Mechanisms of Apoptosis Resistance to NK Cell-Mediated Cytotoxicity in Cancer
Abstract
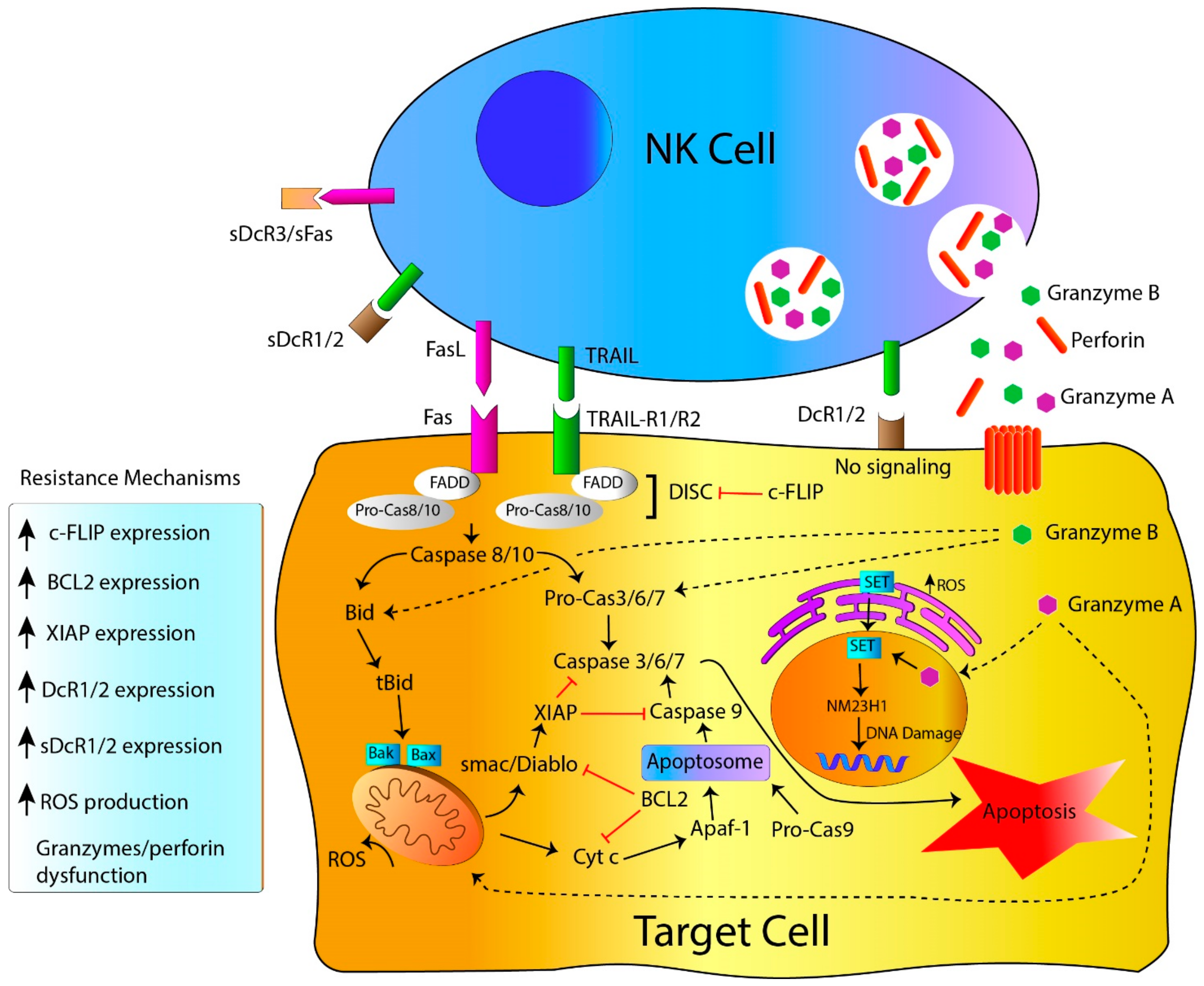
:1. Introduction
2. NK Cell-Killing Mechanisms
2.1. NK Cell Killing Mechanisms: Death Receptors, the Extrinsic Apoptosis Pathway
2.2. NK Cell-Killing Mechanisms: Target Cell Lysis by Perforin, Granzymes and Granulysin Granules
3. Resistance to NK Cell-Mediated Cytotoxicity
3.1. Resistance to Apoptosis: Genetic Alterations Linked to Genes Governing Apoptosis
3.2. Resistance to Death Receptors-Mediated Cell Death
3.3. Resistance to Perforin, Granzymes and Granulysin-Mediated Cell Death
3.4. Resistance to Apoptosis: Influence of the Tumor Microenvironment
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Chiossone, L.; Dumas, P.-Y.; Vienne, M.; Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar] [CrossRef] [PubMed]
- López-Soto, A.; Gonzalez, S.; Smyth, M.J.; Galluzzi, L. Control of Metastasis by NK Cells. Cancer Cell 2017, 32, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Herrero, S.; López-Soto, A.; Sordo-Bahamonde, C.; Gonzalez, A.P.; Vitale, M.; Gonzalez, S. NK Cell-Based Immunotherapy in Cancer Metastasis. Cancers 2018, 11, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.C.; Lanier, L.L. NK cell development, homeostasis and function: Parallels with CD8⁺ T cells. Nat. Rev. Immunol. 2011, 11, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Herrero, S.; Sordo-Bahamonde, C.; González, S.; López-Soto, A. Immunosurveillance of cancer cell stress. Cell Stress 2019, 3, 295–309. [Google Scholar] [CrossRef]
- Sivori, S.; Vacca, P.; Del Zotto, G.; Munari, E.; Mingari, M.C.; Moretta, L. Human NK cells: Surface receptors, inhibitory checkpoints, and translational applications. Cell. Mol. Immunol. 2019, 16, 430–441. [Google Scholar] [CrossRef]
- Villa-Álvarez, M.; Sordo-Bahamonde, C.; Lorenzo-Herrero, S.; Gonzalez-Rodriguez, A.P.; Payer, A.R.; Gonzalez-Garcia, E.; Villa-Álvarez, M.C.; López-Soto, A.; Gonzalez, S. Ig-Like Transcript 2 (ILT2) Blockade and Lenalidomide Restore NK Cell Function in Chronic Lymphocytic Leukemia. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- López-Soto, A.; Huergo-Zapico, L.; Acebes-Huerta, A.; Villa-Alvarez, M.; González, S. NKG2D signaling in cancer immunosurveillance. Int. J. Cancer 2014, 136, 1741–1750. [Google Scholar] [CrossRef]
- Topham, N.J.; Hewitt, E. Natural killer cell cytotoxicity: How do they pull the trigger? Immunol. 2009, 128, 7–15. [Google Scholar] [CrossRef]
- Gutiérrez-Cívicos, R.; Hurtado, A.M.; Torres-Moreno, D.; Sanchez-Blanco, J.J.; Español, I.; Consuegra-Sanchez, L.; Pérez-Ceballos, E.; Gutiérrez-Meca, M.D.; Jerez, A.; Conesa-Zamora, P. A polymorphism in FASL is associated with rituximab response in follicular lymphoma patients. Am. J. Hematol. 2016, 91, E305–E307. [Google Scholar] [CrossRef] [Green Version]
- Vega, M.I.; Huerta-Yepez, S.; Jazirehi, A.R.; Garban, H.; Bonavida, B. Rituximab (chimeric anti-CD20) sensitizes B-NHL cell lines to Fas-induced apoptosis. Oncogene 2005, 24, 8114–8127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyfizadeh, N.; Seyfizadeh, N.; Hasenkamp, J.; Huerta-Yepez, S.; Information, P.E.K.F.C. A molecular perspective on rituximab: A monoclonal antibody for B cell non Hodgkin lymphoma and other affections. Crit. Rev. Oncol. 2016, 97, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Stel, A.J.; Cate, B.T.; Jacobs, S.; Kok, J.W.; Spierings, D.C.; Dondorff, M.; Helfrich, W.; Kluin-Nelemans, H.C.; De Leij, L.F.M.H.; Withoff, S.; et al. Fas receptor clustering and involvement of the death receptor pathway in rituximab-mediated apoptosis with concomitant sensitization of lymphoma B cells to fas-induced apoptosis. J. Immunol. 2007, 178, 2287–2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedi, A.; Chang, X.; Noonan, K.; Pham, V.; Bedi, R.; Fertig, E.J.; Considine, M.; Califano, J.A.; Borrello, I.; Chung, C.H.; et al. Inhibition of TGF-β enhances the in vivo antitumor efficacy of EGF receptor-targeted therapy. Mol. Cancer Ther. 2012, 11, 2429–2439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; A Weinberg, R. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Lowe, S.W.; Cepero, E.; Evan, G. Intrinsic tumour suppression. Nature 2004, 432, 307–315. [Google Scholar] [CrossRef]
- Peter, M.E.; Hadji, A.; E Murmann, A.; Brockway, S.; Putzbach, W.; Pattanayak, A.; Ceppi, P. The role of CD95 and CD95 ligand in cancer. Cell Death Differ. 2015, 22, 549–559. [Google Scholar] [CrossRef]
- Yamada, A.; Arakaki, R.; Saito, M.; Kudo, Y.; Ishimaru, N. Dual Role of Fas/FasL-Mediated Signal in Peripheral Immune Tolerance. Front. Immunol. 2017, 8, 1449. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, S.; Steensma, D.P. On the TRAIL of a new therapy for leukemia. Leukemia 2005, 19, 2195–2202. [Google Scholar] [CrossRef] [Green Version]
- Mirandola, P.; Ponti, C.; Gobbi, G.; Sponzilli, I.; Vaccarezza, M.; Cocco, L.; Zauli, G.; Secchiero, P.; Manzoli, F.A.; Vitale, M. Activated human NK and CD8+ T cells express both TNF-related apoptosis-inducing ligand (TRAIL) and TRAIL receptors but are resistant to TRAIL-mediated cytotoxicity. Blood 2004, 104, 2418–2424. [Google Scholar] [CrossRef]
- Von Karstedt, S.; Montinaro, A.; Walczak, H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat. Rev. Cancer 2017, 17, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Sprick, M.R.; A Weigand, M.; Rieser, E.; Rauch, C.T.; Juo, P.; Blenis, J.; Krammer, P.H.; Walczak, H. FADD/MORT1 and Caspase-8 Are Recruited to TRAIL Receptors 1 and 2 and Are Essential for Apoptosis Mediated by TRAIL Receptor 2. Immunity 2000, 12, 599–609. [Google Scholar] [CrossRef] [Green Version]
- Pitti, R.M.; Marsters, S.A.; Lawrence, D.A.; Roy, M.; Kischkel, F.C.; Dowd, P.; Huang, A.; Donahue, C.J.; Sherwood, S.W.; Baldwin, D.T.; et al. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature 1998, 396, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Neumann, S.; Hasenauer, J.; Pollak, N.; Scheurich, P. Dominant Negative Effects of Tumor Necrosis Factor (TNF)-related Apoptosis-inducing Ligand (TRAIL) Receptor 4 on TRAIL Receptor 1 Signaling by Formation of Heteromeric Complexes. J. Boil. Chem. 2014, 289, 16576–16587. [Google Scholar] [CrossRef] [Green Version]
- Merino, D.; Lalaoui, N.; Morizot, A.; Schneider, P.; Solary, E.; Micheau, O. Differential Inhibition of TRAIL-Mediated DR5-DISC Formation by Decoy Receptors 1 and 2. Mol. Cell. Boil. 2006, 26, 7046–7055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheridan, J.P.; Marsters, S.A.; Pitti, R.M.; Gurney, A.; Skubatch, M.; Baldwin, D.; Ramakrishnan, L.; Gray, C.L.; Baker, K.; Wood, W.I.; et al. Control of TRAIL-Induced Apoptosis by a Family of Signaling and Decoy Receptors. Science 1997, 277, 818–821. [Google Scholar] [CrossRef]
- Sanlioglu, A.D.; Dirice, E.; Aydin, C.; Erin, N.; Koksoy, S.; Sanlioglu, S. Surface TRAIL decoy receptor-4 expression is correlated with TRAIL resistance in MCF7 breast cancer cells. BMC Cancer 2005, 5, 54. [Google Scholar] [CrossRef] [Green Version]
- Leblanc, H.N.; Ashkenazi, A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 2003, 10, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Kischkel, F.C.; Hellbardt, S.; Behrmann, I.; Germer, M.; Pawlita, M.; Krammer, P.H.; Peter, M.E. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995, 14, 5579–5588. [Google Scholar] [CrossRef]
- Schug, Z.T.; Gonzalvez, F.; Houtkooper, R.H.; Vaz, F.M.; Gottlieb, E. BID is cleaved by caspase-8 within a native complex on the mitochondrial membrane. Cell Death Differ. 2010, 18, 538–548. [Google Scholar] [CrossRef]
- Korsmeyer, S.J.; Wei, M.C.; Saito, M.; Weiler, S.; Oh, K.J.; Schlesinger, P.H. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000, 7, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Itai, T.; Adachi, M.; Nagata, S. Downregulation of Fas ligand by shedding. Nat. Med. 1998, 4, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Reilly, L.A.O.; Tai, L.; Lee, L.; Kruse, E.A.; Grabow, S.; Fairlie, W.; Haynes, N.M.; Tarlinton, D.; Zhang, J.-G.; Belz, G.; et al. Membrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature 2009, 461, 659–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, P.; Holler, N.; Bodmer, J.-L.; Hahne, M.; Frei, K.; Fontana, A.; Tschopp, J. Conversion of Membrane-bound Fas(CD95) Ligand to Its Soluble Form Is Associated with Downregulation of Its Proapoptotic Activity and Loss of Liver Toxicity. J. Exp. Med. 1998, 187, 1205–1213. [Google Scholar] [CrossRef]
- Wajant, H.; Moosmayer, D.; Wüest, T.; Bartke, T.; Gerlach, E.; Schönherr, U.; Peters, N.; Scheurich, P.; Pfizenmaier, K. Differential activation of TRAIL-R1 and -2 by soluble and membrane TRAIL allows selective surface antigen-directed activation of TRAIL-R2 by a soluble TRAIL derivative. Oncogene 2001, 20, 4101–4106. [Google Scholar] [CrossRef] [Green Version]
- Maecker, H.L.; Yun, Z.; Maecker, H.T.; Giaccia, A.J. Epigenetic changes in tumor Fas levels determine immune escape and response to therapy. Cancer Cell 2002, 2, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Price, S.; Shaw, P.A.; Seitz, A.; Joshi, G.; Davis, J.; Niemela, J.E.; Perkins, K.; Hornung, R.L.; Folio, L.; Rosenberg, P.S.; et al. Natural history of autoimmune lymphoproliferative syndrome associated with FAS gene mutations. Blood 2014, 123, 1989–1999. [Google Scholar] [CrossRef]
- Zörnig, M.; Grzeschiczek, A.; Kowalski, M.B.; Hartmann, K.U.; Möröy, T. Loss of Fas/Apo-1 receptor accelerates lymphomagenesis in E mu L-MYC transgenic mice but not in animals infected with MoMuLV. Oncogene 1995, 10, 2397–2401. [Google Scholar]
