Metabolomic Fingerprinting of Potato Cultivars Differing in Susceptibility to Spongospora subterranea f. sp. subterranea Root Infection
Abstract
:1. Introduction
2. Results
2.1. Zoosporangia Root Infection
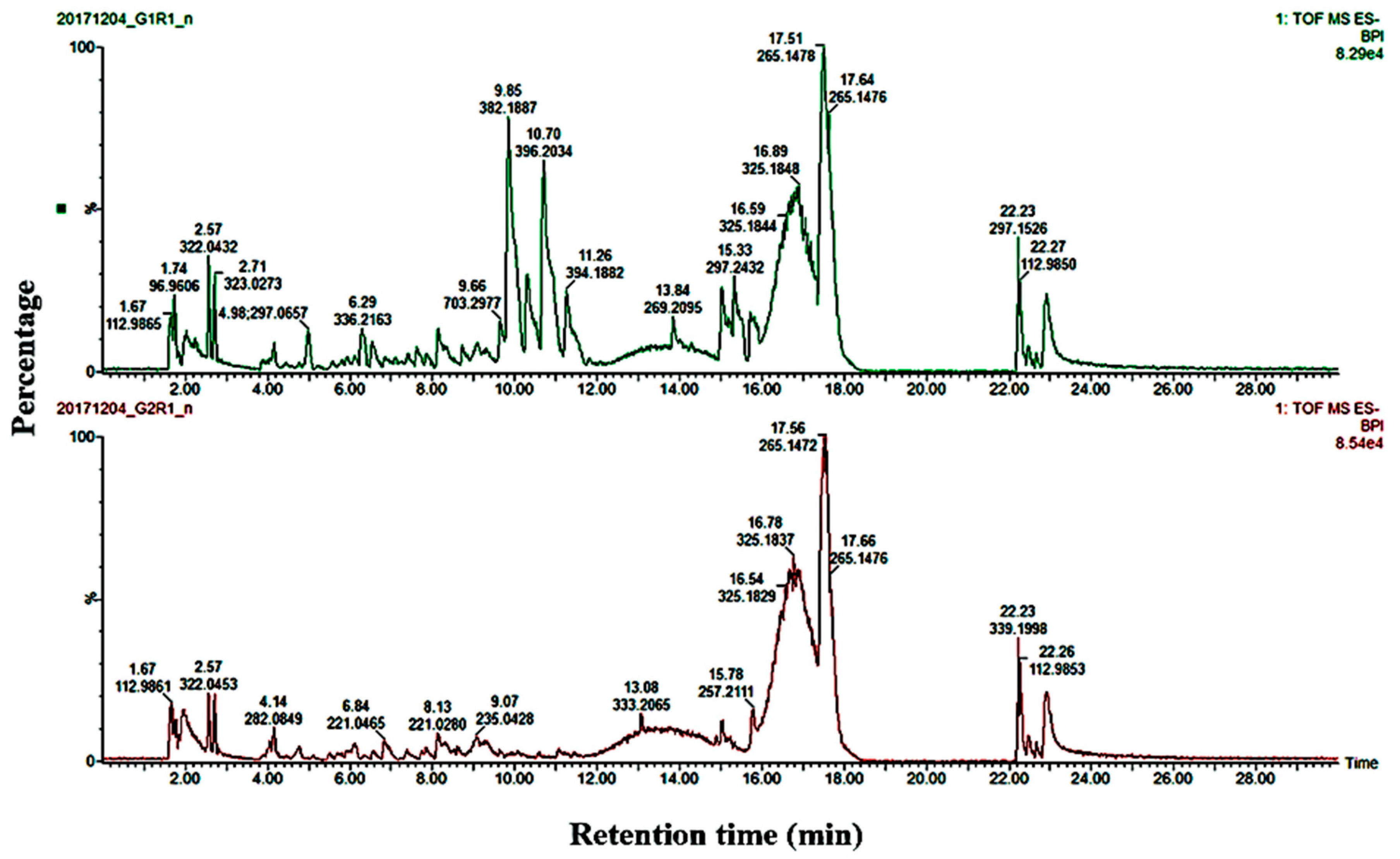
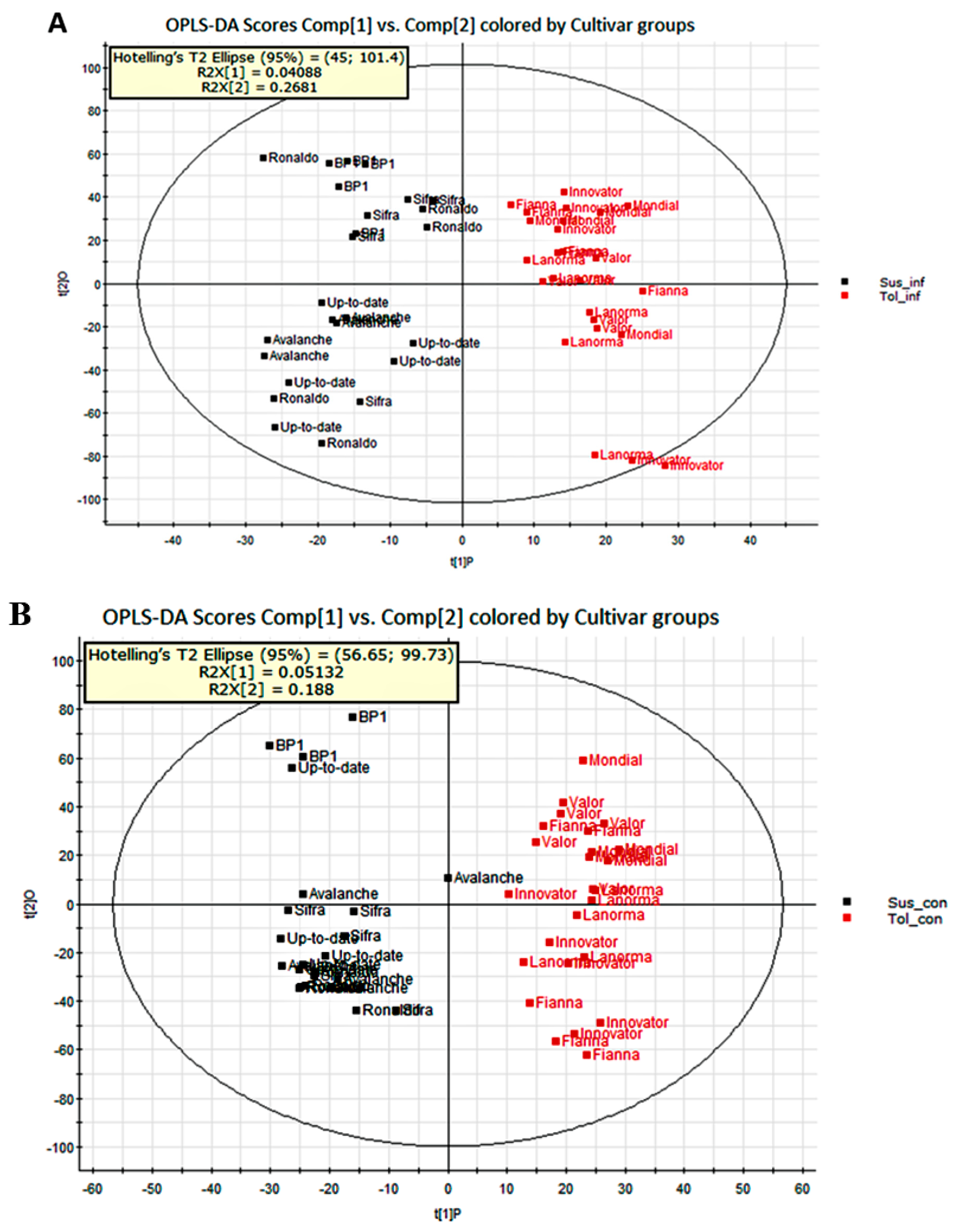
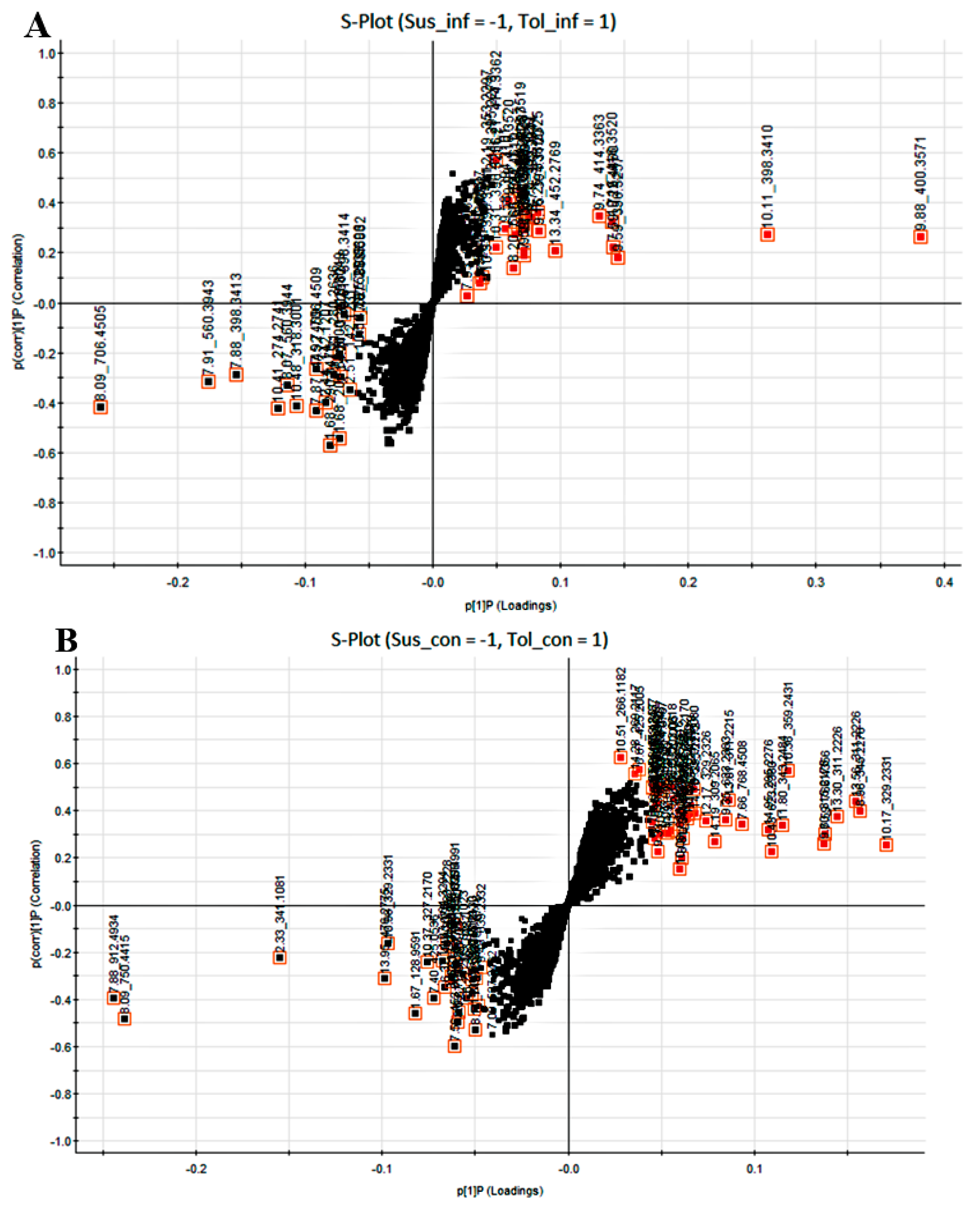
2.2. Determination of Metabolite Levels by Chemometric Models
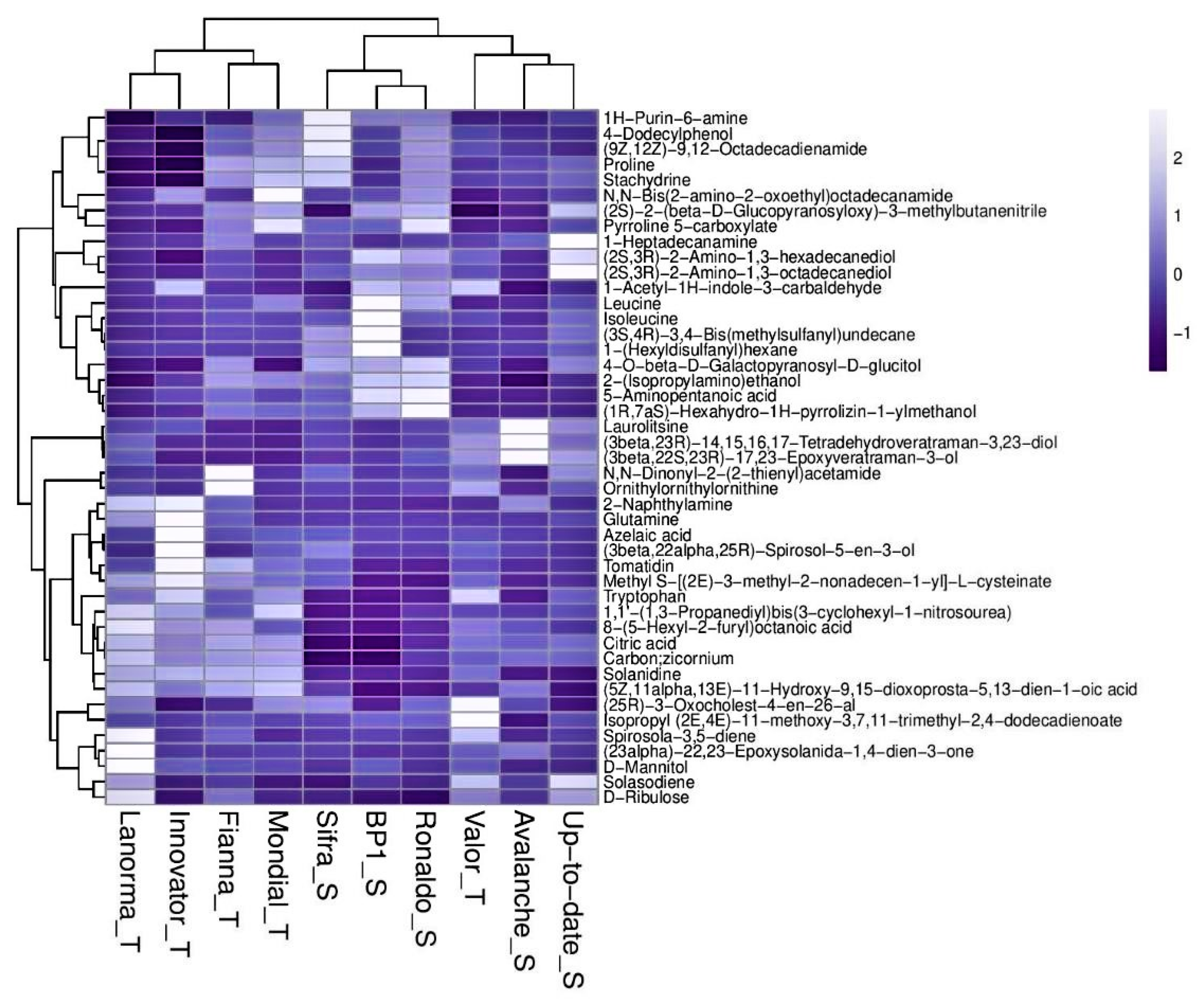
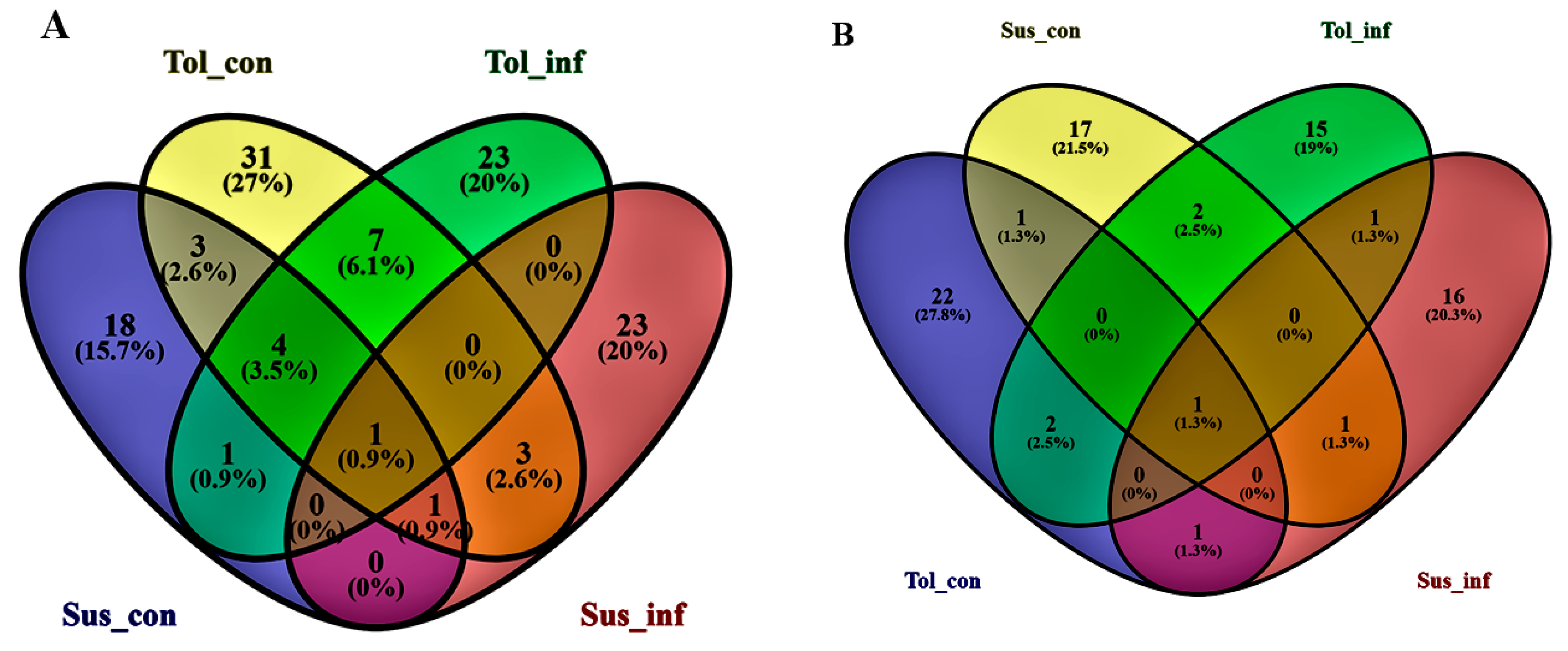
2.3. Metabolic Profiling of Potato Cultivars
3. Discussion
4. Materials and Methods
4.1. Plant Growth and Spongospora subterranea f. sp. subterranea Inoculation of Plants
4.2. Potato Root Exudate Collection and Root Sample Preparation
4.3. Zoosporangia Root Infection Assessment
4.4. Metabolite Extraction
4.5. Ultra-Performance Liquid Chromatography-Time of Flight Coupled with Mass Spectrometry (UPLC–TOF-MS) Analysis
4.6. Multivariate Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Falloon, R.E. Control of powdery scab of potato: Towards integrated disease management. Am. J. Potato Res. 2008, 85, 253–260. [Google Scholar] [CrossRef]
- Falloon, R.E.; Curtin, D.; Lister, R.A.; Butler, R.C. The obligate, soilborne pathogen Spongospora subterranea affects host (Solanum tuberosum) root function. In Proceedings of the 3rd Australasian Soilborne Diseases Symposium; Ophel Keller, K.M., Hall, B.H., Eds.; South Australian Research and Development Institute: Adelaide, Australia, 2004; pp. 30–31. [Google Scholar]
- Falloon, R.E.; Merz, U.; Butler, R.C.; Curtin, D.; Lister, R.A.; Thomas, S.M. Root infection of potato by Spongospora subterranea: Knowledge review and evidence for decreased plant productivity. Plant Pathol. 2016, 65, 422–434. [Google Scholar] [CrossRef]
- Huang, H.; Thu, T.N.T.; He, X.; Gravot, A.; Bernillon, S.; Ballini, E.; Morel, J. Increase of fungal pathogenicity and role of plant glutamine in nitrogen-induced susceptibility (NIS) to rice blast. Front. Plant Sci. 2017, 8, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, F.A.; Falloon, R.E.; Butler, R.C.; Lister, R.A. Low amounts of Spongospora subterranea sporosorus inoculum cause severe powdery scab, root galling and reduced water use in potato (Solanum tuberosum). Australas. Plant Pathol. 2012, 41, 219–228. [Google Scholar] [CrossRef]
