Impact of a Histone Deacetylase Inhibitor—Trichostatin A on Neurogenesis after Hypoxia-Ischemia in Immature Rats
Abstract
1. Introduction
2. Results
2.1. The Effect of TSA on the Acetylation of Histone 3 and Alpha-Tubulin after Hypoxia-Ischemia
2.2. Phenotypic Characterization of Proliferating Cells after Neonatal Hypoxic/Ischemia
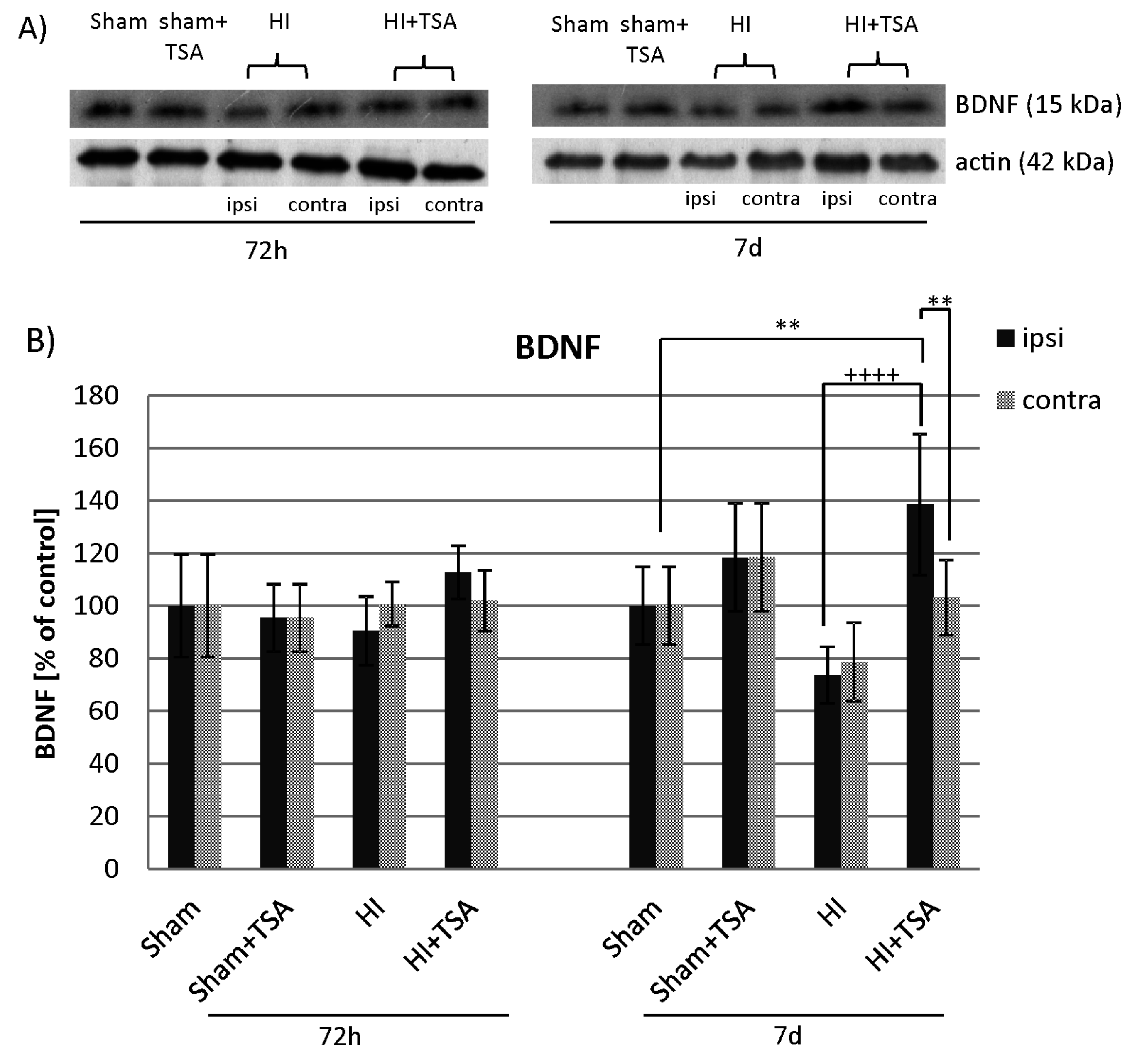
2.3. Contribution of Neurotrophic Growth Factors to TSA-Induced Neurogenesis
2.4. The Effect of TSA on TrkB Receptor
2.5. The Influence of TSA on Transcription Factor Phospho-CREB
3. Discussion
4. Material and Methods
4.1. Experimental Neonatal Hypoxia-Ischemia
4.2. Drug Administration and Bromodeoxyuridine Labeling
4.2.1. Drug Treatment
4.2.2. Bromodeoxyuridine Labeling
4.3. Tissue Preparation
4.4. Immunohistochemical Staining
4.5. Quantitative Polymerase Chain Reaction (Real-Time PCR)
4.6. Western Blot Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Acetyl-H3 | Acetylated Histone H3 |
| BDNF | Brain-derived neurotrophic factor |
| BrdU | 5-bromo-2’-deoxyuridine |
| CREB | cAMP response element-binding protein |
| CA1 | Cornu Ammonis region 1 |
| contra | Contralateral hemisphere (not injured, hypoxic only) |
| DCX | Neuronal migration protein doublecortin |
| GDNF | Glial cell line-derived neurotrophic factor |
| HDACis | Histone deacetylase inhibitors |
| HDACs | Histone deacetylases |
| HI | Hypoxia-ischemia |
| ipsi | Ipsilateral hemisphere (injured, hypoxic-ischemic) |
| LPS | Lipopolysaccharide |
| LSM | Laser Scanning Microscope |
| MBP | Myelin basic protein |
| NG2 | Nerve/glial antigen 2, Chondroitin sulfate proteoglycan 4 |
| NGF | Nerve growth factor |
| O4 | Late oligodendrocyte progenitor-specific marker |
| OPCs | Oligodendrocyte progenitor cells |
| PLP | Proteolipid protein |
| SB | Sodium butyrate |
| SGZ | Subgranular zone |
| Trk | Tropomyosin receptor kinase |
| TSA | Trichostatin A |
| VPA | Valproic acid |
References
- Allen, K.A.; Brandon, D.H. Hypoxic Ischemic Encephalopathy: Pathophysiology and Experimental Treatments. Newborn Infant Nurs. Rev. 2011, 11, 125–133. [Google Scholar] [CrossRef]
- Natarajan, G.; Laptook, A.; Shankaran, S. Therapeutic Hypothermia. Clin. Perinatol. 2018, 45, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Gunn, A.J.; Laptook, A.R.; Robertson, N.J.; Barks, J.; Thoresen, M.; Wassink, G.; Bennet, L. Therapeutic hypothermia translates from ancient history in to practice. Pediatr. Res. 2016, 81, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.; Mulholland, J.; McDonald, J.; Comi, A.M. Neurogenesis and neuronal commitment following ischemia in a new mouse model for neonatal stroke. Brain Res. 2008, 1208, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.; Plane, J.M.; Parent, J.M.; Silverstein, F.S. Hypoxic-Ischemic Injury Stimulates Subventricular Zone Proliferation and Neurogenesis in the Neonatal Rat. Pediatr. Res. 2005, 58, 600–606. [Google Scholar] [CrossRef]
- Eckle, T.; Eltzschig, H.K. Toll-like receptor signaling during myocardial ischemia. Anesthesiology 2011, 114, 490–492. [Google Scholar] [CrossRef]
