Increase in the Number of Bone Marrow Osteoclast Precursors at Different Skeletal Sites, Particularly in Long Bone and Jaw Marrow in Mice Lacking IL-1RA
Abstract
1. Introduction
2. Results
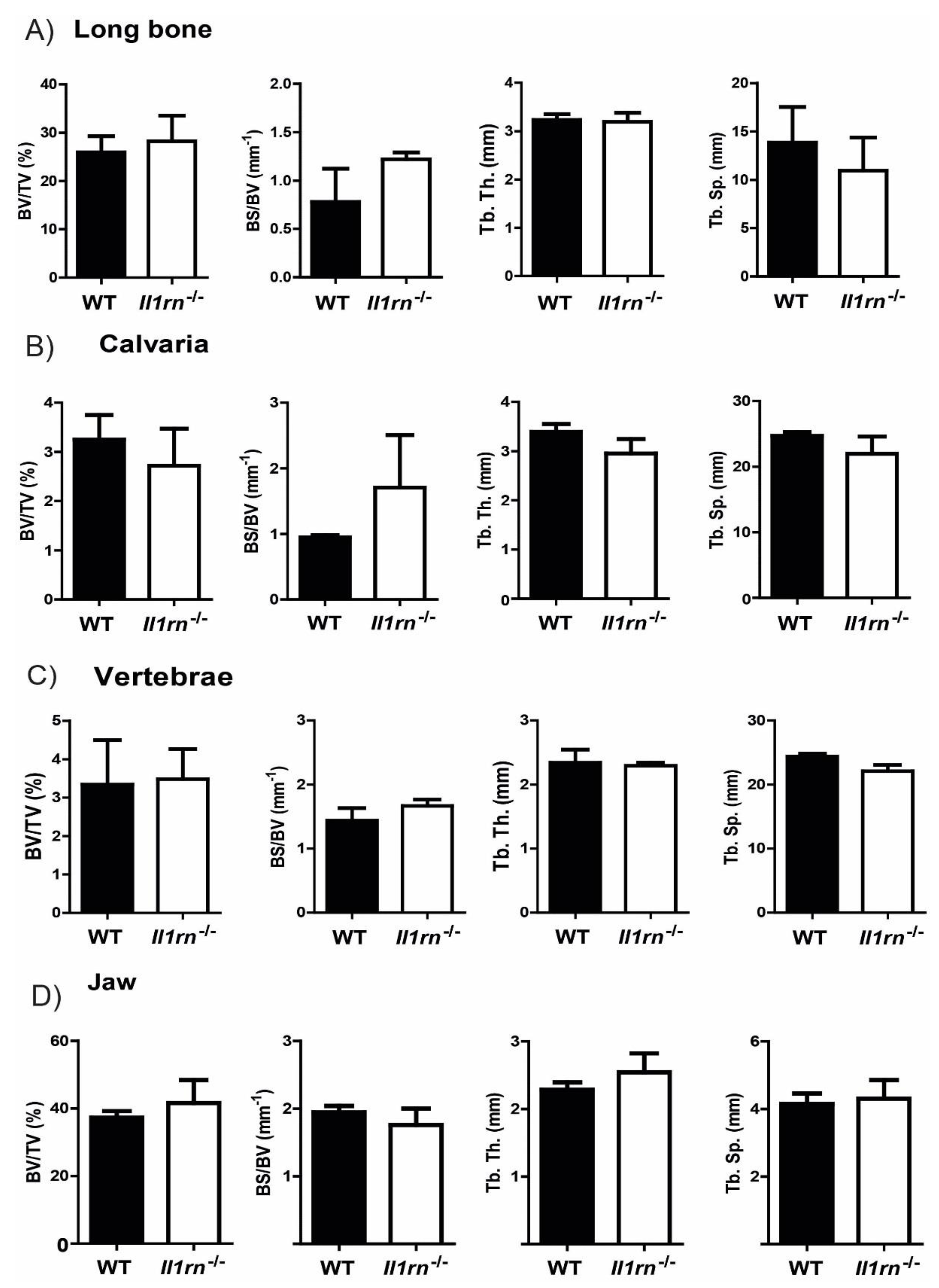
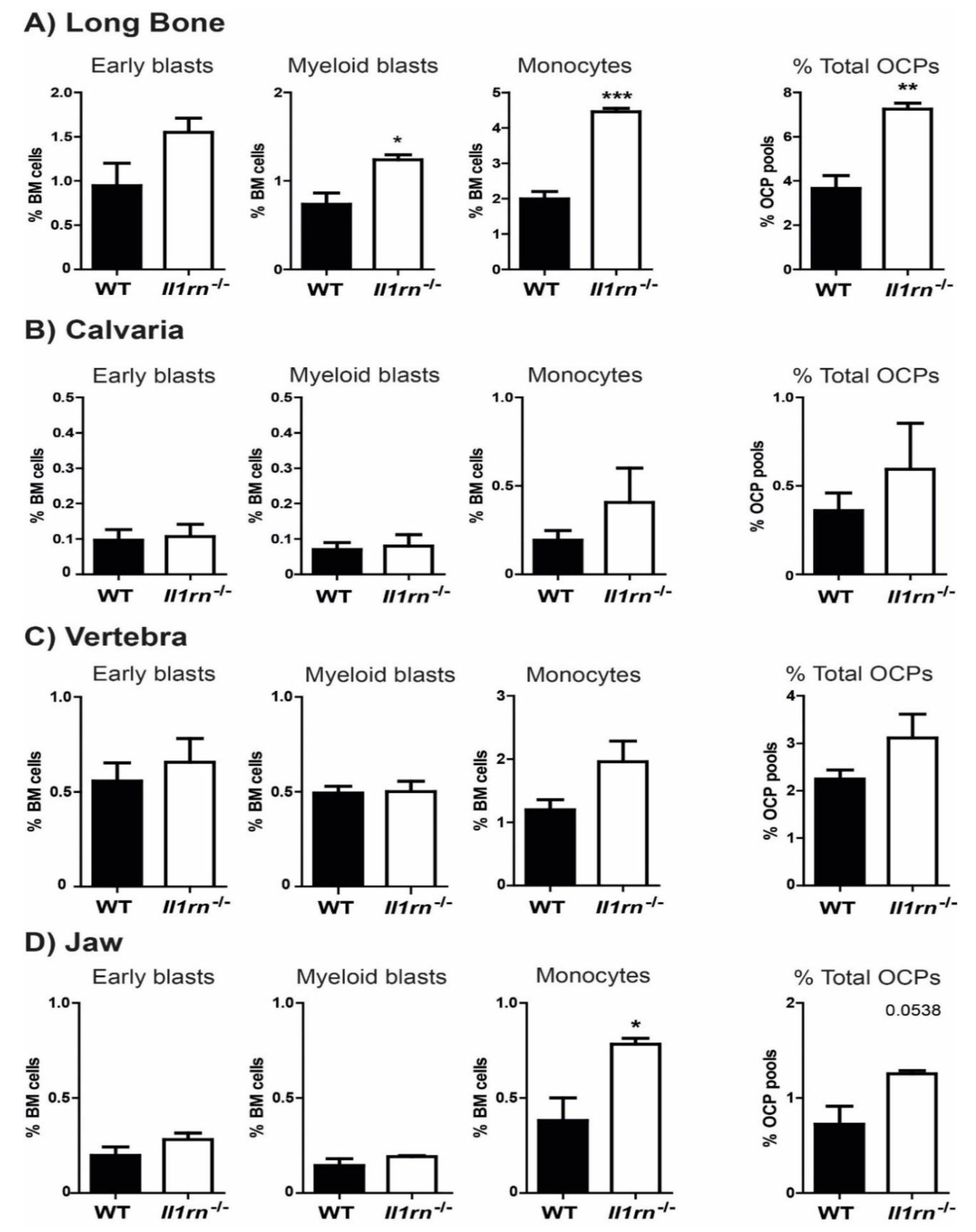
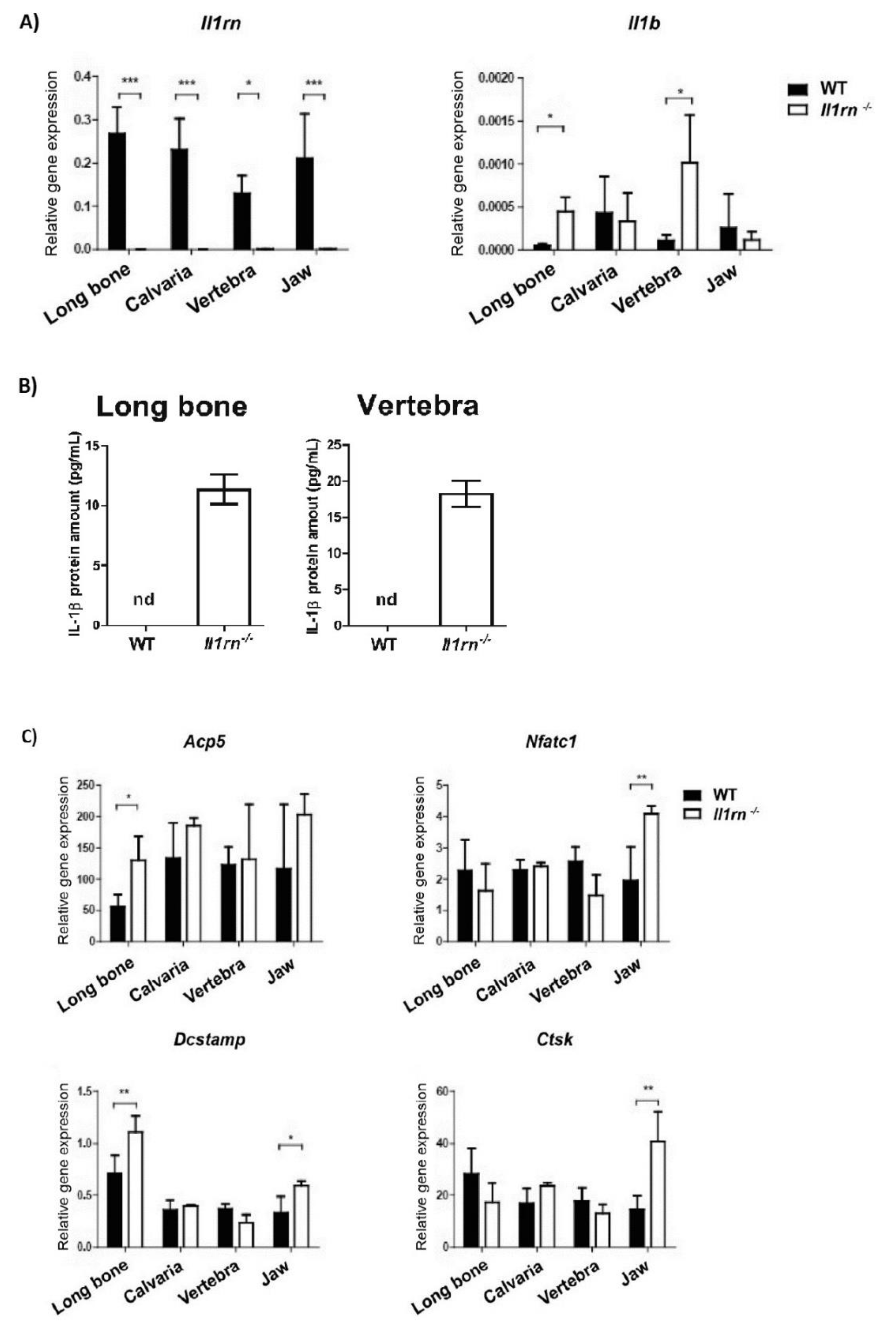
2.1. Absence of Il1rn Significantly Increases the Number of Osteoclast Precursors, Particularly Monocytes, in the Long Bone and Jaw Marrow
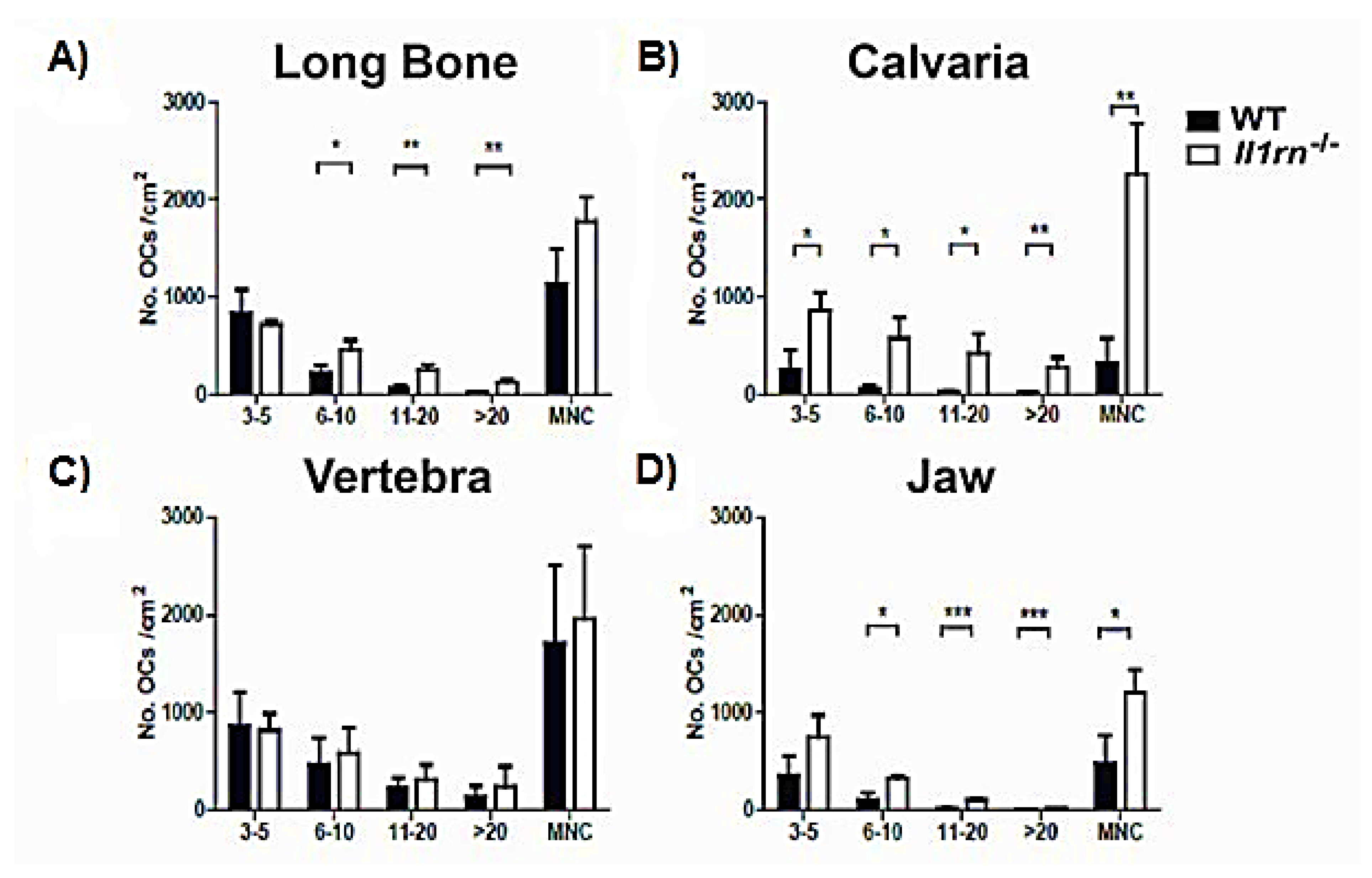
2.2. Enhanced Osteoclast Formation and Multinucleation by Bone Marrow Cells Derived from the Long Bone, Calvaria, and Jaw of Il1rn−/− Mice
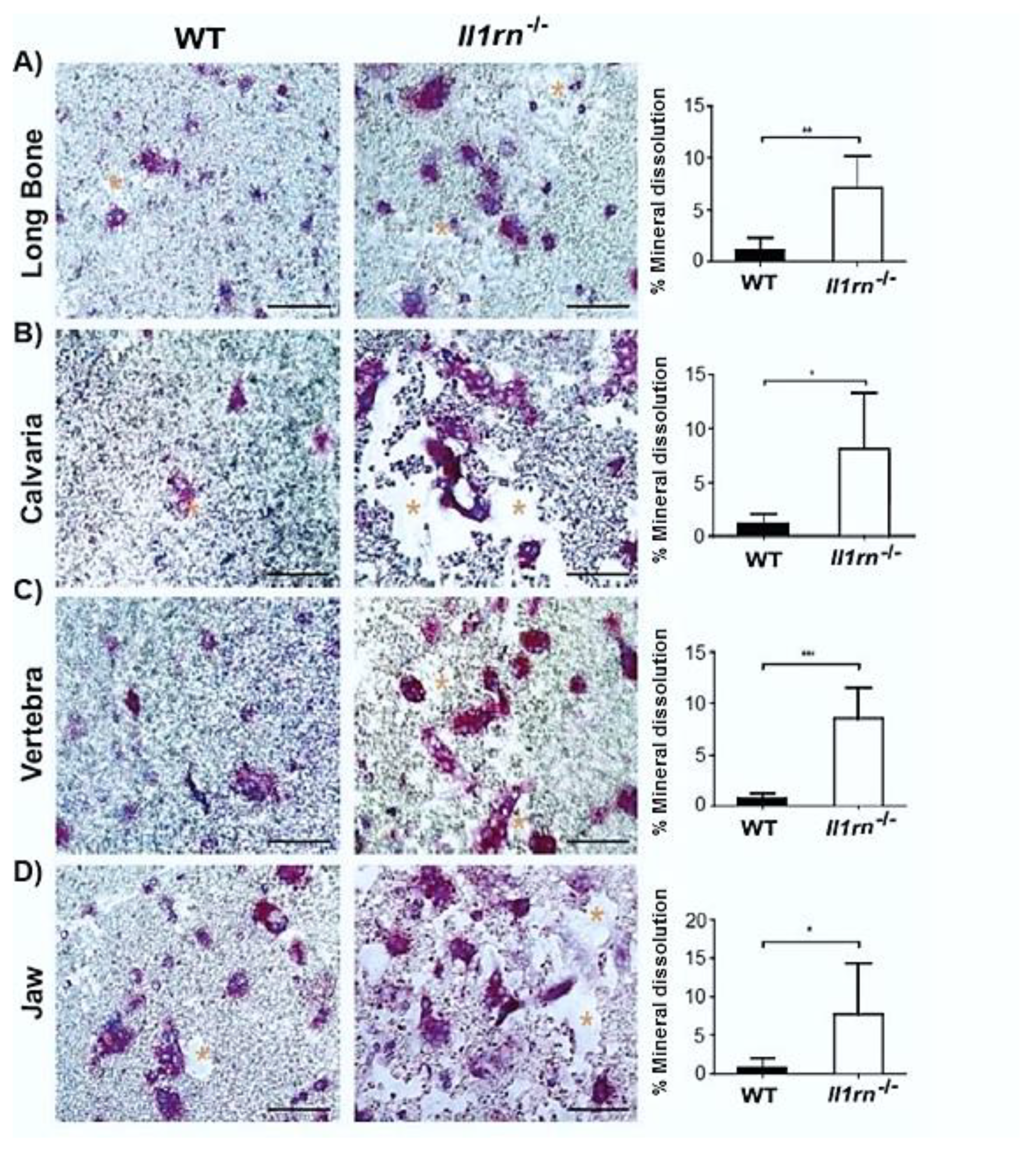
2.3. Il1rn−/− Osteoclasts Show Increased Mineral Dissolving Capacity on Calcium Phosphate-Coated Plates Independently of the Skeletal Site
3. Discussion
4. Materials and Methods
4.1. Il1rn−/− Mice
4.2. Histology
4.3. Microcomputed Tomography (µCT)
4.4. Bone Marrow Isolation
4.5. Immunofluorescence Labeling and Flow Cytometric Analysis
4.6. Cell Culture
4.7. Analysis of Tartrate-Resistant Acid Phosphatase Positive Cells
4.8. Calcium Phosphate Coating and Analysis of Areas of Lysis
4.9. Quantitative RT-PCR
4.10. Luminex
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Vries, T.J.; El Bakkali, I.; Kamradt, T.; Schett, G.; Jansen, I.D.C.; D′Amelio, P. What Are the Peripheral Blood Determinants for Increased Osteoclast Formation in the Various Inflammatory Diseases Associated With Bone Loss? Front. Immunol. 2019, 10, 505. [Google Scholar] [CrossRef] [PubMed]
- Araujo, V.M.; Melo, I.M.; Lima, V. Relationship between Periodontitis and Rheumatoid Arthritis: Review of the Literature. Mediat. Inflamm. 2015, 2015, 259074. [Google Scholar] [CrossRef] [PubMed]
- Detert, J.; Pischon, N.; Burmester, G.R.; Buttgereit, F. The association between rheumatoid arthritis and periodontal disease. Arthritis Res. Ther. 2010, 12, 218. [Google Scholar] [CrossRef]