- Lister, R.A.; Falloon, R.E.; Curtin, D.; Butler, R.C. Spongospora subterranea reduces host (Solanum tuberosum) growth. In Proceedings of the 3rd Australasian Soilborne Diseases Symposium; Ophel Keller, K.M., Hall, B.H., Eds.; South Australian Research and Development Institute: Adelaide, Australia, 2004; pp. 135–136. [Google Scholar]
- Maldonado, M.L.H.; Falloon, R.E.; Butler, R.C.; Conner, A.J.; Bulman, S.R. Spongospora subterranea root infection assessed in two potato cultivars differing in susceptibility to tuber powdery scab. Plant Pathol. 2012, 62, 1089–1096. [Google Scholar] [CrossRef]
- Tawaraya, K.; Horie, R.; Saito, S.; Wagatsuma, T.; Saito, K.; Oikawa, A. Metabolite profiling of root exudates of common bean under phosphorus deficiency. Metabolites 2014, 4, 599–611. [Google Scholar] [CrossRef] [Green Version]
- Balendres, M.A.; Nichols, D.S.; Tegg, R.S.; Wilson, C.R. Metabolomes of potato root exudates: Compounds that stimulate resting spore germination of the soil-borne pathogen Spongospora subterranea. J. Agric. Food Chem. 2016, 64, 7466–7474. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [Green Version]
- Návarová, H.; Bernsdorff, F.; Döring, A.C.; Zeier, J. Pipecolic acid. An endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell 2012, 24, 5123–5141. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.K.; Millard, P.; Whiteley, A.S.; Murrell, J.C. Unravelling rhizosphere-microbial interactions: Opportunities and limitations. Trends Microbiol. 2004, 12, 386–393. [Google Scholar] [CrossRef]
- Baetz, U.; Martinoia, E. Root exudates: The hidden part of plant defense. Trends Plant Sci. 2014, 19, 90–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kombrink, E.; Schmelzer, E. The hypersensitive response and its role in local and systemic disease resistance. Eur. J. Plant Pathol. 2001, 107, 69–78. [Google Scholar] [CrossRef]
- Ahn, I.P.; Kim, S.; Lee, Y.H. Vitamin B1 functions as an activator of plant disease resistance. Plant Physiol. 2005, 138, 1505–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobato, M.; Olivieri, F.; Daleo, G.; Andreu, A. Antimicrobial activity of phosphites against different potato pathogens/antimikrobielle aktivitat von phosphiten gegenüber verschiedenen kartoffelpathogenen. J. Plant Dis. Protect. 2010, 117, 102–109. [Google Scholar] [CrossRef]
