Abstract
Plant stress is a real dilemma; it puzzles plant biologists and is a global problem that negatively affects people’s daily lives. Of particular interest is salinity, because it represents one of the major water-related stress types. We aimed to determine the signals that guide the cellular-related events where various adaptation mechanisms cross-talk to cope with salinity-related water stress in plants. In an attempt to unravel these mechanisms and introduce cellular events in the right context, we expansively discussed how salt-related signals are sensed, with particular emphasis on aquaporins, nonselective cation channels (NSCCs), and glycosyl inositol phosphorylceramide (GIPC). We also elaborated on the critical role Ca2+, H+, and ROS in mediating signal transduction pathways associated with the response and tolerance to salt stress. In addition, the fragmentary results from the literature were compiled to develop a harmonized, informational, and contemplative model that is intended to improve our perception of these adaptative mechanisms and set a common platform for plant biologists to identify intriguing research questions in this area.
Keywords:
salinity; osmotic and ionic stress; salt-related signals; adaptive cellular responses; aquaporins; NSCCs; GIPC; FERONIA; Ca2+; H+; ROS 1. Review Scope: Signaling Signatures Shape the Consequences
Unlike animals that can exhibit the fight or flight response, plants are sessile organisms that have only one approach when encountering stress—adaptation. Plants constantly monitor an ever-changing environment, sense the incoming signals, and then correctly integrate and decode the signals to formulate a battery of signaling molecules that ultimately enable them to cope with stress. Water-related stress (e.g., drought and salinity) represents one of the major types of plant stressors, of which osmotic stress is considered a general component [1]. In the following sections, we focus on salinity, along with its ionic and osmotic components. We elaborate on the main elements of the term “signals on demand” by answering two questions: (1) how do plants sense salt-related signals? and (2) how are these signals correctly decoded and processed to formulate adaptive cellular responses?
2. Sensing the Upcoming Signals
2.1. Aquaporin Water Channels: Debatable Osmosensors
When it comes to plant–water relations, aquaporins (AQPs) play a central role in transport and adaptation processes. AQPs are intrinsic membrane proteins that channel not only H2O, but also a high diversity of small and noncharged substrates, such as H2O2, CO2, and urea [2,3]. Based on their cellular localization and functions, AQPs can be subdivided into at least four subfamilies, including the plasma membrane intrinsic proteins (PIPs) and the tonoplast intrinsic proteins (TIPs) [4]. Under water-related stress, the reduction in plant hydraulic conductivity is a common response mediated by AQPs, which have been suggested to act as osmosensors [5]. AQP gating depends on apoplastic/cellular water potential, but occurs at the cytoplasmic side by de/phosphorylation (and/or protonation, in the case of cellular acidosis), which, along with other downregulation processes (e.g., ubiquitination and internalization), have been reported under water-related stress states [6,7,8]. For example, NaCl treatment decreases phosphorylation of AtPIP2;1 and AtPIP2;2, which are among the most abundant AQPs in Arabidopsis roots [9], as well as AtPIP3, AtPIP2;5 AtPIP2;7 [10,11]. Further investigation using AtPIP2;1 showed that the Ser283 residue, which is required for guiding AtPIP2;1 to the plasma membrane, is the dephosphorylation site targeted by NaCl stress; hence, nonphosphorylated AtPIP2;1 intracellularly accumulates under increased salinity [12]. The inversion of water flux through the AQP aqueous filter (notably PIPs) under osmotic imbalance may represent the earliest osmosensing action (Figure 1 [1O]). In contrast to a steady-state condition, water-related stress raises extracellular osmolarity in conjunction with low apoplastic H2O potential. Therefore, an efflux of water out of the cell will dominate the influx process through AQP aqueous filters, leading to cytosolic osmotic disturbances and the loss of cell turgor pressure [13]. These intracellular changes, in turn, might be sensed by the plasma membrane (PM) itself and/or the cell cytoskeleton (mainly microtubules), which subsequently activate the mechanosensitive cation “Ca2+” channels (MSCCs) (Figure 1 [2O]) [14]. The open MSCCs increase the Ca2+ influx [15], which in turn can result in the gating of AQPs, either directly (per se) or indirectly, to prevent further water loss until osmotic readjustment is achieved (Figure 1 [2C→1]).