- Jimi, E.; Nakamura, I.; Duong, L.T.; Ikebe, T.; Takahashi, N.; Rodan, G.A.; Suda, T. Interleukin 1 induces multinucleation and bone-resorbing activity of osteoclasts in the absence of osteoblasts/stromal cells. Exp. Cell Res. 1999, 247, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jin, H.M.; Kim, K.; Song, I.; Youn, B.U.; Matsuo, K.; Kim, N. The mechanism of osteoclast differentiation induced by IL-1. J. Immunol. 2009, 183, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Jansen, I.D.; Sprangers, S.; Stap, J.; Leenen, P.J.; Everts, V.; De Vries, T.J. IL-1beta differently stimulates proliferation and multinucleation of distinct mouse bone marrow osteoclast precursor subsets. J. Leukoc. Biol. 2016, 100, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Arend, W.P.; Malyak, M.; Guthridge, C.J.; Gabay, C. Interleukin-1 receptor antagonist: Role in biology. Annu. Rev. Immunol. 1998, 16, 27–55. [Google Scholar] [CrossRef] [PubMed]
- McMahan, C.J.; Slack, J.L.; Mosley, B.; Cosman, D.; Lupton, S.D.; Brunton, L.L.; Grubin, C.E.; Wignall, J.M.; Jenkins, N.A.; Brannan, C.I.; et al. A novel IL-1 receptor, cloned from B cells by mammalian expression, is expressed in many cell types. EMBO J. 1991, 10, 2821–2832. [Google Scholar] [CrossRef]
- Kitazawa, R.; Kimble, R.B.; Vannice, J.L.; Kung, V.T.; Pacifici, R. Interleukin-1 receptor antagonist and tumor necrosis factor binding protein decrease osteoclast formation and bone resorption in ovariectomized mice. J. Clin. Investig. 1994, 94, 2397–2406. [Google Scholar] [CrossRef]
- Horai, R.; Saijo, S.; Tanioka, H.; Nakae, S.; Sudo, K.; Okahara, A.; Ikuse, T.; Asano, M.; Iwakura, Y. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J. Exp. Med. 2000, 191, 313–320. [Google Scholar] [CrossRef]
