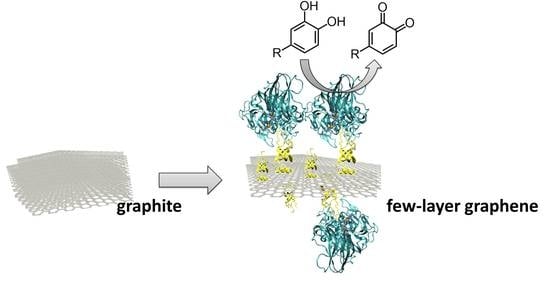
From Graphite to Laccase Biofunctionalized Few-Layer Graphene: A “One Pot” Approach Using a Chimeric Enzyme
Abstract
1. Introduction
2. Results and Discussion
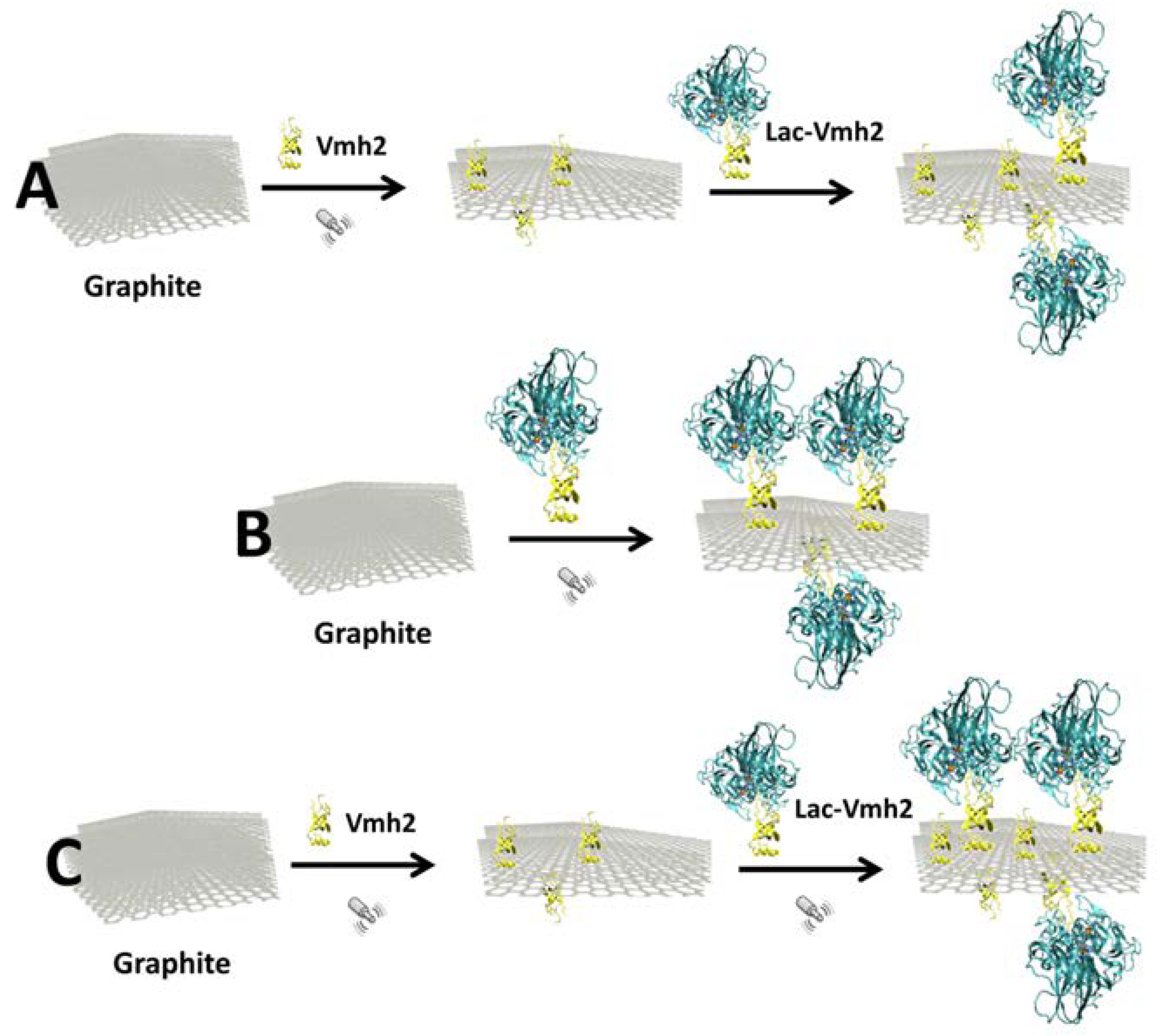
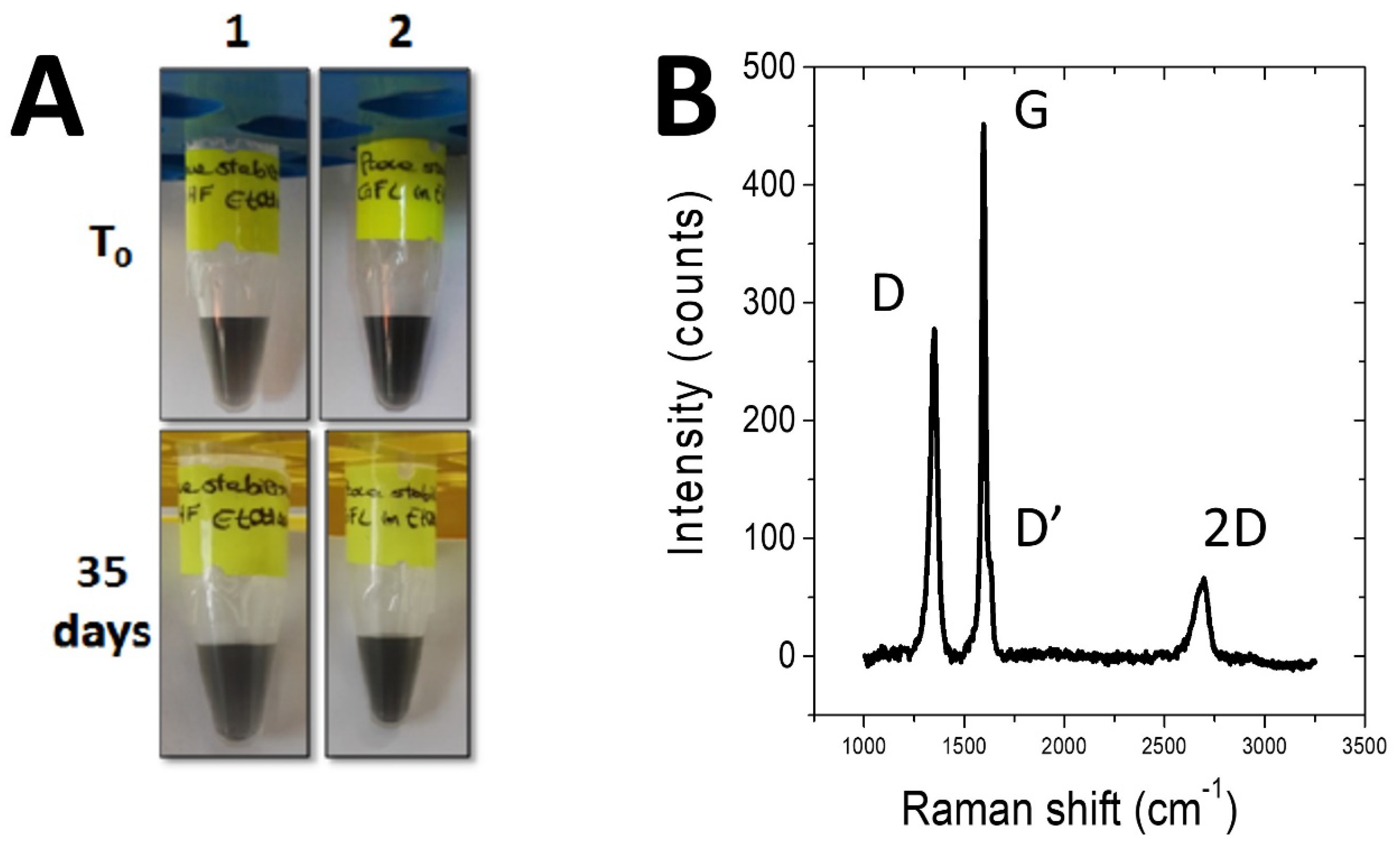
