Participation of Somatic Stem Cells, Labeled by a Unique Antibody (A3) Recognizing Both N-glycan and Peptide, to Hair Follicle Cycle and Cutaneous Wound Healing in Rats
Abstract
1. Introduction
2. Results
2.1. Molecular Biological Analysis of A3-Recognizing Antigen
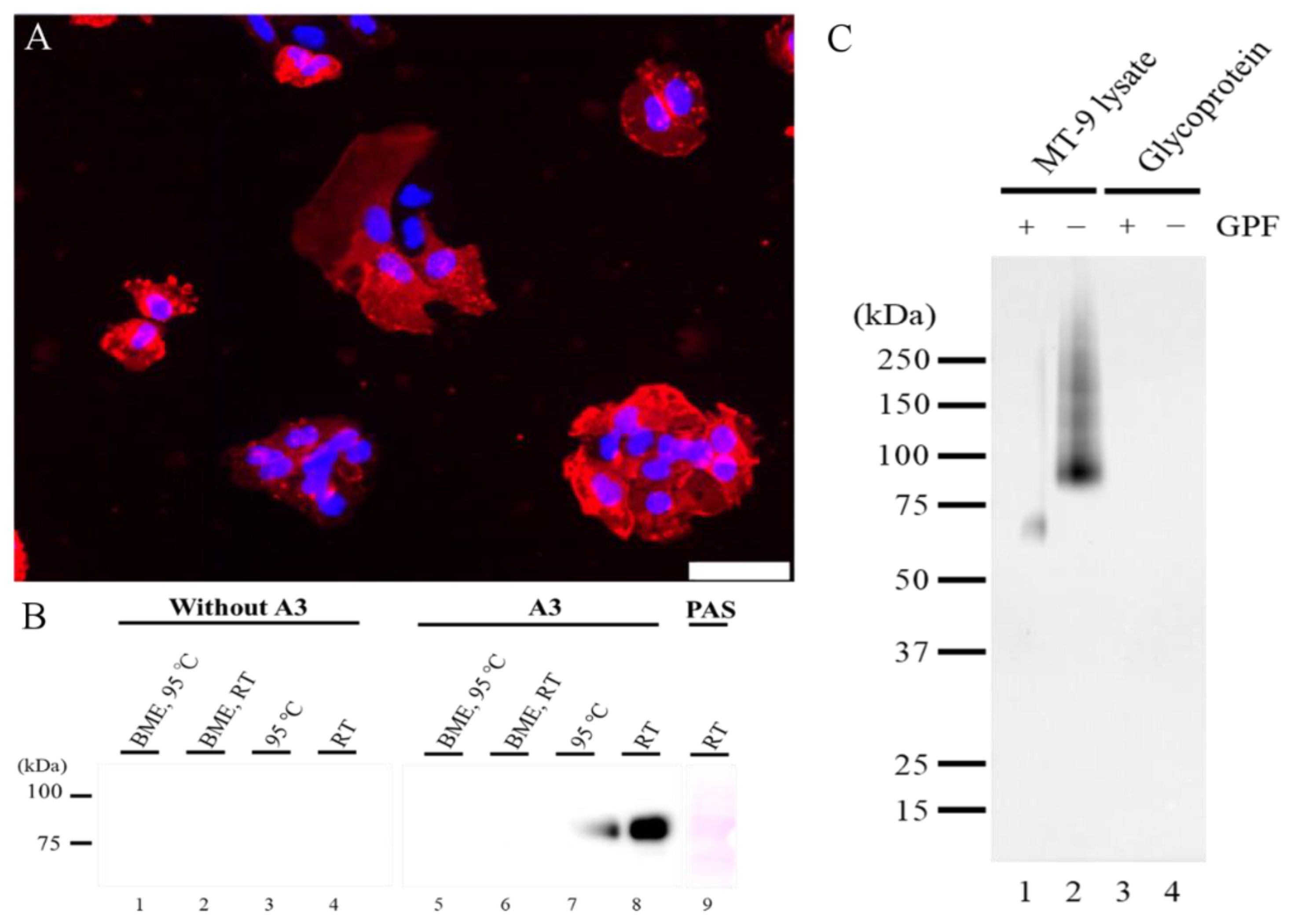
2.1.1. The Characteristic of A3-Recognizing Antigen on MT-9 Cells
2.1.2. A3-Labeled Cells in Bone Marrow of Adult Rats
2.1.3. A3 Immunoexpression in the Hair Cycle
2.1.4. Characteristics of A3-Labeled Cells in the Hair Cycle
2.2. Wound-Healing Process
2.2.1. Histology of the Wound-Healing Process
2.2.2. Distribution of A3-Labeled Cells in Cutaneous Wound Healing
2.2.3. Characteristics of A3-Labeled Epithelial Cells
2.2.4. Characteristics of A3-Labeled Mesenchymal Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Immunofluorescence of MT-9 Cells for A3
4.3. Isolation of A3 Antigen from Cultured MT-9 Cells
4.4. Western Blotting
4.5. Periodic Acid–Schiff (PAS) Stain
4.6. Glycosidase Analysis
4.7. Histological Analysis
4.7.1. Animals
4.7.2. Tissue Preparations
4.7.3. Immunohistochemistry and Double-Immunofluorescence
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMeX | Acetone-Methylbenzoate-Xylen |
| BME | 2-Mercaptoeyhanol |
| BPB | Bromophenol blue |
| CD | Cluster of differentiation |
| CK | Cytokeratin |
| DAB | 3,3′-Diaminobenzidin |
| DAPI | 4′,6-Diamino-2-phenylindole |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DP | Dermal papilla |
| ES cells | Embryonic stem cells |
| FBS | Fetal bovine serum |
| GPF | Glycopeptidase F |
| HP | Hair papilla |
| HS | Hair shaft |
| iPS cells | Induced pluripotent stem cells |
| Lgr6 | Leucine-rich repeat-containing G-protein coupled receptor 6 |
| MFH | Malignant fibrous histiocytoma |
| PAS | Periodic acid-Schiff |
| PBS | Phosphate buffered saline |
| PCNA | Proliferating cell nuclear antigen |
| PLP | Periodate-lysine-paraformaldehyde |
| PVDF | Polyvinylidene difluoride |
| PW | Post wounding |
| RECA | Rat endothelial cell antigen |
| RT | Room temperature |
| SDS | Sodium dodecyl sulfate |
| SMA | Smooth muscle actin |
| SSEA | Stage-specific-embryonic antigen |
References
- Hasehira, K.; Hirabayashi, J.; Tateno, H. Structural and quantitative evidence of α2–6-sialylated N-glycans as markers of the differentiation potential of human mesenchymal stem cells. Glycoconj. J. 2017, 34, 797–806. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kawabe, K.; Tateyama, D.; Toyoda, H.; Kawasaki, N.; Hashii, N.; Nakao, H.; Matsumoto, S.; Nonaka, M.; Matsumura, H.; Hirose, Y.; et al. A novel antibody for human induced pluripotent stem cells and embryonic stem cells recognizes a type of keratan sulfate lacking oversulfated structures. Glycobiology 2013, 23, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, D.; Yamate, J.; Tajima, T.; Tsukamoto, Y.; Yasui, H.; Kuwamura, M.; Kotani, T.; Sakuma, S. Distribution of cells labelled by a monoclonal antibody (A3) against a cloned cell line derived from a rat malignant fibrous histiocytoma. J. Comp. Pathol. 