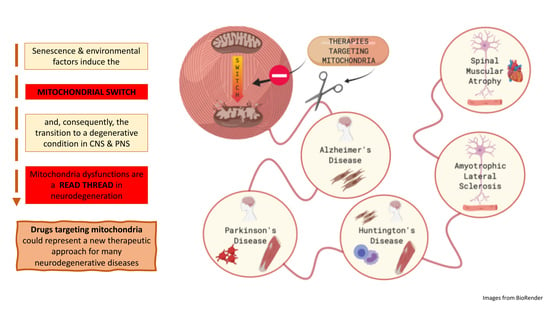
Mitochondrial Dysfunctions: A Red Thread across Neurodegenerative Diseases
Abstract
:1. Introduction: The Peculiarity of Mitochondria
2. The “Mitochondrial Switch” and Its Impact on Neurodegeneration
2.1. Mitochondrial Dysfunctions in AD
2.2. Mitochondrial Dysfunctions in PD
2.3. Mitochondrial Dysfunctions in HD
2.4. Mitochondrial Dysfunctions in ALS
2.5. Mitochondrial Dysfunctions in SMA
3. Are Mitochondria the Red Thread in Neurodegenerative Diseases?
4. Therapies Targeting Mitochondria
4.1. Antioxidant
4.1.1. Synthetic Antioxidants
4.1.2. Natural Antioxidants
4.2. Mitochondrial Biogenesis and Permeability
4.3. Mitochondrial Bioenergetics
4.4. Compounds Targeting Multiple Mitochondrial Dysfunctions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Aco2 | Aconitase 2 |
| CAT | Catalase |
| Cyt C | Cytochrome C |
| DAMPs | Damage-associated molecular patterns |
| DDW | Deuterium depleted water |
| ETC | Electron transport chain |
| Fis1 | Mitochondrial fission 1 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GPX | Glutathione peroxidase |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3pyrin domain-containing 3 |
| NOX | NADPH oxidase |
| Nrf1 | Nuclear respiratory factor 1 |
| OPA1 | Protein optic atrophy 1 |
| PBMCs | Peripheral blood mononuclear cells |
| PGK1 | Phosphoglycerate kinase 1 |
| PINK1 | PTEN-induced kinase 1 |
| SIRT1 | Sirtuin1 |
| SNpc | Substantia nigra pars compacta |
| TCA | Tricarboxylic acid |
| TDP43 | TAR DNA-binding protein 43 |
| TFAM | Mitochondrial transcription factor A |
| TPP+ | Alkyl-triphenylphosphonium |
| TSPO | Translocator protein |
| UCP-2 | Mitochondrial uncoupling protein 2 |
| VDAC | Voltage-dependent ion channel |
| XO | Xanthine oxidase |
References
- Wallace, D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu. Rev. Genet. 2005, 39, 359–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, T.A.; Tkachuk, A.N.; Shtengel, G.; Kopek, B.G.; Bogenhagen, D.F.; Hess, H.F.; Clayton, D.A. Superresolution fluorescence imaging of mitochondrial nucleoids reveals their spatial range, limits, and membrane interaction. Mol. Cell Biol. 2011, 31, 4994–5010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steele, H.E.; Horvath, R.; Lyon, J.J.; Chinnery, P.F. Monitoring clinical progression with mitochondrial disease biomarkers. Brain 2017, 140, 2530–2540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, D.C. Mitochondrial fusion and fission in mammals. Annu. Rev. Cell Dev. Biol. 2006, 22, 79–99. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015; Volume xxxviii, 905p. [Google Scholar]
- Shadel, G.S.; Horvath, T.L. Mitochondrial ROS signaling in organismal homeostasis. Cell 2015, 163, 560–569. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, D.G.; Vesce, S.; Kirk, L.; Chalmers, S. Interactions between mitochondrial bioenergetics and cytoplasmic calcium in cultured cerebellar granule cells. Cell Calcium 2003, 34, 407–424. [Google Scholar] [CrossRef]
- Giacomello, M.; Drago, I.; Pizzo, P.; Pozzan, T. Mitochondrial Ca2+ as a key regulator of cell life and death. Cell Death Differ. 2007, 14, 1267–1274. [Google Scholar] [CrossRef]
- Drago, I.; De Stefani, D.; Rizzuto, R.; Pozzan, T. Mitochondrial Ca2+ uptake contributes to buffering cytoplasmic Ca2+ peaks in cardiomyocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 12986–12991. [Google Scholar] [CrossRef] [Green Version]
- Wang, X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001, 15, 2922–2933. [Google Scholar]
- Gahl, R.F.; Dwivedi, P.; Tjandra, N. Bcl-2 proteins bid and bax form a network to permeabilize the mitochondria at the onset of apoptosis. Cell Death Dis. 2016, 7, e2424. [Google Scholar] [CrossRef] [Green Version]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egan, D.F.; Shackelford, D.B.; Mihaylova, M.M.; Gelino, S.; Kohnz, R.A.; Mair, W.; Vasquez, D.S.; Joshi, A.; Gwinn, D.M.; Taylor, R.; et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 2011, 331, 456–461. [Google Scholar] [CrossRef] [Green Version]
- Lemasters, J.J. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005, 8, 3–5. [Google Scholar] [CrossRef]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 2018, 20, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Kirienko, N.V.; Ausubel, F.M.; Ruvkun, G. Mitophagy confers resistance to siderophore-mediated killing by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2015, 112, 1821–1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, A.P.; Khoury-Hanold, W.; Staron, M.; Tal, M.C.; Pineda, C.M.; Lang, S.M.; Bestwick, M.; Duguay, B.A.; Raimundo, N.; MacDuff, D.A.; et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 2015, 520, 553–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorman, G.S.; Chinnery, P.F.; DiMauro, S.; Hirano, M.; Koga, Y.; McFarland, R.; Suomalainen, A.; Thorburn, D.R.; Zeviani, M.; Turnbull, D.M. Mitochondrial diseases. Nat. Rev. Dis. Primers 2016, 2, 16080. [Google Scholar] [CrossRef]
- Kim, J.A.; Wei, Y.; Sowers, J.R. Role of mitochondrial dysfunction in insulin resistance. Circ. Res. 2008, 102, 401–414. [Google Scholar] [CrossRef]
- Barcelos, I.P.; Troxell, R.M.; Graves, J.S. Mitochondrial dysfunction and multiple sclerosis. Biology 2019, 8, 37. [Google Scholar] [CrossRef] [Green Version]
- Porporato, P.E.; Filigheddu, N.; Pedro, J.M.B.; Kroemer, G.; Galluzzi, L. Mitochondrial metabolism and cancer. Cell Res. 2018, 28, 265–280. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, M.; Jiang, J. Mitochondrial dysfunction in neurodegenerative diseases and drug targets via apoptotic signaling. Mitochondrion 2019, 49, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Kandimalla, R.; Manczak, M.; Yin, X.; Wang, R.; Reddy, P.H. Hippocampal phosphorylated tau induced cognitive decline, dendritic spine loss and mitochondrial abnormalities in a mouse model of Alzheimer’s disease. Hum. Mol. Genet. 2018, 27, 30–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, X.; Manczak, M.; Reddy, P.H. Mitochondria-targeted molecules MitoQ and SS31 reduce mutant huntingtin-induced mitochondrial toxicity and synaptic damage in Huntington’s disease. Hum. Mol. Genet. 2016, 25, 1739–1753. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, T.L. Mitochondrial trafficking in neurons. Cold Spring Harb. Perspect. Biol. 2013, 5, a011304. [Google Scholar] [CrossRef] [Green Version]
- Somlyai, G.; Jancsó, G.; Jákli, G.; Vass, K.; Barna, B.; Lakics, V.; Gaál, T. Naturally occurring deuterium is essential for the normal growth rate of cells. FEBS Lett. 1993, 317, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Olgun, A. Biological effects of deuteration: ATP synthase as an example. Theor. Biol. Med. Model. 2007, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Mladin, C.; Ciobica, A.; Lefter, R.; Popescu, A.; Bild, W. Deuterium-depleted water has stimulating effects on long-term memory in rats. Neurosci. Lett. 2014, 583, 154–158. [Google Scholar] [CrossRef]
- Basov, A.; Fedulova, L.; Baryshev, M.; Dzhimak, S. Deuterium-depleted water infulence on the isotope 2H/1H regulation in body and individual adaptation. Nutrients 2019, 11, 1903. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, N.; Lu, B. Mechanisms and roles of mitophagy in neurodegenerative diseases. CNS Neurosci. Ther. 2019, 25, 859–875. [Google Scholar] [CrossRef]
- Scott, S.V.; Klionsky, D.J. Delivery of proteins and organelles to the vacuole from the cytoplasm. Curr. Opin. Cell Biol. 1998, 10, 523–529. [Google Scholar] [CrossRef]
- Berezhnov, A.V.; Soutar, M.P.M.; Fedotova, E.I.; Frolova, M.S.; Plun-Favreau, H.; Zinchenko, V.P.; Abramov, A.Y. Intracellular pH modulates autophagy and mitophagy. J. Biol. Chem. 2016, 291, 8701–8708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Missiroli, S.; Genovese, I.; Perrone, M.; Vezzani, B.; Vitto, V.A.M.; Giorgi, C. The role of mitochondria in inflammation: From cancer to neurodegenerative disorders. J. Clin. Med. 2020, 9, 740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, A.; Lindsley, T.A.; Sheridan, A.; Bhoiwala, D.L.; Hushmendy, S.F.; Yager, E.J.; Ruggiero, E.A.; Crawford, D.R. Degraded mitochondrial DNA is a newly identified subtype of the damage associated molecular pattern (DAMP) family and possible trigger of neurodegeneration. J. Alzheimers Dis. 2012, 30, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Kepp, O.; Galluzzi, L.; Kroemer, G. Inflammasomes in carcinogenesis and anticancer immune responses. Nat. Immunol. 2012, 13, 343–351. [Google Scholar] [CrossRef]
- Schroder, K.; Zhou, R.; Tschopp, J. The NLRP3 inflammasome: A sensor for metabolic danger? Science 2010, 327, 296–300. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Park, S.; Won, J.H.; Hwang, I.; Hong, S.; Lee, H.K.; Yu, J.W. Defective mitochondrial fission augments NLRP3 inflammasome activation. Sci. Rep. 2015, 5, 15489. [Google Scholar] [CrossRef] [Green Version]
- Schon, E.A.; Manfredi, G. Neuronal degeneration and mitochondrial dysfunction. J. Clin. Invest. 2003, 111, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Trushina, E.; McMurray, C.T. Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience 2007, 145, 1233–1248. [Google Scholar] [CrossRef]