- Eschen-Lippold, L.; Altmann, S.; Rosahl, S.K.J. DL-β-aminobutyric acid–induced resistance of potato against Phytophthora infestans requires salicylic acid but not oxylipins. Mol. Plant Microbe Interact. 2010, 23, 585–592. [Google Scholar] [CrossRef] [Green Version]
- Liljeroth, E.; Bengtsson, T.; Wiik, L.; Andreasson, E. Erratum to: Induced resistance in potato to Phytophthora infestans-effects of BABA in greenhouse and field tests with different potato varieties. Eur. J. Plant Pathol. 2010, 127, 303. [Google Scholar] [CrossRef]
- Kopka, J. Current challenges and developments in GC-MS based metabolite profiling technology. J. Biotechnol. 2006, 124, 312–322. [Google Scholar] [CrossRef]
- van de Haar, J. The powdery scab situation in the Netherlands. In Proceedings of the First European Powdery Scab Workshop; Merz, U., Lees, A.K., Eds.; SAC: Aberdeen, Scotland, 2000; pp. 21–22. Available online: http://www.spongospora.ethz.ch/EUworkshop/proceedings.htm (accessed on 15 May 2019).
- Lekota, M.; Muzhinji, N.; van der Waals, J. Relative susceptibility of selected potato cultivars to diseases caused by Spongospora subterranea f. sp. subterranea, assessed in pot trials in South Africa. (Unpublished; manuscript in preparation).
- Del Pozo, O.; Pedley, K.F.; Martin, G.B. MAPKKK alpha is a positive regulator of cell death associated with both plant immunity and disease. EMBO J. 2004, 23, 3072–3082. [Google Scholar] [CrossRef] [Green Version]
- Olivieri, F.P.; Lobato, M.C.; Altamiranda, E.; Daleo, G.R.; Huarte, M.; Guevara, M.G.; Andreu, A.B. BABA effects on the behaviour of potato cultivars infected by Phytophthora infestans and Fusarium solani. Eur. J. Plant Pathol. 2009, 123, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Yogendra, K.N.; Pushpa, D.; Mosa, K.A.; Kushalappa, A.C.; Murphy, A.; Mosquera, T. Quantitative resistance in potato leaves to late blight associated with induced hydroxycinnamic acid amides. Funct. Integr. Genom. 