2000, 123, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Matushansky, I.; Charytonowicz, E.; Mills, J.; Siddiqi, S.; Hricik, T.; Cordon-Cardo, C. MFH classification: Differentiating undifferentiated pleomorphic sarcoma in the 21(st) century. Expert Rev. Anticancer Ther. 2009, 9, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Yamate, J.; Ogata, K.; Yuasa, T.; Kuwamura, M.; Takenaka, S.; Kumagai, D.; Itoh, K.; LaMarre, J. Adipogenic, osteogenic and myofibrogenic differentiations of a rat malignant fibrous histiocytoma (MFH)-derived cell line, and a relationship of MFH cells with embryonal mesenchymal, perivascular and bone marrow stem cells. Eur. J. Cancer 2007, 43, 2747–2756. [Google Scholar] [CrossRef] [PubMed]
- Kramann, R.; Schneider, R.K.; DiRocco, D.P.; Machado, F.; Fleig, S.; Bondzie, P.A.; Henderson, J.M.; Ebert, B.L.; Humphreys, B.D. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 2015, 16, 51–66. [Google Scholar] [CrossRef]
- Hosaka, K.; Yang, Y.; Seki, T.; Fischer, C.; Dubey, O.; Fredlund, E.; Hartman, J.; Religa, P.; Morikawa, H.; Ishii, Y.; et al. Pericyte–fibroblast transition promotes tumor growth and metastasis. Proc. Natl. Acad. Sci. USA 2016, 113, 5618–5627. [Google Scholar] [CrossRef]
- Ichikawa, C.; Izawa, T.; Juniantito, V.; Tanaka, M.; Hori, M.; Tanaka, K.; Takenaka, S.; Kuwamura, M.; Yamate, J. Rat hair follicle-constituting cells labeled by a newly-developed somatic stem cell-recognizing antibody: A possible marker of hair follicle development. Histol. Histopathol. 2013, 28, 257–268. [Google Scholar] [CrossRef]
- Ito, M.; Liu, Y.; Yang, Z.; Nguyen, J.; Liang, F.; Morris, R.J.; Cotsarelis, G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat. Med. 2005, 11, 1351–1354. [Google Scholar] [CrossRef]
- Roy, E.; Neufeld, Z.; Cerone, L.; Wong, H.Y.; Hodgson, S.; Livet, J.; Khosrotehrani, K. Bimodal behaviour of interfollicular epidermal progenitors regulated by hair follicle position and cycling. EMBO J. 2016, 35, 2658–2670. [Google Scholar] [CrossRef]
- Botchkarev, V.A.; Paus, R. Molecular biology of hair morphogenesis: Development and cycling. J. Exp. Zool. Part B 2003, 298, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Sellheyer, K.; Krahl, D. Cutaneous mesenchymal stem cells: Status of current knowledge, implications for dermatopathology. J. Cutan. Pathol. 2010, 37, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Dezawa, M. Muse cells provide the pluripotency of mesenchymal stem cells: Direct contribution of muse cells to tissue regeneration. Cell Transplant. 2016, 25, 849–861. [Google Scholar] [CrossRef]