- Dell’Aversana, C.; Lepore, I.; Altucci, L. HDAC modulation and cell death in the clinic. Exp. Cell Res. 2012, 318, 1229–1244. [Google Scholar] [CrossRef]
- Formisano, L.; Guida, N.; Valsecchi, V.; Cantile, M.; Cuomo, O.; Vinciguerra, A.; Laudati, G.; Pignataro, G.; Sirabella, R.; Di Renzo, G.; et al. Sp3/REST/HDAC1/HDAC2 Complex Represses and Sp1/HIF-1/p300 Complex Activates ncx1 Gene Transcription, in Brain Ischemia and in Ischemic Brain Preconditioning, by Epigenetic Mechanism. J. Neurosci. 2015, 35, 7332–7348. [Google Scholar] [CrossRef]
- Schweizer, S.; Meisel, A.; Märschenz, S. Epigenetic mechanisms in cerebral ischemia. Br. J. Pharmacol. 2013, 33, 1335–1346. [Google Scholar] [CrossRef]
- Ziemka-Nalecz, M.; Zalewska, T. Neuroprotective effects of histone deacetylase inhibitors in brain ischemia. Acta Neurobiol. Exp. 2014, 74, 383–395. [Google Scholar]
- Covic, M.; Karaca, E.; Lie, D.C. Epigenetic regulation of neurogenesis in the adult hippocampus. Heredity 2010, 105, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Faraco, G.; Pancani, T.; Formentini, L.; Mascagni, P.; Fossati, G.; Leoni, F.; Moroni, F.; Chiarugi, A. Pharmacological Inhibition of Histone Deacetylases by Suberoylanilide Hydroxamic Acid Specifically Alters Gene Expression and Reduces Ischemic Injury in the Mouse Brain. Mol. Pharmacol. 2006, 70, 1876–1884. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Rowe, M.; Ren, M.; Hong, J.-S.; Chen, P.-S.; Chuang, D.-M. Histone Deacetylase Inhibitors Exhibit Anti-Inflammatory and Neuroprotective Effects in a Rat Permanent Ischemic Model of Stroke: Multiple Mechanisms of Action. J. Pharmacol. Exp. Ther. 2007, 321, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Leng, Y.; Jeong, M.; Leeds, P.R.; Chuang, D.-M. Valproic acid reduces brain damage induced by transient focal cerebral ischemia in rats: Potential roles of histone deacetylase inhibition and heat shock protein induction. J. Neurochem. 2004, 89, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Langley, B.; Brochier, C.; Rivieccio, M.A. Targeting Histone Deacetylases as a Multifaceted Approach to Treat the Diverse Outcomes of Stroke. Stroke 2009, 40, 2899–2905. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.S.; Chopp, M.; Kassis, H.; Jia, L.F.; Hozeska-Solgot, A.; Zhang, R.L.; Chen, C.; Cui, Y.S.; Zhang, Z.G. Valproic acid increases white matter repair and neurogenesis after stroke. Neuroscience 2012, 220, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Glozak, M.A.; Sengupta, N.; Zhang, X.; Seto, E. Acetylation and deacetylation of non-histone proteins. Gene 2005, 363, 15–23. [Google Scholar] [CrossRef]
- Kabakus, N.; Ay, I.; Aysun, S.; Söylemezoglu, F.; Ozcan, A.; Celasun, B. Protective effects of valproic acid against hypoxic-ischemic brain injury in neonatal rats. J. Child Neurol. 2005, 20, 582–587. [Google Scholar] [CrossRef]
- Sandner, G.; Host, L.; Angst, M.-J.; Guiberteau, T.; Guignard, B.; Zwiller, J. The HDAC Inhibitor Phenylbutyrate Reverses Effects of Neonatal Ventral Hippocampal Lesion in Rats. Front. Psychol. 2011, 1, 153. [Google Scholar] [CrossRef]