- Ferri, C.P.; Prince, M.; Brayne, C.; Brodaty, H.; Fratiglioni, L.; Ganguli, M.; Hall, K.; Hasegawa, K.; Hendrie, H.; Huang, Y.; et al. Global prevalence of dementia: A Delphi consensus study. Lancet 2005, 366, 2112–2117. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Annaert, W.; De Strooper, B. A cell biological perspective on Alzheimer’s disease. Annu. Rev. Cell Dev. Biol. 2002, 18, 25–51. [Google Scholar] [CrossRef] [PubMed]
- Herholz, K. Use of FDG PET as an imaging biomarker in clinical trials of Alzheimer’s disease. Biomark. Med. 2012, 6, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Praticò, D.; Uryu, K.; Leight, S.; Trojanoswki, J.Q.; Lee, V.M. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J. Neurosci. 2001, 21, 4183–4187. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, R.; LaFerla, F.M. Astrocytes: Conductors of the Alzheimer disease neuroinflammatory symphony. Exp. Neurol. 2013, 239, 133–138. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, C. Decoding Alzheimer’s disease from perturbed cerebral glucose metabolism: Implications for diagnostic and therapeutic strategies. Prog. Neurobiol. 2013, 108, 21–43. [Google Scholar] [CrossRef] [Green Version]
- Hroudová, J.; Singh, N.; Fišar, Z. Mitochondrial dysfunctions in neurodegenerative diseases: Relevance to Alzheimer’s disease. Biomed. Res. Int. 2014, 2014, 175062. [Google Scholar] [CrossRef]
- Carvalho, C.; Correia, S.C.; Santos, R.X.; Cardoso, S.; Moreira, P.I.; Clark, T.A.; Zhu, X.; Smith, M.A.; Perry, G. Role of mitochondrial-mediated signaling pathways in Alzheimer disease and hypoxia. J. Bioenerg. Biomembr. 2009, 41, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Kerr, J.S.; Adriaanse, B.A.; Greig, N.H.; Mattson, M.P.; Cader, M.Z.; Bohr, V.A.; Fang, E.F. Mitophagy and Alzheimer’s disease: Cellular and molecular mechanisms. Trends Neurosci. 2017, 40, 151–166. [Google Scholar] [CrossRef] [Green Version]
- Lanni, C.; Nardinocchi, L.; Puca, R.; Stanga, S.; Uberti, D.; Memo, M.; Govoni, S.; D’Orazi, G.; Racchi, M. Homeodomain interacting protein kinase 2: A target for Alzheimer’s beta amyloid leading to misfolded p53 and inappropriate cell survival. PLoS ONE 2010, 5, e10171. [Google Scholar] [CrossRef] [Green Version]
- Pérez, M.J.; Ponce, D.P.; Aranguiz, A.; Behrens, M.I.; Quintanilla, R.A. Mitochondrial permeability transition pore contributes to mitochondrial dysfunction in fibroblasts of patients with sporadic Alzheimer’s disease. Redox Biol. 2018, 19, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Stanga, S.; Zanou, N.; Audouard, E.; Tasiaux, B.; Contino, S.; Vandermeulen, G.; René, F.; Loeffler, J.P.; Clotman, F.; Gailly, P.; et al. APP-dependent glial cell line-derived neurotrophic factor gene expression drives neuromuscular junction formation. FASEB J. 2016, 30, 1696–1711. [Google Scholar] [CrossRef] [Green Version]
- Contino, S.; Porporato, P.E.; Bird, M.; Marinangeli, C.; Opsomer, R.; Sonveaux, P.; Bontemps, F.; Dewachter, I.; Octave, J.N.; Bertrand, L.; et al. Presenilin 2-dependent maintenance of mitochondrial oxidative capacity and morphology. Front. Physiol. 2017, 8, 796. [Google Scholar] [CrossRef] [Green Version]
- Stanga, S.; Brambilla, L.; Tasiaux, B.; Dang, A.H.; Ivanoiu, A.; Octave, J.N.; Rossi, D.; van Pesch, V.; Kienlen-Campard, P. A Role for GDNF and soluble APP as biomarkers of amyotrophic lateral sclerosis pathophysiology. Front. Neurol. 2018, 9, 384. [Google Scholar] [CrossRef] [PubMed]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardy, J.; Lewis, P.; Revesz, T.; Lees, A.; Paisan-Ruiz, C. The genetics of Parkinson’s syndromes: A critical review. Curr. Opin. Genet. Dev. 2009, 19, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.; Krishnan, K.J.; Morris, C.M.; Taylor, G.A.; Reeve, A.K.; Perry, R.H.; Jaros, E.; Hersheson, J.S.; Betts, J.; Klopstock, T.; et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat. Genet. 2006, 38, 515–517. [Google Scholar] [CrossRef]
- McGeer, P.L.; Itagaki, S.; Boyes, B.E.; McGeer, E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 1988, 38, 1285–1291. [Google Scholar] [CrossRef]
- Gerhard, A.; Pavese, N.; Hotton, G.; Turkheimer, F.; Es, M.; Hammers, A.; Eggert, K.; Oertel, W.; Banati, R.B.; Brooks, D.J. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol. Dis. 2006, 21, 404–412. [Google Scholar] [CrossRef]
- Langston, J.W.; Ballard, P.; Tetrud, J.W.; Irwin, I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 1983, 219, 979–980. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in induced pluripotent stem cell models of Parkinson’s disease. Eur. J. Neurosci. 2019, 49, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Schöndorf, D.C.; Ivanyuk, D.; Baden, P.; Sanchez-Martinez, A.; De Cicco, S.; Yu, C.; Giunta, I.; Schwarz, L.K.; Di Napoli, G.; Panagiotakopoulou, V.; et al. The NAD+ precursor nicotinamide riboside rescues mitochondrial defects and neuronal loss in iPSC and fly models of Parkinson’s disease. Cell Rep. 2018, 23, 2976–2988. [Google Scholar] [CrossRef] [PubMed]
- Mosley, R.L.; Hutter-Saunders, J.A.; Stone, D.K.; Gendelman, H.E. Inflammation and adaptive immunity in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009381. [Google Scholar] [CrossRef] [Green Version]
- Bose, A.; Beal, M.F. Mitochondrial dysfunction in Parkinson’s disease. J. Neurochem. 2016, 139, 216–231. [Google Scholar] [CrossRef]
- Keeney, P.M.; Xie, J.; Capaldi, R.A.; Bennett, J.P. Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J. Neurosci. 2006, 26, 5256–5264. [Google Scholar] [CrossRef]
- Perier, C.; Bové, J.; Dehay, B.; Jackson-Lewis, V.; Rabinovitch, P.S.; Przedborski, S.; Vila, M. Apoptosis-inducing factor deficiency sensitizes dopaminergic neurons to Parkinsonian neurotoxins. Ann. Neurol. 2010, 68, 184–192. [Google Scholar]
- Parihar, M.S.; Parihar, A.; Fujita, M.; Hashimoto, M.; Ghafourifar, P. Mitochondrial association of alpha-synuclein causes oxidative stress. Cell Mol. Life Sci. 2008, 65, 1272–1284. [Google Scholar] [CrossRef]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.S. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jäger, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef] [Green Version]
- Zheng, B.; Liao, Z.; Locascio, J.J.; Lesniak, K.A.; Roderick, S.S.; Watt, M.L.; Eklund, A.C.; Zhang-James, Y.; Kim, P.D.; Hauser, M.A.; et al. PGC-1α, a potential therapeutic target for early intervention in Parkinson’s disease. Sci. Transl. Med. 2010, 2, 52ra73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berthet, A.; Margolis, E.B.; Zhang, J.; Hsieh, I.; Hnasko, T.S.; Ahmad, J.; Edwards, R.H.; Sesaki, H.; Huang, E.J.; Nakamura, K. Loss of mitochondrial fission depletes axonal mitochondria in midbrain dopamine neurons. J. Neurosci. 2014, 34, 14304–14317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarffe, L.A.; Stevens, D.A.; Dawson, V.L.; Dawson, T.M. Parkin and PINK1: Much more than mitophagy. Trends Neurosci. 2014, 37, 315–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaturvedi, R.K.; Beal, M.F. Mitochondrial approaches for neuroprotection. Ann. N. Y. Acad. Sci. 2008, 1147, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Vonsattel, J.P.; Myers, R.H.; Stevens, T.J.; Ferrante, R.J.; Bird, E.D.; Richardson, E.P. Neuropathological classification of Huntington’s disease. J. Neuropathol. Exp. Neurol. 1985, 44, 559–577. [Google Scholar] [CrossRef]
- Reddy, P.H.; Charles, V.; Williams, M.; Miller, G.; Whetsell, W.O.; Tagle, D.A. Transgenic mice expressing mutated full-length HD cDNA: A paradigm for locomotor changes and selective neuronal loss in Huntington’s disease. Philos. Trans. R Soc. Lond. B Biol. Sci. 1999, 354, 1035–1045. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Li, X.J. Multiple pathways contribute to the pathogenesis of Huntington disease. Mol. Neurodegener. 2006, 1, 19. [Google Scholar] [CrossRef] [Green Version]
- Browne, S.E.; Beal, M.F. The energetics of Huntington’s disease. Neurochem. Res. 2004, 29, 531–546. [Google Scholar] [CrossRef]
- Fukui, H.; Moraes, C.T. Extended polyglutamine repeats trigger a feedback loop involving the mitochondrial complex III, the proteasome and huntingtin aggregates. Hum. Mol. Genet. 2007, 16, 783–797. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.; Disatnik, M.H.; Mochly-Rosen, D. Impaired GAPDH-induced mitophagy contributes to the pathology of Huntington’s disease. EMBO Mol. Med. 2015, 7, 1307–1326. [Google Scholar] [CrossRef]
- Acevedo-Torres, K.; Berríos, L.; Rosario, N.; Dufault, V.; Skatchkov, S.; Eaton, M.J.; Torres-Ramos, C.A.; Ayala-Torres, S. Mitochondrial DNA damage is a hallmark of chemically induced and the R6/2 transgenic model of Huntington’s disease. DNA Repair 2009, 8, 126–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banoei, M.M.; Houshmand, M.; Panahi, M.S.; Shariati, P.; Rostami, M.; Manshadi, M.D.; Majidizadeh, T. Huntington’s disease and mitochondrial DNA deletions: Event or regular mechanism for mutant huntingtin protein and CAG repeats expansion?! Cell Mol. Neurobiol. 2007, 27, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Crotti, A.; Glass, C.K. The choreography of neuroinflammation in Huntington’s disease. Trends Immunol. 2015, 36, 364–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duyao, M.P.; Auerbach, A.B.; Ryan, A.; Persichetti, F.; Barnes, G.T.; McNeil, S.M.; Ge, P.; Vonsattel, J.P.; Gusella, J.F.; Joyner, A.L. Inactivation of the mouse Huntington’s disease gene homolog Hdh. Science 1995, 269, 407–410. [Google Scholar] [CrossRef]