- Juniantito, V.; Izawa, T.; Yuasa, T.; Ichikawa, C.; Yamamoto, E.; Kuwamura, M.; Yamate, J. Immunophenotypical analyses of myofibroblasts in rat excisional wound healing: Possible transdifferentiation of blood vessel pericytes and perifollicular dermal sheath cells into myofibroblasts. Histol. Histopathol. 2012, 27, 515–527. [Google Scholar] [CrossRef]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Galli, A.; Bochaton-Piallat, M.L.; Gabbiani, G. The myofibroblast: One function, multiple origins. Am. J. Pathol. 2007, 170, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Gabbiani, G. Fibrosis: Recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol. Rep. 2010, 2, 78. [Google Scholar] [CrossRef]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmouliere, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Kotera, T.; Katou-Ichikawa, C.; Tennakoon, A.H.; Tanaka, M.; Tanaka, N.; Izawa, T.; Kuwamura, M.; Yamate, J. Rat malignant fibrous histiocytoma (MFH)-derived cloned cell lines (MT-8 and MT-9) show different differentiation in mesenchymal stem cell lineage. Exp. Toxicol. Pathol. 2015, 67, 499–507. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, R.C.H.; Tredget, E.E. Concise review: Bone marrow-derived stem/progenitor cells in cutaneous repair and regeneration. Stem Cells 2010, 28, 905–915. [Google Scholar] [CrossRef]
- Nishina, H.; Katou-Ichikawa, C.; Kuramochi, M.; Izawa, T.; Kuwamura, M.; Yamate, J. Participation of somatic stem cells, recognized by a unique A3 antibody, in mucosal epithelial regeneration in Dextran Sulfate Sodium (DSS)-induced rat colonic lesions. Toxicol. Pathol. 2020. [Google Scholar] [CrossRef]
- Kane, N.M.; Meloni, M.; Spencer, H.L.; Craig, M.A.; Strehl, R.; Milligan, G.; Houslay, M.D.; Mountford, J.C.; Emanueli, C.; Baker, A.H. Derivation of endothelial cells from human embryonic stem cells by directed differentiation: Analysis of microRNA and angiogenesis in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1389–1397. [Google Scholar] [CrossRef]
- Lian, X.; Bao, X.; Al-Ahmad, A.; Liu, J.; Wu, Y.; Dong, W.; Dunn, K.K.; Shusta, E.V.; Palecek, S.P. Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling. Stem Cell Rep. 2014, 3, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Snippert, H.J.; Clevers, H. Tracking adult stem cells. EMBO Rep. 2011, 12, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Pasolli, H.A.; Fuchs, E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 2011, 144, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Fuchs, E. A family business: Stem cell progeny join the niche to regulate homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 103–114. [Google Scholar] [CrossRef]
- Purba, T.S.; Haslam, I.S.; Poblet, E.; Jimenez, F.; Gandarillas, A.; Izeta, A.; Paus, R. Human epithelial hair follicle stem cells and their progeny: Current state of knowledge, the widening gap in translational research and future challenges. Bioessays 2014, 36, 513–525. [Google Scholar] [CrossRef]
- Nishina, H.; Katou-Ichikawa, C.; Kuramochi, M.; Izawa, T.; Kuwamura, M.; Yamate, J. The localization and distribution of cells labeled by a somatic stem cell-recognizing antibody (A3) in rat colon development; possible presence of a new cell type forming the intestinal stem cell niche. J. Toxicol. Pathol. 2019, 32, 37–48. [Google Scholar] [CrossRef]