- Davidson, W.F.; Giese, T.; Fredrickson, T.N. Spontaneous Development of Plasmacytoid Tumors in Mice with Defective Fas–Fas Ligand Interactions. J. Exp. Med. 1998, 187, 1825–1838. [Google Scholar] [CrossRef]
- Adachi, M.; Suematsu, S.; Suda, T.; Watanabe, D.; Fukuyama, H.; Ogasawara, J.; Tanaka, T.; Yoshida, N.; Nagata, S. Enhanced and accelerated lymphoproliferation in Fas-null mice. Proc. Natl. Acad. Sci. USA 1996, 93, 2131–2136. [Google Scholar] [CrossRef] [Green Version]
- Fingleton, B.; Carter, K.J.; Matrisian, L.M. Loss of Functional Fas Ligand Enhances Intestinal Tumorigenesis in the Min Mouse Model. Cancer Res. 2007, 67, 4800–4806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedger, L.; Glaccum, M.B.; Schuh, J.C.L.; Kanaly, S.T.; Williamson, E.; Kayagaki, N.; Yun, T.; Smolak, P.; Le, T.; Goodwin, R.; et al. Characterization of the in vivo function of TNF-α-related apoptosis-inducing ligand, TRAIL/Apo2L, using TRAIL/Apo2L gene-deficient mice. Eur. J. Immunol. 2002, 32, 2246. [Google Scholar] [CrossRef]
- Cretney, E.; Takeda, K.; Yagita, H.; Glaccum, M.; Peschon, J.J.; Smyth, M.J. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J. Immunol. 2002, 168, 1356–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diao, Z.; Shi, J.; Zhu, J.; Yuan, H.; Ru, Q.; Liu, S.; Liu, Y.; Zheng, D. TRAIL suppresses tumor growth in mice by inducing tumor-infiltrating CD4+CD25+ Treg apoptosis. Cancer Immunol. Immunother. 2012, 62, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Finnberg, N.; Klein-Szanto, A.J.; El-Deiry, W. TRAIL-R deficiency in mice promotes susceptibility to chronic inflammation and tumorigenesis. J. Clin. Investig. 2007, 118, 111–123. [Google Scholar] [CrossRef]
- Grosse-Wilde, A.; Voloshanenko, O.; Bailey, S.L.; Longton, G.M.; Schaefer, U.; Csernok, A.I.; Schutz, G.; Greiner, E.F.; Kemp, C.J.; Walczak, H. TRAIL-R deficiency in mice enhances lymph node metastasis without affecting primary tumor development. J. Clin. Investig. 2007, 118, 100–110. [Google Scholar] [CrossRef] [Green Version]
- Grosse-Wilde, A.; Kemp, C.J. Metastasis suppressor function of tumor necrosis factor-related apoptosis-inducing ligand-R in mice: Implications for TRAIL-based therapy in humans? Cancer Res. 2008, 68, 6035–6037. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Lostao, L.; Anel, A.; Pardo, J. How Do Cytotoxic Lymphocytes Kill Cancer Cells? Clin. Cancer Res. 2015, 21, 5047–5056. [Google Scholar] [CrossRef] [Green Version]
- Ewen, C.L.; Kane, K.P.; Bleackley, R.C. A quarter century of granzymes. Cell Death Differ. 2011, 19, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Voskoboinik, I.; Smyth, M.J.; Trapani, J.A. Perforin-mediated target-cell death and immune homeostasis. Nat. Rev. Immunol. 2006, 6, 940–952. [Google Scholar] [CrossRef]
- Basile, G.D.S.; Ménasché, G.; Fischer, A. Molecular mechanisms of biogenesis and exocytosis of cytotoxic granules. Nat. Rev. Immunol. 2010, 10, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Praper, T.; Sonnen, A.F.-P.; Kladnik, A.; Andrighetti, A.O.; Viero, G.; Morris, K.J.; Volpi, E.; Lunelli, L.; Serra, M.D.; Froelich, C.J.; et al. Perforin activity at membranes leads to invaginations and vesicle formation. Proc. Natl. Acad. Sci. USA 2011, 108, 21016–21021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trapani, J.A.; Sutton, V.R.; Thia, K.Y.; Li, Y.Q.; Froelich, C.J.; Jans, D.A.; Sandrin, M.S.; Browne, K.A. A clathrin/dynamin- and mannose-6-phosphate receptor–independent pathway for granzyme B–induced cell death. J. Cell Boil. 2003, 160, 223–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veugelers, K.; Motyka, B.; Frantz, C.; Shostak, I.; Sawchuk, T.; Bleackley, R.C. The granzyme B–serglycin complex from cytotoxic granules requires dynamin for endocytosis. Blood 2004, 103, 3845–3853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolitho, P.; Voskoboinik, I.; Trapani, J.; Smyth, M.J.; Trapani, J.A. Apoptosis induced by the lymphocyte effector molecule perforin. Curr. Opin. Immunol. 2007, 19, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Keefe, D.; Durand, E.; Feng, H.; Zhang, N.; Lieberman, J. Granzyme B binds to target cells mostly by charge and must be added at the same time as perforin to trigger apoptosis. J. Immunol. 2005, 174, 5456–5461. [Google Scholar] [CrossRef] [Green Version]
- Kägi, D.; Ledermann, B.; Bürki, K.; Seiler, P.; Odermatt, B.; Olsen, K.J.; Podack, E.R.; Zinkernagel, R.M.; Hengartner, H.; K, B.L.D. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 1994, 369, 31–37. [Google Scholar] [CrossRef]
- Smyth, M.J.; Thia, K.Y.; Street, S.E.; MacGregor, D.; Godfrey, D.; Trapani, J.A. Perforin-Mediated Cytotoxicity Is Critical for Surveillance of Spontaneous Lymphoma. J. Exp. Med. 2000, 192, 755–760. [Google Scholar] [CrossRef] [Green Version]
- Broek, M.E.V.D.; Kägi, D.; Ossendorp, F.; Toes, R.; Vamvakas, S.; Lutz, W.K.; Melief, C.J.; Zinkernagel, R.M.; Hengartner, H. Decreased tumor surveillance in perforin-deficient mice. J. Exp. Med. 1996, 184, 1781–1790. [Google Scholar] [CrossRef] [Green Version]
- Smyth, M.J.; Thia, K.Y.; Cretney, E.; Kelly, J.M.; Snook, M.B.; A Forbes, C.; A Scalzo, A. Perforin is a major contributor to NK cell control of tumor metastasis. J. Immunol. 1999, 162, 6658–6662. [Google Scholar]
- Chia, J.; Yeo, K.P.; Whisstock, J.C.; Dunstone, M.A.; Trapani, J.A.; Voskoboinik, I. Temperature sensitivity of human perforin mutants unmasks subtotal loss of cytotoxicity, delayed FHL, and a predisposition to cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 9809–9814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcenaro, S.; Gallo, F.; Martini, S.; Santoro, A.; Griffiths, G.M.; Aricò, M.; Moretta, L.; Pende, D. Analysis of natural killer–cell function in familial hemophagocytic lymphohistiocytosis (FHL): Defective CD107a surface expression heralds Munc13-4 defect and discriminates between genetic subtypes of the disease. Blood 2006, 108, 2316–2323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soung, Y.H.; Lee, J.W.; Kim, S.Y.; Jang, J.; Park, Y.G.; Park, W.S.; Nam, S.W.; Lee, J.Y.; Yoo, N.J.; Lee, S.H. CASPASE-8 gene is inactivated by somatic mutations in gastric carcinomas. Cancer Res. 2005, 65, 815–821. [Google Scholar] [PubMed]
- Liu, B.; Peng, D.; Lu, Y.; Jin, W.; Fan, Z. A Novel Single Amino Acid Deletion Caspase-8 Mutant in Cancer Cells That Lost Proapoptotic Activity. J. Boil. Chem. 2002, 277, 30159–30164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soung, Y.H.; Lee, J.W.; Kim, S.Y.; Sung, Y.J.; Park, W.S.; Nam, S.W.; Kim, S.H.; Lee, J.Y.; Yoo, N.J.; Lee, S.H. Caspase-8 gene is frequently inactivated by the frameshift somatic mutation 1225_1226delTG in hepatocellular carcinomas. Oncogene 2004, 24, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Kim, M.R.; Soung, Y.H.; Nam, S.W.; Kim, S.H.; Lee, J.Y.; Yoo, N.J.; Lee, S.H. Mutational analysis of the CASP6 gene in colorectal and gastric carcinomas. APMIS 2006, 114, 646–650. [Google Scholar] [CrossRef]
- Soung, Y.; Lee, J.; Kim, S.; Park, W.; Nam, S.; Lee, J.; Yoo, N.; Lee, S. Somatic mutations of CASP3 gene in human cancers. Qual. Life Res. 2004, 115, 112–115. [Google Scholar] [CrossRef]
- Kim, Y.R.; Kim, K.M.; Yoo, N.J.; Lee, S.H. Mutational analysis of CASP1, 2, 3, 4, 5, 6, 7, 8, 9, 10, and 14 genes in gastrointestinal stromal tumors. Hum. Pathol. 2009, 40, 868–871. [Google Scholar] [CrossRef]
- Soung, Y.H.; Jeong, E.G.; Ahn, C.H.; Kim, S.S.; Song, S.Y.; Yoo, N.J.; Lee, S.H. Mutational analysis of caspase 1, 4, and 5 genes in common human cancers. Hum. Pathol. 2008, 39, 895–900. [Google Scholar] [CrossRef]
- Soung, Y.H.; Lee, J.W.; Kim, H.S.; Park, W.S.; Kim, S.Y.; Lee, J.-H.; Park, J.Y.; Cho, Y.G.; Kim, C.J.; Park, Y.G.; et al. Inactivating mutations of CASPASE-7 gene in human cancers. Oncogene 2003, 22, 8048–8052. [Google Scholar] [CrossRef] [Green Version]
- Soung, Y.H.; Lee, J.W.; Kim, S.Y.; Park, W.S.; Nam, S.W.; Lee, J.Y.; Yoo, N.J.; Lee, S.H. Mutational analysis of proapoptotic caspase-9 gene in common human carcinomas. APMIS 2006, 114, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Kageyama, H.; Takada, N.; Kawamato, T.; Takayasu, H.; Isogai, E.; Ohira, M.; Hashizume, K.; Kobayashi, H.; Kaneko, Y.; et al. High expression of Survivin, mapped to 17q25, is significantly associated with poor prognostic factors and promotes cell survival in human neuroblastoma. Oncogene 2000, 19, 617–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasula, S.M.; Ashwell, J.D. IAPs: What’s in a Name? Mol. Cell 2008, 30, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Zender, L.; Spector, M.S.; Xue, W.; Flemming, P.; Cordon-Cardo, C.; Silke, J.; Fan, S.-T.; Luk, J.; Wigler, M.; Hannon, G.J.; et al. Identification and Validation of Oncogenes in Liver Cancer Using an Integrative Oncogenomic Approach. Cell 2006, 125, 1253–1267. [Google Scholar] [CrossRef] [Green Version]
- Dierlamm, J.; Baens, M.; Wlodarska, I.; Stefanova-Ouzounova, M.; Hernandez, J.M.; Hossfeld, D.K.; De Wolf-Peeters, C.; Hagemeijer, A.; Berghe, H.V.D.; Marynen, P. The apoptosis inhibitor gene API2 and a novel 18q gene, MLT, are recurrently rearranged in the t(11;18)(q21;q21) associated with mucosa-associated lymphoid tissue lymphomas. Blood 1999, 93, 3601–3609. [Google Scholar] [CrossRef]
- Miquel, C.; Borrini, F.; Grandjouan, S.; Aupérin, A.; Viguier, J.; Velasco, V.; Duvillard, P.; Praz, F.; Sabourin, J.-C. Role of bax Mutations in Apoptosis in Colorectal Cancers With Microsatellite Instability. Am. J. Clin. Pathol. 2005, 123, 562–570. [Google Scholar] [CrossRef]
- Ionov, Y.; Yamamoto, H.; Krajewski, S.; Reed, J.C.; Perucho, M. Mutational inactivation of the proapoptotic gene BAX confers selective advantage during tumor clonal evolution. Proc. Natl. Acad. Sci. USA 2000, 97, 10872–10877. [Google Scholar] [CrossRef] [Green Version]