- Borovecki, F.; Lovrecic, L.; Zhou, J.; Jeong, H.; Then, F.; Rosas, H.D.; Hersch, S.M.; Hogarth, P.; Bouzou, B.; Jensen, R.V.; et al. Genome-wide expression profiling of human blood reveals biomarkers for Huntington’s disease. Proc. Natl. Acad. Sci. USA 2005, 102, 11023–11028. [Google Scholar] [CrossRef] [Green Version]
- Mihm, M.J.; Amann, D.M.; Schanbacher, B.L.; Altschuld, R.A.; Bauer, J.A.; Hoyt, K.R. Cardiac dysfunction in the R6/2 mouse model of Huntington’s disease. Neurobiol. Dis. 2007, 25, 297–308. [Google Scholar] [CrossRef] [Green Version]
- Farrer, L.A. Diabetes mellitus in Huntington disease. Clin. Genet. 1985, 27, 62–67. [Google Scholar] [CrossRef]
- Busse, M.E.; Hughes, G.; Wiles, C.M.; Rosser, A.E. Use of hand-held dynamometry in the evaluation of lower limb muscle strength in people with Huntington’s disease. J. Neurol. 2008, 255, 1534–1540. [Google Scholar] [CrossRef] [Green Version]
- Jędrak, P.; Mozolewski, P.; Węgrzyn, G.; Więckowski, M.R. Mitochondrial alterations accompanied by oxidative stress conditions in skin fibroblasts of Huntington’s disease patients. Metab. Brain Dis. 2018, 33, 2005–2017. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.M.; Wu, Y.R.; Chang, K.H. Altered aconitase 2 activity in Huntington’s disease peripheral blood cells and mouse model striatum. Int. J. Mol. Sci. 2017, 18, 2480. [Google Scholar] [CrossRef] [Green Version]
- Turner, M.R.; Bowser, R.; Bruijn, L.; Dupuis, L.; Ludolph, A.; McGrath, M.; Manfredi, G.; Maragakis, N.; Miller, R.G.; Pullman, S.L.; et al. Mechanisms, models and biomarkers in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 2013, 14, 19–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robberecht, W.; Philips, T. The changing scene of amyotrophic lateral sclerosis. Nat. Rev. Neurosci. 2013, 14, 248–264. [Google Scholar] [CrossRef] [PubMed]
- Vance, C.; Rogelj, B.; Hortobágyi, T.; De Vos, K.J.; Nishimura, A.L.; Sreedharan, J.; Hu, X.; Smith, B.; Ruddy, D.; Wright, P.; et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 2009, 323, 1208–1211. [Google Scholar] [CrossRef] [Green Version]
- Sreedharan, J.; Blair, I.P.; Tripathi, V.B.; Hu, X.; Vance, C.; Rogelj, B.; Ackerley, S.; Durnall, J.C.; Williams, K.L.; Buratti, E.; et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 2008, 319, 1668–1672. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Ferri, A.; Carrì, M.T. Amyotrophic lateral sclerosis: From current developments in the laboratory to clinical implications. Antioxid. Redox Signal. 2008, 10, 405–443. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, D.; Cordera, S.; Cavalla, P.; Migheli, A. Reactive astrogliosis of the spinal cord in amyotrophic lateral sclerosis. J. Neurol. Sci. 1996, 139, 27–33. [Google Scholar] [CrossRef]
- Kawamata, T.; Akiyama, H.; Yamada, T.; McGeer, P.L. Immunologic reactions in amyotrophic lateral sclerosis brain and spinal cord tissue. Am. J. Pathol. 1992, 140, 691–707. [Google Scholar]
- Sasaki, S.; Iwata, M. Mitochondrial alterations in the spinal cord of patients with sporadic amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 2007, 66, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.F.; Shaw, P.J.; De Vos, K.J. The role of mitochondria in amyotrophic lateral sclerosis. Neurosci. Lett. 2019, 710, 132933. [Google Scholar] [CrossRef]
- Shi, P.; Gal, J.; Kwinter, D.M.; Liu, X.; Zhu, H. Mitochondrial dysfunction in amyotrophic lateral sclerosis. Biochim. Biophys. Acta 2010, 1802, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Pasinelli, P.; Belford, M.E.; Lennon, N.; Bacskai, B.J.; Hyman, B.T.; Trotti, D.; Brown, R.H. Amyotrophic lateral sclerosis-associated SOD1 mutant proteins bind and aggregate with Bcl-2 in spinal cord mitochondria. Neuron 2004, 43, 19–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupuis, L.; Gonzalez de Aguilar, J.L.; Oudart, H.; de Tapia, M.; Barbeito, L.; Loeffler, J.P. Mitochondria in amyotrophic lateral sclerosis: A trigger and a target. Neurodegener. Dis. 2004, 1, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Pollari, E.; Goldsteins, G.; Bart, G.; Koistinaho, J.; Giniatullin, R. The role of oxidative stress in degeneration of the neuromuscular junction in amyotrophic lateral sclerosis. Front. Cell Neurosci. 2014, 8, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, W.; Pasinelli, P.; Trotti, D. Role of mitochondria in mutant SOD1 linked amyotrophic lateral sclerosis. Biochim. Biophys. Acta 2014, 1842, 1295–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elfawy, H.A.; Das, B. Crosstalk between mitochondrial dysfunction, oxidative stress, and age related neurodegenerative disease: Etiologies and therapeutic strategies. Life Sci. 2019, 218, 165–184. [Google Scholar] [CrossRef] [PubMed]
- Lorson, C.L.; Hahnen, E.; Androphy, E.J.; Wirth, B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl. Acad. Sci. USA 1999, 96, 6307–6311. [Google Scholar] [CrossRef] [Green Version]
- Ripolone, M.; Ronchi, D.; Violano, R.; Vallejo, D.; Fagiolari, G.; Barca, E.; Lucchini, V.; Colombo, I.; Villa, L.; Berardinelli, A.; et al. Impaired muscle mitochondrial biogenesis and myogenesis in spinal muscular atrophy. JAMA Neurol. 2015, 72, 666–675. [Google Scholar] [CrossRef]
- Vaidya, S.; Boes, S. Correction to: Measuring quality of life in children with spinal muscular atrophy: A systematic literature review. Qual. Life Res. 2018, 27, 3095. [Google Scholar] [CrossRef]
- Fuller, H.R.; Gillingwater, T.H.; Wishart, T.M. Commonality amid diversity: Multi-study proteomic identification of conserved disease mechanisms in spinal muscular atrophy. Neuromuscul. Disord. 2016, 26, 560–569. [Google Scholar] [CrossRef] [Green Version]
- Boyd, P.J.; Tu, W.Y.; Shorrock, H.K.; Groen, E.J.N.; Carter, R.N.; Powis, R.A.; Thomson, S.R.; Thomson, D.; Graham, L.C.; Motyl, A.A.L.; et al. Bioenergetic status modulates motor neuron vulnerability and pathogenesis in a zebrafish model of spinal muscular atrophy. PLoS Genet. 2017, 13, e1006744. [Google Scholar] [CrossRef] [Green Version]
- Pagliardini, S.; Giavazzi, A.; Setola, V.; Lizier, C.; Di Luca, M.; DeBiasi, S.; Battaglia, G. Subcellular localization and axonal transport of the survival motor neuron (SMN) protein in the developing rat spinal cord. Hum. Mol. Genet. 2000, 9, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Lotti, F.; Imlach, W.L.; Saieva, L.; Beck, E.S.; Hao, L.T.; Li, D.K.; Jiao, W.; Mentis, G.Z.; Beattie, C.E.; McCabe, B.D.; et al. An SMN-dependent U12 splicing event essential for motor circuit function. Cell 2012, 151, 440–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boido, M.; Vercelli, A. Neuromuscular junctions as key contributors and therapeutic targets in spinal muscular atrophy. Front. Neuroanat. 2016, 10, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadimitriou, D.; Le Verche, V.; Jacquier, A.; Ikiz, B.; Przedborski, S.; Re, D.B. Inflammation in ALS and SMA: Sorting out the good from the evil. Neurobiol. Dis. 2010, 37, 493–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simone, C.; Ramirez, A.; Bucchia, M.; Rinchetti, P.; Rideout, H.; Papadimitriou, D.; Re, D.B.; Corti, S. Is spinal muscular atrophy a disease of the motor neurons only: Pathogenesis and therapeutic implications? Cell Mol. Life Sci. 2016, 73, 1003–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubowitz, V. Muscle disorders in childhood. Major Probl. Clin. Pediatr. 1978, 16, 1–282. [Google Scholar]
- Xu, C.C.; Denton, K.R.; Wang, Z.B.; Zhang, X.; Li, X.J. Abnormal mitochondrial transport and morphology as early pathological changes in human models of spinal muscular atrophy. Dis. Model. Mech. 2016, 9, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Borgia, D.; Malena, A.; Spinazzi, M.; Desbats, M.A.; Salviati, L.; Russell, A.P.; Miotto, G.; Tosatto, L.; Pegoraro, E.; Sorarù, G.; et al. Increased mitophagy in the skeletal muscle of spinal and bulbar muscular atrophy patients. Hum. Mol. Genet. 2017, 26, 1087–1103. [Google Scholar] [CrossRef] [Green Version]
- Acsadi, G.; Lee, I.; Li, X.; Khaidakov, M.; Pecinova, A.; Parker, G.C.; Hüttemann, M. Mitochondrial dysfunction in a neural cell model of spinal muscular atrophy. J. Neurosci. Res. 2009, 87, 2748–2756. [Google Scholar] [CrossRef]
- Stifani, N. Motor neurons and the generation of spinal motor neuron diversity. Front. Cell Neurosci. 2014, 8, 293. [Google Scholar] [CrossRef] [Green Version]
- Wijngaarde, C.A.; Blank, A.C.; Stam, M.; Wadman, R.I.; van den Berg, L.H.; van der Pol, W.L. Cardiac pathology in spinal muscular atrophy: A systematic review. Orphanet. J. Rare Dis. 2017, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Fortune, M.D.; Guo, H.; Burren, O.; Schofield, E.; Walker, N.M.; Ban, M.; Sawcer, S.J.; Bowes, J.; Worthington, J.; Barton, A.; et al. Statistical colocalization of genetic risk variants for related autoimmune diseases in the context of common controls. Nat. Genet. 2015, 47, 839–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, B.C.; Ye, C.J.; Price, A.L.; Zaitlen, N.; Asian Genetic Epidemiology Network Type 2 Diabetes Consortium. Transethnic genetic-correlation estimates from summary statistics. Am. J. Hum. Genet. 2016, 99, 76–88. [Google Scholar] [CrossRef] [Green Version]