- Tobin, D.J.; Magerl, M.; Gunin, A.; Handijski, B.; Paus, R. Plasticity and cytokinetic dynamics of the hair follicle mesenchyme: Implications for hair growth control. J. Investig. Dermatol. 2003, 120, 895–904. [Google Scholar] [CrossRef][Green Version]
- Ito, M.; Cotsarelis, G. Is the hair follicle necessary for normal wound healing? J. Investig. Dermatol. 2008, 128, 1059–1061. [Google Scholar] [CrossRef]
- Vagnozzi, A.N.; Reiter, J.F.; Wong, S.Y. Hair follicle and interfollicular epidermal stem cells make varying contributions to wound regeneration. Cell Cycle 2015, 14, 3408–3417. [Google Scholar] [CrossRef]
- Hoffman, R.M. The pluripotency of hair follicle stem cells. Cell Cycle 2006, 5, 232–233. [Google Scholar] [CrossRef] [PubMed]
- Rompolas, P.; Mesa, K.R.; Greco, V. Spatial organization within a niche as a determinant of stem-cell fate. Nature 2013, 502, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Sidney, L.E.; Branch, M.J.; Dunphy, S.E.; Dua, H.S.; Hopkinson, A. Concise review: Evidence for CD34 as a common marker for diverse progenitors. Stem Cells 2014, 32, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Schepeler, T.; Page, M.E.; Jensen, K.B. Heterogeneity and plasticity of epidermal stem cells. Development 2014, 141, 2559–2567. [Google Scholar] [CrossRef]
- Amoh, Y.; Li, L.; Katsuoka, K.; Hoffman, R.M. Multipotent nestin-expressing hair follicle stem cells. J. Dermatol. 2009, 36, 1–9. [Google Scholar] [CrossRef]
- Trempus, C.; Morris, R.J.; Bortner, C.D.; Cotsarelis, G.; Faircloth, R.S.; Reece, J.M.; Tennant, R.W. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J. Investig. Dermatol. 2003, 120, 501–511. [Google Scholar] [CrossRef]
- Jahoda, C.A.; Whitehouse, C.J.; Reynolds, A.J.; Hole, N. Hair follicle dermal cells differentiate into adipogenic and osteogenic lineages. Exp. Dermatol. 2003, 12, 849–859. [Google Scholar] [CrossRef]
- Montiel-Eulefi, E.; Nery, A.A.; Rodrigues, L.C.; Sánchez, R.; Romero, F.; Ulrich, H. Neural differentiation of rat aorta pericyte cells. Cytom. Part A 2012, 81, 65–71. [Google Scholar] [CrossRef]
- Mercati, F.; Pascucci, L.; Ceccarelli, P.; Dall’Aglio, C.; Pedini, V.; Gargiulo, A.M. Expression of mesenchymal stem cell marker CD90 on dermal sheath cells of the anagen hair follicle in canine species. Eur. J. Histochem. 2009, 53, 159–166. [Google Scholar] [CrossRef][Green Version]
- Pervin, M.; Golbar, H.M.; Bondoc, A.; Izawa, T.; Kuwamura, M.; Yamate, J. Transient effects of empty liposomes on hepatic macrophage populations in rats. J. Toxicol. Pathol. 2016, 29, 139–144. [Google Scholar] [CrossRef]










| PrimaryAntibody | Clone | Poly/Mono | Dilution | Source of Antibody | Specificity |
|---|---|---|---|---|---|
| A3 | A3 | Mouse mono | 1000 | TransGenic Inc.,Hyogo, JPN | - |