2.1. Laccase Immobilization on FLG
2.2. Exploitation of Biofunctionalized FLG in Electrochemical Biosensing
3. Materials and Methods
3.1. Electrochemical Measurements
3.2. Laccase Enzymes
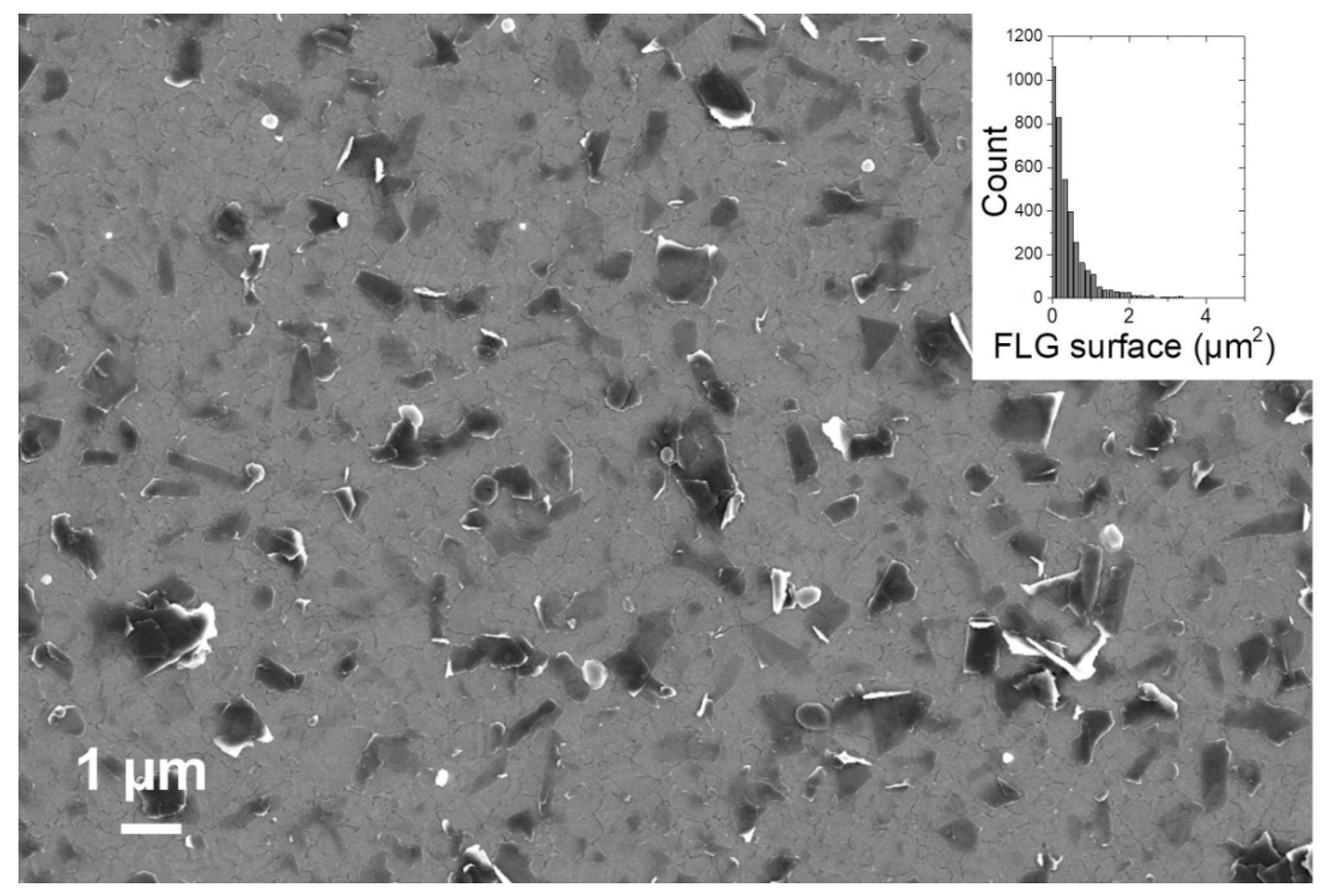
3.3. In Situ Exfoliation of Graphite
- A 7 mL solution of PoxA1b or Lac-Vmh2 in 40% EtOH was added to Vmh2-exfoliated graphene and incubated at 4 °C whilst stirring continuously.
- The immobilization was performed by adding the wild-type or chimeric enzyme solution to graphite powder at the beginning of the exfoliation.