2014, 14, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.R.; Achnine, L.; Kota, P.; Liu, C.J.; Reddy, M.S.S.; Wang, L. The phenylpropanoid pathway and plant defense—A genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Abu-Nada, Y.; Kushalappa, A.C.; Marshall, W.D.; Al-Mughrabi, K.; Murphy, A. Temporal dynamics of pathogenesis-related metabolites and their plausible pathways of induction in potato leaves following inoculation with Phytophthora infestans. Eur. J. Plant Pathol. 2007, 118, 375–391. [Google Scholar] [CrossRef]
- Barabasi, A.L.; Oltvai, Z.N. Network biology: Understanding the cell’s functional organization. Nat. Rev. Genet. 2004, 5, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Lachman, J.; Hamouz, K.; Orsak, M.; Pivec, V. Potato glycoalkaloids and their significance in plant protection and human nutrition-review. Rostl. Vyrob. 2001, 47, 181–191. [Google Scholar]
- Aliferis, K.A.; Jabaji, S. FT-ICR/MS and GC-EI/MS metabolomics networking unravels global potato sprout’s responses to Rhizoctonia solani infection. PLoS ONE 2012, 7, e42576. [Google Scholar] [CrossRef]
- Fatland, B.L.; Nikolau, B.J.; Wurtel, E.S. Reverse genetic characterization of cytosolic acetyl-CoA generation by ATP-citrate lyase in arabidopsis. Plant Cell 2005, 17, 182–203. [Google Scholar] [CrossRef] [Green Version]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry, 5th ed.; W H Freeman: New York, NY, USA, 2002; ISBN 10: 0-7167-3051-0. [Google Scholar]
- Martinez-Pacheco, M.M.; Flores-Garcia, A.; Gonzalez, E.V.; Cepeda-Villegas, A. Effect of citric acid on the proteolytic activity of Zea mays L. Ciênc Agrotec 2011, 35, 908–915. [Google Scholar] [CrossRef] [Green Version]
- Djami-Tchatchou, A.T.; Ncube, E.N.; Steenkamp, P.A.; Dubery, I.A. Similar, but different: Structurally related azelaic acid and hexanoic acid trigger differential metabolomic and transcriptomic responses in tobacco cells. BMC Plant Biol. 2017, 17, 227. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, H.; Kanayama, Y.; Zheng, M.S.; Kusano, T.; Hase, S.; Ikegami, M. Antagonistic interactions between the SA and JA signaling pathways in Arabidopsis modulated expression of defense genes and gene for gene resistance to cucumber mosaic virus. Plant Cell Physiol. 2004, 45, 803–809. [Google Scholar] [CrossRef]