- Rampino, N.; Yamamoto, H.; Ionov, Y.; Li, Y.; Sawai, H.; Reed, J.C.; Perucho, M. Somatic Frameshift Mutations in theBAXGene in Colon Cancers of the Microsatellite Mutator Phenotype. Science 1997, 275, 967–969. [Google Scholar] [CrossRef]
- Inoue, K.; Kohno, T.; Takakura, S.; Hayashi, Y.; Mizoguchi, H.; Yokota, J. Frequent microsatellite instability and BAX mutations in T cell acute lymphoblastic leukemia cell lines. Leuk. Res. 2000, 24, 255–262. [Google Scholar] [CrossRef]
- Moshynska, O.; Sankaran, K.; Saxena, A. Molecular detection of the G(−248)A BAX promoter nucleotide change in B cell chronic lymphocytic leukaemia. Mol. Pathol. 2003, 56, 205–209. [Google Scholar] [CrossRef] [Green Version]
- Brimmell, M.; Mendiola, R.; Mangion, J.; Packham, G. BAX frameshift mutations in cell lines derived from human haemopoietic malignancies are associated with resistance to apoptosis and microsatellite instability. Oncogene 1998, 16, 1803–1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuetz, J.M.; A Johnson, N.; Morin, R.D.; Scott, D.W.; Tan, K.; Ben-Nierah, S.; Boyle, M.; Slack, G.W.; A Marra, M.; Connors, J.M.; et al. BCL2 mutations in diffuse large B-cell lymphoma. Leukemia 2011, 26, 1383–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarid, R.; Sato, T.; Bohenzky, R.A.; Russo, J.J.; Chang, Y. Kaposi’s sarcoma-associated herpesvirus encodes a functional Bcl-2 homologue. Nat. Med. 1997, 3, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Stürzl, M.; Hohenadl, C.; Zietz, C.; Castaños-Vèlez, E.; Wunderlich, A.; Ascherl, G.; Biberfeld, P.; Monini, P.; Browning, P.J.; Ensoli, B. Expression of K13/v-FLIP Gene of Human Herpesvirus 8 and Apoptosis in Kaposi’s Sarcoma Spindle Cells. J. Natl. Cancer Inst. 1999, 91, 1725–1733. [Google Scholar] [CrossRef] [Green Version]
- Valnet-Rabier, M.-B.; Challier, B.; Thiébault, S.; Angonin, R.; Margueritte, G.; Mougin, C.; Kantelip, B.; Deconinck, E.; Cahn, J.-Y.; Fest, T. c-Flip protein expression in Burkitt’s lymphomas is associated with a poor clinical outcome. Br. J. Haematol. 2005, 128, 767–773. [Google Scholar] [CrossRef]
- McLornan, D.; Hay, J.; McLaughlin, K.; Holohan, C.; Burnett, A.; Hills, R.K.; Johnston, P.G.; Mills, K.I.; McMullin, M.F.; Longley, D.B. Prognostic and therapeutic relevance of c-FLIP in acute myeloid leukaemia. Br. J. Haematol. 2012, 160, 188–198. [Google Scholar] [CrossRef]
- Ullenhag, G.J.; Mukherjee, A.; Watson, N.J.; Al-Attar, A.H.; Scholefield, J.H.; Durrant, L. Overexpression of FLIPL Is an Independent Marker of Poor Prognosis in Colorectal Cancer Patients. Clin. Cancer Res. 2007, 13, 5070–5075. [Google Scholar] [CrossRef] [Green Version]
- Tagawa, H.; Karnan, S.; Suzuki, R.; Matsuo, K.; Zhang, X.; Ota, A.; Morishima, Y.; Nakamura, S.; Seto, M. Genome-wide array-based CGH for mantle cell lymphoma: Identification of homozygous deletions of the proapoptotic gene BIM. Oncogene 2004, 24, 1348–1358. [Google Scholar] [CrossRef]
- Mestre-Escorihuela, C.; Rubio-Moscardó, F.; Richter, J.A.; Siebert, R.; Climent, J.; Fresquet, V.; Beltran, E.; Agirre, X.; Marugan, I.; Mariín, M.; et al. Homozygous deletions localize novel tumor suppressor genes in B-cell lymphomas. Blood 2006, 109, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Fisher, M.J.; Virmani, A.K.; Wu, L.; Aplenc, R.; Harper, J.C.; Powell, S.M.; Rebbeck, T.R.; Sidransky, D.; Gazdar, A.F.; El-Deiry, W.S. Nucleotide substitution in the ectodomain of trail receptor DR4 is associated with lung cancer and head and neck cancer. Clin. Cancer Res. 2001, 7, 1688–1697. [Google Scholar]
- Lee, S.H.; Shin, M.S.; Kim, H.S.; Lee, H.K.; Park, W.S.; Kim, S.Y.; Lee, J.-H.; Han, S.Y.; Park, J.Y.; Oh, R.R.; et al. Somatic mutations of TRAIL-receptor 1 and TRAIL-receptor 2 genes in non-Hodgkin’s lymphoma. Oncogene 2001, 20, 399–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubio-Moscardó, F.; Blesa, D.; Mestre, C.; Siebert, R.; Balasas, T.; Benito, A.; Rosenwald, A.; Climent, J.; Martínez, J.I.; Schilhabel, M.; et al. Characterization of 8p21.3 chromosomal deletions in B-cell lymphoma: TRAIL-R1 and TRAIL-R2 as candidate dosage-dependent tumor suppressor genes. Blood 2005, 106, 3214–3222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, M.S.; Kim, H.S.; Lee, S.H.; Park, W.S.; Kim, S.Y.; Park, J.Y.; Lee, J.H.; Lee, S.K.; Lee, S.N.; Jung, S.S.; et al. Mutations of tumor necrosis factor-related apoptosis-inducing ligand receptor 1 (TRAIL-R1) and receptor 2 (TRAIL-R2) genes in metastatic breast cancers. Cancer Res. 2001, 61, 4942–4946. [Google Scholar] [PubMed]
- McDonald, E.R.; Chui, P.C.; Martelli, P.F.; Dicker, D.T.; El-Deiry, W. Death Domain Mutagenesis of KILLER/DR5 Reveals Residues Critical for Apoptotic Signaling. J. Boil. Chem. 2001, 276, 14939–14945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Shin, M.S.; Kim, H.S.; Lee, H.K.; Park, W.S.; Kim, S.Y.; Lee, J.H.; Han, S.Y.; Park, J.Y.; Oh, R.R.; et al. Alterations of the DR5/TRAIL Receptor 2 Gene in Non-Small Cell Lung Cancers. Cancer Res. 1999, 59, 5683–5686. [Google Scholar] [PubMed]
- Park, W.S.; Lee, J.-H.; Shin, M.S.; Park, J.Y.; Kim, H.S.; Kim, Y.S.; Park, C.H.; Lee, S.K.; Lee, S.H.; Lee, S.N.; et al. Inactivating mutations of KILLER/DR5 gene in gastric cancers. Gastroenterol. 2001, 121, 1219–1225. [Google Scholar] [CrossRef]
- Tauzin, S.; Debure, L.; Moreau, J.-F.; Legembre, P. CD95-mediated cell signaling in cancer: Mutations and post-translational modulations. Cell. Mol. Life Sci. 2011, 69, 1261–1277. [Google Scholar] [CrossRef]
- Shipman, C.M.; I Croucher, P. Osteoprotegerin is a soluble decoy receptor for tumor necrosis factor-related apoptosis-inducing ligand/Apo2 ligand and can function as a paracrine survival factor for human myeloma cells. Cancer Res. 2003, 63, 912–916. [Google Scholar]
- Roth, W.; Isenmann, S.; Nakamura, M.; Platten, M.; Wick, W.; Kleihues, P.; Bähr, M.; Ohgaki, H.; Ashkenazi, A.; Weller, M. Soluble decoy receptor 3 is expressed by malignant gliomas and suppresses CD95 ligand-induced apoptosis and chemotaxis. Cancer Res. 2001, 61, 2759–2765. [Google Scholar]
- Wu, Q.; Zheng, Y.; Chen, D.; Li, X.; Lu, C.; Zhang, Z.-M. Aberrant expression of decoy receptor 3 in human breast cancer: Relevance to lymphangiogenesis. J. Surg. Res. 2014, 188, 459–465. [Google Scholar] [CrossRef] [Green Version]
- Takahama, Y.; Yamada, Y.; Emoto, K.; Fujimoto, H.; Takayama, T.; Ueno, M.; Uchida, H.; Hirao, S.; Mizuno, T.; Nakajima, Y. The prognostic significance of overexpression of the decoy receptor for Fas ligand (DcR3) in patients with gastric carcinomas. Gastric Cancer 2002, 5, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-P.; Zhu, H.-F.; Liu, D.-L.; Hu, Z.-Y.; Li, S.-N.; Kan, H.-P.; Wang, X.-Y.; Li, Z.-G. DcR3 induces epithelial-mesenchymal transition through activation of the TGF-β3/SMAD signaling pathway in CRC. Oncotarget 2016, 7, 77306–77318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamuleau, M.E.; Ossenkoppele, G.; Van Rhenen, A.; Van Dreunen, L.; Jirka, S.; Zevenbergen, A.; Schuurhuis, G.; Van De Loosdrecht, A. High TRAIL-R3 expression on leukemic blasts is associated with poor outcome and induces apoptosis-resistance which can be overcome by targeting TRAIL-R2. Leuk. Res. 2011, 35, 741–749. [Google Scholar] [CrossRef] [Green Version]
- De Almodóvar, C.R.; Ruiz-Ruiz, C.; Rodríguez, A.; Ortiz-Ferrón, G.; Redondo, J.M.; Lopez-Rivas, A. Tumor Necrosis Factor-related Apoptosis-inducing Ligand (TRAIL) Decoy Receptor TRAIL-R3 Is Up-regulated by p53 in Breast Tumor Cells through a Mechanism Involving an Intronic p53-binding Site. J. Boil. Chem. 2003, 279, 4093–4101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koksal, I.T.; Sanlioglu, A.D.; Karaçay, B.; Griffith, T.S.; Sanlioglu, S. Tumor necrosis factor-related apoptosis inducing ligand-R4 decoy receptor expression is correlated with high Gleason scores, prostate-specific antigen recurrence, and decreased survival in patients with prostate carcinoma. Urol. Oncol. Semin. Orig. Investig. 2008, 26, 158–165. [Google Scholar] [CrossRef]
- Peng, Y.-P.; Zhu, Y.; Zhang, J.-J.; Xu, Z.; Qian, Z.; Dai, C.-C.; Jiang, K.-R.; Wu, J.; Gao, W.; Li, Q.; et al. Comprehensive analysis of the percentage of surface receptors and cytotoxic granules positive natural killer cells in patients with pancreatic cancer, gastric cancer, and colorectal cancer. J. Transl. Med. 2013, 11, 262. [Google Scholar] [CrossRef] [Green Version]
- Hodge, G.; Barnawi, J.; Jurisevic, C.; Moffat, D.; Holmes, M.; Reynolds, P.N.; Jersmann, H.; Hodge, S. Lung cancer is associated with decreased expression of perforin, granzyme B and interferon (IFN)-γ by infiltrating lung tissue T cells, natural killer (NK) T-like and NK cells. Clin. Exp. Immunol. 2014, 178, 79–85. [Google Scholar] [CrossRef]
- Sun, C.; Xu, J.; Huang, Q.; Huang, M.; Wen, H.; Zhang, C.; Wang, J.; Song, J.; Zheng, M.; Sun, H.; et al. High NKG2A expression contributes to NK cell exhaustion and predicts a poor prognosis of patients with liver cancer. Oncoimmunology 2016, 6, e1264562. [Google Scholar] [CrossRef]
- Liu, C.; Yu, S.; Kappes, J.; Wang, J.; Grizzle, W.E.; Zinn, K.; Zhang, H.-G. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood 2007, 109, 4336–4342. [Google Scholar] [CrossRef]
- Li, T.; Yang, Y.; Hua, X.; Wang, G.; Liu, W.; Jia, C.; Tai, Y.; Zhang, Q.; Chen, G. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett. 2012, 318, 154–161. [Google Scholar] [CrossRef]