- Fleiss, B.; Nilsson, M.K.; Blomgren, K.; Mallard, C. Neuroprotection by the histone deacetylase inhibitor trichostatin A in a model of lipopolysaccharide-sensitised neonatal hypoxic-ischaemic brain injury. J. Neuroinflamm. 2012, 9, 70. [Google Scholar] [CrossRef]
- George, S.; Kadam, S.D.P.; Irving, N.D.; Markowitz, G.J.; Raja, S.; Kwan, A.; Tu, Y.; Chen, H.; Rohde, C.P.; Smith, D.R.P.; et al. Impact of trichostatin A and sodium valproate treatment on post-stroke neurogenesis and behavioral outcomes in immature mice. Front. Cell. Neurosci. 2013, 7, 123. [Google Scholar] [CrossRef]
- Ziemka-Nalecz, M.; Jaworska, J.; Sypecka, J.; Polowy, R.; Filipkowski, R.K.; Zalewska, T. Sodium Butyrate, a Histone Deacetylase Inhibitor, Exhibits Neuroprotective/Neurogenic Effects in a Rat Model of Neonatal Hypoxia-Ischemia. Mol. Neurobiol. 2016, 54, 5300–5318. [Google Scholar] [CrossRef]
- Jaworska, J.; Zalewska, T.; Sypecka, J.; Ziemka-Nalecz, M. Effect of the HDAC Inhibitor, Sodium Butyrate, on Neurogenesis in a Rat Model of Neonatal Hypoxia–Ischemia: Potential Mechanism of Action. Mol. Neurobiol. 2019, 56, 6341–6370. [Google Scholar] [CrossRef]
- Holtzman, D.M.; Sheldon, R.A.; Jaffe, W.; Cheng, Y.; Ferriero, D.M. Nerve growth factor protects the neonatal brain against hypoxic-ischemic injury. Ann. Neurol. 1996, 39, 114–122. [Google Scholar] [CrossRef]
- Schäbitz, W.-R.; Sommer, C.; Zoder, W.; Kiessling, M.; Schwaninger, M.; Schwab, S.; Finklestein, S.P. Intravenous Brain-Derived Neurotrophic Factor Reduces Infarct Size and Counterregulates Bax and Bcl-2 Expression after Temporary Focal Cerebral Ischemia. Stroke 2000, 31, 2212–2217. [Google Scholar] [CrossRef] [PubMed]
- Wu, D. Neuroprotection in experimental stroke with targeted neurotrophins. NeuroRX 2005, 2, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Wang, C.-P.; Cong, N.; Gu, Y.-Y.; Ma, R.; Chi, F.-L. Therapeutic value of nerve growth factor in promoting neural stem cell survival and differentiation and protecting against neuronal hearing loss. Mol. Cell. Biochem. 2017, 428, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Endres, M.; Meisel, A.; Biniszkiewicz, D.; Namura, S.; Prass, K.; Ruscher, K.; Lipski, A.; Jaenisch, R.; Moskowitz, M.A.; Dirnagl, U. DNA Methyltransferase Contributes to Delayed Ischemic Brain Injury. J. Neurosci. 2000, 20, 3175–3181. [Google Scholar] [CrossRef]
- Arvidsson, A.; Collin, T.; Kirik, D.; Kokaia, Z.; Lindvall, O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002, 8, 963–970. [Google Scholar] [CrossRef]
- Snyder, E.Y.; Park, K.I. Limitations in brain repair. Nat. Med. 2002, 8, 928–930. [Google Scholar] [CrossRef]
- Plane, J.M.; Liu, R.; Wang, T.-W.; Silverstein, F.S.; Parent, J.M. Neonatal hypoxic–ischemic injury increases forebrain subventricular zone neurogenesis in the mouse. Neurobiol. Dis. 2004, 16, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Madri, J.A. Modeling the neurovascular niche: Implications for recovery from CNS injury. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2009, 60, 95–104. [Google Scholar]