- Yogendra, K.N.; Kushalappa, A.C.; Sarmiento, F.; Rodriguez, E.; Mosquera, T. Metabolomics deciphers quantitative resistance mechanisms in diploid potato clones against late blight. Funct. Plant Biol. 2014, 42, 284–298. [Google Scholar] [CrossRef]
- Showalter, A.M. Structure and function of plant cell wall proteins. Plant Cell 1993, 5, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.A.P.; Galinha, C.I.R.; Pereira, C.S.; Fortunato, A.; Soares, N.C.; Amancio, S.B.Q.; Ricardo, C.P.P. Rapid deposition of extensin during the elicitation of grapevine callus cultures is specifically catalyzed by a 40-kilodalton peroxidase. Plant Physiol. 2001, 127, 1065–1076. [Google Scholar] [CrossRef]
- Coruzzi, G.M.; Last, R.L. Amino acids. In Biochemistry and Molecular Biology of Plants; Buchanan, R.B., Gruissem, W., Jones, R., Eds.; American Society of Plant Physiology Press: Rockville, MD, USA, 2000; pp. 358–410. [Google Scholar]
- Taiz, L.; Zeiger, E. Plant Physiology, 3rd ed.; Sinauer Associates, Inc.: Sunderland, UK, 2002; pp. 67–86. [Google Scholar]
- Altamiranda, E.G.; Andreu, A.B.; Daleo, G.R.; Olivieri, F.P. Effect of beta-aminobutyric acid (BABA) on protection against Phytophthora infestans throughout the potato crop cycle. Australas. Plant Pathol. 2008, 37, 421–427. [Google Scholar] [CrossRef]
- Bengtsson, T.; Holefors, A.; Witzell, J.; Andreasson, E.; Liljeroth, E. Activation of defense responses to Phytophthora infestans in potato by BABA. Plant Pathol. 2014, 63, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Li, Y.C.; Bi, Y.; Chen, S.J.; Yuan, L.; Wang, Y.; Wang, D. Postharvest treatment with beta-aminobutyric acid induces resistance against dry rot caused by Fusarium sulphureum in potato tuber. Agric. Sci. China 2010, 9, 1372–1380. [Google Scholar] [CrossRef]
- Lim, G.H.; Singhal, R.; Kachroo, A.; Kachroo, P. Fatty acid- and lipid-mediated signalling in plant defense. Annu. Rev. Phytopathol. 2017, 4, 505–536. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Wright, L.P.; Gershenzon, J.; Wasternack, C.; Hause, B.; Schaller, A.; Stintzi, A. Jasmonic acid and its precursor 12-oxophytodienoic acid control different aspects of constitutive and induced herbivore defenses in tomato. Plant Physiol. 2014, 166, 396–410. [Google Scholar] [CrossRef] [Green Version]