- The wild-type or chimeric enzyme solution was added during the last 10 min of exfoliation. Indeed, the inactivation of the enzyme when higher sonication time was used has been previously verified. The process was performed normalizing the activity units (4 Utot for both) between wild-type POXA1b and Lac-Vmh2 (0.16 mg and 0.44 mg, respectively).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pumera, M. Voltammetry of carbon nanotubes and graphenes: Excitement, disappointment, and reality. Chem. Rec. 2012, 12, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Brownson, D.A.C.; Lacombe, A.C.; Gómez-Mingot, M.; Banks, C.E. Graphene oxide gives rise to unique and intriguing voltammetry. Rsc. Adv. 2011, 2, 665–668. [Google Scholar] [CrossRef]
- Brownson, D.A.C.; Munro, L.J.; Kampouris, D.K.; Banks, C.E. Electrochemistry of graphene: Not such a beneficial electrode material? Rsc. Adv. 2011, 1, 978–988. [Google Scholar] [CrossRef]
- Kampouris, D.K.; Banks, C.E. Exploring the physicoelectrochemical properties of graphene. Chem. Commun. 2010, 46, 8986–8988. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Bonaccorso, F.; Fal’ko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.L.; Palermo, V.; Pugno, N.; et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2015, 7, 4598–4810. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.-H.; Li, J.-Y.; Tang, Z.-R.; Xu, Y.-J. 3D graphene-based gel photocatalysts for environmental pollutants degradation. Environ. Pollut. 2019, 253, 365–376. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, M.-Q.; Liu, S.; Sun, Y.; Xu, Y.-J. Waltzing with the Versatile Platform of Graphene to Synthesize Composite Photocatalysts. Chem. Rev. 2015, 115, 10307–10377. [Google Scholar] [CrossRef]