- Pathania, M.; Yan, L.D.; Bordey, A. A symphony of signals conducts early and late stages of adult neurogenesis. Neuropharmacology 2010, 58, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Van Velthoven, C.T.J.; Kavelaars, A.; Van Bel, F.; Heijnen, C.J. Mesenchymal stem cell transplantation changes the gene expression profile of the neonatal ischemic brain. Brain, Behav. Immun. 2011, 25, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Leeds, P.; Chuang, D.-M. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J. Neurochem. 2009, 110, 1226–1240. [Google Scholar] [CrossRef]
- Qiu, L.; Zhu, C.; Wang, X.; Xu, F.; Eriksson, P.S.; Nilsson, M.; Cooper-Kuhn, C.M.; Kuhn, H.-G.; Blomgren, K. Less Neurogenesis and Inflammation in the Immature than in the Juvenile Brain after Cerebral Hypoxia-Ischemia. Br. J. Pharmacol. 2006, 27, 785–794. [Google Scholar] [CrossRef]
- Scheepens, A.; Wassink, G.; Piersma, M.J.; Van De Berg, W.D.; Blanco, C.E. A delayed increase in hippocampal proliferation following global asphyxia in the neonatal rat. Dev. Brain Res. 2003, 142, 67–76. [Google Scholar] [CrossRef]
- Simon, C.; Götz, M.; Dimou, L. Progenitors in the adult cerebral cortex: Cell cycle properties and regulation by physiological stimuli and injury. Glia 2011, 59, 869–881. [Google Scholar] [CrossRef]
- Dai, X.; Lercher, L.D.; Clinton, P.M.; Du, Y.; Livingston, D.L.; Vieira, C.; Yang, L.; Shen, M.; Dreyfus, C.F. The Trophic Role of Oligodendrocytes in the Basal Forebrain. J. Neurosci. 2003, 23, 5846–5853. [Google Scholar] [CrossRef]
- Ubhi, K.; Rockenstein, E.; Mante, M.; Inglis, C.; Adame, A.; Patrick, C.; Whitney, K.; Masliah, E. Neurodegeneration in a transgenic mouse model of multiple system atrophy is associated with altered expression of oligodendroglial-derived neurotrophic factors. J. Neurosci. 2010, 30, 6236–6246. [Google Scholar] [CrossRef][Green Version]
- Joanna, S.; Sarnowska, A. Heterogeneity of local tissue microenvironment influences differentiation of oligodendroglial progenitors. Folia Neuropathol. 2013, 2, 103–110. [Google Scholar] [CrossRef]
- Ziemka-Nalecz, M.; Janowska, J.; Strojek, L.; Jaworska, J.; Zalewska, T.; Frontczak-Baniewicz, M.; Joanna, S. Impact of neonatal hypoxia-ischaemia on oligodendrocyte survival, maturation and myelinating potential. J. Cell. Mol. Med. 2017, 22, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Li, J.; Casaccia-Bonnefil, P. Histone modifications affect timing of oligodendrocyte progenitor differentiation in the developing rat brain. J. Cell Boil. 2005, 169, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.; Consiglio, A.; Ray, J.; Sawai, T.; D’Amour, K.A.; Gage, F.H. In Vivo Fate Analysis Reveals the Multipotent and Self-Renewal Capacities of Sox2+ Neural Stem Cells in the Adult Hippocampus. Cell Stem Cell 2007, 1, 515–528. [Google Scholar] [CrossRef]
