TLR2, TLR4 and TLR10 Shape the Cytokine and Chemokine Release of H. pylori-Infected Human DCs
Abstract
1. Introduction
2. Results
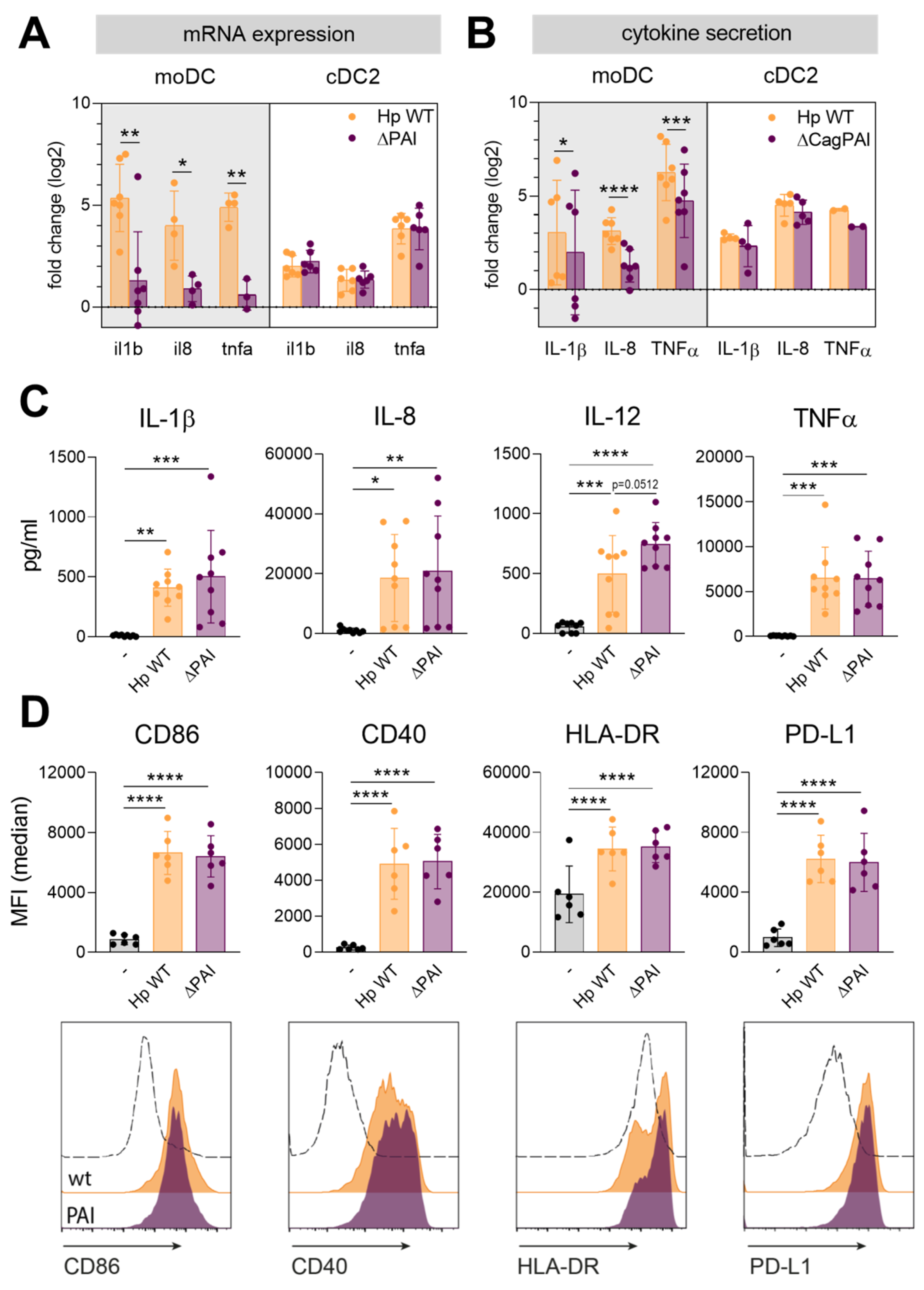
2.1. The Type IV Secretion System Plays Only a Minor Role during Infection of Human CD1c+ Conventional DCs (cDC2s) by H. pylori
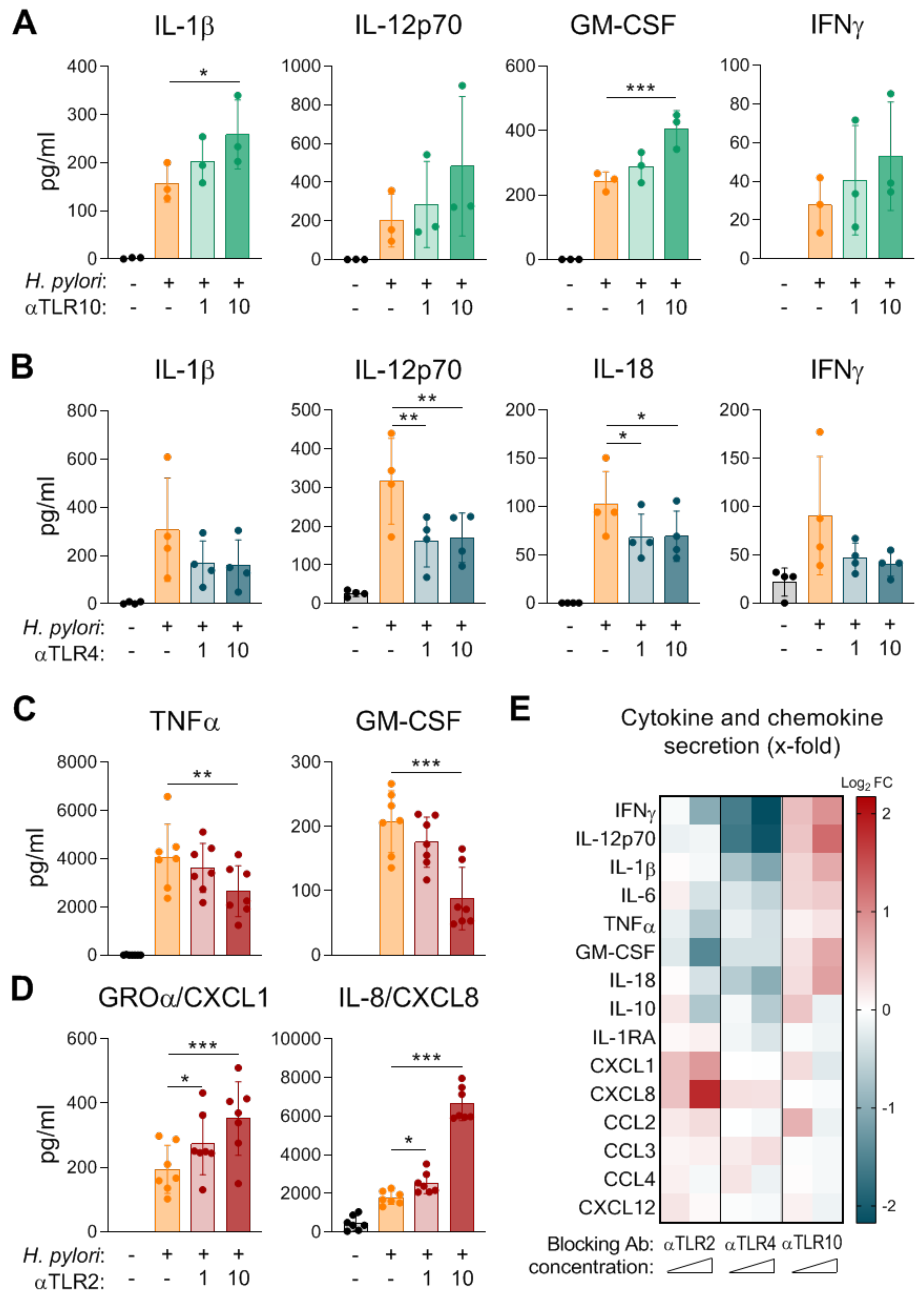
2.2. Contribution of Toll-Like Receptors (TLRs) to H. pylori-Induced Cytokine and Chemokine Secretion by Human cDC2s
3. Discussion
4. Materials and Methods
4.1. Isolation of Primary CD1c+ Dendritic Cells (cDC2s)
4.2. Generation of moDCs
4.3. Bacterial Culture and Infection Experiments
4.4. Flow Cytometry
4.5. Multiplex Assay
4.6. ELISA
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CagPAI | Cag pathogenicity island |
| CCL | CC chemokine ligand |
| CD | Cluster of differentiation |
| cDC | Conventional dendritic cell |
| CXCL | CXC-motif ligand |
| GM-CSF | Granulocyte macrophage stimulating factor |
| H. pylori | Helicobacter pylori |
| IL | Interleukin |
| LPS | Lipopolysaccharide |
| PD-L1 | Programmed-death ligand 1 |
| TNF | Tumor necrosis factor |
| TLR | Toll.like receptor |
| T4SS | Type IV secretion system |
References
- Necchi, V.; Manca, R.; Ricci, V.; Solcia, E. Evidence for transepithelial dendritic cells in human H. pylori active gastritis. Helicobacter 2009, 14, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Sebrell, T.A.; Hashimi, M.; Sidar, B.; Wilkinson, R.A.; Kirpotina, L.; Quinn, M.T.; Malkoc, Z.; Taylor, P.J.; Wilking, J.N.; Bimczok, D. A Novel Gastric Spheroid Co-culture Model Reveals Chemokine-Dependent Recruitment of Human Dendritic Cells to the Gastric Epithelium. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 157–171.e3. [Google Scholar] [CrossRef] [PubMed]
- Arnold, I.C.; Zhang, X.; Artola-Boran, M.; Fallegger, A.; Sander, P.; Johansen, P.; Muller, A. BATF3-dependent dendritic cells drive both effector and regulatory T-cell responses in bacterially infected tissues. PLoS Pathog. 2019, 15, e1007866. [Google Scholar] [CrossRef] [PubMed]
- Khamri, W.; Walker, M.M.; Clark, P.; Atherton, J.C.; Thursz, M.R.; Bamford, K.B.; Lechler, R.I.; Lombardi, G. Helicobacter pylori stimulates dendritic cells to induce interleukin-17 expression from CD4+ T lymphocytes. Infect. Immun. 2010, 78, 845–853. [Google Scholar] [CrossRef]