| α-SMA | 1A4 | Mouse mono | 500 | Dako, Carpinteria, CA, USA | Smooch musclecells, myofibroblasts |
| Cytokeratin 6 | LHK6B | Mouse mono | 200 | Thermo Fisher Scientific Inc.,Waltham, MA, USA | Epithelial cell, inner root sheathcells |
| Cytokeratin 15 | LHK15 | Mouse mono | 200 | Thermo Fisher Scientific Inc.,Waltham, MA, USA | Follicular basalcells (stem cells) inthe bulge |
| Cytokeratin 19 | B170 | Mouse mono | 200 | Leica Biosystems, Eisfeld, GER | Follicular stem cell |
| CD34 | - | Goat poly | 200 | R&D Systems, Minneapolis, MN, USA | Stem cells |
| CD44 FITC | OX49 | Mouse mono | 500 | BD Pharmingen Inc. San Jose, CA, USA | Stem cells |
| CD73 | 5F/B9 | Mouse mono | 500 | BD Pharmingen Inc. San Jose, CA, USA | Immaturemesenchymal cells |
| CD90 Alexa Fluor 488 | OX-7 | Mouse mono | 500 | Bio-Rad Laboratories Inc., Hercules, CA, USA | Mesenchymal stemcells |
| CD105 Alexa Fluor 488 | SN6 | Mouse mono | 500 | Bio-Rad Laboratories Inc., Hercules, CA, USA | Mesenchymal stemcells |
| Lgr6 | EPR6874 | Rabbit mono | 200 | Abcam, Cambridge, UK | Stem cell in theisthmus |
| Nestin | 25 | Mouse mono | 500 | BD Pharmingen Inc. San Jose, CA, USA | Stem cells |
| RECA-1 labeled by Alexa Fluor 555 Mouse IgG1 labeling kit | RECA-1 | Mouse mono | 200 | Abcam, Cambridge, UK | Endothelial cells |
| PCNA | PC-10 | Mouse mono | 50,000 | Dako, Carpinteria, CA, USA | Proliferating cells |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katou-Ichikawa, C.; Nishina, H.; Tanaka, M.; Takenaka, S.; Izawa, T.; Kuwamura, M.; Yamate, J. Participation of Somatic Stem Cells, Labeled by a Unique Antibody (A3) Recognizing Both N-glycan and Peptide, to Hair Follicle Cycle and Cutaneous Wound Healing in Rats. Int. J. Mol. Sci. 2020, 21, 3806. https://doi.org/10.3390/ijms21113806
Katou-Ichikawa C, Nishina H, Tanaka M, Takenaka S, Izawa T, Kuwamura M, Yamate J. Participation of Somatic Stem Cells, Labeled by a Unique Antibody (A3) Recognizing Both N-glycan and Peptide, to Hair Follicle Cycle and Cutaneous Wound Healing in Rats. International Journal of Molecular Sciences. 2020; 21(11):3806. https://doi.org/10.3390/ijms21113806
Chicago/Turabian StyleKatou-Ichikawa, Chisa, Hironobu Nishina, Miyuu Tanaka, Shigeo Takenaka, Takeshi Izawa, Mitsuru Kuwamura, and Jyoji Yamate. 2020. "Participation of Somatic Stem Cells, Labeled by a Unique Antibody (A3) Recognizing Both N-glycan and Peptide, to Hair Follicle Cycle and Cutaneous Wound Healing in Rats" International Journal of Molecular Sciences 21, no. 11: 3806. https://doi.org/10.3390/ijms21113806
APA StyleKatou-Ichikawa, C., Nishina, H., Tanaka, M., Takenaka, S., Izawa, T., Kuwamura, M., & Yamate, J. (2020). Participation of Somatic Stem Cells, Labeled by a Unique Antibody (A3) Recognizing Both N-glycan and Peptide, to Hair Follicle Cycle and Cutaneous Wound Healing in Rats. International Journal of Molecular Sciences, 21(11), 3806. https://doi.org/10.3390/ijms21113806