- Pickrell, J.K.; Berisa, T.; Liu, J.Z.; Ségurel, L.; Tung, J.Y.; Hinds, D.A. Detection and interpretation of shared genetic influences on 42 human traits. Nat. Genet. 2016, 48, 709–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, L.; Chan, K.H.K.; Zhang, G.; Huan, T.; Kurt, Z.; Zhao, Y.; Codoni, V.; Trégouët, D.A.; Yang, J.; Wilson, J.G.; et al. Shared genetic regulatory networks for cardiovascular disease and type 2 diabetes in multiple populations of diverse ethnicities in the United States. PLoS Genet. 2017, 13, e1007040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arneson, D.; Zhang, Y.; Yang, X.; Narayanan, M. Shared mechanisms among neurodegenerative diseases: From genetic factors to gene networks. J. Genet. 2018, 97, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.C.; Lockwood, A.H.; Sonawane, B.R. Neurodegenerative diseases: An overview of environmental risk factors. Environ. Health Perspect. 2005, 113, 1250–1256. [Google Scholar] [CrossRef] [Green Version]
- Myhre, O.; Utkilen, H.; Duale, N.; Brunborg, G.; Hofer, T. Metal dyshomeostasis and inflammation in Alzheimer’s and Parkinson’s diseases: Possible impact of environmental exposures. Oxid. Med. Cell Longev. 2013, 2013, 726954. [Google Scholar] [CrossRef] [Green Version]
- Cutler, N.R.; Sramek, J.J. Review of the next generation of Alzheimer’s disease therapeutics: Challenges for drug development. Prog. Neuropsychopharmacol. Biol. Psychiatry 2001, 25, 27–57. [Google Scholar] [CrossRef]
- Pogačnik, L.; Ota, A.; Ulrih, N.P. An overview of crucial dietary substances and their modes of action for prevention of neurodegenerative diseases. Cells 2020, 9, 576. [Google Scholar] [CrossRef] [Green Version]
- Wilkins, H.M.; Morris, J.K. New therapeutics to modulate mitochondrial function in neurodegenerative disorders. Curr. Pharm. Des. 2017, 23, 731–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoy, S.M. Nusinersen: First global approval. Drugs 2017, 77, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Potkin, K.T.; Potkin, S.G. New directions in therapeutics for Huntington disease. Future Neurol. 2018, 13, 101–121. [Google Scholar] [CrossRef] [PubMed]
- Atri, A. Current and future treatments in Alzheimer’s disease. Semin. Neurol. 2019, 39, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Onasemnogene abeparvovec: First global approval. Drugs 2019, 79, 1255–1262. [Google Scholar] [CrossRef]
- Mejzini, R.; Flynn, L.L.; Pitout, I.L.; Fletcher, S.; Wilton, S.D.; Akkari, P.A. ALS genetics, mechanisms, and therapeutics: Where are we now? Front. Neurosci. 2019, 13, 1310. [Google Scholar] [CrossRef] [Green Version]
- Reich, S.G.; Savitt, J.M. Parkinson’s disease. Med. Clin. North. Am. 2019, 103, 337–350. [Google Scholar] [CrossRef]
- Cenini, G.; Voos, W. Mitochondria as potential targets in Alzheimer disease therapy: An update. Front. Pharmacol. 2019, 10, 902. [Google Scholar] [CrossRef]
- Iturria-Medina, Y.; Carbonell, F.M.; Sotero, R.C.; Chouinard-Decorte, F.; Evans, A.C.; Alzheimer’s Disease Neuroimaging Initiative. Multifactorial causal model of brain (dis)organization and therapeutic intervention: Application to Alzheimer’s disease. Neuroimage 2017, 152, 60–77. [Google Scholar] [CrossRef] [Green Version]
- Veitch, D.P.; Weiner, M.W.; Aisen, P.S.; Beckett, L.A.; Cairns, N.J.; Green, R.C.; Harvey, D.; Jack, C.R.; Jagust, W.; Morris, J.C.; et al. Understanding disease progression and improving Alzheimer’s disease clinical trials: Recent highlights from the Alzheimer’s disease neuroimaging initiative. Alzheimers Dement. 2019, 15, 106–152. [Google Scholar] [CrossRef]
- Moss, D.E.; Perez, R.G.; Kobayashi, H. Cholinesterase inhibitor therapy in Alzheimer’s disease: The limits and tolerability of irreversible CNS-selective acetylcholinesterase inhibition in primates. J. Alzheimers Dis. 2017, 55, 1285–1294. [Google Scholar] [CrossRef] [Green Version]
- Folch, J.; Busquets, O.; Ettcheto, M.; Sánchez-López, E.; Castro-Torres, R.D.; Verdaguer, E.; Garcia, M.L.; Olloquequi, J.; Casadesús, G.; Beas-Zarate, C.; et al. Memantine for the treatment of dementia: A review on its current and future applications. J. Alzheimers Dis. 2018, 62, 1223–1240. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef] [Green Version]
- Rascol, O.; Payoux, P.; Ory, F.; Ferreira, J.J.; Brefel-Courbon, C.; Montastruc, J.L. Limitations of current Parkinson’s disease therapy. Ann. Neurol 2003, 53, S3–S12. [Google Scholar] [CrossRef]
- Kong, M.; Ba, M.; Ren, C.; Yu, L.; Dong, S.; Yu, G.; Liang, H. An updated meta-analysis of amantadine for treating dyskinesia in Parkinson’s disease. Oncotarget 2017, 8, 57316–57326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khairoalsindi, O.A.; Abuzinadah, A.R. Maximizing the survival of amyotrophic lateral sclerosis patients: Current perspectives. Neurol. Res. Int. 2018, 2018, 6534150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dangouloff, T.; Servais, L. Clinical evidence supporting early treatment of patients with spinal muscular atrophy: Current perspectives. Ther. Clin. Risk Manag. 2019, 15, 1153–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Morató, L.; Bertini, E.; Verrigni, D.; Ardissone, A.; Ruiz, M.; Ferrer, I.; Uziel, G.; Pujol, A. Mitochondrial dysfunction in central nervous system white matter disorders. Glia 2014, 62, 1878–1894. [Google Scholar] [CrossRef]
- Carrera-Juliá, S.; Moreno, M.L.; Barrios, C.; de la Rubia Ortí, J.E.; Drehmer, E. Antioxidant alternatives in the treatment of amyotrophic lateral sclerosis: A comprehensive review. Front. Physiol. 2020, 11, 63. [Google Scholar] [CrossRef] [Green Version]
- Innamorato, N.G.; Lastres-Becker, I.; Cuadrado, A. Role of microglial redox balance in modulation of neuroinflammation. Curr. Opin. Neurol. 2009, 22, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Dheen, S.T.; Kaur, C.; Ling, E.A. Microglial activation and its implications in the brain diseases. Curr. Med. Chem. 2007, 14, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Kelso, G.F.; Porteous, C.M.; Coulter, C.V.; Hughes, G.; Porteous, W.K.; Ledgerwood, E.C.; Smith, R.A.; Murphy, M.P. Selective targeting of a redox-active ubiquinone to mitochondria within cells: Antioxidant and antiapoptotic properties. J. Biol. Chem. 2001, 276, 4588–4596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, M.L.; Franklin, J.L. The mitochondria-targeted antioxidant MitoQ inhibits memory loss, neuropathology, and extends lifespan in aged 3xTg-AD mice. Mol. Cell Neurosci. 2019, 101, 103409. [Google Scholar] [CrossRef]
- Xi, Y.; Feng, D.; Tao, K.; Wang, R.; Shi, Y.; Qin, H.; Murphy, M.P.; Yang, Q.; Zhao, G. MitoQ protects dopaminergic neurons in a 6-OHDA induced PD model by enhancing Mfn2-dependent mitochondrial fusion via activation of PGC-1α. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2859–2870. [Google Scholar] [CrossRef]
- Grünewald, A.; Kumar, K.R.; Sue, C.M. New insights into the complex role of mitochondria in Parkinson’s disease. Prog. Neurobiol. 2019, 177, 73–93. [Google Scholar] [CrossRef]
- Miquel, E.; Cassina, A.; Martínez-Palma, L.; Souza, J.M.; Bolatto, C.; Rodríguez-Bottero, S.; Logan, A.; Smith, R.A.; Murphy, M.P.; Barbeito, L.; et al. Neuroprotective effects of the mitochondria-targeted antioxidant MitoQ in a model of inherited amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2014, 70, 204–213. [Google Scholar] [CrossRef]
- Pinho, B.R.; Duarte, A.I.; Canas, P.M.; Moreira, P.I.; Murphy, M.P.; Oliveira, J.M.A. The interplay between redox signalling and proteostasis in neurodegeneration: In vivo effects of a mitochondria-targeted antioxidant in Huntington’s disease mice. Free Radic. Biol. Med. 2020, 146, 372–382. [Google Scholar] [CrossRef]
- Weissig, V. Drug development for the therapy of mitochondrial diseases. Trends Mol. Med. 2020, 26, 40–57. [Google Scholar] [CrossRef]
- Stefanova, N.A.; Muraleva, N.A.; Maksimova, K.Y.; Rudnitskaya, E.A.; Kiseleva, E.; Telegina, D.V.; Kolosova, N.G. An antioxidant specifically targeting mitochondria delays progression of Alzheimer’s disease-like pathology. Aging 2016, 8, 2713–2733. [Google Scholar] [CrossRef] [Green Version]
- Brenza, T.M.; Ghaisas, S.; Ramirez, J.E.V.; Harischandra, D.; Anantharam, V.; Kalyanaraman, B.; Kanthasamy, A.G.; Narasimhan, B. Neuronal protection against oxidative insult by polyanhydride nanoparticle-based mitochondria-targeted antioxidant therapy. Nanomedicine 2017, 13, 809–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, A.; Langley, M.R.; Harischandra, D.S.; Neal, M.L.; Jin, H.; Anantharam, V.; Joseph, J.; Brenza, T.; Narasimhan, B.; Kanthasamy, A.; et al. Mitoapocynin treatment protects against neuroinflammation and dopaminergic neurodegeneration in a preclinical animal model of Parkinson’s disease. J. Neuroimmune Pharmacol. 2016, 11, 259–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langley, M.; Ghosh, A.; Charli, A.; Sarkar, S.; Ay, M.; Luo, J.; Zielonka, J.; Brenza, T.; Bennett, B.; Jin, H.; et al. Mito-apocynin prevents mitochondrial dysfunction, microglial activation, oxidative damage, and progressive neurodegeneration in MitoPark transgenic mice. Antioxid. Redox Signal. 2017, 27, 1048–1066. [Google Scholar] [CrossRef] [PubMed]