- Balsamo, M.; Scordamaglia, F.; Pietra, G.; Manzini, C.; Cantoni, C.; Boitano, M.; Queirolo, P.; Vermi, W.; Facchetti, F.; Moretta, A.; et al. Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 20847–20852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Yi, S.; Liu, W.; Jia, C.; Wang, G.; Hua, X.; Tai, Y.; Zhang, Q.; Chen, G. Colorectal carcinoma-derived fibroblasts modulate natural killer cell phenotype and antitumor cytotoxicity. Med Oncol. 2013, 30, 663. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, C.; Zeis, M.; Schmitz, N.; Uharek, L. Impaired binding of perforin on the surface of tumor cells is a cause of target cell resistance against cytotoxic effector cells. Blood 2000, 96, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Donatelli, S.S.; Zhou, J.-M.; Gilvary, D.L.; Eksioglu, E.A.; Chen, X.; Cress, W.D.; Haura, E.B.; Schabath, M.B.; Coppola, M.; Wei, S.; et al. TGF-β-inducible microRNA-183 silences tumor-associated natural killer cells. Proc. Natl. Acad. Sci. USA 2014, 111, 4203–4208. [Google Scholar] [CrossRef] [Green Version]
- Platonova, S.; Cherfils-Vicini, J.; Damotte, D.; Crozet, L.; Vieillard, V.; Validire, P.; Alifano, M.; Sautès-Fridman, C.; Cremer, I.; André, P.; et al. Profound Coordinated Alterations of Intratumoral NK Cell Phenotype and Function in Lung Carcinoma. Cancer Res. 2011, 71, 5412–5422. [Google Scholar] [CrossRef] [Green Version]
- Baginska, J.; Viry, E.; Berchem, G.; Poli, A.; Noman, M.Z.; Van Moer, K.; Medves, S.; Zimmer, J.; Oudin, A.; Niclou, S.P.; et al. Granzyme B degradation by autophagy decreases tumor cell susceptibility to natural killer-mediated lysis under hypoxia. Proc. Natl. Acad. Sci. USA 2013, 110, 17450–17455. [Google Scholar] [CrossRef] [Green Version]
- Viry, E.; Baginska, J.; Berchem, G.; Noman, M.Z.; Medves, S.; Chouaib, S.; Janji, B. Autophagic degradation of GZMB/granzyme B. Autophagy 2013, 10, 173–175. [Google Scholar] [CrossRef] [Green Version]
- Bladergroen, B.A.; Meijer, C.J.L.M.; Berge, R.L.T.; Hack, C.E.; Muris, J.J.F.; Dukers, D.F.; Chott, A.; Kazama, Y.; Oudejans, J.J.; Van Berkum, O.; et al. Expression of the granzyme B inhibitor, protease inhibitor 9, by tumor cells in patients with non-Hodgkin and Hodgkin lymphoma: A novel protective mechanism for tumor cells to circumvent the immune system? Blood 2002, 99, 232–237. [Google Scholar] [CrossRef] [Green Version]
- Rousalova, I.; Krepela, E.; Prochazka, J.; Cermak, J.; Benkova, K. Expression of proteinase inhibitor-9/serpinB9 in non-small cell lung carcinoma cells and tissues. Int. J. Oncol. 2010, 36, 275–283. [Google Scholar]
- Soriano, C.; Mukaro, V.; Hodge, G.; Ahern, J.; Holmes, M.; Jersmann, H.; Moffat, D.; Meredith, D.; Jurisevic, C.; Reynolds, P.N.; et al. Increased proteinase inhibitor-9 (PI-9) and reduced granzyme B in lung cancer: Mechanism for immune evasion? Lung Cancer 2012, 77, 38–45. [Google Scholar] [CrossRef]
- Chechlinska, M.; Kowalewska, M.; Brzoska, E.; Radziszewski, J.; Ptaszyński, K.; Ryś, J.; Kaminska, J.; Nowak, R. Squamous cell carcinoma antigen 1 and 2 expression in cultured normal peripheral blood mononuclear cells and in vulvar squamous cell carcinoma. Tumor Boil. 2010, 31, 559–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cataltepe, S.; Gornstein, E.R.; Schick, C.; Kamachi, Y.; Chatson, K.; Fries, J.; Silverman, G.A.; Upton, M.P. Co-expression of the squamous cell carcinoma antigens 1 and 2 in normal adult human tissues and squamous cell carcinomas. J. Histochem. Cytochem. 2000, 48, 113–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saidak, Z.; Morisse, M.C.; Chatelain, D.; Sauzay, C.; Houessinon, A.; Guilain, N.; Soyez, M.; Chauffert, B.; Dakpe, S.; Galmiche, A. Squamous Cell Carcinoma Antigen-encoding Genes SERPINB3/B4 as Potentially Useful Markers for the Stratification of HNSCC Tumours. Anticancer. Res. 2018, 38, 1343–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, S.; Kulkarni, N.; Shilpi; Lal, G. Intratumoral natural killer cells show reduced effector and cytolytic properties and control the differentiation of effector Th1 cells. Oncoimmunology 2016, 5, e1235106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Absi, A.A.; Wurzer, H.; Guerin, C.; Hoffmann, C.; Moreau, F.; Mao, X.; Brown-Clay, J.; Petrolli, R.; Casellas, C.P.; Dieterle, M.; et al. Actin Cytoskeleton Remodeling Drives Breast Cancer Cell Escape from Natural Killer–Mediated Cytotoxicity. Cancer Res. 2018, 78, 5631–5643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tittarelli, A.; Janji, B.; Van Moer, K.; Noman, M.Z.; Chouaib, S. The Selective Degradation of Synaptic Connexin 43 Protein by Hypoxia-induced Autophagy Impairs Natural Killer Cell-mediated Tumor Cell Killing. J. Boil. Chem. 2015, 290, 23670–23679. [Google Scholar] [CrossRef] [Green Version]
- Wensink, A.C.; Hack, C.E.; Bovenschen, N. Granzymes Regulate Proinflammatory Cytokine Responses. J. Immunol. 2015, 194, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Voskoboinik, I.; Whisstock, J.C.; Trapani, J.A. Perforin and granzymes: Function, dysfunction and human pathology. Nat. Rev. Immunol. 2015, 15, 388–400. [Google Scholar] [CrossRef]
- Bovenschen, N.; Kummer, J.A. Orphan granzymes find a home. Immunol. Rev. 2010, 235, 117–127. [Google Scholar] [CrossRef]
- Martinvalet, D.; Dykxhoorn, D.M.; Ferrini, R.; Lieberman, J. Granzyme A Cleaves a Mitochondrial Complex I Protein to Initiate Caspase-Independent Cell Death. Cell 2008, 133, 681–692. [Google Scholar] [CrossRef] [Green Version]
- Fan, Z.; Beresford, P.J.; Oh, D.Y.; Zhang, D.; Lieberman, J. Tumor Suppressor NM23-H1 Is a Granzyme A-Activated DNase during CTL-Mediated Apoptosis, and the Nucleosome Assembly Protein SET Is Its Inhibitor. Cell 2003, 112, 659–672. [Google Scholar] [CrossRef] [Green Version]
- Martinvalet, D.; Zhu, P.; Lieberman, J. Granzyme A Induces Caspase-Independent Mitochondrial Damage, a Required First Step for Apoptosis. Immun. 2005, 22, 355–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, D.; Beresford, P.J.; Zhu, P.; Zhang, N.; Sung, J.-S.; Demple, B.; Perrino, F.W.; Lieberman, J. The Exonuclease TREX1 Is in the SET Complex and Acts in Concert with NM23-H1 to Degrade DNA during Granzyme A-Mediated Cell Death. Mol. Cell 2006, 23, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Zhang, D.; Chowdhury, D.; Martinvalet, D.; Keefe, D.; Shi, L.; Lieberman, J. Granzyme A, which causes single-stranded DNA damage, targets the double-strand break repair protein Ku70. EMBO Rep. 2006, 7, 431–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Pasternack, M.S.; Beresford, P.J.; Wagner, L.; Greenberg, A.H.; Lieberman, J. Induction of Rapid Histone Degradation by the Cytotoxic T Lymphocyte Protease Granzyme A. J. Boil. Chem. 2000, 276, 3683–3690. [Google Scholar] [CrossRef] [Green Version]
- Beresford, P.J.; Xia, Z.; Greenberg, A.H.; Lieberman, J. Granzyme A Loading Induces Rapid Cytolysis and a Novel Form of DNA Damage Independently of Caspase Activation. Immun. 1999, 10, 585–595. [Google Scholar] [CrossRef] [Green Version]
- Rissoan, M.-C.; Duhen, T.; Bridon, J.-M.; Bendriss-Vermare, N.; Péronne, C.; Vis, B.D.S.; Brière, F.; Bates, E.E.M. Subtractive hybridization reveals the expression of immunoglobulinlike transcript 7, Eph-B1, granzyme B, and 3 novel transcripts in human plasmacytoid dendritic cells. Blood 2002, 100, 3295–3303. [Google Scholar] [CrossRef] [Green Version]
- Wagner, C.; Iking-Konert, C.; Denefleh, B.; Stegmaier, S.; Hug, F.; Hänsch, G.M. Granzyme B and perforin: Constitutive expression in human polymorphonuclear neutrophils. Blood 2004, 103, 1099–1104. [Google Scholar] [CrossRef] [Green Version]
- Tschopp, C.M.; Spiegl, N.; Didichenko, S.; Lutmann, W.; Julius, P.; Virchow, J.C.; Hack, C.E.; Dahinden, C.A. Granzyme B, a novel mediator of allergic inflammation: Its induction and release in blood basophils and human asthma. Blood 2006, 108, 2290–2299. [Google Scholar] [CrossRef]
- Strik, M.C.; De Koning, P.J.; Kleijmeer, M.J.; Bladergroen, B.A.; Wolbink, A.M.; Griffith, J.M.; Wouters, D.; Fukuoka, Y.; Schwartz, L.B.; Hack, C.E.; et al. Human mast cells produce and release the cytotoxic lymphocyte associated protease granzyme B upon activation. Mol. Immunol. 2007, 44, 3462–3472. [Google Scholar] [CrossRef]
- Trapani, J.A.; Sutton, V.R. Granzyme B: Pro-apoptotic, antiviral and antitumor functions. Curr. Opin. Immunol. 2003, 15, 533–543. [Google Scholar] [CrossRef]
- Kelly, J.M.; Waterhouse, N.; Cretney, E.; Browne, K.; Ellis, S.; Trapani, J.A.; Smyth, M.J. Granzyme M Mediates a Novel Form of Perforin-dependent Cell Death. J. Boil. Chem. 2004, 279, 22236–22242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bovenschen, N.; De Koning, P.J.A.; Quadir, R.; Broekhuizen, R.; Damen, J.M.A.; Froelich, C.J.; Slijper, M.; Kummer, J.A. NK Cell Protease Granzyme M Targets α-Tubulin and Disorganizes the Microtubule Network. J. Immunol. 2008, 180, 8184–8191. [Google Scholar] [CrossRef] [PubMed]
- Pao, L.I.; Sumaria, N.; Kelly, J.M.; Van Dommelen, S.; Cretney, E.; Wallace, M.E.; Anthony, D.A.; Uldrich, A.P.; Godfrey, D.; Papadimitriou, J.M.; et al. Functional Analysis of Granzyme M and Its Role in Immunity to Infection. J. Immunol. 2005, 175, 3235–3243. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Sun, Q.; Wu, Y.; Wang, L.; Zhou, C.; Ma, W.; Zhang, Y.; Wang, S.; Zhang, S. Granzyme M expressed by tumor cells promotes chemoresistance and EMT in vitro and metastasis in vivo associated with STAT3 activation. Oncotarget 2015, 6, 5818–5831. [Google Scholar] [CrossRef] [Green Version]
- Pegram, H.J.; Haynes, N.M.; Smyth, M.J.; Kershaw, M.; Darcy, P.K. Characterizing the anti-tumor function of adoptively transferred NK cells in vivo. Cancer Immunol. Immunother. 2010, 59, 1235–1246. [Google Scholar] [CrossRef]