- Planck, S.R.; Woods, A.; Clowers, J.S.; Nicklin, M.J.; Rosenbaum, J.T.; Rosenzweig, H.L. Impact of IL-1 signalling on experimental uveitis and arthritis. Ann. Rheum. Dis. 2012, 71, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.H.; Farrell, E.; Vis, M.; Colin, E.M.; Lubberts, E. Animal Models of Bone Loss in Inflammatory Arthritis: From Cytokines in the Bench to Novel Treatments for Bone Loss in the Bedside-a Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 51, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.H.; Cho, M.L.; Moon, Y.M.; Oh, H.J.; Park, J.S.; Jhun, J.Y.; Min, S.Y.; Cho, Y.G.; Park, K.S.; Yoon, C.H.; et al. IL-23 induces receptor activator of NF-kappaB ligand expression on CD4+ T cells and promotes osteoclastogenesis in an autoimmune arthritis model. J. Immunol. 2008, 181, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Abramson, S.B.; Amin, A. Blocking the effects of IL-1 in rheumatoid arthritis protects bone and cartilage. Rheumatology (Oxford) 2002, 41, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.L.; Jordan-Mahy, N.; Nicklin, M.J.; Le Maitre, C.L. Interleukin-1 receptor antagonist deficient mice provide insights into pathogenesis of human intervertebral disc degeneration. Ann. Rheum. Dis. 2013, 72, 1860–1867. [Google Scholar] [CrossRef]
- Jacquin, C.; Gran, D.E.; Lee, S.K.; Lorenzo, J.A.; Aguila, H.L. Identification of multiple osteoclast precursor populations in murine bone marrow. J. Bone Miner. Res. 2006, 21, 67–77. [Google Scholar] [CrossRef]
- De Vries, T.J.; Schoenmaker, T.; Hooibrink, B.; Leenen, P.J.; Everts, V. Myeloid blasts are the mouse bone marrow cells prone to differentiate into osteoclasts. J. Leukoc. Biol. 2009, 85, 919–927. [Google Scholar] [CrossRef]