- Mitchell, P.J.; Afzali, B.; Fazekasova, H.; Chen, D.; Ali, N.; Powell, N.; Lord, G.M.; Lechler, R.I.; Lombardi, G. Helicobacter pylori induces in-vivo expansion of human regulatory T cells through stimulating interleukin-1beta production by dendritic cells. Clin. Exp. Immunol. 2012, 170, 300–309. [Google Scholar] [CrossRef]
- Ansari, S.; Yamaoka, Y. Helicobacter pylori Virulence Factors Exploiting Gastric Colonization and its Pathogenicity. Toxins (Basel) 2019, 11, 677. [Google Scholar] [CrossRef]
- Noto, J.M.; Peek, R.M., Jr. The Helicobacter pylori cag Pathogenicity Island. Methods Mol. Biol. 2012, 921, 41–50. [Google Scholar]
- Sgouras, D.; Tegtmeyer, N.; Wessler, S. Activity and Functional Importance of Helicobacter pylori Virulence Factors. Adv. Exp. Med. Biol. 2019, 1149, 35–56. [Google Scholar]
- Guillemin, K.; Salama, N.R.; Tompkins, L.S.; Falkow, S. Cag pathogenicity island-specific responses of gastric epithelial cells to Helicobacter pylori infection. Proc. Natl. Acad. Sci. USA 2002, 99, 15136–15141. [Google Scholar] [CrossRef]
- Granot, T.; Senda, T.; Carpenter, D.J.; Matsuoka, N.; Weiner, J.; Gordon, C.L.; Miron, M.; Kumar, B.V.; Griesemer, A.; Ho, S.H.; et al. Dendritic Cells Display Subset and Tissue-Specific Maturation Dynamics over Human Life. Immunity 2017, 46, 504–515. [Google Scholar] [CrossRef]
- Schreibelt, G.; Tel, J.; Sliepen, K.H.; Benitez-Ribas, D.; Figdor, C.G.; Adema, G.J.; de Vries, I.J. Toll-like receptor expression and function in human dendritic cell subsets: Implications for dendritic cell-based anti-cancer immunotherapy. Cancer Immunol. Immunother. 2010, 59, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Cullen, T.W.; Giles, D.K.; Wolf, L.N.; Ecobichon, C.; Boneca, I.G.; Trent, M.S. Helicobacter pylori versus the host: Remodeling of the bacterial outer membrane is required for survival in the gastric mucosa. PLoS Pathog. 2011, 7, e1002454. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.; Ohnishi, T.; Muroi, M.; Tanamoto, K.; Fujii, N.; Amano, K. Highly-purified Helicobacter pylori LPS preparations induce weak inflammatory reactions and utilize Toll-like receptor 2 complex but not Toll-like receptor 4 complex. FEMS Immunol. Med. Microbiol. 2007, 51, 140–148. [Google Scholar] [CrossRef]