- Mahrus, S.; Kisiel, W.; Craik, C.S. Granzyme M Is a Regulatory Protease That Inactivates Proteinase Inhibitor 9, an Endogenous Inhibitor of Granzyme B. J. Boil. Chem. 2004, 279, 54275–54282. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.E.; Smyth, M.J.; Trapani, J.A. Granzyme A and B-deficient killer lymphocytes are defective in eliciting DNA fragmentation but retain potent in vivo anti-tumor capacity. Eur. J. Immunol. 2001, 31, 39–47. [Google Scholar] [CrossRef]
- Smyth, M.J.; Street, S.E.A.; Trapani, J.A. Cutting Edge: Granzymes A and B Are Not Essential for Perforin-Mediated Tumor Rejection. J. Immunol. 2003, 171, 515–518. [Google Scholar] [CrossRef] [Green Version]
- Revell, P.A.; Grossman, W.J.; Thomas, D.A.; Cao, X.; Behl, R.; Ratner, J.A.; Lu, Z.H.; Ley, T.J. Granzyme B and the downstream granzymes C and/or F are important for cytotoxic lymphocyte functions. J. Immunol. 2005, 174, 2124–2131. [Google Scholar] [CrossRef] [Green Version]
- Fehniger, T.A.; Cai, S.; Cao, X.; Bredemeyer, A.J.; Presti, R.; French, A.R.; Ley, T.J. Acquisition of Murine NK Cell Cytotoxicity Requires the Translation of a Pre-existing Pool of Granzyme B and Perforin mRNAs. Immunity 2007, 26, 798–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, N.L.; Alencar, R.D.C.G.; Valadares, M.C.; Da Silva, T.A.; De Mendonça, E.F.; Batista, A.C. The clinicopathological significance of the expression of Granzyme B in oral squamous cell carcinoma. Oral Oncol. 2010, 46, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.X.; Wang, S.; Wang, J.P.; Mills, G.B.; Zhou, Y.; Xu, H.-J. Expression of endogenous granzyme B in a subset of human primary breast carcinomas. Br. J. Cancer 2003, 89, 135–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindner, S.; Dahlke, K.; Sontheimer, K.; Hagn, M.; Kaltenmeier, C.; Barth, T.F.E.; Beyer, T.; Reister, F.; Fabricius, D.; Lotfi, R.; et al. Interleukin 21-Induced Granzyme B-Expressing B Cells Infiltrate Tumors and Regulate T Cells. Cancer Res. 2013, 73, 2468–2479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieckowski, E.; Wang, G.-Q.; Gastman, B.R.; Goldstein, L.A.; Rabinowich, H. Granzyme B-mediated degradation of T-cell receptor zeta chain. Cancer Res. 2002, 62, 4884–4889. [Google Scholar] [PubMed]
- Grossman, W.J.; Verbsky, J.W.; Barchet, W.; Colonna, M.; Atkinson, J.P.; Ley, T.J. Human T Regulatory Cells Can Use the Perforin Pathway to Cause Autologous Target Cell Death. Immun. 2004, 21, 589–601. [Google Scholar] [CrossRef] [Green Version]
- Gondek, D.C.; Lu, L.-F.; Quezada, S.A.; Sakaguchi, S.; Noelle, R.J. Cutting Edge: Contact-Mediated Suppression by CD4+CD25+ Regulatory Cells Involves a Granzyme B-Dependent, Perforin-Independent Mechanism. J. Immunol. 2005, 174, 1783–1786. [Google Scholar] [CrossRef] [Green Version]
- Salama, P.; Phillips, M.; Platell, C.; Iacopetta, B. Low expression of Granzyme B in colorectal cancer is associated with signs of early metastastic invasion. Histopathol. 2011, 59, 207–215. [Google Scholar] [CrossRef]
- Gamen, S.; A Hanson, D.; Kaspar, A.; Naval, J.; Krensky, A.M.; Anel, A. Granulysin-induced apoptosis. I. Involvement of at least two distinct pathways. J. Immunol. 1998, 161, 1758–1764. [Google Scholar]
- Krensky, A.M.; Clayberger, C. Biology and clinical relevance of granulysin. Tissue Antigens 2009, 73, 193–198. [Google Scholar] [CrossRef]
- Kaspar, A.A.; Okada, S.; Kumar, J.; Poulain, F.R.; Drouvalakis, K.A.; Kelekar, A.; Hanson, D.A.; Kluck, R.M.; Hitoshi, Y.; Johnson, D.E.; et al. A distinct pathway of cell-mediated apoptosis initiated by granulysin. J. Immunol. 2001, 167, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.; Wilson, C.; Finn, M.W.; Wang, T.; Krensky, A.M.; Clayberger, C. Granulysin Delivered by Cytotoxic Cells Damages Endoplasmic Reticulum and Activates Caspase-7 in Target Cells. J. Immunol. 2011, 186, 3497–3504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, D.H.; Sawaya, M.R.; Cascio, D.; Ernst, W.; Modlin, R.L.; Krensky, A.; Eisenberg, D.S. Granulysin crystal structure and a structure-derived lytic mechanism. J. Mol. Boil. 2003, 325, 355–365. [Google Scholar] [CrossRef]
- Zhang, H.; Zhong, C.; Shi, L.; Guo, Y.; Fan, Z. Granulysin Induces Cathepsin B Release from Lysosomes of Target Tumor Cells to Attack Mitochondria through Processing of Bid Leading to Necroptosis. J. Immunol. 2009, 182, 6993–7000. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, N.; Katagiri, Y.U.; Itagaki, M.; Miyagawa, Y.; Onda, K.; Okita, H.; Mori, A.; Fujimoto, J.; Kiyokawa, N. The expression of granulysin in systemic anaplastic large cell lymphoma in childhood. Leuk. Res. 2009, 33, 908–912. [Google Scholar] [CrossRef]
- Saigusa, S.; Ichikura, T.; Tsujimoto, H.; Sugasawa, H.; Majima, T.; Kawarabayashi, N.; Chochi, K.; Ono, S.; Kinoshita, M.; Seki, S.; et al. Serum granulysin level as a novel prognostic marker in patients with gastric carcinoma. J. Gastroenterol. Hepatol. 2007, 22, 1322–1327. [Google Scholar] [CrossRef]
- Bruno, M.E.; Kaiser, A.; Montville, T.J. Depletion of proton motive force by nisin in Listeria monocytogenes cells. Appl. Environ. Microbiol. 1992, 58, 2255–2259. [Google Scholar] [CrossRef] [Green Version]
- Kishi, A.; Takamori, Y.; Ogawa, K.; Takano, S.; Tomita, S.; Tanigawa, M.; Niman, M.; Kishida, T.; Fujita, S. Differential expression of granulysin and perforin by NK cells in cancer patients and correlation of impaired granulysin expression with progression of cancer. Cancer Immunol. Immunother. 2002, 50, 604–614. [Google Scholar] [CrossRef]
- Furudate, S.; Fujimura, T.; Kambayashi, Y.; Aiba, S. Profiles of Cytotoxic T Lymphocytes in Cutaneous Lymphoid Hyperplasia of the Face. Case Rep. Dermatol. 2013, 5, 88–92. [Google Scholar] [CrossRef]
- Bello, G.L.; Akarca, A.U.; Ambrosio, M.R.; Agostinelli, C.; Molina-Kirsch, H.; Ramsay, A.; Rodriguez-Justo, M.; Pugh, M.; Zhao, S.; Delisser, M.; et al. Granulysin, a novel marker for extranodal NK/T cell lymphoma, nasal type. Virchows Arch. 2018, 473, 749–757. [Google Scholar] [CrossRef] [Green Version]
- Aporta, A.; Catalán, E.; Galan-Malo, P.; Ramirez-Labrada, A.G.; Pérez, M.; Azaceta, G.; Palomera, L.; Naval, J.; Marzo, I.; Pardo, J.; et al. Granulysin induces apoptotic cell death and cleavage of the autophagy regulator Atg5 in human hematological tumors. Biochem. Pharmacol. 2014, 87, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Al-Wasaby, S.; De Miguel, D.; Aporta, A.; Naval, J.; Conde, B.; Martinez-Lostao, L.; Anel, A. In vivo potential of recombinant granulysin against human tumors. Oncoimmunology 2015, 4, e1036213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibáñez-Pérez, R.; Guerrero-Ochoa, P.; Al-Wasaby, S.; Navarro, R.; Tapia-Galisteo, A.; De Miguel, D.; Gonzalo, O.; Conde, B.; Martínez-Lostao, L.; Hurtado-Guerrero, R.; et al. Anti-tumoral potential of a human granulysin-based, CEA-targeted cytolytic immunotoxin. Oncoimmunology 2019, 8, 1641392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupack, D.G. Caspase-8 as a therapeutic target in cancer. Cancer Lett. 2010, 332, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dagbay, K.B.; Hill, M.E.; Barrett, E.; Hardy, J.A. Tumor-Associated Mutations in Caspase-6 Negatively Impact Catalytic Efficiency. Biochem. 2017, 56, 4568–4577. [Google Scholar] [CrossRef]
- Hosokawa, Y.; Suzuki, H.; Takahashi, R.; Seto, M. Antiapoptotic Function of Apoptosis Inhibitor 2-MALT1 Fusion Protein Involved in t(11;18)(q21;q21) Mucosa-Associated Lymphoid Tissue Lymphoma. Cancer Res. 2004, 64, 3452–3457. [Google Scholar] [CrossRef] [Green Version]
- Stoffel, A.; Chaurushiya, M.; Singh, B.; Levine, A.J. Activation of NF-κB and inhibition of p53-mediated apoptosis by API2/mucosa-associated lymphoid tissue 1 fusions promote oncogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 9079–9084. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, H.; Gil, J.; Schwartz, S.; Perucho, M. Frameshift mutations in Fas, Apaf-1, and Bcl-10 in gastro-intestinal cancer of the microsatellite mutator phenotype. Cell Death Differ. 2000, 7, 238–239. [Google Scholar] [CrossRef]
- Stolz, C.; Hess, G.; Hähnel, P.S.; Grabellus, F.; Hoffarth, S.; Schmid, K.W.; Schuler, M. Targeting Bcl-2 family proteins modulates the sensitivity of B-cell lymphoma to rituximab-induced apoptosis. Blood 2008, 112, 3312–3321. [Google Scholar] [CrossRef]
- Wobser, M.; Voigt, H.; O Eggert, A.; Houben, R.; Kauczok, C.S.; Bröcker, E.B.; Becker, J.C. Bcl-2 expression in rituximab refractory cutaneous B-cell lymphoma. Br. J. Cancer 2007, 96, 1540–1543. [Google Scholar] [CrossRef]
- Bin, L.; Thorburn, J.; Thomas, L.R.; Clark, P.E.; Humphreys, R.; Thorburn, A. Tumor-derived Mutations in the TRAIL Receptor DR5 Inhibit TRAIL Signaling through the DR4 Receptor by Competing for Ligand Binding. J. Boil. Chem. 2007, 282, 28189–28194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, L.X.; Tang, Z.-Y.; Sham, J.S.; Ma, Z.C.; Ye, S.L.; Zhou, X.D.; Wu, Z.Q.; Trent, J.M.; Guan, X.Y. The association of chromosome 8p deletion and tumor metastasis in human hepatocellular carcinoma. Cancer Res. 1999, 59, 5662–5665. [Google Scholar] [PubMed]
- Cai, Y.; Crowther, J.; Pastor, T.; Asbagh, L.A.; Baietti, M.F.; De Troyer, M.; Vazquez, I.; Talebi, A.; Renzi, F.; Dehairs, J.; et al. Loss of Chromosome 8p Governs Tumor Progression and Drug Response by Altering Lipid Metabolism. Cancer Cell 2016, 29, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Kluth, M.; Amschler, N.N.; Galal, R.; Möller-Koop, C.; Barrow, P.; Tsourlakis, M.C.; Jacobsen, F.; Hinsch, A.; Wittmer, C.; Steurer, S.; et al. Deletion of 8p is an independent prognostic parameter in prostate cancer. Oncotarget 2016, 8, 379–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichenauer, T.; Bannenberg, D.C.; Kluth, M.; Wittmer, C.; Büscheck, F.; Möller, K.; Dum, D.; Fraune, C.; Hube-Magg, C.; Möller-Koop, C.; et al. 8p deletions in renal cell carcinoma are associated with unfavorable tumor features and poor overall survival. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 43.e13-43.e20. [Google Scholar] [CrossRef]