- Sprangers, S.; Schoenmaker, T.; Cao, Y.; Everts, V.; De Vries, T.J. Different Blood-Borne Human Osteoclast Precursors Respond in Distinct Ways to IL-17A. J. Cell. Physiol. 2016, 231, 1249–1260. [Google Scholar] [CrossRef]
- Everts, V.; De Vries, T.J.; Helfrich, M.H. Osteoclast heterogeneity: Lessons from osteopetrosis and inflammatory conditions. Biochim. Biophys. Acta 2009, 1792, 757–765. [Google Scholar] [CrossRef]
- Everts, V.; Korper, W.; Hoeben, K.A.; Jansen, I.D.; Bromme, D.; Cleutjens, K.B.; Heeneman, S.; Peters, C.; Reinheckel, T.; Saftig, P.; et al. Osteoclastic bone degradation and the role of different cysteine proteinases and matrix metalloproteinases: Differences between calvaria and long bone. J. Bone Miner. Res. 2006, 21, 1399–1408. [Google Scholar] [CrossRef]
- Shorey, S.; Heersche, J.N.; Manolson, M.F. The relative contribution of cysteine proteinases and matrix metalloproteinases to the resorption process in osteoclasts derived from long bone and scapula. Bone 2004, 35, 909–917. [Google Scholar] [CrossRef] [PubMed]
- De Souza Faloni, A.P.; Schoenmaker, T.; Azari, A.; Katchburian, E.; Cerri, P.S.; De Vries, T.J.; Everts, V. Jaw and long bone marrows have a different osteoclastogenic potential. Calcif. Tissue Int. 2011, 88, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, T.; De Bruijn, M.F.; Lutz, M.B.; Leenen, P.J. Developmental stages of myeloid dendritic cells in mouse bone marrow. Int. Immunol. 2003, 15, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Izawa, A.; Ishihara, Y.; Mizutani, H.; Kobayashi, S.; Goto, H.; Okabe, E.; Takeda, H.; Ozawa, Y.; Kamiya, Y.; Sugita, Y.; et al. Inflammatory bone loss in experimental periodontitis induced by Aggregatibacter actinomycetemcomitans in interleukin-1 receptor antagonist knockout mice. Infect. Immun. 2014, 82, 1904–1913. [Google Scholar] [CrossRef]