- Fritea, L.; Tertis, M.; Sandulescu, R.; Cristea, C. Enzyme–Graphene Platforms for Electrochemical Biosensor Design with Biomedical Applications. In Methods in Enzymology; Kumar, C.V., Ed.; Academic Press: Cambridge, MA, USA, 2018; Chapter Eleven; Volume 609, pp. 293–333. [Google Scholar]
- Pumera, M. Graphene in biosensing. Mater. Today 2011, 14, 308–315. [Google Scholar] [CrossRef]
- Zhang, W.; Jia, B.; Furumai, H. Fabrication of graphene film composite electrochemical biosensor as a pre-screening algal toxin detection tool in the event of water contamination. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Lalaoui, N.; Le Goff, A.; Holzinger, M.; Mermoux, M.; Cosnier, S. Wiring Laccase on Covalently Modified Graphene: Carbon Nanotube Assemblies for the Direct Bio-electrocatalytic Reduction of Oxygen. Chem. Eur. J. 2015, 21, 3198–3201. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, A.; Reuillard, B.; Cosnier, S. A Pyrene-Substituted Tris(bipyridine)osmium(II) Complex as a Versatile Redox Probe for Characterizing and Functionalizing Carbon Nanotube- and Graphene-Based Electrodes. Langmuir 2013, 29, 8736–8742. [Google Scholar] [CrossRef] [PubMed]
- Fritea, L.; Le Goff, A.; Putaux, J.-L.; Tertis, M.; Cristea, C.; Sandulescu, R.; Cosnier, S. Design of a reduced-graphene-oxide composite electrode from an electropolymerizable graphene aqueous dispersion using a cyclodextrin-pyrrole monomer. Application to dopamine biosensing. Electrochim. Acta 2015, 178, 108–112. [Google Scholar] [CrossRef]
- Keeley, G.P.; O’Neill, A.; Holzinger, M.; Cosnier, S.; Coleman, J.N.; Duesberg, G.S. DMF-exfoliated graphene for electrochemical NADH detection. Phys. Chem. Chem. Phys. 2011, 13, 7747–7750. [Google Scholar] [CrossRef]
- Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, I.T.; et al. Liquid Phase Production of Graphene by Exfoliation of Graphite in Surfactant/Water Solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef]
- Smith, R.J.; Lotya, M.; Coleman, J.N. The importance of repulsive potential barriers for the dispersion of graphene using surfactants. New J. Phys. 2010, 12, 125008. [Google Scholar] [CrossRef]
- Ramakrishna, T.R.B.; Nalder, T.D.; Yang, W.; Marshall, S.N.; Barrow, C.K. Controlling enzyme function through immobilisation on graphene, graphene derivatives and other two dimensional nanomaterials. J. Mater. Chem. B 2018, 6, 3200–3218. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Gravagnuolo, A.M.; Morales-Narváez, E.; Longobardi, S.; da Silva, E.T.; Giardina, P.; Merkoçi, A. In Situ Production of Biofunctionalized Few-Layer Defect-Free Microsheets of Graphene. Adv. Funct. Mater. 2015, 25, 2771–2779. [Google Scholar] [CrossRef]
- Zampieri, F.; Wösten, H.A.B.; Scholtmeijer, K. Creating Surface Properties Using a Palette of Hydrophobins. Materials 2010, 3, 4607–4625. [Google Scholar] [CrossRef]
- Piscitelli, A.; Pennacchio, A.; Longobardi, S.; Velotta, R.; Giardina, P. Vmh2 hydrophobin as a tool for the development of “self-immobilizing” enzymes for biosensing. Biotechnol. Bioeng. 2017, 114, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, I.; Giardina, P.; Piscitelli, A. Development of a biosensing platform based on a laccase-hydrophobin chimera. Appl. Microbiol. Biotechnol. 2019, 103, 3061–3071. [Google Scholar] [CrossRef] [PubMed]
- Pezzella, C.; Guarino, L.; Piscitelli, A. How to enjoy laccases. Cell. Mol. Life Sci. 2015, 72, 923–940. [Google Scholar] [CrossRef] [PubMed]