- Luebke, T.; Schwarz, L.; Beer, Y.Y.; Schumann, S.; Misterek, M.; Sander, F.E.; Plaza-Sirvent, C.; Schmitz, I. c-FLIP and CD95 signaling are essential for survival of renal cell carcinoma. Cell Death Dis. 2019, 10, 384. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Zhang, Y.; Zhan, Y.; Liu, S.; Lu, J.; Wen, Q.; Fan, S. Expression of DR5 and c-FLIP proteins as novel prognostic biomarkers for non-small cell lung cancer patients treated with surgical resection and chemotherapy. Oncol. Rep. 2019, 42, 2363–2370. [Google Scholar] [CrossRef]
- Lee, S.; Yoon, C.Y.; Byun, S.-S.; Lee, E.; Lee, S.E. The Role of c-FLIP in Cisplatin Resistance of Human Bladder Cancer Cells. J. Urol. 2013, 189, 2327–2334. [Google Scholar] [CrossRef]
- Alkurdi, L.; Virard, F.; Vanbervliet, B.; Weber, K.; Toscano, F.; Bonnin, M.; Le Stang, N.; Lantuejoul, S.; Micheau, O.; Renno, T.; et al. Release of c-FLIP brake selectively sensitizes human cancer cells to TLR3-mediated apoptosis. Cell Death Dis. 2018, 9, 874. [Google Scholar] [CrossRef]
- Longley, D.B.; Wilson, T.R.; McEwan, M.; Allen, W.L.; McDermott, M.W.; Galligan, L.; Johnston, P.G. c-FLIP inhibits chemotherapy-induced colorectal cancer cell death. Oncogene 2005, 25, 838–848. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, T.; Schröter, M.; Hahne, M.; Schneider, P.; Irmler, M.; Thome, M.; Froelich, C.J.; Tschopp, J. FLIP prevents apoptosis induced by death receptors but not by perforin/granzyme B, chemotherapeutic drugs, and gamma irradiation. J. Immunol. 1998, 161, 3936–3942. [Google Scholar] [PubMed]
- Taylor, M.A.; Chaudhary, P.M.; Klem, J.; Kumar, V.; Schatzle, J.D.; Bennett, M. Inhibition of the death receptor pathway by cFLIP confers partial engraftment of MHC class I-deficient stem cells and reduces tumor clearance in perforin-deficient mice. J. Immunol. 2001, 167, 4230–4237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krueger, A.; Schmitz, I.; Baumann, S.; Krammer, P.H.; Kirchhoff, S. Cellular FLICE-inhibitory Protein Splice Variants Inhibit Different Steps of Caspase-8 Activation at the CD95 Death-inducing Signaling Complex. J. Boil. Chem. 2001, 276, 20633–20640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golks, A.; Brenner, D.; Fritsch, C.; Krammer, P.H.; Lavrik, I.N. c-FLIPR, a New Regulator of Death Receptor-induced Apoptosis. J. Boil. Chem. 2005, 280, 14507–14513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, D.W.; Xing, Z.; Pan, Y.; Algeciras-Schimnich, A.; Barnhart, B.C.; Yaish-Ohad, S.; Peter, M.E.; Yang, X. c-FLIPL is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 2002, 21, 3704–3714. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, X.; Li, N.; Cui, L.; Ye, X.; Wan, X. DcR3 promotes hepatoma cell migration by downregulating E-cadherin expression. Oncol. Rep. 2017, 38, 377–383. [Google Scholar] [CrossRef] [Green Version]
- Ge, H.; Liang, C.; Li, Z.; An, D.; Ren, S.; Yue, C.; Wu, J. DcR3 induces proliferation, migration, invasion, and EMT in gastric cancer cells via the PI3K/AKT/GSK-3β/β-catenin signaling pathway. Onco. Targets Ther. 2018, 11, 4177–4187. [Google Scholar] [CrossRef] [Green Version]
- Ganten, T.M.; Sykora, J.; Koschny, R.; Batke, E.; Aulmann, S.; Mansmann, U.; Stremmel, W.; Sinn, H.-P.; Walczak, H. Prognostic significance of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) receptor expression in patients with breast cancer. J. Mol. Med. 2009, 87, 995–1007. [Google Scholar] [CrossRef]
- Sanlioglu, A.D.; Dirice, E.; Elpek, O.; Korcum, A.F.; Ozdogan, M.; Süleymanlar, I.; Balcı, M.K.; Griffith, T.S.; Sanlioglu, S. High TRAIL Death Receptor 4 and Decoy Receptor 2 Expression Correlates With Significant Cell Death in Pancreatic Ductal Adenocarcinoma Patients. Pancreas 2009, 38, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Leblanc, F.; Dighe, S.A.; Hamele, C.E.; Olson, T.; Feith, D.J.; Loughran, T.P. TRAIL mediates and sustains constitutive NF-κB activation in LGL leukemia. Blood 2018, 131, 2803–2815. [Google Scholar] [CrossRef]
- Degli-Esposti, M.A.; Dougall, W.C.; Smolak, P.J.; Waugh, J.Y.; Smith, C.A.; Goodwin, R.G. The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity 1997, 7, 813–820. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Park, S.-M.; Tumanov, A.V.; Hau, A.; Sawada, K.; Feig, C.; Turner, J.R.; Fu, Y.-X.; Romero, I.L.; Lengyel, E.; et al. CD95 promotes tumour growth. Nature 2010, 465, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Ceppi, P.; Hadji, A.; Kohlhapp, F.J.; Pattanayak, A.; Hau, A.; Liu, X.; Liu, H.; Murmann, A.E.; Peter, M.E. CD95 and CD95L promote and protect cancer stem cells. Nat. Commun. 2014, 5, 5238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peter, M.E. DICE. Cell Cycle 2014, 13, 1373–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walczak, H.; Miller, R.E.; Ariail, K.; Gliniak, B.; Griffith, T.S.; Kubin, M.; Chin, W.; Jones, J.; Woodward, A.; Le, T.; et al. Tumoricidal activity of tumor necrosis factor–related apoptosis–inducing ligand in vivo. Nat. Med. 1999, 5, 157–163. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Pai, R.C.; Fong, S.; Leung, S.; Lawrence, D.A.; Marsters, S.A.; Blackie, C.; Chang, L.; McMurtrey, A.E.; Hebert, A.; et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Investig. 1999, 104, 155–162. [Google Scholar] [CrossRef]
- Ouyang, X.; Shi, M.; Jie, F.; Bai, Y.; Shen, P.; Yu, Z.; Wang, X.; Huang, C.; Tao, M.; Wang, Z.; et al. Phase III study of dulanermin (recombinant human tumor necrosis factor-related apoptosis-inducing ligand/Apo2 ligand) combined with vinorelbine and cisplatin in patients with advanced non-small-cell lung cancer. Investig. New Drugs 2017, 36, 315–322. [Google Scholar] [CrossRef]
- Han, H.R.; Park, S.A.; Ahn, S.; Jeun, S.-S.; Ryu, C.H. Evaluation of Combination Treatment Effect With TRAIL-secreting Mesenchymal Stem Cells and Compound C Against Glioblastoma. Anticancer. Res. 2019, 39, 6635–6643. [Google Scholar] [CrossRef]
- Eng, J.W.-L.; Mace, T.A.; Sharma, R.; Twum, D.Y.F.; Peng, P.; Gibbs, J.F.; Pitoniak, R.; Reed, C.B.; Abrams, S.I.; Repasky, E.A.; et al. Pancreatic cancer stem cells in patient pancreatic xenografts are sensitive to drozitumab, an agonistic antibody against DR5. J. Immunother. Cancer 2016, 4, 33. [Google Scholar] [CrossRef] [Green Version]
- Dine, J.L.; O’Sullivan, C.C.; Voeller, D.; Greer, Y.E.; Chavez, K.J.; Conway, C.M.; Sinclair, S.; Stone, B.; Amiri-Kordestani, L.; Merchant, A.S.; et al. The TRAIL receptor agonist drozitumab targets basal B triple-negative breast cancer cells that express vimentin and Axl. Breast Cancer Res. Treat. 2016, 155, 235–251. [Google Scholar] [CrossRef] [Green Version]
- Cheah, C.Y.; Belada, D.; Fanale, M.A.; Janikova, A.; Czucman, M.S.; Flinn, I.W.; Kapp, A.V.; Ashkenazi, A.; Kelley, S.; Bray, G.L.; et al. Dulanermin with rituximab in patients with relapsed indolent B-cell lymphoma: An open-label phase 1b/2 randomised study. Lancet Haematol. 2015, 2, e166–e174. [Google Scholar] [CrossRef]
- Soria, J.-C.; Márk, Z.; Zatloukal, P.; Szima, B.; Albert, I.; Juhász, E.; Pujol, J.-L.; Kozielski, J.; Baker, N.; Smethurst, D.; et al. Randomized Phase II Study of Dulanermin in Combination With Paclitaxel, Carboplatin, and Bevacizumab in Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2011, 29, 4442–4451. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Lima, C.M.; Bayraktar, S.; Flores, A.M.; MacIntyre, J.; Montero, A.; Baranda, J.C.; Wallmark, J.; Portera, C.; Raja, R.; Stern, H.; et al. Phase Ib Study of Drozitumab Combined With First-Line mFOLFOX6 Plus Bevacizumab in Patients with Metastatic Colorectal Cancer. Cancer Investig. 2012, 30, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Von Pawel, J.; Harvey, J.H.; Spigel, D.R.; Dediu, M.; Reck, M.; Cebotaru, C.L.; Humphreys, R.C.; Gribbin, M.J.; Fox, N.L.; Camidge, D.R. Phase II Trial of Mapatumumab, a Fully Human Agonist Monoclonal Antibody to Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand Receptor 1 (TRAIL-R1), in Combination With Paclitaxel and Carboplatin in Patients With Advanced Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2014, 15, 188–196.e2. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Bálint, B.; De Boer, R.H.; Van Meerbeeck, J.P.; Wierzbicki, R.; De Souza, P.; Galimi, F.; Haddad, V.; Sabin, T.; Hei, Y.-J.; et al. A Randomized Phase 2 Study of Paclitaxel and Carboplatin with or without Conatumumab for First-Line Treatment of Advanced Non–Small-Cell Lung Cancer. J. Thorac. Oncol. 2013, 8, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Forero-Torres, A.; Infante, J.R.; Waterhouse, D.; Wong, L.; Vickers, S.; Arrowsmith, E.; He, A.R.; Hart, L.; Trent, D.; Wade, J.; et al. Phase 2, multicenter, open-label study of tigatuzumab (CS-1008), a humanized monoclonal antibody targeting death receptor 5, in combination with gemcitabine in chemotherapy-naive patients with unresectable or metastatic pancreatic cancer. Cancer Med. 2013, 2, 925–932. [Google Scholar] [CrossRef]
- Macfarlane, M.; Inoue, S.; Kohlhaas, S.L.; Majid, A.; Harper, N.; Kennedy, D.B.J.; Dyer, M.J.; Cohen, G.M. Chronic lymphocytic leukemic cells exhibit apoptotic signaling via TRAIL-R1. Cell Death Differ. 2005, 12, 773–782. [Google Scholar] [CrossRef]