- Ghozlani, I.; Ghazi, M.; Nouijai, A.; Mounach, A.; Rezqi, A.; Achemlal, L.; Bezza, A.; El Maghraoui, A. Prevalence and risk factors of osteoporosis and vertebral fractures in patients with ankylosing spondylitis. Bone 2009, 44, 772–776. [Google Scholar] [CrossRef]
- Boyce, B.F.; Schwarz, E.M.; Xing, L. Osteoclast precursors: Cytokine-stimulated immunomodulators of inflammatory bone disease. Curr. Opin. Rheumatol. 2006, 18, 427–432. [Google Scholar] [CrossRef]
- Nevius, E.; Gomes, A.C.; Pereira, J.P. Inflammatory Cell Migration in Rheumatoid Arthritis: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 51, 59–78. [Google Scholar] [CrossRef]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef]
- Li, P.; Schwarz, E.M.; O′Keefe, R.J.; Ma, L.; Looney, R.J.; Ritchlin, C.T.; Boyce, B.F.; Xing, L. Systemic tumor necrosis factor alpha mediates an increase in peripheral CD11bhigh osteoclast precursors in tumor necrosis factor alpha-transgenic mice. Arthritis Rheumatol. 2004, 50, 265–276. [Google Scholar] [CrossRef]
- Walsh, M.C.; Choi, Y. Biology of the RANKL-RANK-OPG System in Immunity, Bone, and Beyond. Front. Immunol. 2014, 5, 511. [Google Scholar] [CrossRef]
- Arranz, L.; Arriero, M.D.M.; Villatoro, A. Interleukin-1beta as emerging therapeutic target in hematological malignancies and potentially in their complications. Blood Rev. 2017, 31, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Biologic basis for interleukin-1 in disease. Blood 1996, 87, 2095–2147. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, H.; Ishihara, Y.; Izawa, A.; Fujihara, Y.; Kobayashi, S.; Gotou, H.; Okabe, E.; Takeda, H.; Ozawa, Y.; Kamiya, Y.; et al. Lipopolysaccharide of Aggregatibacter actinomycetemcomitans up-regulates inflammatory cytokines, prostaglandin E2 synthesis and osteoclast formation in interleukin-1 receptor antagonist-deficient mice. J. Periodontal Res. 