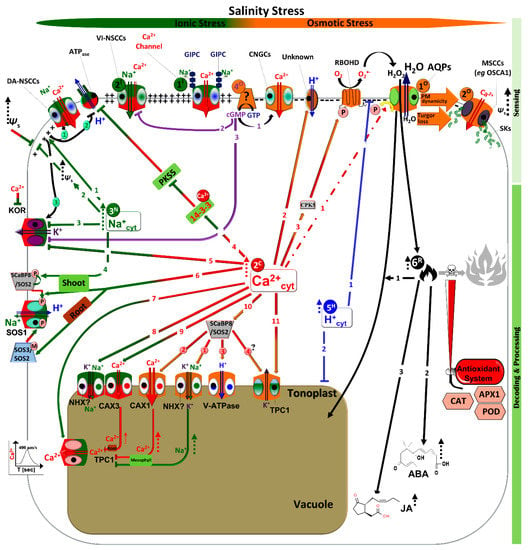
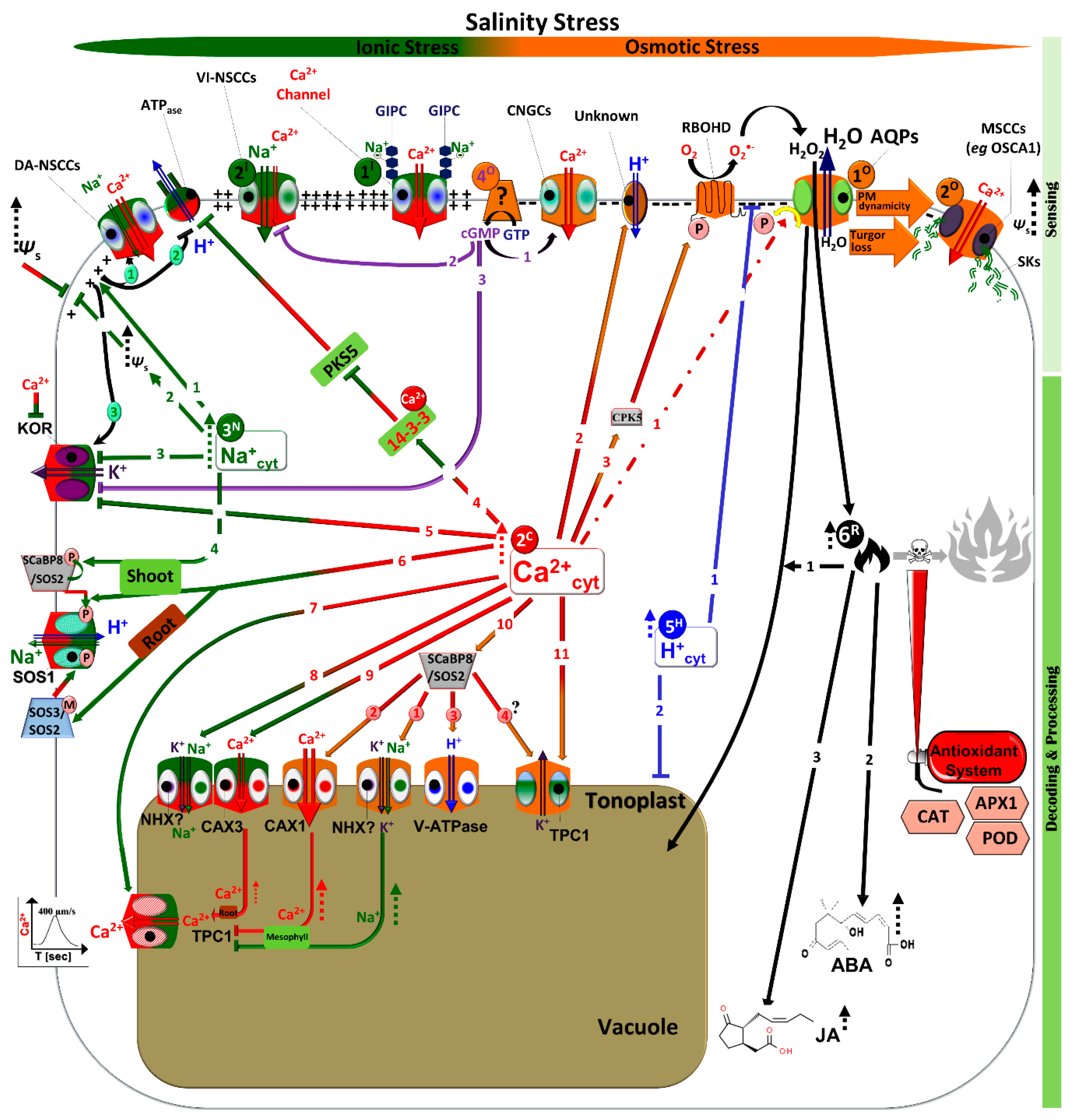
Figure 1.