- Rad, R.; Brenner, L.; Krug, A.; Voland, P.; Mages, J.; Lang, R.; Schwendy, S.; Reindl, W.; Dossumbekova, A.; Ballhorn, W.; et al. Toll-like receptor-dependent activation of antigen-presenting cells affects adaptive immunity to Helicobacter pylori. Gastroenterology 2007, 133, 150–163.e3. [Google Scholar] [CrossRef]
- Ishihara, S.; Rumi, M.A.; Kadowaki, Y.; Ortega-Cava, C.F.; Yuki, T.; Yoshino, N.; Miyaoka, Y.; Kazumori, H.; Ishimura, N.; Amano, Y.; et al. Essential role of MD-2 in TLR4-dependent signaling during Helicobacter pylori-associated gastritis. J. Immunol. 2004, 173, 1406–1416. [Google Scholar] [CrossRef]
- Nagashima, H.; Iwatani, S.; Cruz, M.; Jimenez Abreu, J.A.; Uchida, T.; Mahachai, V.; Vilaichone, R.K.; Graham, D.Y.; Yamaoka, Y. Toll-like Receptor 10 in Helicobacter pylori Infection. J. Infect. Dis. 2015, 212, 1666–1676. [Google Scholar] [CrossRef]
- Smith, M.F., Jr.; Mitchell, A.; Li, G.; Ding, S.; Fitzmaurice, A.M.; Ryan, K.; Crowe, S.; Goldberg, J.B. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-kappa B activation and chemokine expression by epithelial cells. J. Biol. Chem. 2003, 278, 32552–32560. [Google Scholar] [CrossRef]
- Geissmann, F.; Manz, M.G.; Jung, S.; Sieweke, M.H.; Merad, M.; Ley, K. Development of monocytes, macrophages, and dendritic cells. Science 2010, 327, 656–661. [Google Scholar] [CrossRef]
- Altobelli, A.; Bauer, M.; Velez, K.; Cover, T.L.; Muller, A. Helicobacter pylori VacA Targets Myeloid Cells in the Gastric Lamina Propria To Promote Peripherally Induced Regulatory T-Cell Differentiation and Persistent Infection. MBio 2019, 10, e00261-19. [Google Scholar] [CrossRef]
- Engler, D.B.; Reuter, S.; van Wijck, Y.; Urban, S.; Kyburz, A.; Maxeiner, J.; Martin, H.; Yogev, N.; Waisman, A.; Gerhard, M.; et al. Effective treatment of allergic airway inflammation with Helicobacter pylori immunomodulators requires BATF3-dependent dendritic cells and IL-10. Proc. Natl. Acad. Sci. USA 2014, 111, 11810–11815. [Google Scholar] [CrossRef] [PubMed]
- Kabisch, R.; Semper, R.P.; Wustner, S.; Gerhard, M.; Mejias-Luque, R. Helicobacter pylori gamma-Glutamyltranspeptidase Induces Tolerogenic Human Dendritic Cells by Activation of Glutamate Receptors. J. Immunol. 2016, 196, 4246–4252. [Google Scholar] [CrossRef] [PubMed]