- Moosmann, B.; Skutella, T.; Beyer, K.; Behl, C. Protective activity of aromatic amines and imines against oxidative nerve cell death. Biol. Chem. 2001, 382, 1601–1612. [Google Scholar] [CrossRef] [Green Version]
- Bachurin, S.O.; Makhaeva, G.F.; Shevtsova, E.F.; Boltneva, N.P.; Kovaleva, N.V.; Lushchekina, S.V.; Rudakova, E.V.; Dubova, L.G.; Vinogradova, D.V.; Sokolov, V.B.; et al. Conjugates of methylene blue with γ-carboline derivatives as new multifunctional agents for the treatment of neurodegenerative diseases. Sci. Rep. 2019, 9, 4873. [Google Scholar] [CrossRef]
- Smith, E.S.; Clark, M.E.; Hardy, G.A.; Kraan, D.J.; Biondo, E.; Gonzalez-Lima, F.; Cormack, L.K.; Monfils, M.; Lee, H.J. Daily consumption of methylene blue reduces attentional deficits and dopamine reduction in a 6-OHDA model of Parkinson’s disease. Neuroscience 2017, 359, 8–16. [Google Scholar] [CrossRef]
- Rocha, M.; Hernandez-Mijares, A.; Garcia-Malpartida, K.; Bañuls, C.; Bellod, L.; Victor, V.M. Mitochondria-targeted antioxidant peptides. Curr. Pharm. Des. 2010, 16, 3124–3131. [Google Scholar] [CrossRef]
- Szeto, H.H.; Birk, A.V. Serendipity and the discovery of novel compounds that restore mitochondrial plasticity. Clin. Pharmacol. Ther. 2014, 96, 672–683. [Google Scholar] [CrossRef] [Green Version]
- Searls, R.L.; Sanadi, D.R. α-Ketoglutaric dehydrogenase. VIII. Isolation and some properties of a flavoprotein component. J. Biol. Chem. 1960, 235, 2485–2491. [Google Scholar]
- Dos Santos, S.M.; Romeiro, C.F.R.; Rodrigues, C.A.; Cerqueira, A.R.L.; Monteiro, M.C. Mitochondrial dysfunction and alpha-lipoic acid: Beneficial or harmful in Alzheimer’s disease? Oxid. Med. Cell Longev. 2019, 2019, 8409329. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Shu, H.Y.; Huang, T.; Zhang, Q.L.; Li, D.; Zhang, G.Q.; Peng, X.Y.; Liu, C.F.; Luo, W.F.; Hu, L.F. Nrf2 signaling contributes to the neuroprotective effects of urate against 6-OHDA toxicity. PLoS ONE 2014, 9, e100286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paganoni, S.; Schwarzschild, M.A. Urate as a marker of risk and progression of neurodegenerative disease. Neurotherapeutics 2017, 14, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Schwarzschild, M.A.; Ascherio, A.; Beal, M.F.; Cudkowicz, M.E.; Curhan, G.C.; Hare, J.M.; Hooper, D.C.; Kieburtz, K.D.; Macklin, E.A.; Oakes, D.; et al. Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: A randomized clinical trial. JAMA Neurol. 2014, 71, 141–150. [Google Scholar] [CrossRef]
- Nicholson, K.; Chan, J.; Macklin, E.A.; Levine-Weinberg, M.; Breen, C.; Bakshi, R.; Grasso, D.L.; Wills, A.M.; Jahandideh, S.; Taylor, A.A.; et al. Pilot trial of inosine to elevate urate levels in amyotrophic lateral sclerosis. Ann. Clin. Transl. Neurol. 2018, 5, 1522–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal. Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Plummer, B.F.; Hardies, L.J.; Weintraub, S.T.; Vijayalaxmi; Shepherd, A.M. A novel melatonin metabolite, cyclic 3-hydroxymelatonin: A biomarker of in vivo hydroxyl radical generation. Biochem. Biophys. Res. Commun. 1998, 253, 614–620. [Google Scholar] [CrossRef]
- Cheng, Y.; Feng, Z.; Zhang, Q.Z.; Zhang, J.T. Beneficial effects of melatonin in experimental models of Alzheimer disease. Acta Pharmacol. Sin. 2006, 27, 129–139. [Google Scholar] [CrossRef]
- Weishaupt, J.H.; Bartels, C.; Pölking, E.; Dietrich, J.; Rohde, G.; Poeggeler, B.; Mertens, N.; Sperling, S.; Bohn, M.; Hüther, G.; et al. Reduced oxidative damage in ALS by high-dose enteral melatonin treatment. J. Pineal. Res. 2006, 41, 313–323. [Google Scholar] [CrossRef]
- Rudnitskaya, E.A.; Muraleva, N.A.; Maksimova, K.Y.; Kiseleva, E.; Kolosova, N.G.; Stefanova, N.A. Melatonin attenuates memory impairment, amyloid-β accumulation, and neurodegeneration in a rat model of sporadic Alzheimer’s disease. J. Alzheimers Dis. 2015, 47, 103–116. [Google Scholar] [CrossRef]
- Lee, J.G.; Woo, Y.S.; Park, S.W.; Seog, D.H.; Seo, M.K.; Bahk, W.M. The neuroprotective effects of melatonin: Possible role in the pathophysiology of neuropsychiatric disease. Brain Sci. 2019, 9, 285. [Google Scholar] [CrossRef] [Green Version]
- Cardinali, D.P. Melatonin: Clinical perspectives in neurodegeneration. Front. Endocrinol. 2019, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; BaHammam, A.S.; Brown, G.M.; Spence, D.W.; Bharti, V.K.; Kaur, C.; Hardeland, R.; Cardinali, D.P. Melatonin antioxidative defense: Therapeutical implications for aging and neurodegenerative processes. Neurotox. Res. 2013, 23, 267–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta 2013, 1830, 4117–4129. [Google Scholar] [CrossRef]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Tardiolo, G.; Bramanti, P.; Mazzon, E. Overview on the effects of N-acetylcysteine in neurodegenerative diseases. Molecules 2018, 23, 3305. [Google Scholar] [CrossRef] [Green Version]
- Bavarsad Shahripour, R.; Harrigan, M.R.; Alexandrov, A.V. N-acetylcysteine (NAC) in neurological disorders: Mechanisms of action and therapeutic opportunities. Brain Behav. 2014, 4, 108–122. [Google Scholar] [CrossRef]
- Remington, R.; Bechtel, C.; Larsen, D.; Samar, A.; Doshanjh, L.; Fishman, P.; Luo, Y.; Smyers, K.; Page, R.; Morrell, C.; et al. A phase II randomized clinical trial of a nutritional formulation for cognition and mood in Alzheimer’s disease. J. Alzheimers Dis. 2015, 45, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Stahl, W.; Sies, H. Antioxidant defense: Vitamins E and C and carotenoids. Diabetes 1997, 46, S14–S18. [Google Scholar] [CrossRef]
- Medina, S.; Martínez, M.; Hernanz, A. Antioxidants inhibit the human cortical neuron apoptosis induced by hydrogen peroxide, tumor necrosis factor alpha, dopamine and beta-amyloid peptide 1-42. Free Radic. Res. 2002, 36, 1179–1184. [Google Scholar] [CrossRef]
- Kook, S.Y.; Lee, K.M.; Kim, Y.; Cha, M.Y.; Kang, S.; Baik, S.H.; Lee, H.; Park, R.; Mook-Jung, I. High-dose of vitamin C supplementation reduces amyloid plaque burden and ameliorates pathological changes in the brain of 5XFAD mice. Cell Death Dis. 2014, 5, e1083. [Google Scholar] [CrossRef] [Green Version]
- Dixit, S.; Fessel, J.P.; Harrison, F.E. Mitochondrial dysfunction in the APP/PSEN1 mouse model of Alzheimer’s disease and a novel protective role for ascorbate. Free Radic. Biol. Med. 2017, 112, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, T.R.; Khaza’ai, H.; Vidyadaran, S.; Abd Mutalib, M.S.; Vasudevan, R. The neuroprotective effects of tocotrienol rich fraction and alpha tocopherol against glutamate injury in astrocytes. Bosn. J. Basic Med. Sci. 2014, 14, 195–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schloesser, A.; Esatbeyoglu, T.; Piegholdt, S.; Dose, J.; Ikuta, N.; Okamoto, H.; Ishida, Y.; Terao, K.; Matsugo, S.; Rimbach, G. Dietary tocotrienol/γ-cyclodextrin complex increases mitochondrial membrane potential and ATP concentrations in the brains of aged mice. Oxid. Med. Cell Longev. 2015, 2015, 789710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, S.; Huang, X.; Zhen, J.; Van Halm-Lutterodt, N.; Wang, J.; Zhou, C.; Yuan, L. Dietary vitamin E status dictates oxidative stress outcomes by modulating effects of fish oil supplementation in Alzheimer disease model APP. Mol. Neurobiol. 2018, 55, 9204–9219. [Google Scholar] [CrossRef]
- Browne, D.; McGuinness, B.; Woodside, J.V.; McKay, G.J. Vitamin E and Alzheimer’s disease: What do we know so far? Clin. Interv. Aging 2019, 14, 1303–1317. [Google Scholar] [CrossRef] [Green Version]
- Gurney, M.E.; Cutting, F.B.; Zhai, P.; Doble, A.; Taylor, C.P.; Andrus, P.K.; Hall, E.D. Benefit of vitamin E, riluzole, and gabapentin in a transgenic model of familial amyotrophic lateral sclerosis. Ann. Neurol. 1996, 39, 147–157. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [Green Version]
- Al-Amin, M.M.; Akhter, S.; Hasan, A.T.; Alam, T.; Nageeb Hasan, S.M.; Saifullah, A.R.; Shohel, M. The antioxidant effect of astaxanthin is higher in young mice than aged: A region specific study on brain. Metab. Brain Dis. 2015, 30, 1237–1246. [Google Scholar] [CrossRef]
- Lobos, P.; Bruna, B.; Cordova, A.; Barattini, P.; Galáz, J.L.; Adasme, T.; Hidalgo, C.; Muñoz, P.; Paula-Lima, A. Astaxanthin protects primary hippocampal neurons against noxious effects of Aβ-oligomers. Neural Plast. 2016, 2016, 3456783. [Google Scholar] [CrossRef] [Green Version]
- Uittenbogaard, M.; Chiaramello, A. Mitochondrial biogenesis: A therapeutic target for neurodevelopmental disorders and neurodegenerative diseases. Curr. Pharm. Des. 2014, 20, 5574–5593. [Google Scholar] [CrossRef] [Green Version]
- Eschbach, J.; von Einem, B.; Müller, K.; Bayer, H.; Scheffold, A.; Morrison, B.E.; Rudolph, K.L.; Thal, D.R.; Witting, A.; Weydt, P.; et al. Mutual exacerbation of peroxisome proliferator-activated receptor γ coactivator 1α deregulation and α-synuclein oligomerization. Ann. Neurol. 2015, 77, 15–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baines, C.P.; Kaiser, R.A.; Purcell, N.H.; Blair, N.S.; Osinska, H.; Hambleton, M.A.; Brunskill, E.W.; Sayen, M.R.; Gottlieb, R.A.; Dorn, G.W.; et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 2005, 434, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Bordet, T.; Berna, P.; Abitbol, J.L.; Pruss, R.M. Olesoxime (TRO19622): A novel mitochondrial-targeted neuroprotective compound. Pharmaceuticals 2010, 3, 345–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrov, D.; Mansfield, C.; Moussy, A.; Hermine, O. ALS clinical trials review: 20 years of failure. Are we any closer to registering a new treatment? Front. Aging Neurosci. 2017, 9, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, J.J.; Clemensson, L.E.; Schiöth, H.B.; Nguyen, H.P. Olesoxime in neurodegenerative diseases: Scrutinising a promising drug candidate. Biochem. Pharmacol. 2019, 168, 305–318. [Google Scholar] [CrossRef]