Molecular events and respective responsive cellular organelles/compartments under salt- and osmotic-related stress signaling. Half dark green-half red colored bars represent ionic-related stress and the orange color refers to osmotic stress as a common water-related stress component. Under different water-related stress types, fluxes of Na+, Ca2+, H+, and ROS take place with different spatiotemporal signatures, channeling plant cells to either adaptation or cell death. Details are given in the discussion. Green-colored arrows indicate specificity to ionic events (3N), while half green-half red colored arrows indicate Ca2+-activated signals (2C) that are related to ion stress. On the other hand, Ca2+-activated signals (2C) under osmotic stress are represented by red-orange colored arrows. Tow-line colored arrows indicate activation of ion fluxes on the plasma membrane and tonoplast by salinity/osmotic stress, where tow-line arrows colored with green, red, and blue represent Na+, Ca2+, H+, and K+ flux, while tow-lines with dark blue colored arrows represent the H2O flux. Dashed arrows refer to a significant induction under stress. Green, red, blue, and black dashed arrows indicate activation of Ca2+, H+, and ROS signaling adaptation pathways, respectively, under water-related stress. Abbreviations: ROS: reactive oxygen species; Ψs osmotic potential; KOR: K+ outward rectifiers; NSCCs: nonselective cation channels; SOS: salt overly sensitive; NHX1: vacuolar Na+/H+ exchanger 1; TPC1: two pore channel 1 ; GIPC: Glycosyl Inositol Phospho Ceramides; ABA: abscisic acid; JA: jasmonic acid; CAT: catalase; POD: peroxidase; GST: Glutathione S-transferase; AQPs: aquaporins; CAXs: Ca2+/H+ exchangers; VI-NSCCs: voltage-insensitive- NSCCs; DA-NSCCs: depolarization-activated-NSCCs; SCaBP8: SOS3-like calcium binding protein 8; PKS5: SOS2-like protein kinase; V-ATPases: V-type H+-ATPases; OSCAs: reduced hyperosmolality-induced [Ca2+]i increase; MSCCs: mechanosensitive NSCCs; CNGCs: cyclic nucleotide gated NSCCs; cAMP: cyclic adenosine monophosphate; cGMP: cyclic guanosine monophosphate; RBOHD: respiratory burst oxidase homolog D; CPK5: calcium-dependent protein kinase 5.
On the contrary, the role of AQPs in stomatal behavior is pivotal for the regulation of the plant’s water status. For example, increasing the water activity (water efflux) of the guard cell via AQPs is a prerequisite for stomatal closure under water stress [4]. Unfortunately, owing to overlapping function of the PIP homologs, a direct link is still missing. Recently, Grondin et al. [16] showed that open stomata 1 (OST1; also known as Snf1-related protein kinase 2.6; SnRK2.6) can phosphorylate PIP2;1 at Ser121 in an ABA-dependent manner. Furthermore, transgenic Arabidopsis pip2;1-1 and pip2;1-2 mutants exhibit ABA-specific defects in their stomatal movement [16]. However, by using the same mutants, Wang et al. [17] found that the PIP2;1 mutation alone was insufficient to impair ABA, as well as CO2, and that the regulation of stomatal movements in the latter response was comparable to that in pip2;1 mutant and wild type leaves. In drought-stressed Vitis vinifera, stomatal closure occurs before the significant increase in foliar ABA. However, ABA induction under water-related stress activates two types of anion channels in a Ca2+-dependent manner, subsequently depolarizing the guard cell membranes [18]. The latter consequence inhibits H+-ATPase pump activation, resulting in the bioenergetic loss of membranes, activating K+ outward rectifiers (KORs), and thereby causing stomatal closure [19]. Interestingly, the involvement of ABA in stomatal regulation was shown to be absent in the early-diverging vascular plant lineages (lycophytes and ferns), and active stomatal control has evolved in seed plants to enhance water regulation and adaptation [20]. In light of these data, stomatal closure is suggested to be induced by passive hydraulic signals, but is maintained by ABA [21]. This might be another evidence of AQPs operating as osmosensors; however, unequivocal proof is still needed.