- Hoces de la Guardia, A.; Staedel, C.; Kaafarany, I.; Clement, A.; Roubaud Baudron, C.; Megraud, F.; Lehours, P. Inflammatory cytokine and microRNA responses of primary human dendritic cells cultured with Helicobacter pylori strains. Front. Microbiol. 2013, 4, 236. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Park, J.H.; Franchi, L.; Backert, S.; Nunez, G. The Cag pathogenicity island and interaction between TLR2/NOD2 and NLRP3 regulate IL-1beta production in Helicobacter pylori infected dendritic cells. Eur. J. Immunol. 2013, 43, 2650–2658. [Google Scholar] [CrossRef] [PubMed]
- Kumar Pachathundikandi, S.; Brandt, S.; Madassery, J.; Backert, S. Induction of TLR-2 and TLR-5 expression by Helicobacter pylori switches cagPAI-dependent signalling leading to the secretion of IL-8 and TNF-alpha. PLoS ONE 2011, 6, e19614. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Li, X.; Hess, N.J.; Guan, Y.; Tapping, R.I. TLR10 Is a Negative Regulator of Both MyD88-Dependent and -Independent TLR Signaling. J. Immunol. 2016, 196, 3834–3841. [Google Scholar] [CrossRef] [PubMed]
- Pachathundikandi, S.K.; Backert, S. Differential Expression of Interleukin 1beta During Helicobacter pylori Infection of Toll-like Receptor 2 (TLR2)- and TLR10-Expressing HEK293 Cell Lines. J. Infect. Dis. 2016, 214, 166–167. [Google Scholar] [CrossRef][Green Version]
- Ravishankar Ram, M.; Goh, K.L.; Leow, A.H.; Poh, B.H.; Loke, M.F.; Harrison, R.; Shankar, E.M.; Vadivelu, J. Polymorphisms at Locus 4p14 of Toll-Like Receptors TLR-1 and TLR-10 Confer Susceptibility to Gastric Carcinoma in Helicobacter pylori Infection. PLoS ONE 2015, 10, e0141865. [Google Scholar] [CrossRef]
- Tang, F.B.; Li, Z.X.; Wang, Y.M.; Zhang, L.; Ma, J.L.; Zhou, T.; Zhang, Y.; Gao, J.J.; Wu, S.; Yang, T.; et al. Toll-like receptor 1 and 10 polymorphisms, Helicobacter pylori susceptibility and risk of gastric lesions in a high-risk Chinese population. Infect. Genet. Evol. 2015, 31, 263–269. [Google Scholar] [CrossRef]
- Oosting, M.; Cheng, S.C.; Bolscher, J.M.; Vestering-Stenger, R.; Plantinga, T.S.; Verschueren, I.C.; Arts, P.; Garritsen, A.; van Eenennaam, H.; Sturm, P.; et al. Human TLR10 is an anti-inflammatory pattern-recognition receptor. Proc. Natl. Acad. Sci. USA 2014, 111, E4478–E4484. [Google Scholar] [CrossRef]