- Kopach, O.; Rybachuk, O.; Krotov, V.; Kyryk, V.; Voitenko, N.V.; Pivneva, T. Maturation of neural stem cells and integration into hippocampal circuits—A functional study in an in situ model of cerebral ischemia. J. Cell Sci. 2018, 131, jcs210989. [Google Scholar] [CrossRef]
- Jaworska, J.; Ziemka-Nalecz, M.; Joanna, S.; Zalewska, T. The potential neuroprotective role of a histone deacetylase inhibitor, sodium butyrate, after neonatal hypoxia-ischemia. J. Neuroinflamm. 2017, 14, 34. [Google Scholar] [CrossRef]
- Langley, B.; Gensert, J.M.; Beal, M.F.; Ratan, R.R. Remodeling chromatin and stress resistance in the central nervous system: Histone deacetylase inhibitors as novel and broadly effective neuroprotective agents. Curr. Drug Target -CNS Neurol. Disord. 2005, 4, 41–50. [Google Scholar] [CrossRef]
- Marks, P.A.; Xu, W.-S. Histone deacetylase inhibitors: Potential in cancer therapy. J. Cell. Biochem. 2009, 107, 600–608. [Google Scholar] [CrossRef]
- Yam, P.S.; Dewar, D.; McCulloch, J. Axonal Injury Caused by Focal Cerebral Ischemia in the Rat. J. Neurotrauma 1998, 15, 441–450. [Google Scholar] [CrossRef]
- Northington, F.J.; Ferriero, N.M.; Martin, L.J. Neurodegeneration in the thalamus following neonatal hypoxia-ischemia is programmed cell death. Dev. Neurosci. 2001, 23, 186–191. [Google Scholar] [CrossRef]
- Fan, L.-W.; Pang, Y.; Lin, S.; Tien, L.-T.; Ma, T.; Rhodes, P.G.; Cai, Z. Minocycline reduces lipopolysaccharide-induced neurological dysfunction and brain injury in the neonatal rat. J. Neurosci. Res. 2005, 82, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Godena, V.K.; Brookes-Hocking, N.; Moller, A.; Shaw, G.; Oswald, M.; Sancho, R.M.; Miller, C.C.J.; Whitworth, A.J.; De Vos, K. Increasing microtubule acetylation rescues axonal transport and locomotor deficits caused by LRRK2 Roc-COR domain mutations. Nat. Commun. 2014, 5, 5245. [Google Scholar] [CrossRef] [PubMed]
- Rind, H.B.; Von Bartheld, C.S. Anterograde axonal transport of internalized GDNF in sensory and motor neurons. NeuroReport 2002, 13, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Pencea, V.; Bingaman, K.D.; Wiegand, S.J.; Luskin, M.B. Infusion of Brain-Derived Neurotrophic Factor into the Lateral Ventricle of the Adult Rat Leads to New Neurons in the Parenchyma of the Striatum, Septum, Thalamus, and Hypothalamus. J. Neurosci. 2001, 21, 6706–6717. [Google Scholar] [CrossRef]
- Benraiss, A.; Chmielnicki, E.; Lerner, K.; Roh, D.; Goldman, S.A. Adenoviral Brain-Derived Neurotrophic Factor Induces Both Neostriatal and Olfactory Neuronal Recruitment from Endogenous Progenitor Cells in the Adult Forebrain. J. Neurosci. 2001, 21, 6718–6731. [Google Scholar] [CrossRef]
- Nakatomi, H.; Kuriu, T.; Okabe, S.; Yamamoto, S.-I.; Hatano, O.; Kawahara, N.; Tamura, A.; Kirino, T.; Nakafuku, M. Regeneration of Hippocampal Pyramidal Neurons after Ischemic Brain Injury by Recruitment of Endogenous Neural Progenitors. Cell 2002, 110, 429–441. [Google Scholar] [CrossRef]
- Schäbitz, W.R.; Steigleder, T.; Cooper-Kuhn, C.M.; Schwab, S.; Sommer, C.; Schneider, A.; Kuhn, H.-G. Intravenous Brain-Derived Neurotrophic Factor Enhances Poststroke Sensorimotor Recovery and Stimulates Neurogenesis. Stroke 2007, 38, 2165–2172. [Google Scholar] [CrossRef]