2013, 48, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.M.; Payne, J.B.; Yu, F.; Thiele, G.M.; Erickson, A.R.; Johnson, P.G.; Schmid, M.J.; Cannon, G.W.; Kerr, G.S.; Reimold, A.M.; et al. Alveolar bone loss is associated with circulating anti-citrullinated protein antibody (ACPA) in patients with rheumatoid arthritis. J. Periodontol. 2015, 86, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Sofat, N.; Wait, R.; Robertson, S.D.; Baines, D.L.; Baker, E.H. Interaction between extracellular matrix molecules and microbial pathogens: Evidence for the missing link in autoimmunity with rheumatoid arthritis as a disease model. Front. Microbiol. 2014, 5, 783. [Google Scholar] [CrossRef] [PubMed]
- Beyer, K.; Zaura, E.; Brandt, B.W.; Buijs, M.J.; Brun, J.G.; Crielaard, W.; Bolstad, A.I. Subgingival microbiome of rheumatoid arthritis patients in relation to their disease status and periodontal health. PLoS ONE 2018, 13, e0202278. [Google Scholar] [CrossRef]
- Bingham, C.O., 3rd; Moni, M. Periodontal disease and rheumatoid arthritis: The evidence accumulates for complex pathobiologic interactions. Curr. Opin. Rheumatol. 2013, 25, 345–353. [Google Scholar] [CrossRef]
- Chen, H.H.; Huang, N.; Chen, Y.M.; Chen, T.J.; Chou, P.; Lee, Y.L.; Chou, Y.J.; Lan, J.L.; Lai, K.L.; Lin, C.H.; et al. Association between a history of periodontitis and the risk of rheumatoid arthritis: A nationwide, population-based, case-control study. Ann. Rheum. Dis. 2013, 72, 1206–1211. [Google Scholar] [CrossRef]
- Griffith, J.F. Identifying osteoporotic vertebral fracture. Quant. Imaging Med. Surg. 2015, 5, 592–602. [Google Scholar] [CrossRef]
- Wei, S.; Kitaura, H.; Zhou, P.; Ross, F.P.; Teitelbaum, S.L. IL-1 mediates TNF-induced osteoclastogenesis. J. Clin. Investig. 2005, 115, 282–290. [Google Scholar] [CrossRef]