- Demarin, V.; Podobnik, S.S.; Storga-Tomic, D.; Kay, G. Treatment of Alzheimer’s disease with stabilized oral nicotinamide adenine dinucleotide: A randomized, double-blind study. Drugs Exp. Clin. Res. 2004, 30, 27–33. [Google Scholar]
- Harlan, B.A.; Pehar, M.; Sharma, D.R.; Beeson, G.; Beeson, C.C.; Vargas, M.R. Enhancing NAD+ salvage pathway reverts the toxicity of primary astrocytes expressing amyotrophic lateral sclerosis-linked mutant superoxide dismutase 1 (SOD1). J. Biol. Chem. 2016, 291, 10836–10846. [Google Scholar] [CrossRef] [Green Version]
- Roe, C.R.; Mochel, F. Anaplerotic diet therapy in inherited metabolic disease: Therapeutic potential. J. Inherit. Metab. Dis. 2006, 29, 332–340. [Google Scholar] [CrossRef]
- Adanyeguh, I.M.; Rinaldi, D.; Henry, P.G.; Caillet, S.; Valabregue, R.; Durr, A.; Mochel, F. Triheptanoin improves brain energy metabolism in patients with Huntington disease. Neurology 2015, 84, 490–495. [Google Scholar] [CrossRef]
- Tefera, T.W.; Wong, Y.; Barkl-Luke, M.E.; Ngo, S.T.; Thomas, N.K.; McDonald, T.S.; Borges, K. Triheptanoin protects motor neurons and delays the onset of motor symptoms in a mouse model of amyotrophic lateral sclerosis. PLoS ONE 2016, 11, e0161816. [Google Scholar] [CrossRef] [Green Version]
- Kolaj, I.; Imindu Liyanage, S.; Weaver, D.F. Phenylpropanoids and Alzheimer’s disease: A potential therapeutic platform. Neurochem. Int. 2018, 120, 99–111. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar] [PubMed]
- Moskaug, J.; Carlsen, H.; Myhrstad, M.C.; Blomhoff, R. Polyphenols and glutathione synthesis regulation. Am. J. Clin. Nutr. 2005, 81, 277S–283S. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.L.; Wilhelmus, M.M.; de Vries, H.E.; Drukarch, B.; Hoozemans, J.J.; van Horssen, J. Antioxidative defense mechanisms controlled by Nrf2: State-of-the-art and clinical perspectives in neurodegenerative diseases. Arch. Toxicol. 2014, 88, 1773–1786. [Google Scholar] [CrossRef] [PubMed]
- Fiorani, M.; Guidarelli, A.; Blasa, M.; Azzolini, C.; Candiracci, M.; Piatti, E.; Cantoni, O. Mitochondria accumulate large amounts of quercetin: Prevention of mitochondrial damage and release upon oxidation of the extramitochondrial fraction of the flavonoid. J. Nutr. Biochem. 2010, 21, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.H.; Kwon, H.; Kim, K.S.; Kim, J.; Kim, M.H.; Yu, H.J.; Kim, M.; Lee, K.W.; Do, B.R.; Jung, H.K.; et al. Epigallocatechin gallate prevents oxidative-stress-induced death of mutant Cu/Zn-superoxide dismutase (G93A) motoneuron cells by alteration of cell survival and death signals. Toxicology 2004, 202, 213–225. [Google Scholar] [CrossRef]
- Jiang, W.; Luo, T.; Li, S.; Zhou, Y.; Shen, X.Y.; He, F.; Xu, J.; Wang, H.Q. Quercetin protects against okadaic acid-induced injury via MAPK and PI3K/Akt/GSK3β signaling pathways in HT22 hippocampal neurons. PLoS ONE 2016, 11, e0152371. [Google Scholar] [CrossRef]
- Huang, D.S.; Yu, Y.C.; Wu, C.H.; Lin, J.Y. Protective effects of wogonin against Alzheimer’s disease by inhibition of amyloidogenic pathway. Evid. Based Complement. Alternat. Med. 2017, 2017, 3545169. [Google Scholar] [CrossRef] [Green Version]
- Mancuso, R.; del Valle, J.; Modol, L.; Martinez, A.; Granado-Serrano, A.B.; Ramirez-Núñez, O.; Pallás, M.; Portero-Otin, M.; Osta, R.; Navarro, X. Resveratrol improves motoneuron function and extends survival in SOD1(G93A) ALS mice. Neurotherapeutics 2014, 11, 419–432. [Google Scholar]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflammation 2017, 14, 1. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.W.; Grossman, H.; Neugroschl, J.; Parker, S.; Burden, A.; Luo, X.; Sano, M. A randomized, double-blind, placebo-controlled trial of resveratrol with glucose and malate (RGM) to slow the progression of Alzheimer’s disease: A pilot study. Alzheimers Dement. 2018, 4, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Ferrari-Toninelli, G.; Maccarinelli, G.; Uberti, D.; Buerger, E.; Memo, M. Mitochondria-targeted antioxidant effects of S(-) and R(+) pramipexole. BMC Pharmacol. 2010, 10, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassarino, D.S.; Fall, C.P.; Smith, T.S.; Bennett, J.P. Pramipexole reduces reactive oxygen species production in vivo and in vitro and inhibits the mitochondrial permeability transition produced by the parkinsonian neurotoxin methylpyridinium ion. J. Neurochem. 1998, 71, 295–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayeed, I.; Parvez, S.; Winkler-Stuck, K.; Seitz, G.; Trieu, I.; Wallesch, C.W.; Schönfeld, P.; Siemen, D. Patch clamp reveals powerful blockade of the mitochondrial permeability transition pore by the D2-receptor agonist pramipexole. FASEB J. 2006, 20, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Nam, Y.; Lee, J.W.; Nguyen, P.T.; Yoo, J.E.; Tran, T.V.; Jeong, J.H.; Jang, C.G.; Oh, Y.J.; Youdim, M.B.H.; et al. N-Methyl, N-propynyl-2-phenylethylamine (MPPE), a selegiline analog, attenuates MPTP-induced dopaminergic toxicity with guaranteed behavioral safety: Involvement of inhibitions of mitochondrial oxidative burdens and p53 gene-elicited pro-apoptotic change. Mol. Neurobiol. 2016, 53, 6251–6269. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Qiu, K.; Liu, H.; He, Q.; Bai, J.H.; Lu, W. Application and prospects of butylphthalide for the treatment of neurologic diseases. Chin. Med. J. 2019, 132, 1467–1477. [Google Scholar] [CrossRef]
- Kapoor, S. Dl-3-n-butylphthalide and its emerging beneficial effects in neurology. Chin. Med. J. 2012, 125, 3360. [Google Scholar]
- Xiong, N.; Huang, J.; Chen, C.; Zhao, Y.; Zhang, Z.; Jia, M.; Hou, L.; Yang, H.; Cao, X.; Liang, Z.; et al. Dl-3-n-butylphthalide, a natural antioxidant, protects dopamine neurons in rotenone models for Parkinson’s disease. Neurobiol. Aging 2012, 33, 1777–1791. [Google Scholar] [CrossRef]
- Zhou, H.; Ye, M.; Xu, W.; Yu, M.; Liu, X.; Chen, Y. DL-3-n-butylphthalide therapy for Parkinson’s disease: A randomized controlled trial. Exp. Ther. Med. 2019, 17, 3800–3806. [Google Scholar] [CrossRef] [Green Version]
- Paumgartner, G.; Beuers, U. Ursodeoxycholic acid in cholestatic liver disease: Mechanisms of action and therapeutic use revisited. Hepatology 2002, 36, 525–531. [Google Scholar] [CrossRef]
- Ackerman, H.D.; Gerhard, G.S. Bile acids in neurodegenerative disorders. Front. Aging Neurosci. 2016, 8, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keene, C.D.; Rodrigues, C.M.; Eich, T.; Chhabra, M.S.; Steer, C.J.; Low, W.C. Tauroursodeoxycholic acid, a bile acid, is neuroprotective in a transgenic animal model of Huntington’s disease. Proc. Natl. Acad. Sci. USA 2002, 99, 10671–10676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaz, A.R.; Cunha, C.; Gomes, C.; Schmucki, N.; Barbosa, M.; Brites, D. Glycoursodeoxycholic acid reduces matrix metalloproteinase-9 and caspase-9 activation in a cellular model of superoxide dismutase-1 neurodegeneration. Mol. Neurobiol. 2015, 51, 864–877. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.M.; Barnes, K.; Clemmens, H.; Al-Rafiah, A.R.; Al-Ofi, E.A.; Leech, V.; Bandmann, O.; Shaw, P.J.; Blackburn, D.J.; Ferraiuolo, L.; et al. Ursodeoxycholic acid improves mitochondrial function and redistributes Drp1 in fibroblasts from patients with either sporadic or familial Alzheimer’s disease. J. Mol. Biol. 2018, 430, 3942–3953. [Google Scholar] [CrossRef]
- Elia, A.E.; Lalli, S.; Monsurrò, M.R.; Sagnelli, A.; Taiello, A.C.; Reggiori, B.; La Bella, V.; Tedeschi, G.; Albanese, A. Tauroursodeoxycholic acid in the treatment of patients with amyotrophic lateral sclerosis. Eur. J. Neurol. 2016, 23, 45–52. [Google Scholar] [CrossRef]
- Daruich, A.; Picard, E.; Boatright, J.H.; Behar-Cohen, F. Review: The bile acids urso- and tauroursodeoxycholic acid as neuroprotective therapies in retinal disease. Mol. Vis. 2019, 25, 610–624. [Google Scholar]
- Wirth, B.; Karakaya, M.; Kye, M.J.; Mendoza-Ferreira, N. Twenty-five years of spinal muscular atrophy research: From phenotype to genotype to therapy, and what comes next. Annu. Rev. Genomics Hum. Genet. 2020, 21. [Google Scholar] [CrossRef] [Green Version]


| Pathology | Drug | Mechanism of Action | Main Effects | Main Limitations | References |
|---|---|---|---|---|---|
| AD | Donepezil, Galantamine, and Rivastigmine | Cholinesterase inhibitors | Acetylcholine increase at synaptic level | Low CNS selectivity; high doses cause gastrointestinal toxicity | [135,142,143,144] |
| Memantine | Noncompetitive NMDA antagonist | Reduction of neuronal dysfunctions due to glutamate downregulation | Low beneficial effects in clinical trials (maybe due to late administration) | ||
| PD | Levodopa + Carbidopa/Levodopa + Benserazide | DA precursor + DOPA decarboxylase inhibitor | SNC DA level increase | Ineffective in mitigating some motor and non-motor symptoms; dyskinesia | [138,145,146] |