2.2. MSCCs Translate Mechanical Stress into the Ca2+ Signal
Nonselective cation channels (NSCCs) are a diverse group of integral membrane proteins that passively flux cations across the PM and other cellular endomembranes to their electrochemical gradients, which are tightly controlled by gating. Although the NSCC classification is problematic, they play a critical role in plant signaling and development, including during stress responses [22]. MSCCs, a class of mechanosensors, directly transduce mechanical stimuli (e.g., osmotic pressure) into the Ca2+ influx and are of particular interest.
For instance, the PM-localized two stretch-activated Ca2+-permeable MCA (Mid1-Complementing Activity) channels mediate osmotic stress responses and Ca2+ homeostasis in plants [15]. Although both MCA1 and MCA2 mediate PM Ca2+, they have overlapping and distinct spatial expression patterns and functions, as mca1 and mca2 null mutant roots exhibit root deficiency while penetrating into hard agar and taking up Ca2+, respectively [23,24]. Interestingly, the mca1;mca2 double mutant, which has a compromised phenotype, does not show any significant Ca2+ alternation under NaCl stress compared to the wild type [25]. In the same line, the PM mechanosensitive channel of small conductance (MscS)-like family msl4,5,6,9,10 quintuple mutant showed the same Ca2+ induction pattern [25], and no distinct phenotype was observed when challenged by a variety of stressors, including osmotic, NaCl, and dehydration stressors [26]. Therefore, other subclasses of MSCCs might act in concert with AQPs—and maybe with PM per se and/or the cell cytoskeleton—to handle common water-related osmotic stress (Figure 1 [2O]), thereby enhancing the Ca2+ influx (Figure 1 [2C→1]). Of particular interest are the newly identified MSCCs termed the OSCAs (reduced hyperosmolality-induced [Ca2+]i increase), in which the osmotically induced Ca2+ signaling is impaired in the guard cells and root cells of the knock-down osca1 mutant [27]. Therefore, OSCA1 is suggested to act as an osmosensor [27,28]. The emerging role of OSCAs and/or other MSCCs may help elucidate Ca2+ signaling under water-related stress [29].
One intriguing question is by which mechanism do these MSCCs open and close? Hamilton et al. [15] reviewed two speculated mechanisms. In the context of the mechanical force derived by water-related stress, another essential query involves the possibility that other cellular components are involved, such as the cytoskeleton, the cell wall (exoskeleton), and the PM–cell wall anchors, known as receptor-like kinases (RLKs) [30]. The dependency of AQP PIP activity on water potential differences highlights the importance of the cytoskeleton—in combination with PM dynamicity—in gating MSCCs, and hence activating the downstream Ca2+ signaling cascade (Figure 1 [1O and 2O]). Indeed, hypo- and hyperosmotic challenges promote cytoskeletal reorganization (actin “AFs” or microtubules “MTs”), which in turn results in modifying cell hydraulic conductivity [31,32,33]. In addition, the MTs-induced MSCC activity upstream of the Ca2+ influx is further supported, since the sole application of either a Ca2+ blocker (GdCl3) or a Ca2+ chelator (EGTA) does not affect the Lp value [30]. Notably, the cytoskeletal components are not only involved in cell signaling, but also in plant vesicle endocytosis and exocytosis [34]. Moreover, MTs are essential for plant cell wall formation by guiding cellulose synthase enzymes to the PM, the mechanism that is inhibited by salinity-induced MTs depolarization [35]. In contrast, two companion of cellulose synthase (CC1 and CC2) proteins promote MTs assembly, restarting cellulose synthesizing machinery and improving salt adaptation [36]. Recently, the CC1 was found to bind to MTs via its N-terminal hydrophobic domains [37]. Future research on AQPs, cytoskeleton, RLKs, and MSCCs and their levels of sensing the osmotic signal, may unravel these aspects.