- O’Leary, L.; Van Der Sloot, A.M.; Reis, C.R.; Deegan, S.; Ryan, A.; Dhami, S.P.S.; Murillo, L.S.; Cool, R.; De Sampaio, P.C.; Thompson, K.; et al. Decoy receptors block TRAIL sensitivity at a supracellular level: The role of stromal cells in controlling tumour TRAIL sensitivity. Oncogene 2015, 35, 1261–1270. [Google Scholar] [CrossRef]
- Kelley, R.F.; Totpal, K.; Lindstrom, S.H.; Mathieu, M.; Billeci, K.; Deforge, L.; Pai, R.; Hymowitz, S.G.; Ashkenazi, A. Receptor-selective Mutants of Apoptosis-inducing Ligand 2/Tumor Necrosis Factor-related Apoptosis-inducing Ligand Reveal a Greater Contribution of Death Receptor (DR) 5 than DR4 to Apoptosis Signaling. J. Boil. Chem. 2004, 280, 2205–2212. [Google Scholar] [CrossRef] [Green Version]
- Macfarlane, M.; Kohlhaas, S.L.; Sutcliffe, M.; Dyer, M.J.; Cohen, G.M. TRAIL Receptor-Selective Mutants Signal to Apoptosis via TRAIL-R1 in Primary Lymphoid Malignancies. Cancer Res. 2005, 65, 11265–11270. [Google Scholar] [CrossRef] [Green Version]
- Lemke, J.; Von Karstedt, S.; Zinngrebe, J.; Walczak, H. Getting TRAIL back on track for cancer therapy. Cell Death Differ. 2014, 21, 1350–1364. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, D.; Shahrokh, Z.; Marsters, S.; Achilles, K.; Shih, D.; Mounho, B.; Hillan, K.; Totpal, K.; Deforge, L.; Schow, P.; et al. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat. Med. 2001, 7, 383–385. [Google Scholar] [CrossRef]
- Ganten, T.M.; Koschny, R.; Sykora, J.; Schulze-Bergkamen, H.; Büchler, P.; Haas, T.; Schader, M.B.; Untergasser, A.; Stremmel, W.; Walczak, H. Preclinical Differentiation between Apparently Safe and Potentially Hepatotoxic Applications of TRAIL Either Alone or in Combination with Chemotherapeutic Drugs. Clin. Cancer Res. 2006, 12, 2640–2646. [Google Scholar] [CrossRef] [Green Version]
- Nihira, K.; Nan-Ya, K.-I.; Kakuni, M.; Ono, Y.; Yoshikawa, Y.; Ota, T.; Hiura, M.; Yoshinari, K. Chimeric Mice With Humanized Livers Demonstrate Human-Specific Hepatotoxicity Caused by a Therapeutic Antibody Against TRAIL-Receptor 2/Death Receptor 5. Toxicol. Sci. 2018, 167, 190–201. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, K.P.; Isaacs, R.; Bilic, S.; Kentsch, K.; Huet, H.A.; Hofmann, M.; Rasco, D.; Kundamal, N.; Tang, Z.; Cooksey, J.; et al. Unexpected hepatotoxicity in a phase I study of TAS266, a novel tetravalent agonistic Nanobody® targeting the DR5 receptor. Cancer Chemother. Pharmacol. 2015, 75, 887–895. [Google Scholar] [CrossRef]
- Lemke, J.; Von Karstedt, S.; El Hay, M.A.; Conti, A.; Arce, F.; Montinaro, A.; Papenfuss, K.; A El-Bahrawy, M.; Walczak, H. Selective CDK9 inhibition overcomes TRAIL resistance by concomitant suppression of cFlip and Mcl-1. Cell Death Differ. 2013, 21, 491–502. [Google Scholar] [CrossRef]
- Gallouet, A.-S.; Travert, M.; Bresson-Bepoldin, L.; Guilloton, F.; Pangault, C.; Caulet-Maugendre, S.; Lamy, T.; Tarte, K.; Guillaudeux, T. COX-2-Independent Effects of Celecoxib Sensitize Lymphoma B Cells to TRAIL-Mediated Apoptosis. Clin. Cancer Res. 2014, 20, 2663–2673. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; McEachern, N.; Sun, H.; Bai, L.; Peng, Y.; Qiu, S.; Miller, R.; Liao, J.; Yi, H.; Liu, M.; et al. Therapeutic potential and molecular mechanism of a novel, potent, nonpeptide, Smac mimetic SM-164 in combination with TRAIL for cancer treatment. Mol. Cancer Ther. 2011, 10, 902–914. [Google Scholar] [CrossRef] [Green Version]
- Fulda, S. Promises and Challenges of Smac Mimetics as Cancer Therapeutics. Clin. Cancer Res. 2015, 21, 5030–5036. [Google Scholar] [CrossRef] [Green Version]
- Jo, E.B.; Lee, Y.S.; Lee, H.; Park, J.B.; Park, H.; Choi, Y.-L.; Hong, D.; Kim, S.J. Combination therapy with c-met inhibitor and TRAIL enhances apoptosis in dedifferentiated liposarcoma patient-derived cells. BMC Cancer 2019, 19, 496. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, G.-; Villa-Álvarez, M.; Bahamonde, S.-; Herrero, L.-; Gonzalez, S.; Gonzalez, A.P.; Sordo-Bahamonde, C.; Lorenzo-Herrero, S. NK Cells in the Treatment of Hematological Malignancies. J. Clin. Med. 2019, 8, 1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sordo-Bahamonde, C.; Vitale, M.; Lorenzo-Herrero, S.; López-Soto, A.; Gonzalez, S. Mechanisms of Resistance to NK Cell Immunotherapy. Cancers 2020, 12, 893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudnick, S.I.; Lou, J.; Shaller, C.C.; Tang, Y.; Klein-Szanto, A.J.; Weiner, L.M.; Marks, J.D.; Adams, G.P. Influence of Affinity and Antigen Internalization on the Uptake and Penetration of Anti-HER2 Antibodies in Solid Tumors. Cancer Res. 2011, 71, 2250–2259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Yu, S.; Zinn, K.; Wang, J.; Zhang, L.; Jia, Y.; Kappes, J.C.; Barnes, S.; Kimberly, R.; Grizzle, W.E.; et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J. Immunol. 2006, 176, 1375–1385. [Google Scholar] [CrossRef] [Green Version]
- Jun, E.; Song, A.Y.; Choi, J.-W.; Lee, H.H.; Kim, M.-Y.; Ko, D.-H.; Kang, H.J.; Kim, S.W.; Bryceson, Y.; Kim, S.C.; et al. Progressive Impairment of NK Cell Cytotoxic Degranulation Is Associated With TGF-β1 Deregulation and Disease Progression in Pancreatic Cancer. Front. Immunol. 2019, 10, 1354. [Google Scholar] [CrossRef]
- Bird, C.H.; Sutton, V.R.; Sun, J.; Hirst, C.; Novak, A.; Kumar, S.; Trapani, J.A.; Bird, P. Selective Regulation of Apoptosis: The Cytotoxic Lymphocyte Serpin Proteinase Inhibitor 9 Protects against Granzyme B-Mediated Apoptosis without Perturbing the Fas Cell Death Pathway. Mol. Cell. Boil. 1998, 18, 6387–6398. [Google Scholar] [CrossRef] [Green Version]
- Medema, J.P.; De Jong, J.; Peltenburg, L.T.C.; Verdegaal, E.M.E.; Gorter, A.; Bres, S.A.; Franken, K.L.M.C.; Hahne, M.; Albar, J.P.; Melief, C.J.M.; et al. Blockade of the granzyme B/perforin pathway through overexpression of the serine protease inhibitor PI-9/SPI-6 constitutes a mechanism for immune escape by tumors. Proc. Natl. Acad. Sci. USA 2001, 98, 11515–11520. [Google Scholar] [CrossRef] [Green Version]
- De Koning, P.J.A.; Kummer, J.A.; De Poot, S.A.H.; Quadir, R.; Broekhuizen, R.; McGettrick, A.F.; Higgins, W.J.; Devreese, B.; Worrall, D.M.; Bovenschen, N. Intracellular Serine Protease Inhibitor SERPINB4 Inhibits Granzyme M-Induced Cell Death. PLoS ONE 2011, 6, e22645. [Google Scholar] [CrossRef]
- Aydin, E.; Johansson, J.; Nazir, F.H.; Martner, A.; Hellstrand, K. Role of NOX2-Derived Reactive Oxygen Species in NK Cell–Mediated Control of Murine Melanoma Metastasis. Cancer Immunol. Res. 2017, 5, 804–811. [Google Scholar] [CrossRef] [Green Version]
- Mellqvist, U.H.; Hansson, M.; Brune, M.; Dahlgren, C.; Hermodsson, S.; Hellstrand, K. Natural killer cell dysfunction and apoptosis induced by chronic myelogenous leukemia cells: Role of reactive oxygen species and regulation by histamine. Blood 2000, 96, 1961–1968. [Google Scholar] [CrossRef]
- Romero, A.I.; Thorén, F.B.; Brune, M.; Hellstrand, K. NKp46 and NKG2D receptor expression in NK cells with CD56dim and CD56bright phenotype: Regulation by histamine and reactive oxygen species. Br. J. Haematol. 2006, 132, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Balsamo, M.; Manzini, C.; Pietra, G.; Raggi, F.; Blengio, F.; Mingari, M.C.; Varesio, L.; Moretta, L.; Bosco, M.C.; Vitale, M. Hypoxia downregulates the expression of activating receptors involved in NK-cell-mediated target cell killing without affecting ADCC. Eur. J. Immunol. 2013, 43, 2756–2764. [Google Scholar] [CrossRef]
- Sceneay, J.; Chow, M.T.; Chen, A.; Halse, H.M.; Wong, C.S.; Andrews, D.; Sloan, E.K.; Parker, B.S.; Bowtell, D.; Smyth, M.J.; et al. Primary Tumor Hypoxia Recruits CD11b+/Ly6Cmed/Ly6G+Immune Suppressor Cells and Compromises NK Cell Cytotoxicity in the Premetastatic Niche. Cancer Res. 2012, 72, 3906–3911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melaiu, O.; Lucarini, V.; Cifaldi, L.; Fruci, D. Influence of the Tumor Microenvironment on NK Cell Function in Solid Tumors. Front. Immunol. 2020, 10, 3038. [Google Scholar] [CrossRef] [PubMed]
- Chiu, D.K.-C.; Xu, I.M.-J.; Lai, R.K.-H.; Tse, A.P.-W.; Wei, L.L.; Koh, H.-Y.; Li, L.L.; Lee, D.; Lo, C.L.R.; Wong, C.-M.; et al. Hypoxia induces myeloid-derived suppressor cell recruitment to hepatocellular carcinoma through chemokine (C-C motif) ligand 26. Hepatol. 2016, 64, 797–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henze, A.-T.; Mazzone, M. The impact of hypoxia on tumor-associated macrophages. J. Clin. Investig. 2016, 126, 3672–3679. [Google Scholar] [CrossRef] [PubMed]
- Facciabene, A.; Peng, X.; Hagemann, I.S.; Balint, K.; Barchetti, A.; Wang, L.-P.; Gimotty, P.A.; Gilks, C.B.; Lal, P.; Zhang, L.; et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature 2011, 475, 226–230. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Menard, C.; Terme, M.; Flament, C.; Taieb, J.; Chaput, N.; Puig, P.E.; Novault, S.; Escudier, B.; Vivier, E.; et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J. Exp. Med. 2005, 202, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Trotta, R.; Col, J.D.; Yu, J.; Ciarlariello, D.; Thomas, B.; Zhang, X.; Allard, J.; Wei, M.; Mao, H.; Byrd, J.C.; et al. TGF-β Utilizes SMAD3 to Inhibit CD16-Mediated IFN-γ Production and Antibody-Dependent Cellular Cytotoxicity in Human NK Cells1. J. Immunol. 2008, 181, 3784–3792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-C.; Lee, K.-M.; Kim, D.-W.; Heo, D.S. Elevated TGF-β1 Secretion and Down-Modulation of NKG2D Underlies Impaired NK Cytotoxicity in Cancer Patients. J. Immunol. 2004, 172, 7335–7340. [Google Scholar] [CrossRef] [PubMed]