| Levodopa + Carbidopa + Entacapone | DA precursor + DOPA decarboxylase inhibitor + COMT inhibitor | SNC DA level increase | Motor fluctuations; dyskinesia | ||
| Pramipexole and Apomorphine | DA agonists | Activation of DA receptors | Less effective than Levodopa; dyskinesia; expensive | ||
| Selegiline, Rasagiline, and Safinamide | MAO-B inhibitors | Prevention of DA metabolism | Mild efficacy in monotherapy | ||
| Amantadine | Antiviral drug | Bradykinesia, tremor and rigidity mitigation, and Levodopa-induced dyskinesia reduction | Several side effects such as hallucination, confusion, blurred vision, and edema | ||
| Trihexyphenidyl | Anticholinergic | Tremor reduction | Mild effects on motor symptoms | ||
| HD | Tetrabenazine (XENAZINETM) and Deutetrabenazine (AUSTEDOTM) | Vesicular monoamine transporter (VMAT) type 2 inhibitor | Treatment of pathology- associated chorea (synaptic DA reduction) | Only symptomatic treatment | [134] |
| ALS | Riluzole | Glutamatergic transmission blocker | Antiexcitotoxic effects | Effectiveness limited to the first six months of therapy | [137,147] |
| Edaravone (RADICAVATM) | Antioxidant | Free radical scavenger (neuroprotection) | Prescription only for limited cohort of patients | ||
| SMA | Nusinersen (SPINRAZATM) | ASO acting on SMN2 pre-mRNA splicing | Increase of full-length SMN production | Limited efficacy in milder or late-treated patients; expensive; repetitive intrathecal injections | [133,136,148] |
| Onasemnogene Abeparvovec (ZOLGENSMATM) | Smn1 delivering by the adeno-associated virus AAV9 (only FDA approved, not yet in therapy) | Increase of full-length SMN production | Limited efficacy in milder or late-treated patients; expensive |
| Therapeutic Function | Drug / Molecule | Pathology | Preclinical Studies / PMCID | Preclinical Results | Clinical Trials / Trial ID | Clinical Results |
|---|---|---|---|---|---|---|
| Synthetic antioxidant | α-Lipoic acid | AD | Preclinical in vitro and in vivo PMC6914903 [171] | In vitro studies: mitigation of cytotoxic effects (reduction of ROS production and lipid peroxidation). | Clinical trials information is collected here PMC6914903 [171] | Safety and neuroprotection are confirmed in combination with other antioxidants conventional treatments but further studies on interactions between them are needed. Isolated α-lipoic acid activity has to be tested |
| In vivo studies: memory and learning improvement | ||||||
| Inosine | ALS | Under evaluation | Clinical trial Phase 1 Completed, PMC6292193 [175] NCT02288091 | Safety, tolerability, and efficacy in increasing urate serum levels | ||
| Inosine/Urate | PD | Preclinical in vitro and in vivo PMC5233635 [173] | In vitro: neuroprotection (Nrf2 transcription and nuclear translocation; GSH increasing) | Clinical trial Phase 2 Completed, PMC3940333 [174] NCT00833690 | Safety, tolerability, and effectiveness in increasing urate serum levels | |
| In vivo: behavioral improvement; reduction of dopaminergic neurons loss | ||||||
| Melatonin | AD | Preclinical in vitro and in vivo PMC6826722 [181] | In vitro: protection from apoptosis and neuroinflammation. | Meta-analysis of controlled trials information is collected in the following work PMC6826722 [181] | Improvement in sleep quality but no ameliorations in cognitive functions when melatonin is administered not in combinations with other AD treatments | |
| In vivo: improvement in cognitive functions and behavioral activities (reduction of neuronal death and beneficial effects on synapses), protection against neuroinflammation | ||||||
| Melatonin | ALS | Preclinical in vitro and in vivo PMC7016185 [151] | In vitro: apoptosis inhibition | Clinical safety trial information is collected in the following work PMID: 22739839 [183] | Safety, improvement of sleep quality, and reduction of oxidative stress biomarkers. Further studies to confirm its efficacy alone or combined to other drugs different from Riluzole are needed | |
| In vivo: survival extension and delay in disease progression (oxidative damage reduction and protection against neuroinflammation) | ||||||
| Melatonin | PD | Preclinical in vivo PMC6646522 [182] | Reduction of locomotor deficit (downregulation of lipid peroxidation and dopaminergic cells loss) and of neuroinflammation | Clinical trials information is collected in the following work PMC6646522 [182] | Improvement in sleep quality but no benefits on motor activity | |
| Methylene Blue | PD | Preclinical in vivo PMID: 30219247 [157] | Attentional functions and motor improvement and neuroprotection | |||
| Mito-Apo | AD | Preclinical in vitro PMC5392427 [162] | Mito-Apo on dopaminergic neuronal cell line, mouse primary cortical neurons, and a human mesencephalic cell line: reduction of neuronal degeneration and of neuroinflammation | |||
| Mito-Apo | PD | Preclinical in vitro and in vivo PMC4995106 [163] PMC5651937 [164] | In vitro: neuroprotection against oxidative stress | |||
| In vivo: motor deficit and neuroinflammation attenuation (neuroprotection) | ||||||
| MitoQ | AD | Preclinical in vitro and in vivo PMC6716473 [139] | In vitro: neuroprotection against oxidative stress and neurites outgrowth | |||
| In vivo: mitigation of cognitive decline and elongation of lifespan | ||||||
| MitoQ | ALS | Preclinical in vivo PMID: 24582549 [158] | MitoQ increases hindlimb strength and promotes lifespan elongation of SOD1G93A mice | |||
| MitoQ | HD | Preclinical in vivo PMC6970224 [159] | MitoQ on R6/2 HD mouse model: reduction of ROS-induced autophagy | |||
| MitoQ | PD | Preclinical in vivo PMID: 29842922 [156] | MitoQ prevents dopaminergic neurons loss in a 6-OHDA PD mouse model promoting mitochondrial fusion | |||
| N-Acetylcysteine | AD | Preclinical in vitro and in vivo PMC6320789 [186] | In vitro: apoptosis inhibition and protection against neuroinflammation | Clinical Trial Phase 2 Completed PMID: 25589719 [184] NCT01320527 | Cognitive and behavioral improvement | |
| In vivo: increase of brain connections, GSH levels, TH and Complex 1 activity and protection against neuroinflammation | ||||||
| N-Acetylcysteine | HD | Preclinical in vivo PMC3967529 [187] | Cognitive and motor deficits improvement | |||
| N-Acetylcysteine | PD | Preclinical in vitro and in vivo, PMC6320789 [186] | In vitro: apoptosis inhibition | Clinical trials information is collected in the following work PMC6320789 [186] | Increase of GSH brain levels | |
| In vivo: increase of GSH levels and reduction of lipid peroxidation | ||||||
| N-Acetylcysteine | SMA | Preclinical in vitro PMC4728333 [119] | NAC on iPSCs: mitigation of motor neuron degeneration (increasing in mitochondrial number and axonal transport, reduction of axonal swelling, and apoptosis inhibition) | |||
| SkQ1 | AD | Preclinical in vivo PMC6716473 [139] | Cognitive and behavioral improvement (reduction of ROS formation, improvement of mitochondrial biogenesis and bioenergetics and mitochondrial structure protection) | |||
| Szeto-Schiller tetrapeptides | AD | Preclinical in vitro and in vivo PMC6716473 [139] | In vitro: mitochondrial biogenesis, bioenergetics and dynamics improvement, and apoptosis inhibition | |||
| In vivo: anterograde axonal transport and synaptic activity enhancement | ||||||
| Szeto-Schiller tetrapeptides | ALS | Preclinical in vitro and in vivo PMC4267688 [169] | In vitro: mutant cells apoptosis inhibition | |||
| In vivo: increase of survival and behavioral improvement in SOD1G93A mice (neuroprotection) | ||||||
| Szeto-Schiller tetrapeptides | PD | Preclinical in vivo PMC4267688 [169] | Lifespan extension and motor performances improvement (neuroprotection) | |||
| Natural antioxidant | Carotenoids (Astaxanthin) | AD | Preclinical in vitro PMC4791503 [200] | Astaxanthin on Aβ1-42 oligomers-treated hippocampal neurons: protection against ROS production reducing synaptotoxic events and neuroinflammation | ||
| Vitamin C | AD | Preclinical in vitro, PMID: 12592670 [190], and in vivo PMC5623070 [192] PMC3944243 [191] | In vitro: inhibition of apoptosis due to mitochondrial membrane depolarization and DNA fragmentation | |||
| In vivo: Preservation of mitochondrial morphology (attenuation of oxidative stress damage) and apoptosis inhibition | ||||||
| Vitamin E | AD | Preclinical in vitro, PMC4333972 [193] and in vivo PMC4537756 [194] and PMID: 29656360 [195] | Vit.E on astrocytes treated with glutamate: mitochondrial injuries recovering (MMP stabilization and lipid peroxidation reduction) | Epidemiological studies information is collected in the following work PMC6645610 [196] | Results insufficient. Additional studies on AD patients are needed | |
| Vit.E in aged mice: increase of TFAM, MMP, and ATP levels | ||||||
| Vit.E on APP/PS1 mice: cognitive and behavioral performances improvement (Aß accumulation prevention, oxidative stress reduction) | ||||||
| Vitamin E | ALS | Preclinical in vivo PMID: 8967745 [197] | Vit.E determines ALS delay onset and slows its progression | Clinical trials information is collected in the following work PMC7016185 [151] | Several clinical studies have shown conflicting outcomes in slowing ALS onset and progression, but further studies are needed |
| Therapeutic Function | Drug / Molecule | Pathology | Preclinical Studies / PMCID | Preclinical Results | Clinical Trials / Trial ID | Clinical Results |