2.3. GIPC Sphingolipids: Ionic (Na+) Sensors
Under increased salinity, and in contrast to other water stress types, the increase in water and soil ions (mainly NaCl) leads to salt-specific ionic stress that can disrupt cell wall integrity. Indeed, Na+ can displace pectin-bound Ca2+ and hence interrupt pectin cross-linking in vitro [38,39]. Interestingly, the malectin-like-domain RK FERONIA (FER) can physically interact with pectin via its extracellular domain, and in combination with its co-receptor glycosylphosphatidylinositol-anchored protein (GPI-AP) LORELEI-like GPI-AP1 (LLG1) can sense such NaCl disturbance [39]. In the same vein, the loss-of-function Arabidopsis fer mutants (fer-4) exhibit strong reduction in the late-stage of Ca2+ spikes compared to wildtype. This FER-dependent late-induction of Ca2+ activates cell wall reinforcement and maintains its integrity during growth recovery [39]. The essential role of RLKs FERONIA in salt adaptation is unquestionable. However, the late increase in cytosolic Ca2+ downstream of FER-dependent signals under salt stress makes one wonder about the potential role of FERONIA as an ionic sensor, particularly with knowing that rapid apoplastic alkalinization takes place within seconds under salinity (see Section 3.2) [40]. The extracellular alkalinization that is triggered by secreted peptide RALF (rapid alkalinization factor) regulates cell expansion in response to different developmental and stress stimuli [41,42]. Notably, RALF inhibits cell expansion of Arabidopsis primary root via interacting with FERONIA, and subsequently deactivates the PM H+-ATPase, resulting in apoplastic alkalinization [42]. Further research is still needed to clarify whether FERONIA acts downstream of RALF in response to salinity, as well as elucidating its up- and downstream signals.
Interestingly, by utilizing forward genetic screening, Jiang et al. [43] successfully isolated an Arabidopsis mutant, monocation-induced [Ca2+]i increases 1 (moca1), that is defective in salt-induced Ca2+ spikes. The authors identified MOCA1 as a glucuronosyltransferase for glycosyl inositol phosphorylceramide (GIPC) sphingolipids located on the plasma membrane. The authors also showed that MOCA1 is essential for NaCl-triggering depolarization of the cell-surface potential. In essence, Na+ binds to GIPCs, and facilitates Ca2+ influx that ultimately leads to Ca2+ spikes and activation of the downstream signals (Figure 1 [1I] and [2C→1]; see Section 3.1; [43]). Although the type of channel that fluxes Ca2+ has not been yet identified, the depolarization-activated DA-NSCCs could be plausible candidates. Indeed, subsequent rapid influx of Na+ into the cortical cytoplasm of plant roots occurs through the voltage-insensitive VI-NSCCs (Figure 1 [2I]), thereby depolarizing the cytosolic surface of the PM (Figure 1 [3N→1]) [44]. Therefore, further activation of the DA-NSCCs is established, which in turn enhances Ca2+ (as well as Na+ but to lesser extent) influx, and in combination with VI-NSCCs and MSCCs, shapes its cytosolic signature (Figure 1 [1I, 2I, 2O, 3N→1→1]) [22]. Therefore, the fluxing activity of different types of NSCCs play a crucial role in stress adaptation mechanisms. The PM of salt-sensitive plants are suggested to be predominantly equipped with late-activated, weakly selective hyperpolarization-activated NSCCs (HA-NSCCs), while the PM of salt-tolerant plants may contain more active NSCCs (DA-NSCCs and VI-NSCCs) [45,46]. For example, moderately salt-tolerant Vitis rupestris cells exhibit vigorous and rapid Na+ influx under saline conditions compared to salt-sensitive Vitis riparia cells [40,47]. This transiently rapid influx of Na+ ions could partially inhibit the K+ outward rectifiers (KORs) to maintain cellular K+/Na+ homeostasis (Figure 1 [3N→3]) [48]. Furthermore, it may act as a cheap osmolyte to counter salt-imposed osmotic stress (Figure 1 [3N→2]) [49].