- Stralberg, F.; Kassem, A.; Kasprzykowski, F.; Abrahamson, M.; Grubb, A.; Lindholm, C.; Lerner, U.H. Inhibition of lipopolysaccharide-induced osteoclast formation and bone resorption in vitro and in vivo by cysteine proteinase inhibitors. J. Leukoc. Biol. 2017, 101, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Melton, L.J., 3rd; Tiegs, R.D.; Atkinson, E.J.; O′Fallon, W.M. Fracture risk among patients with Paget’s disease: A population-based cohort study. J. Bone Miner. Res. 2000, 15, 2123–2128. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, N.M.; Kesavan, C.; Mohan, S. Long-term Consequences of Traumatic Brain Injury in Bone Metabolism. Front. Neurol. 2018, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Everts, V.; Korper, W.; Jansen, D.C.; Steinfort, J.; Lammerse, I.; Heera, S.; Docherty, A.J.; Beertsen, W. Functional heterogeneity of osteoclasts: Matrix metalloproteinases participate in osteoclastic resorption of calvarial bone but not in resorption of long bone. FASEB J. 1999, 13, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Jansen, I.D.; Mardones, P.; Lecanda, F.; De Vries, T.J.; Recalde, S.; Hoeben, K.A.; Schoenmaker, T.; Ravesloot, J.H.; Van Borren, M.M.; Van Eijden, T.M.; et al. Ae2(a,b)-deficient mice exhibit osteopetrosis of long bones but not of calvaria. FASEB J. 2009, 23, 3470–3481. [Google Scholar] [CrossRef] [PubMed]
- Nicklin, M.J.; Hughes, D.E.; Barton, J.L.; Ure, J.M.; Duff, G.W. Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. J. Exp. Med. 2000, 191, 303–312. [Google Scholar] [CrossRef]
- Ten Harkel, B.; Schoenmaker, T.; Picavet, D.I.; Davison, N.L.; De Vries, T.J.; Everts, V. The Foreign Body Giant Cell Cannot Resorb Bone, But Dissolves Hydroxyapatite Like Osteoclasts. PLoS ONE 2015, 10, e0139564. [Google Scholar] [CrossRef]