- Pezzella, C.; Giacobelli, V.G.; Lettera, V.; Olivieri, G.; Cicatiello, P.; Sannia, G.; Piscitelli, A. A step forward in laccase exploitation: Recombinant production and evaluation of techno-economic feasibility of the process. J. Biotechnol. 2017, 259, 175–181. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Yaropolov, A.I.; Kharybin, A.N.; Emnéus, J.; Marko-Varga, G.; Gorton, L. Flow-injection analysis of phenols at a graphite electrode modified with co-immobilised laccase and tyrosinase. Anal. Chim. Acta 1995, 308, 137–144. [Google Scholar] [CrossRef]
- Freire, R.S.; Thongngamdee, S.; Durán, N.; Wang, J.; Kubota, L.T. Mixed enzyme (laccase/tyrosinase)-based remote electrochemical biosensor for monitoring phenolic compounds. Analyst 2002, 127, 258–261. [Google Scholar] [CrossRef][Green Version]
- Rodríguez-Delgado, M.M.; Alemán-Nava, G.S.; Rodríguez-Delgado, J.M.; Dieck-Assad, G.; Martínez-Chapa, S.O.; Barceló, D.; Parra, R. Laccase-based biosensors for detection of phenolic compounds. Trac. Trends Anal. Chem. 2015, 74, 21–45. [Google Scholar] [CrossRef]
- Ferry, Y.; Leech, D. Amperometric Detection of Catecholamine Neurotransmitters Using Electrocatalytic Substrate Recycling at a Laccase Electrode. Electroanalysis 2005, 17, 113–119. [Google Scholar] [CrossRef]
- Palanisamy, S.; Ramaraj, S.K.; Chen, S.-M.; Yang, T.C.K.; Yi-Fan, P.; Chen, T.-W.; Velusamy, V.; Selvam, S. A novel Laccase Biosensor based on Laccase immobilized Graphene-Cellulose Microfiber Composite modified Screen-Printed Carbon Electrode for Sensitive Determination of Catechol. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Boujakhrout, A.; Jimenez-Falcao, S.; Martínez-Ruiz, P.; Sánchez, A.; Díez, P.; Pingarrón, J.M.; Villalonga, R. Novel reduced graphene oxide–glycol chitosan nanohybrid for the assembly of an amperometric enzyme biosensor for phenols. Analyst 2016, 141, 4162–4169. [Google Scholar] [CrossRef] [PubMed]