- Lazarova, M.; Steinle, A. Impairment of NKG2D-Mediated Tumor Immunity by TGF-β. Front. Immunol. 2019, 10, 2689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Han, Y.; Guo, Q.; Zhang, M.; Cao, X. Cancer-Expanded Myeloid-Derived Suppressor Cells Induce Anergy of NK Cells through Membrane-Bound TGF-β1. J. Immunol. 2008, 182, 240–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corzo, C.A.; Cotter, M.J.; Cheng, P.; Cheng, F.; Kusmartsev, S.; Sotomayor, E.; Padhya, T.; McCaffrey, T.V.; McCaffrey, J.C.; Gabrilovich, D.I. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J. Immunol. 2009, 182, 5693–5701. [Google Scholar] [CrossRef]
- Erler, J.T.; Cawthorne, C.; Williams, K.J.; Koritzinsky, M.; Wouters, B.G.; Wilson, C.; Miller, C.J.; Demonacos, C.; Stratford, I.J.; Dive, C. Hypoxia-Mediated Down-Regulation of Bid and Bax in Tumors Occurs via Hypoxia-Inducible Factor 1-Dependent and -Independent Mechanisms and Contributes to Drug Resistance. Mol. Cell. Boil. 2004, 24, 2875–2889. [Google Scholar] [CrossRef] [Green Version]
- Sermeus, A.; Genin, M.; Maincent, A.; Fransolet, M.; Notte, A.; LeClere, L.; Riquier, H.; Arnould, T.; Michiels, C. Hypoxia-Induced Modulation of Apoptosis and BCL-2 Family Proteins in Different Cancer Cell Types. PLoS ONE 2012, 7, e47519. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-Y.; Billiar, T.R.; Seol, D.-W. Hypoxia Inhibition of Apoptosis Induced by Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL). Biochem. Biophys. Res. Commun. 2002, 291, 150–153. [Google Scholar] [CrossRef]
- Piret, J.-P.; Minet, E.; Ninane, N.; Debacq, C.; Raes, M.; Cosse, J.-P.; Michiels, C. Hypoxia-inducible Factor-1-dependent Overexpression of Myeloid Cell Factor-1 Protects Hypoxic Cells against tert-Butyl Hydroperoxide-induced Apoptosis. J. Boil. Chem. 2004, 280, 9336–9344. [Google Scholar] [CrossRef] [Green Version]
- Koundouros, N.; Poulogiannis, G. Phosphoinositide 3-Kinase/Akt Signaling and Redox Metabolism in Cancer. Front. Oncol. 2018, 8, 160. [Google Scholar] [CrossRef]
- Chang, F.; Lee, J.T.; Navolanic, P.M.; Steelman, L.S.; Shelton, J.G.; Blalock, W.L.; Franklin, R.A.; McCubrey, J. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: A Target for cancer chemotherapy. Leukemia 2003, 17, 590–603. [Google Scholar] [CrossRef] [Green Version]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Qi, F.; Zhao, F.; Li, G.; Shao, S.; Zhang, X.; Yuan, L.; Feng, Y. Cancer-associated fibroblasts enhance tumor-associated macrophages enrichment and suppress NK cells function in colorectal cancer. Cell Death Dis. 2019, 10, 273. [Google Scholar] [CrossRef] [Green Version]
- Ziani, L.; Ben Safta-Saadoun, T.; Gourbeix, J.; Cavalcanti, A.; Robert, C.; Favre, G.; Chouaib, S.; Thiery, J. Melanoma-associated fibroblasts decrease tumor cell susceptibility to NK cell-mediated killing through matrix-metalloproteinases secretion. Oncotarget 2017, 8, 19780–19794. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, H.; Sakakura, K.; Kudo, T.; Toyoda, M.; Kaira, K.; Oyama, T.; Chikamatsu, K. Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages. Oncotarget 2016, 8, 8633–8647. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.-T.; Deng, Y.-N.; Yi, H.-M.; Wang, G.-Y.; Fu, B.-S.; Chen, W.-J.; Liu, W.; Tai, Y.; Peng, Y.-W.; Zhang, Q. Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis 2016, 5, e198. [Google Scholar] [CrossRef] [Green Version]
- Ziani, L.; Chouaib, S.; Thiery, J. Alteration of the Antitumor Immune Response by Cancer-Associated Fibroblasts. Front. Immunol. 2018, 9, 414. [Google Scholar] [CrossRef]
- Pietra, G.; Manzini, C.; Rivara, S.; Vitale, M.; Cantoni, C.; Petretto, A.; Balsamo, M.; Conte, R.; Benelli, R.; Minghelli, S.; et al. Melanoma Cells Inhibit Natural Killer Cell Function by Modulating the Expression of Activating Receptors and Cytolytic Activity. Cancer Res. 2012, 72, 1407–1415. [Google Scholar] [CrossRef] [Green Version]
- Park, A.; Lee, Y.; Kim, M.S.; Kang, Y.J.; Park, Y.-J.; Jung, H.; Kim, T.-D.; Lee, H.G.; Choi, I.; Yoon, S.R. Prostaglandin E2 Secreted by Thyroid Cancer Cells Contributes to Immune Escape Through the Suppression of Natural Killer (NK) Cell Cytotoxicity and NK Cell Differentiation. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Galland, S.; Vuille, J.; Martin, P.; Letovanec, I.; Caignard, A.; Fregni, G.; Stamenkovic, I. Tumor-Derived Mesenchymal Stem Cells Use Distinct Mechanisms to Block the Activity of Natural Killer Cell Subsets. Cell Rep. 2017, 20, 2891–2905. [Google Scholar] [CrossRef] [Green Version]
- Spaggiari, G.M.; Capobianco, A.; Abdelrazik, H.; Becchetti, F.; Mingari, M.C.; Moretta, L. Mesenchymal stem cells inhibit natural killer–cell proliferation, cytotoxicity, and cytokine production: Role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 2008, 111, 1327–1333. [Google Scholar] [CrossRef]
| Receptor Name | Alternative Name | Ligands | Death Domains |
|---|---|---|---|
| TNF-R1 | P55/P60 | TNF-α | Y |
| Fas | CD95/APO-1 | FasL (CD95L) | Y |
| TRAIL-R1 | DR4 | TRAIL | Y |
| TRAIL-R2 | DR5 | TRAIL | Y |
| TRAIL-R3 | DcR1 | TRAIL | N |
| TRAIL-R4 | DcR2 | TRAIL | N |
| Osteoprotegerin | OPG | TRAIL | N |
| EDAR | Ectodysplasin-A receptor | Ectodysplasin A (EDA) | N |
| NGFR | P75(NTR) | NGF, BDNF, NTF3, NTF4 | Y |
| Protein | Mechanism of Resistance | Cancer | Reference |
|---|---|---|---|
| Caspase-3 | Gene mutations that interfere with caspase activity | Gastric carcinoma | [63] |
| Vulvar squamous carcinoma | [64] | ||
| Hepatocellular carcinoma | [65] | ||
| Caspase-4 | Advanced gastric adenocarcinoma and colorectal cancer | [66] | |
| Caspase-5 | MM, NHL, NSCLC, hepatocellular, colorectal and gastric carcinomas | [67] | |
| Caspase-6 | GIST | [68] | |
| Caspase-7 | NSCLC, colorectal and gastric carcinomas | [69] | |
| Caspase-8 | Colorectal, esophageal and head and neck carcinomas | [70] | |
| Caspase-9 | Colorectal and gastric carcinomas | [71] | |
| Survivin | Aberrant expression by chromosomal amplification | Neuroblastoma | [72] |
| c-IAP1 | Esophageal, liver, lung and ovarian carcinomas | [73,74] | |
| c-IAP2 | Aberrant activity by chromosomal translocation | MALT lymphomas | [75] |
| Bax | Inactivating gene mutations | Colon and gastric carcinomas with microsatellite instability | [76,77,78] |
| T-ALL | [79] | ||
| CLL | [80] | ||
| Burkitt’s lymphoma | [81] | ||
| BCL2 | Aberrant expression by chromosomal translocation | DLBCL | [82] |
| Homologue protein expression by cancer-associated viruses | Kaposi’s sarcoma | [83] | |
| c-FLIP | Homologue protein expression by cancer-associated viruses | Kaposi’s sarcoma | [84] |
| Aberrant protein expression | Burkitt’s lymphoma | [85] | |
| AML | [86] | ||
| Colorectal cancer | [87] | ||
| Bim | Gene deletion | MCL | [88,89] |
| Noxa | Silencing gene mutations | DLBCL | [89] |
| TRAIL-R1 | Gene mutations that interfere with receptor activity | Lung, head and neck and gastric carcinomas | [90] |
| NHL | [91] | ||
| Allelic deletion | B-NHL | [92] | |
| Breast cancer | [93] | ||
| TRAIL-R2 | Allelic deletion | B-NHL | [92] |
| Breast cancer | [93] | ||
| Loss-of-function mutations | Head and neck and lung carcinomas | [94] | |
| NSCLC | [95] | ||
| Gastric cancer | [96] | ||
| Gene mutations that interfere with receptor activity | NHL | [91] | |
| Fas | Loss-of-function mutations | Hematological malignancies | [97] |
| Osteoprotegerin | Aberrant protein expression | MM | [98] |
| DcR3 | Glioblastoma | [99] | |
| Breast cancer | [100] | ||
| Gastric cancer | [101] | ||
| Colorectal cancer | [102] | ||
| TRAIL-R3 | AML | [103] | |
| Breast cancer | [104] | ||
| TRAIL-R4 | Prostate cancer | [105] | |
| PRF1 | Downregulation of protein expression | Pancreatic, gastric and colorectal carcinomas | [106] |
| Lung cancer | [107] | ||
| Hepatocellular carcinoma | [108] | ||
| Reduction of protein levels by tumor-associated cells | T-cell lymphoma | [109] | |
| Hepatocellular carcinoma | [110] | ||
| Melanoma | [111] | ||
| Colorectal cancer | [112] | ||
| Impaired cell surface binding | AML | [113] | |
| Impaired protein mobilization to the immune synapse | Burkitt’s lymphoma | [114] | |
| GMZB | Downregulation of protein expression | Lung cancer | [115] |
| Hepatocellular carcinoma | [108] | ||
| Lung cancer | [107] | ||
| Increased protein degradation | Breast cancer | [116,117] | |
| Serpin B9 | Aberrant protein expression | DLBCL | [118] |
| NSCLC | [119] | ||
| Lung cancer | [120] | ||
| Serpin B4 | Squamous cell carcinomas | [121,122,123] | |
| CD107a | Downregulation of protein expression | Pancreatic cancer | [124] |
| F-actin | Intracellular accumulation | Breast cancer | [125] |
| Connexin-43 | Increased protein degradation | Melanoma | [126] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sordo-Bahamonde, C.; Lorenzo-Herrero, S.; Payer, Á.R.; Gonzalez, S.; López-Soto, A. Mechanisms of Apoptosis Resistance to NK Cell-Mediated Cytotoxicity in Cancer. Int. J. Mol. Sci. 2020, 21, 3726. https://doi.org/10.3390/ijms21103726
Sordo-Bahamonde C, Lorenzo-Herrero S, Payer ÁR, Gonzalez S, López-Soto A. Mechanisms of Apoptosis Resistance to NK Cell-Mediated Cytotoxicity in Cancer. International Journal of Molecular Sciences. 2020; 21(10):3726. https://doi.org/10.3390/ijms21103726
Chicago/Turabian StyleSordo-Bahamonde, Christian, Seila Lorenzo-Herrero, Ángel R. Payer, Segundo Gonzalez, and Alejandro López-Soto. 2020. "Mechanisms of Apoptosis Resistance to NK Cell-Mediated Cytotoxicity in Cancer" International Journal of Molecular Sciences 21, no. 10: 3726. https://doi.org/10.3390/ijms21103726
APA StyleSordo-Bahamonde, C., Lorenzo-Herrero, S., Payer, Á. R., Gonzalez, S., & López-Soto, A. (2020). Mechanisms of Apoptosis Resistance to NK Cell-Mediated Cytotoxicity in Cancer. International Journal of Molecular Sciences, 21(10), 3726. https://doi.org/10.3390/ijms21103726