|---|---|---|---|---|---|---|
| Mitochondrial biogenesis | PGC-1α | PD | Preclinical in vitro and in vivo PMC4293280 [202] | PGC-1α restoration in a cell culture model for α-synuclein oligomerization | ||
| In A30P α-syn transgenic animals: α-synuclein oligomerization reduction | ||||||
| Mitochondrial permeability | Olesoxime | HD | Preclinical in vitro and in vivo PMID: 31283931 [206] | Mitochondrial membrane stabilization; cognitive and behavioral improvement | ||
| Olesoxime | PD | Preclinical in vitro and in vivo PMID: 31283931 [206] | Mitochondrial activity enhancement and apoptosis inhibition | |||
| Olesoxime | SMA | Preclinical in vivo PMC4033913 [204] | Lifespan elongation | Clinical trial Phase 2 Completed PMID: 31283931 [206] NCT01302600 | Efficacy in motor improvement and safety have been confirmed | |
| It could be administered in combinatorial therapy |
| Therapeutic Function | Drug / Molecule | Pathology | Preclinical Studies / PMCID | Preclinical Results | Clinical Trials / Trial ID | Clinical Results |
|---|---|---|---|---|---|---|
| Mitochondrial bioenergetics | NAD | AD | Preclinical in vitro and in vivo PMC6716473 [139] | Mitochondrial bioenergetics and dynamics enhancement and mitophagy stimulation; cognitive functions improvement | Clinical trial information is collected in the following work PMID:15134388 [207] | Lower cognitive impairment than patients treated with placebo |
| Nicotinamide Riboside | AD | Preclinical in vivo PMC7016185 [151] | Learning and memory improvement (synaptic plasticity amelioration, neurogenesis enhancement, and apoptosis reduction) | |||
| Nicotinamide Riboside | ALS | Preclinical in vitro PMC4865928 [208] | Protection against oxidative stress | |||
| Nicotinamide Riboside | PD | Preclinical in vitro PMID: 29874584 [64] | Mitochondrial biogenesis and bioenergetics enhancement; MMP reduction; downregulation of ROS formation | |||
| Triheptanoin | ALS | Preclinical in vivo PMC5001695 [211] | Motor symptoms onset delay in SOD1G93A mice thanks to mitigation of motor neuron loss | |||
| Triheptanoin | HD | Not found | Clinical trial Phase 2 completed PMC4336068 [210] NCT0188206 | Brain metabolic profile enhancement |
| Therapeutic Function | Drug / Molecule | Pathology | Preclinical Studies / PMCID | Preclinical Results | Clinical Trials / Trial ID | Clinical Results |
|---|---|---|---|---|---|---|
| Antioxidant, mitochondrial dynamics, permeability and bioenergetics, MMP stabilization, and apoptosis inhibition | 3-N-butylphthalide | AD | Preclinical in vitro and in vivo PMID: 30103000 [228] | In vitro: neuroprotection (apoptosis reduction and neuronal proliferation) | ||
| In vivo: cognitive impairment amelioration (synaptic protection, apoptosis inhibition, and antioxidant activity) | ||||||
| 3-N-butylphthalide | ALS | Preclinical in vivo PMID: 30103000 [228] | Lifetime extension and motor performances improvement (motor neuron loss reduction) | |||
| 3-N-butylphthalide | PD | Preclinical in vitro and in vivo PMID: 30103000 [228] PMID: 21524431 [229] | Neuroprotection (oxidative stress mitigation, MMP stabilization, and mPTP opening prevention) | Randomized controlled trial information is collected in the following work PMC6447885 [230] (ChiCTR1800018892) | Motor and sleep quality improvement | |
| Antioxidant, mitochondrial biogenesis, bioenergetics, permeability, and dynamics | Curcumin | AD | Preclinical in vitro and in vivo PMC6716473 [139] | Oxidative stress reduction, mitochondrial biogenesis and bioenergetics enhancement, and MMP stabilization | ||
| Curcumin | ALS | Preclinical in vitro PMC7016185 [151] | Cytotoxicity reduction (antioxidant activity) | Clinical trials information is collected in the following work PMC7016185 [151] | Lifespan prolongation and delaying diseases progression but further studies on different delivery methods are needed | |
| Antioxidant, mitochondrial bioenergetics, and MMP stabilization | Epigallocatechin-Gallate | AD | Preclinical in vitro and in vivo PMC6716473 [139] | In vitro: antioxidant activity and MMP restoration | ||
| In vivo: improvement of cognitive functions in rats injected with streptozotocin | ||||||
| Antioxidant and apoptosis inhibition | Epigallocatechin- Gallate | ALS | Preclinical in vitr, and in vivo PMC7016185 [151] | In vitro: oxidative stress and lipid peroxidation reduction; apoptosis inhibition | ||
| In vivo: motor performances enhancement (increase of survival signal and reduction of death signal) | ||||||
| Antioxidant, mitochondrial bioenergetics, MMP stabilization, and apoptosis inhibition | Flavonoids | ALS | Preclinical in vitro in vivo PMC7016185 [151] | In vitro: antioxidant activity | ||
| In vivo: motor performances improvement (prevention of MN loss) | ||||||
| Antioxidant and apoptosis inhibition | N-Methyl,N-propynyl-2-phenylethylamine | PD | Preclinical studies in vivo PMID: 26563498 [226] | MPPE in MPTP-treated mice: neuroprotection (increase of Complex I activity and UCP-2 expression and antiapoptotic activity) and motor function enhancement | ||
| Antioxidant, mitochondrial dynamics and bioenergetics, MMP stabilization, and apoptosis inhibition | Quercetin | AD | Preclinical in vitro and in vivo PMC6716473 [139] | In vitro: oxidative stress reduction and apoptosis inhibition | ||
| In vivo: cognitive functions improvement; antioxidant activity, MMP and mitochondrial morphology restoration, ROS reduction, ATP levels increase, and apoptosis inhibition | ||||||
| Antioxidant and mitochondrial permeability | R(+) and S(-) Pramipexole | PD | Preclinical studies in vitro and in vivo PMID: 9648878 [224] PMID: 16407457 [225] | Neuroprotection (reduction of ROS generation and mPTP opening prevention) | ||
| Antioxidant, mitochondrial biogenesis and bioenergetics, MMP stabilization, and apoptosis inhibition | Resveratrol | AD | Preclinical in vitro, and in vivo PMC6716473 [139] | In vitro: antioxidant activity, MMP restoration, apoptosis inhibition and mitophagy stimulation | Clinical trial phase Completed PMC5234138 [121] NCT0150485 | Cognitive decline mitigation |
| In vivo: memory loss prevention | ||||||
| Antioxidant, mitochondrial biogenesis and bioenergetics, MMP stabilization, and apoptosis inhibition | Resveratrol with Glucose and Malate | AD | Not found | Clinical trial phase 3Completed PMC6240843 [222] NCT0067843 | Safety and tolerability of low doses are confirmed | |
| Antioxidant, mitochondrial biogenesis and bioenergetics, MMP stabilization, and apoptosis inhibition | Resveratrol | ALS | Preclinical in vivo PMC3996124 [220] | Resveratrol in SOD1G93A ALS mice: delay in pathology onset and progression and lower and upper MNs preservation; increase of mitochondria biogenesis and regulation of autophagic flux | ||
| Antioxidant, mitochondrial dynamics, MMP stabilization, and apoptosis inhibition | Tauroursodeoxycholic acid | ALS | Preclinical in vitro PMID: 24848512 [234] | Glycine-conjugated UDCA exerts an antiapoptotic activity on NSC34 cells carrying G93A mutation | Clinical trial Phase 2 Completed PMC5024041 [236] NCT0087760 | Safety and disease progression decline |
| Ursodeoxycholic acid | ALS | Preclinical in vitro PMID: 24848512 [234] | Already described for TUDCA | Randomized, non-controlled trial, PMC6817734 [237] | Safety and tolerability are confirmed | |
| Ursodeoxycholic acid | ALS | Preclinical in vitro PMID: 24848512 [234] | Already described for TUDCA | Clinical trial Phase 3, PMC6817734 [237] | ALS progression decline | |
| Ursodeoxycholic acid | AD | Preclinical studies in vitro PMC6193139 [235] | UDCA on fibroblasts from AD patients: MMP restoration involving Drp1 | |||
| Tauroursodeoxycholic acid | HD | Preclinical in vivo PMC125009 [233] | Motor and sensory improvement on R6/2 mice (neuroprotection) | |||
| Ursodeoxycholic acid | PD | Preclinical in vitro and in vivo PMID: 30219247 [157] | Apoptosis inhibition; motor performances enhancement (low striatal dopamine decline) | |||
| MMP stabilization and apoptosis inhibition | Wogonin | AD | Preclinical in vitro and in vivo PMC5478820 [219] | Wogonin on Tet-On Aβ42-GFP SH-SY5Y neuroblastoma cells: MMP stabilization and apoptosis inhibition | ||
| Wogonin on 3xTg mice: cognitive functions improvement (neuroprotective and neurotrophic activity) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanga, S.; Caretto, A.; Boido, M.; Vercelli, A. Mitochondrial Dysfunctions: A Red Thread across Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 3719. https://doi.org/10.3390/ijms21103719
Stanga S, Caretto A, Boido M, Vercelli A. Mitochondrial Dysfunctions: A Red Thread across Neurodegenerative Diseases. International Journal of Molecular Sciences. 2020; 21(10):3719. https://doi.org/10.3390/ijms21103719
Chicago/Turabian StyleStanga, Serena, Anna Caretto, Marina Boido, and Alessandro Vercelli. 2020. "Mitochondrial Dysfunctions: A Red Thread across Neurodegenerative Diseases" International Journal of Molecular Sciences 21, no. 10: 3719. https://doi.org/10.3390/ijms21103719
APA StyleStanga, S., Caretto, A., Boido, M., & Vercelli, A. (2020). Mitochondrial Dysfunctions: A Red Thread across Neurodegenerative Diseases. International Journal of Molecular Sciences, 21(10), 3719. https://doi.org/10.3390/ijms21103719