- Gargallo-Garriga, A.; Preece, C.; Sardans, J.; Oravec, M.; Urban, O.; Peñuelas, J. Root exudate metabolomes change under drought and show limited capacity for recovery. Sci. Rep. 2018, 8, 12696. [Google Scholar] [CrossRef] [Green Version]
- Walker, T.S.; Bais, H.P.; Grotewold, E.; Vivanco, J.M. Root exudation and rhizosphere biology. Plant Physiol. 2003, 132, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Lanoue, A.; Burlat, V.; Henkes, G.J.; Koch, I.; Schurr, U.; Röse, U.S. De novo biosynthesis of defense root exudates in response to Fusarium attack in barley. New Phytol. 2010, 185, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Vukovic´, R.; Bauer, N.; Curković-Perica, M. Genetic elicitation by inducible expression of b-cryptogein stimulates secretion of phenolics from coleus blumei hairy roots. Plant Sci. 2013, 199, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Wurst, S.; Wagenaar, R.; Biere, A.; van der Putten, W.H. Microorganisms and nematodes increase levels of secondary metabolites in roots and root exudates of Plantago lanceolata. Plant Soil 2010, 329, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Badri, D.V.; Chaparro, J.M.; Zhang, R.; Shen, Q.; Vivanco, J.M. Application of natural blends of phytochemicals derived from the root exudates of Arabidopsis to the soil reveal that phenolic-related compounds predominantly modulate the soil microbiome. J. Biol. Chem. 2013, 288, 4502–4512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, H.W.; Tschaplinski, T.J.; Wang, L.; Glazebrook, J.; Greenberg, J.T. Priming in systemic plant immunity. Science 2009, 324, 89–91. [Google Scholar] [CrossRef]
- Neumann, G.; Bott, S.; Ohler, M.A.; Mock, H.P.; Lippmann, R.; Grosch, R.; Smalla, K. Root exudation and root development of lettuce (Lactuca sativa L.cv. Tizian) as affected by different soils. Front. Microbiol. 2014, 5, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, S.L.; Nicolaisen, M. National potato production and the powdery scab situation in Denmark. In Proceedings of the First European Powdery Scab Workshop; Merz, U., Lees, A.K., Eds.; SAC: Aberdeen, Scotland, 2000; p. 13. Available online: http://www.spongospora.ethz.ch/EUworkshop/proceedings.htm (accessed on 20 June 2019).