- Chen, J.; Zacharek, A.; Zhang, C.; Jiang, H.; Li, Y.; Roberts, C.; Lü, M.; Kapke, A.; Chopp, M. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J. Neurosci. 2005, 25, 2366–2375. [Google Scholar] [CrossRef]
- Schneider, A.; Krüger, C.; Steigleder, T.; Weber, D.; Pitzer, C.; Laage, R.; Aronowski, J.; Maurer, M.; Gassler, N.; Mier, W.; et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J. Clin. Investig. 2005, 115, 2083–2098. [Google Scholar] [CrossRef]
- Denton, K.R.; Lei, L.; Grenier, J.; Rodionov, V.; Blackstone, C.; Li, X.-J. Loss of spastin function results in disease-specific axonal defects in human pluripotent stem cell-based models of hereditary spastic paraplegia. Stem Cells 2014, 32, 414–423. [Google Scholar] [CrossRef]
- Sorbara, C.D.; Wagner, N.E.; Ladwig, A.; Nikić-Spiegel, I.; Merkler, D.; Kleele, T.; Marinković, P.; Naumann, R.; Godinho, L.; Bareyre, F.M.; et al. Pervasive Axonal Transport Deficits in Multiple Sclerosis Models. Neuron 2014, 84, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Scardigli, R.; Capelli, P.; Vignone, M.; Brandi, R.; Ceci, M.; La Regina, F.; Piras, E.; Cintoli, S.; Berardi, N.; Capsoni, S.; et al. Neutralization of Nerve Growth Factor Impairs Proliferation and Differentiation of Adult Neural Progenitors in the Subventricular Zone. Stem Cells 2014, 32, 2516–2528. [Google Scholar] [CrossRef] [PubMed]
- Katsuragi, S.; Ikeda, T.; Date, I.; Shingo, T.; Yasuhara, T.; Mishima, K.; Aoo, N.; Harada, K.; Egashira, N.; Iwasaki, K.; et al. Implantation of encapsulated glial cell line-derived neurotrophic factor-secreting cells prevents long-lasting learning impairment following neonatal hypoxic-ischemic brain insult in rats. Am. J. Obstet. Gynecol. 2005, 192, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Liang, M.-H.; Marinova, Z.; Yahyavi, A.; Chuang, D.-M. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol. Psychiatry 2007, 14, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Duan, W.; Mattson, M.P. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. 2002, 82, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Chuang, D.-M. HDAC inhibitors mitigate ischemia-induced oligodendrocyte damage: Potential roles of oligodendrogenesis, VEGF, and anti-inflammation. Am. J. Transl. Res. 2014, 6, 206–223. [Google Scholar]
- Matsumoto, T.; Rauskolb, S.; Polack, M.; Klose, J.; Kolbeck, R.; Korte, M.; Barde, Y.-A. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat. Neurosci. 2008, 11, 131–133. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Lau, L.; Liu, S.H.; Wei, J.S.; Lu, Y.M. Activation of cAMP-response-element-binding protein (CREB) after focal cerebral ischemia stimulates neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. USA 2004, 101, 9453–9457. [Google Scholar] [CrossRef]
- Barnabé-Heider, F.; Miller, F.D. Endogenously Produced Neurotrophins Regulate Survival and Differentiation of Cortical Progenitors via Distinct Signaling Pathways. J. Neurosci. 2003, 23, 5149–5160. [Google Scholar] [CrossRef]
