oil. J. Chromatogr. A 2016, 1437, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Visioli, F.; Davalos, A.; López de Las Hazas, M.C.; Crespo, M.C.; Tomé-Carneiro, J. An overview of the pharmacology of olive oil and its active ingredients. Br. J. Pharmacol. 2020, 177, 1316–1330. [Google Scholar] [CrossRef] [Green Version]
- Zrelli, H.; Matsuoka, M.; Kitazaki, S.; Araki, M.; Kusunoki, M.; Zarrouk, M.; Miyazaki, H. Hydroxytyrosol induces proliferation and cytoprotection against oxidative injury in vascular endothelial cells: Role of Nrf2 activation and HO-1 induction. J. Agric. Food Chem. 2011, 59, 4473–4482. [Google Scholar] [CrossRef]
- Zrelli, H.; Kusunoki, M.; Miyazaki, H. Role of Hydroxytyrosol-dependent Regulation of HO-1 Expression in Promoting Wound Healing of Vascular Endothelial Cells via Nrf2 De Novo Synthesis and Stabilization. Phytother. Res. 2015, 29, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Zrelli, H.; Wu, C.W.; Zghonda, N.; Shimizu, H.; Miyazaki, H. Combined treatment of hydroxytyrosol with carbon monoxide-releasing molecule-2 prevents TNF α-induced vascular endothelial cell dysfunction through NO production with subsequent NFκB inactivation. BioMed Res. Int. 2013, 2013, 912431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, F.; Wang, Y.C.; Zhao, T.Z.; Zhang, S.; Du, T.Y.; Yang, C.B.; Li, Y.H.; Sun, X.Q. Effects of simulated microgravity on human umbilical vein endothelial cell angiogenesis and role of the PI3K-Akt-eNOS signal pathway. PLoS ONE 2012, 7, e40365. [Google Scholar] [CrossRef] [Green Version]
- Ishii, M.; Nakahara, T.; Ikeuchi, S.; Nishimura, M. β-Amyrin induces angiogenesis in vascular endothelial cells through the Akt/endothelial nitric oxide synthase signaling pathway. Biochem. Biophys. Res. Commun. 2015, 467, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.H.; Cho, B.J.; Kim, M.J.; Lim, S.; Park, Y.J.; Jang, H.C.; Choi, S.H. Rosiglitazone increases endothelial cell migration and vascular permeability through Akt phosphorylation. BMC Pharmacol. Toxicol. 2017, 18, 62. [Google Scholar] [CrossRef]
- Wang, J.; He, L.; Huwatibieke, B.; Liu, L.; Lan, H.; Zhao, J.; Li, Y.; Zhang, W. Ghrelin Stimulates Endothelial Cells Angiogenesis through Extracellular Regulated Protein Kinases (ERK) Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 2530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Zhang, Z.; Xu, T.; Lou, Y.; Wang, Q.; Jin, H.; Zhang, L.; Feng, Y.; Xu, H.; Mao, C. Upregulating mTOR/ERK signaling with leonurine for promoting angiogenesis and tissue regeneration in a full-thickness cutaneous wound model. Food Funct. 2018, 9, 2374–2385. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.H.; Ma, Y.; Ruan, C.C.; Yang, Y.; Liu, X.H.; Ge, Q.; Kong, L.R.; Zhang, J.W.; Yan, C.; Gao, P.J. Loss of osteoglycin promotes angiogenesis in limb ischaemia mouse models via modulation of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 signalling pathway. Cardiovasc. Res. 2017, 113, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Chiu, J.H.; Wu, I.H.; Wang, B.W.; Pan, C.M.; Chen, Y.H. Ferulic acid augments angiogenesis via VEGF, PDGF and HIF-1 alpha. J. Nutr. Biochem. 2010, 21, 627–633. [Google Scholar] [CrossRef]
- Arnaoutova, I.; George, J.; Kleinman, H.K.; Benton, G. The endothelial cell tube formation assay on basement membrane turns 20: State of the science and the art. Angiogenesis 2009, 12, 267–274. [Google Scholar] [CrossRef]
- Echeverría, F.; Ortiz, M.; Valenzuela, R.; Videla, L.A. Hydroxytyrosol and Cytoprotection: A Projection for Clinical Interventions. Int. J. Mol. Sci. 2017, 28, E930. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, L.; Chen, A.; Xu, C.; Feng, Q. Protective Effects of Olive Leaf Extract on Acrolein-Exacerbated Myocardial Infarction via an Endoplasmic Reticulum Stress Pathway. Int. J. Mol. Sci. 2018, 7, 493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siefer, S.; Wacker, R.; Wilhelm, M.; Schoen, C. Absorption of Hydroxytyrosol from Different Sources and its Impact on Lipid Status in Human Subjects. J. Nutri. Med. Diet Care 2018, 4, 025. [Google Scholar]
- Pisanti, S.; Picardi, P.; Ciaglia, E.; Margarucci, L.; Ronca, R.; Giacomini, A.; Malfitano, A.M.; Casapullo, A.; Laezza, C.; Gazzerro, P.; et al. Antiangiogenic effects of N6-isopentenyladenosine, an endogenous isoprenoid end product, mediated by AMPK activation. FASEB J. 2014, 28, 1132–1144. [Google Scholar] [CrossRef] [PubMed]
- Ciaglia, E.; Abate, M.; Laezza, C.; Pisanti, S.; Vitale, M.; Seneca, V.; Torelli, G.; Franceschelli, S.; Catapano, G.; Gazzerro, P.; et al. Antiglioma effects of N6-isopentenyladenosine, an endogenous isoprenoid end product, through the downregulation of epidermal growth factor receptor. Int. J. Cancer 2017, 140, 959–972. [Google Scholar] [CrossRef] [Green Version]
- Abate, M.; Laezza, C.; Pisanti, S.; Torelli, G.; Seneca, V.; Catapano, G.; Montella, F.; Ranieri, R.; Notarnicola, M.; Gazzerro, P.; et al. Deregulated expression and activity of Farnesyl Diphosphate Synthase (FDPS) in Glioblastoma. Sci. Rep. 2017, 7, 14123. [Google Scholar] [CrossRef] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abate, M.; Pisanti, S.; Caputo, M.; Citro, M.; Vecchione, C.; Martinelli, R. 3-Hydroxytyrosol Promotes Angiogenesis In Vitro by Stimulating Endothelial Cell Migration. Int. J. Mol. Sci. 2020, 21, 3657. https://doi.org/10.3390/ijms21103657
Abate M, Pisanti S, Caputo M, Citro M, Vecchione C, Martinelli R. 3-Hydroxytyrosol Promotes Angiogenesis In Vitro by Stimulating Endothelial Cell Migration. International Journal of Molecular Sciences. 2020; 21(10):3657. https://doi.org/10.3390/ijms21103657
Chicago/Turabian StyleAbate, Mario, Simona Pisanti, Mariella Caputo, Marianna Citro, Carmine Vecchione, and Rosanna Martinelli. 2020. "3-Hydroxytyrosol Promotes Angiogenesis In Vitro by Stimulating Endothelial Cell Migration" International Journal of Molecular Sciences 21, no. 10: 3657. https://doi.org/10.3390/ijms21103657
APA StyleAbate, M., Pisanti, S., Caputo, M., Citro, M., Vecchione, C., & Martinelli, R. (2020). 3-Hydroxytyrosol Promotes Angiogenesis In Vitro by Stimulating Endothelial Cell Migration. International Journal of Molecular Sciences, 21(10), 3657. https://doi.org/10.3390/ijms21103657