- Shakoor, M.A.; Ahmad, M.; Shahid, M.R.; Hafiz, W.A.; Ahsan, M.; Mumtaz, H. Genetic resistance and correlation between black scurf and powdery scab disease of potato. Int. J. Adv. 2015, 2, 106–110. [Google Scholar]
- EL-Naggar, M.A.; AL-Rajhi, A.M.; Abdelkareem, E.M.; Mahmoud, M.A.; Abdlgany, R.E. Biochemical defense mechanisms in potato against stem canker and black scurf disease. Asian J. Plant Sci. 2013, 12, 165–170. [Google Scholar] [CrossRef]
- Viant, M.R.; Kurland, I.J.; Jones, M.R.; Dunn, W.B. How close are we to complete annotation of metabolomes? Curr. Opin. Chem. Biol. 2017, 36, 64–69. [Google Scholar] [CrossRef]

| Cultivar | Root Infection Severity | |
|---|---|---|
| s-BP 1 | 3.6 * | a |
| s-Up-to-date | 3.0 | ab |
| t-Fianna | 2.6 | ab |
| s-Avalanche | 2.4 | ab |
| t-Innovator | 2.0 | bc |
| s-Ronaldo | 2.0 | bc |
| s-Sifra | 2.0 | bc |
| t-Lanorma | 1.6 | bc |
| t-Mondial | 1.6 | bc |
| t-Valor | 0.8 | c |
| LSD | 1.5 | |
| Standard error | 0.6 | |
| Putative Identity | ESI * | m/z | Rt (min) | Roots | Root Exudates | Tolerant Inoculated | Tolerant Un-Inoculated | Susceptible Inoculated | Susceptible Un-Inoculated |
|---|---|---|---|---|---|---|---|---|---|
| Sugars | |||||||||
| D-mannitol | + | 181.0716 | 1.94 | + | + | ||||
| D-ribulose | − | 149.0454 | 2.34 | + | + | ||||
| Heterodendrin | + | 262.1291 | 2.57 | + | + | + | + | + | + |
| Linamarin | + | 248.1132 | 2.01 | + | + | ||||
| Nystose | − | 341.1081 | 2.33 | + | + | + | |||
| Sugar alcohol | |||||||||
| Lactitol dehydrate | − | 343.1237 | 2.15 | + | + | ||||
| Amino acids | |||||||||
| Arginine | − | 175.1197 | 1.85 | + | + | + | + | ||
| 4-Aminobutanoic acid | − | 102.0556 | 2.17 | + | + | ||||
| Glutamine | − | 145.0617 | 1.85 | + | + | ||||
| Kinetin | + | 136.0623 | 2.58 | + | + | ||||
| Leucine | − | 130.0870 | 2.66 | + | + | + | + | + | |
| Methionine | − | 150.0603 | 2.76 | + | + | ||||
| Methionine sulfoxide | − | 166.0543 | 4.40 | + | + | ||||
| 5-Oxoproline | − | 130.0506 | 3.95 | + | + | ||||
| Phenylalanine | − | 166.0846 | 4.77 | + | + | ||||
| Proline | + | 116.0711 | 2.15 | + | + | + | + | ||
| Pyrroline 5-carboxylate | + | 130.0504 | 1.84 | + | + | ||||
| Stachydrine | + | 144.1025 | 2.63 | + | + | + | |||
| Tryptophan | − | 203.0820 | 5.55 | + | + | + | + | ||
| Valine | − | 118.0869 | 2.82 | + | + | + | + | ||
| Fatty acids | |||||||||
| (6Z)-6-Decosenamide | + | 338.3443 | 6.22 | + | + | + | |||
| Linoleic acid amide | + | 280.2636 | 11.55 | + | + | + | |||
| Laestisaric acid | − | 295.2276 | 14.95 | + | + | ||||
| Octanoic acid | − | 145.1259 | 2.73 | + | + | ||||
| 16-Oxohexadecanoic acid | − | 269.2117 | 14.28 | + | + | ||||
| C16 sphinganine | + | 274.2741 | 10.41 | + | + | + | |||
| (11E)-9,10,13-Trihydroxy-11-Octadecenoic acid | + | 329.2329 | 10.98 | + | + | ||||
| Alkaloids | |||||||||
| Cyclopamine | + | 706.4505 | 8.09 | + | + | + | |||
| Dehydrosolasodine | + | 398.3254 | 9.93 | + | + | ||||
| Laurolitsine | + | 314.1389 | 8.91 | + | + | ||||
| Melicopicine | − | 328.1187 | 7.45 | + | + | + | |||
| Solanine | + | 722.4457 | 7.59 | + | + | ||||
| Solanidine | + | 398.3412 | 10.10 | + | + | + | + | + | |
| Solanidane | + | 383.6895 | 9.10 | + | + | ||||
| Solasodiene | + | 396.3255 | 10.31 | + | + | + | + | + | |
| Solasodine | + | 414.3361 | 8.53 | + | + | + | + | + | |
| Swainsonine | + | 174.1113 | 2.74 | + | + | ||||