- Sairanen, M.; Lucas, G.; Ernfors, P.; Castrén, M.L.; Castren, E. Brain-Derived Neurotrophic Factor and Antidepressant Drugs Have Different But Coordinated Effects on Neuronal Turnover, Proliferation, and Survival in the Adult Dentate Gyrus. J. Neurosci. 2005, 25, 1089–1094. [Google Scholar] [CrossRef]
- Hu, B.R.; Fux, C.; Martone, M.; Zivin, J.; Ellisman, M.H. Persistent phosphorylation of cyclic amp responsive element-binding protein and activating transcription factor-2 transcription factors following transient cerebral ischemia in rat brain. Neuroscience 1999, 89, 437–452. [Google Scholar] [CrossRef]
- Tanaka, K.; Nogawa, S.; Nagata, E.; Suzuki, S.; Dembo, T.; Kosakai, A.; Fukuuchi, Y. Effects of blockade of voltage-sensitive Ca(2+)/Na(+) channels by a novel phenylpyrimidine derivative, NS-7, on CREB phosphorylation in focal cerebral ischemia in the rat. Brain Res. 2000, 873, 83–93. [Google Scholar] [CrossRef]
- Mabuchi, T.; Kitagawa, K.; Kuwabara, K.; Takasawa, K.; Ohtsuki, T.; Xia, Z.; Storm, D.; Yanagihara, T.; Hori, M.; Matsumoto, M. Phosphorylation of cAMP Response Element-Binding Protein in Hippocampal Neurons as a Protective Response after Exposure to Glutamate In Vitro and Ischemia In Vivo. J. Neurosci. 2001, 21, 9204–9213. [Google Scholar] [CrossRef]
- Rice, J.E.; Vannucci, R.C.; Brierley, J.B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 1981, 9, 131–141. [Google Scholar] [CrossRef] [PubMed]











| Forward Primer Sequence | Reverse Primer Sequence | Product Length (bp) | |
|---|---|---|---|
| BDNF | 5′-CGGCTGGTGCAGGAAAGCAA-3′ | 5′-TCAGGTCACACCTGGGGCTG-3′ | 136 |
| NGF | 5′-CCCGAATCCTGTAGAGAGTGG-3′ | 5′-GACAAAGGTGTGAGTCGTGG-3′ | 82 |
| GDNF | 5′-AAGGTCGCAGAGGCCAGAGG-3′ | 5′-TCTCGGCCGCTTCACAGGAA-3′ | 144 |
| SDHA | 5′-CCCTGAGCATTGCAGAATC-3′ | 5′-CATTTGCCTTAATCGGAGGA-3′ | 60 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zalewska, T.; Jaworska, J.; Sypecka, J.; Ziemka-Nalecz, M. Impact of a Histone Deacetylase Inhibitor—Trichostatin A on Neurogenesis after Hypoxia-Ischemia in Immature Rats. Int. J. Mol. Sci. 2020, 21, 3808. https://doi.org/10.3390/ijms21113808
Zalewska T, Jaworska J, Sypecka J, Ziemka-Nalecz M. Impact of a Histone Deacetylase Inhibitor—Trichostatin A on Neurogenesis after Hypoxia-Ischemia in Immature Rats. International Journal of Molecular Sciences. 2020; 21(11):3808. https://doi.org/10.3390/ijms21113808
Chicago/Turabian StyleZalewska, Teresa, Joanna Jaworska, Joanna Sypecka, and Malgorzata Ziemka-Nalecz. 2020. "Impact of a Histone Deacetylase Inhibitor—Trichostatin A on Neurogenesis after Hypoxia-Ischemia in Immature Rats" International Journal of Molecular Sciences 21, no. 11: 3808. https://doi.org/10.3390/ijms21113808
APA StyleZalewska, T., Jaworska, J., Sypecka, J., & Ziemka-Nalecz, M. (2020). Impact of a Histone Deacetylase Inhibitor—Trichostatin A on Neurogenesis after Hypoxia-Ischemia in Immature Rats. International Journal of Molecular Sciences, 21(11), 3808. https://doi.org/10.3390/ijms21113808