- Yang, L.; Perez-Amodio, S.; Barrere-de Groot, F.Y.; Everts, V.; Van Blitterswijk, C.A.; Habibovic, P. The effects of inorganic additives to calcium phosphate on in vitro behavior of osteoblasts and osteoclasts. Biomaterials 2010, 31, 2976–2989. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ascone, G.; Cao, Y.; Jansen, I.D.C.; Di Ceglie, I.; van den Bosch, M.H.J.; Blom, A.B.; van Lent, P.L.E.M.; Everts, V.; de Vries, T.J. Increase in the Number of Bone Marrow Osteoclast Precursors at Different Skeletal Sites, Particularly in Long Bone and Jaw Marrow in Mice Lacking IL-1RA. Int. J. Mol. Sci. 2020, 21, 3774. https://doi.org/10.3390/ijms21113774
Ascone G, Cao Y, Jansen IDC, Di Ceglie I, van den Bosch MHJ, Blom AB, van Lent PLEM, Everts V, de Vries TJ. Increase in the Number of Bone Marrow Osteoclast Precursors at Different Skeletal Sites, Particularly in Long Bone and Jaw Marrow in Mice Lacking IL-1RA. International Journal of Molecular Sciences. 2020; 21(11):3774. https://doi.org/10.3390/ijms21113774
Chicago/Turabian StyleAscone, Giuliana, Yixuan Cao, Ineke D.C. Jansen, Irene Di Ceglie, Martijn H.J. van den Bosch, Arjen B. Blom, Peter L.E.M. van Lent, Vincent Everts, and Teun J. de Vries. 2020. "Increase in the Number of Bone Marrow Osteoclast Precursors at Different Skeletal Sites, Particularly in Long Bone and Jaw Marrow in Mice Lacking IL-1RA" International Journal of Molecular Sciences 21, no. 11: 3774. https://doi.org/10.3390/ijms21113774
APA StyleAscone, G., Cao, Y., Jansen, I. D. C., Di Ceglie, I., van den Bosch, M. H. J., Blom, A. B., van Lent, P. L. E. M., Everts, V., & de Vries, T. J. (2020). Increase in the Number of Bone Marrow Osteoclast Precursors at Different Skeletal Sites, Particularly in Long Bone and Jaw Marrow in Mice Lacking IL-1RA. International Journal of Molecular Sciences, 21(11), 3774. https://doi.org/10.3390/ijms21113774