| Trachelanthamidine | + | 142.1207 | 2.44 | + | + | + | + | ||
| Veratramine | + | 410.3049 | 9.00 | + | + | ||||
| Tomatidine | + | 416.3519 | 11.28 | + | + | + | |||
| Organic acids | |||||||||
| Azelaic acid | − | 187.0971 | 8.26 | + | + | + | + | ||
| Citric acid | − | 191.0194 | 2.58 | + | + | ||||
| Erythronic acid | + | 136.1032 | 2.72 | + | + | ||||
| Phenolics | |||||||||
| p-coumaric acid | + | 165.0557 | 2.91 | + | + | ||||
| 6-Hydroxymellein | + | 193.0501 | 7.63 | + | + | ||||
| Quinic Acid | − | 191.0536 | 2.22 | + | + |
| Putative Identity | m/z | P-Value a | Fold-Change b |
|---|---|---|---|
| Sugars | |||
| D-mannitol | 181.0716 | 0.0426* | 2.43 |
| D-ribulose | 149.0454 | 0.0272* | 1.68 |
| Heterodendrin | 262.1291 | 0.0100** | 2.17 |
| Linamarin | 248.1132 | 0.0049** | 3.17 |
| Nystose | 341.1081 | 0.0229* | 0.62 |
| Amino acids | |||
| 4-Aminobutanoic acid | 102.0556 | 0.0086** | 3.58 |
| Glutamine | 145.0617 | 0.0141* | 0.74 |
| Kinetin | 136.0623 | 0.0198* | 0.63 |
| Phenylalanine | 166.0846 | 0.0331* | 1.58 |
| Proline | 116.0711 | 0.0393* | 1.67 |
| Tryptophan | 203.0820 | 0.0019** | 2.56 |
| Valine | 118.0869 | 0.0024* | 1.50 |
| Fatty acids | |||
| Linoleic acid amide | 280.2636 | 0.0315* | 1.75 |
| Laestisaric acid | 295.2276 | 0.0275* | 1.58 |
| C16 Sphinganine | 274.2741 | 0.0102** | 0.55 |
| Alkaloids | |||
| Solanidine | 398.3412 | 0.0003*** | 3.11 |
| Solanidane | 368.6895 | 0.0145* | 2.04 |
| Solasodiene | 396.3255 | 0.0092** | 2.87 |
| Trachelanthamidine | 142.1207 | 0.0473* | 0.41 |
| Veratramine | 410.3049 | 0.0091** | 4.15 |
| Tomatidine | 416.3519 | 0.0025** | 4.91 |
| Organic acids | |||
| Azelaic acid | 187.0971 | 0.0136* | 1.72 |
| Citric acid | 191.0194 | 0.0017** | 2.04 |
| Phenolics | |||
| Quinic Acid | 191.0536 | 0.0225* | 0.52 |
| Cultivar | Root Infection Response * |
|---|---|
| Fianna | Tolerant |
| Innovator | Tolerant |
| Lanorma | Tolerant |
| Mondial | Tolerant |
| Valor | Tolerant |
| Avalanche | Susceptible |
| Sifra | Susceptible |
| BP1 | Susceptible |
| Ronaldo | Susceptible |
| Up-to-date | Susceptible |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lekota, M.; Modisane, K.J.; Apostolides, Z.; van der Waals, J.E. Metabolomic Fingerprinting of Potato Cultivars Differing in Susceptibility to Spongospora subterranea f. sp. subterranea Root Infection. Int. J. Mol. Sci. 2020, 21, 3788. https://doi.org/10.3390/ijms21113788
Lekota M, Modisane KJ, Apostolides Z, van der Waals JE. Metabolomic Fingerprinting of Potato Cultivars Differing in Susceptibility to Spongospora subterranea f. sp. subterranea Root Infection. International Journal of Molecular Sciences. 2020; 21(11):3788. https://doi.org/10.3390/ijms21113788
Chicago/Turabian StyleLekota, Moleboheng, Kehumile J. Modisane, Zeno Apostolides, and Jacquie E. van der Waals. 2020. "Metabolomic Fingerprinting of Potato Cultivars Differing in Susceptibility to Spongospora subterranea f. sp. subterranea Root Infection" International Journal of Molecular Sciences 21, no. 11: 3788. https://doi.org/10.3390/ijms21113788
APA StyleLekota, M., Modisane, K. J., Apostolides, Z., & van der Waals, J. E. (2020). Metabolomic Fingerprinting of Potato Cultivars Differing in Susceptibility to Spongospora subterranea f. sp. subterranea Root Infection. International Journal of Molecular Sciences, 21(11), 3788. https://doi.org/10.3390/ijms21113788