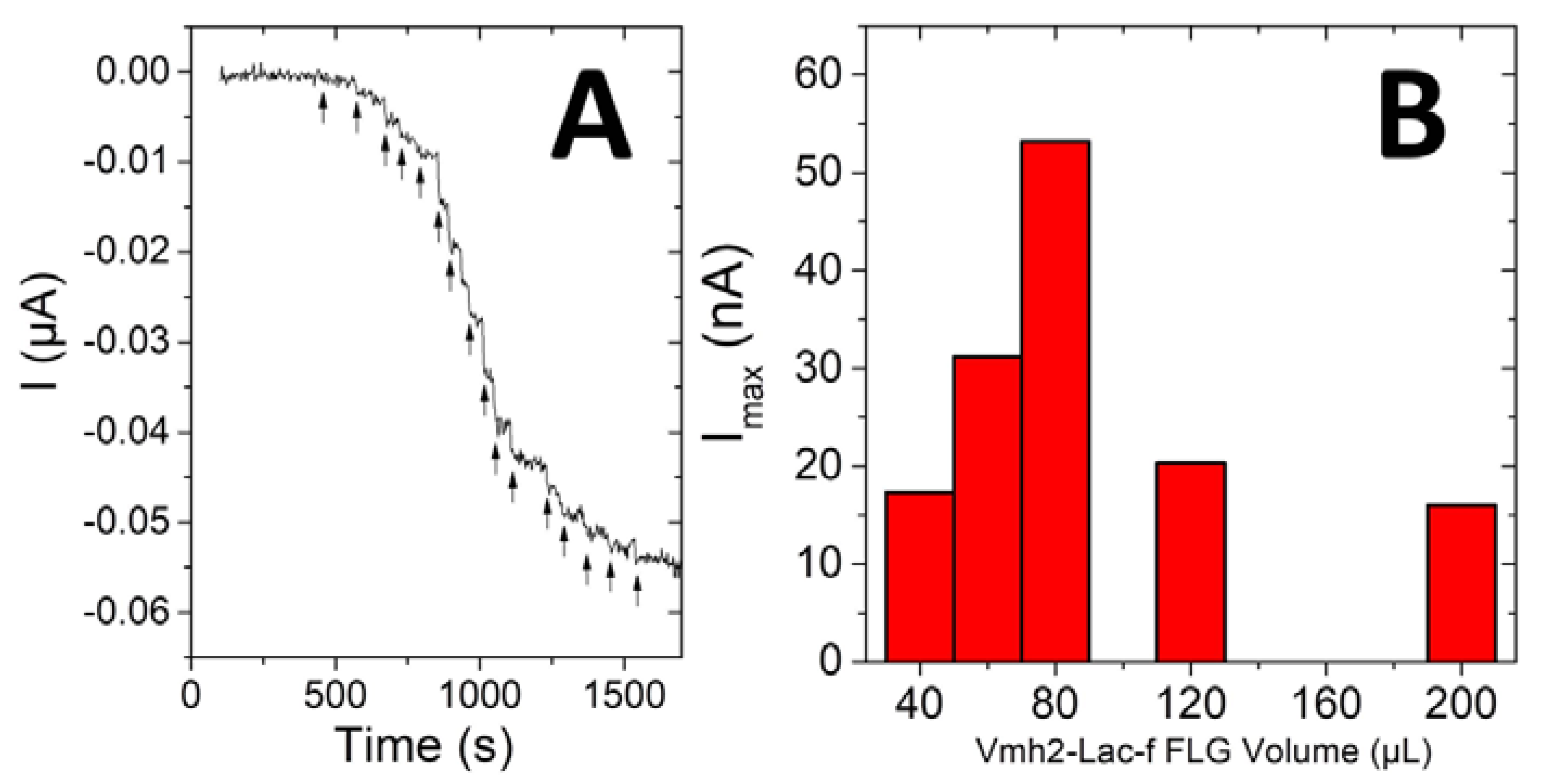
| Samples | Units in Milligrams of Graphene (U mg−1) | t1/2 (Days) | Activity after Washing |
|---|---|---|---|
| Graphene/PoxA1b | 0.3 ± 0.1 | 17 | Stable up to the second wash |
| Graphene/Lac-Vmh2 | 0.4 ± 0.1 | 26 | Stable up to the fourth wash |
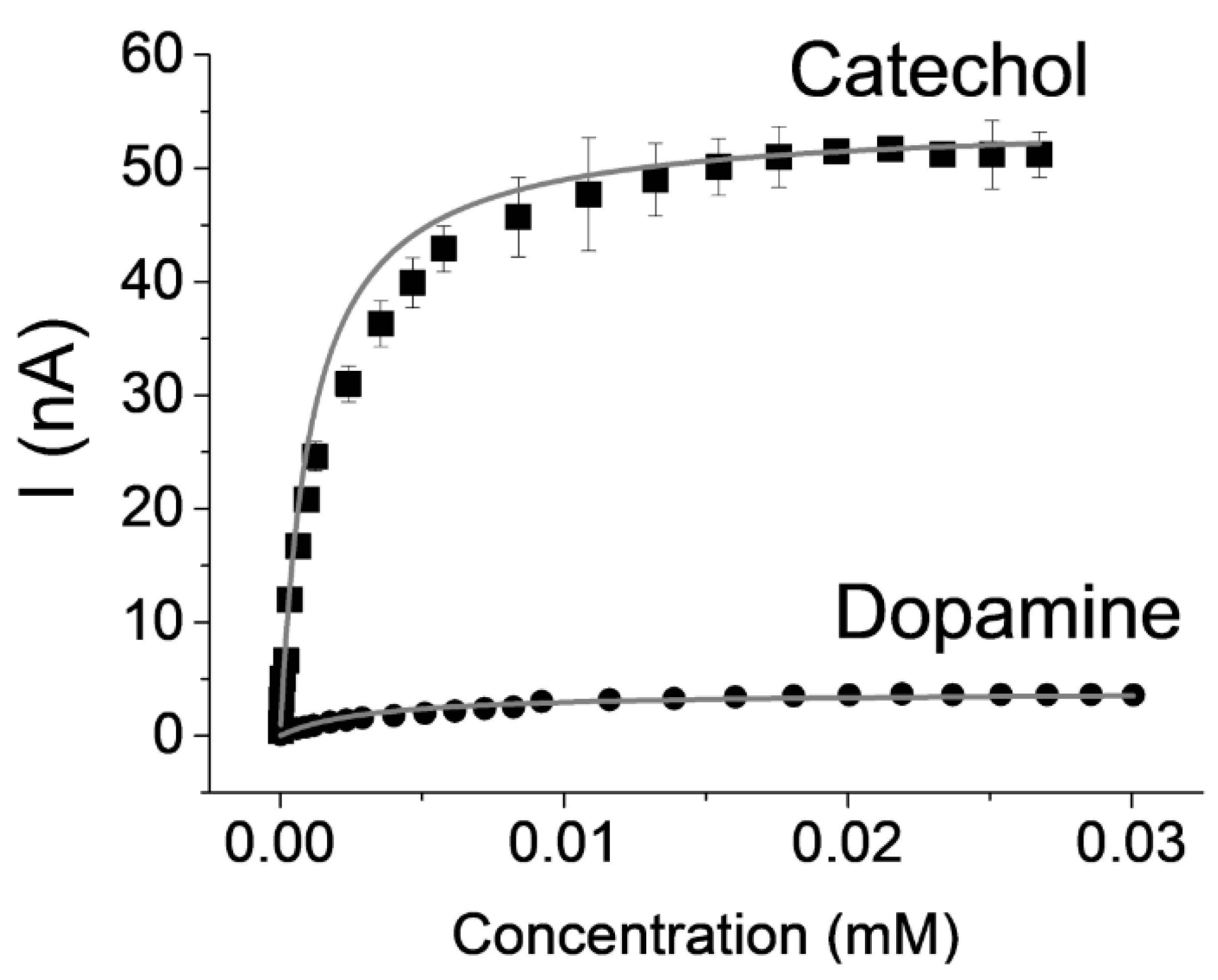
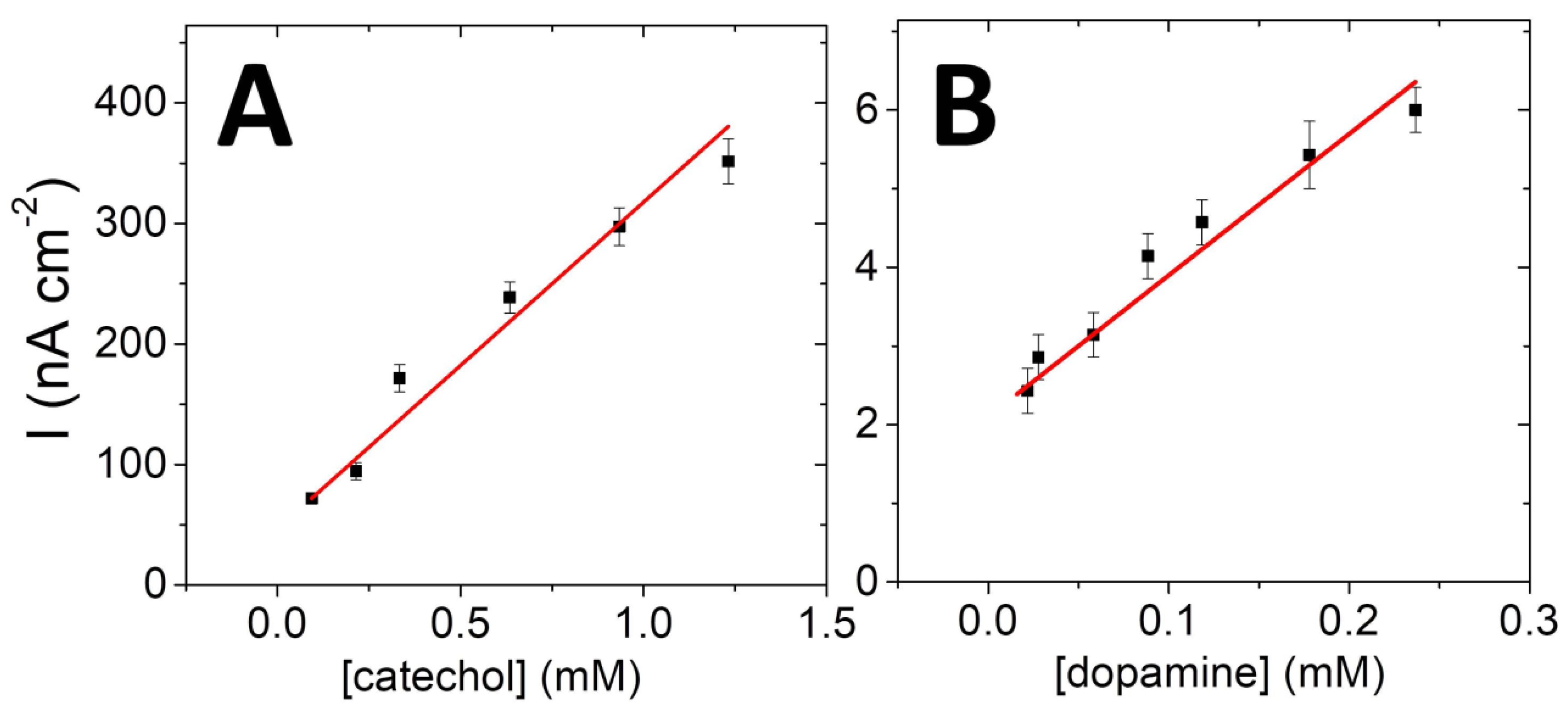
| Substrate | KM (mM) | IMAX (nA cm−2) | R2 |
|---|---|---|---|
| Catechol | 1.1 | 775.7 | 0.99 |
| Dopamine | 3.0 | 55.5 | 0.95 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorrentino, I.; Stanzione, I.; Nedellec, Y.; Piscitelli, A.; Giardina, P.; Le Goff, A. From Graphite to Laccase Biofunctionalized Few-Layer Graphene: A “One Pot” Approach Using a Chimeric Enzyme. Int. J. Mol. Sci. 2020, 21, 3741. https://doi.org/10.3390/ijms21113741
Sorrentino I, Stanzione I, Nedellec Y, Piscitelli A, Giardina P, Le Goff A. From Graphite to Laccase Biofunctionalized Few-Layer Graphene: A “One Pot” Approach Using a Chimeric Enzyme. International Journal of Molecular Sciences. 2020; 21(11):3741. https://doi.org/10.3390/ijms21113741
Chicago/Turabian StyleSorrentino, Ilaria, Ilaria Stanzione, Yannig Nedellec, Alessandra Piscitelli, Paola Giardina, and Alan Le Goff. 2020. "From Graphite to Laccase Biofunctionalized Few-Layer Graphene: A “One Pot” Approach Using a Chimeric Enzyme" International Journal of Molecular Sciences 21, no. 11: 3741. https://doi.org/10.3390/ijms21113741
APA StyleSorrentino, I., Stanzione, I., Nedellec, Y., Piscitelli, A., Giardina, P., & Le Goff, A. (2020). From Graphite to Laccase Biofunctionalized Few-Layer Graphene: A “One Pot” Approach Using a Chimeric Enzyme. International Journal of Molecular Sciences, 21(11), 3741. https://doi.org/10.3390/ijms21113741