- Kabisch, R.; Mejias-Luque, R.; Gerhard, M.; Prinz, C. Involvement of Toll-like receptors on Helicobacter pylori-induced immunity. PLoS ONE 2014, 9, e104804. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Tao, L.; Jing, L.; Liu, D.; Hu, S.; Liu, W.; Zhou, N.; Xie, Y. Association of TLR4 and Treg in Helicobacter pylori Colonization and Inflammation in Mice. PLoS ONE 2016, 11, e0149629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, M.; Luther, J.; Kao, J.Y. Helicobacter pylori directs tolerogenic programming of dendritic cells. Gut Microbes 2010, 1, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.N.; Hartung, M.L.; Urban, S.; Kyburz, A.; Bahlmann, A.S.; Lind, J.; Backert, S.; Taube, C.; Muller, A. Helicobacter urease-induced activation of the TLR2/NLRP3/IL-18 axis protects against asthma. J. Clin. Investig. 2015, 125, 3297–3302. [Google Scholar] [CrossRef]
- Rad, R.; Ballhorn, W.; Voland, P.; Eisenacher, K.; Mages, J.; Rad, L.; Ferstl, R.; Lang, R.; Wagner, H.; Schmid, R.M.; et al. Extracellular and intracellular pattern recognition receptors cooperate in the recognition of Helicobacter pylori. Gastroenterology 2009, 136, 2247–2257. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, M.; El-Zataari, M.; Owyang, S.Y.; Eaton, K.A.; Liu, M.; Chang, Y.M.; Zou, W.; Kao, J.Y. TLR2 mediates Helicobacter pylori-induced tolerogenic immune response in mice. PLoS ONE 2013, 8, e74595. [Google Scholar] [CrossRef][Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neuper, T.; Frauenlob, T.; Sarajlic, M.; Posselt, G.; Wessler, S.; Horejs-Hoeck, J. TLR2, TLR4 and TLR10 Shape the Cytokine and Chemokine Release of H. pylori-Infected Human DCs. Int. J. Mol. Sci. 2020, 21, 3897. https://doi.org/10.3390/ijms21113897
Neuper T, Frauenlob T, Sarajlic M, Posselt G, Wessler S, Horejs-Hoeck J. TLR2, TLR4 and TLR10 Shape the Cytokine and Chemokine Release of H. pylori-Infected Human DCs. International Journal of Molecular Sciences. 2020; 21(11):3897. https://doi.org/10.3390/ijms21113897
Chicago/Turabian StyleNeuper, Theresa, Tobias Frauenlob, Muamera Sarajlic, Gernot Posselt, Silja Wessler, and Jutta Horejs-Hoeck. 2020. "TLR2, TLR4 and TLR10 Shape the Cytokine and Chemokine Release of H. pylori-Infected Human DCs" International Journal of Molecular Sciences 21, no. 11: 3897. https://doi.org/10.3390/ijms21113897
APA StyleNeuper, T., Frauenlob, T., Sarajlic, M., Posselt, G., Wessler, S., & Horejs-Hoeck, J. (2020). TLR2, TLR4 and TLR10 Shape the Cytokine and Chemokine Release of H. pylori-Infected Human DCs. International Journal of Molecular Sciences, 21(11), 3897. https://doi.org/10.3390/ijms21113897