On the contrary, although salt-adapting plants can derive benefits from initially influxing ions, overaccumulated Na+ in the cytosol is detrimental [50]. Treatment of grapes or tobacco BY-2 cells and Arabidopsis roots with high concentrations of NaCl results in increased reactive oxygen species (ROS) accumulation, leading to programmed cell death (PCD) [40,51,52]. Furthermore, salt-induced membrane depolarization inhibits PM ATPase (Figure 1 [3N→1→2]), but promotes KORs that subsequently efflux K+ out of the cell (Figure 1 [3N→1→3]) [43,53]. When the latter depletion is irrevocable, cells launch the PCD process [54]. Salt-tolerant plants therefore have to block additional Na+ influx by rapidly deactivating NSCCs (notably VI-NSCCs and DA-NSCCs) and remove excessive cytosolic concentrations. To achieve this, cyclic adenosine monophosphate (cAMP) or cyclic guanosine monophosphate (cGMP), which are produced via the mechanosensitive membrane-located cyclase, is induced within seconds by salt and osmotic stress, which enhances plant adaptation under increased salinity, but not during osmotic stress, via inhibiting VI-NSCC activity (Figure 1 [4O→2]) [55,56]. This rapid ionic-induced cyclic nucleotide monophosphate (cNMP) can activate the cyclic nucleotide gated (nonselective) cation channels (CNGCs) for further Ca2+ influxes, but it deactivates KOR (Figure 1 [4O→1 & 4O→3]) [48]. Subsequent signals of Ca2+ act upstream of the salt overly sensitive (SOS) pathway, which removes Na+ from the cytosol (Figure 1 [2C→6&9]) [44,45]. In addition, Oryza sativa phospholipase C (OsPLC1) was shown to trigger Ca2+ induction under NaCl, and the latter signal is substantial for controlling Na+ accumulation in leaf blades, hence improving salt adaptation [57].
Moreover, a plant regulates Na+ distribution at the systematic level through the high affinity K+ transporters (HKTs), with only one representing the gene AtHKT1;1 in the Arabidopsis genome [58]. The expression site of AtHKT1;1, as well as Oryza sativa OsHKT1;5 in the vasculature in roots and shoots, allows the plant to protect leaves against salinity via uploading K+ instead of Na+ in the xylem vessels [59,60]. The plant utilizes the negative effect of Na+-induced membrane depolarization as an adaption strategy. The specific overexpression of the AtHKT1;1 in the Arabidopsis mature root stele, and in the outer cells of the root in both Arabidopsis and rice, results in decreasing Na+ accumulation in the shoot and improved salinity tolerance [61,62]. Furthermore, the salinity sensitivity of durum wheat is attributed to its poor capability in excluding Na+ from the leaf when compared to the salt-tolerant wheat relative Triticum monococcum, which contains Nax2 (TmHKT1;5-A). Thus, introducing TmHKT1;5-A into the durum wheat cultivar Tamaroi was sufficient to significantly reduce Na+ contents in leaves while increasing grain yield by 25% under increased salinity compared to near-isogenic lines without Nax2 (TmHKT1;5-A) [63]. In contrast, both constitutive 35S-overexpression of AtHKT1;1 and athkt1 single mutant show Na+ hypersensitivity as more Na+ ions overaccumulate in shoots under NaCl stress [61,64]. However, in the presence of 3 mM Ca2+ concentration, (but not 1 mM), T-DNA insertion mutations in AtHKT1 (hkt1–1 and hkt1–2) result in reducing the intracellularly accumulated Na+ and enhancing salinity tolerance of sos3-1 mutant seedlings [65,66]. Interestingly, the Ca2+-dependent calmodulin-binding transcription activator 6 (CAMTA6) regulates the spatial expression of HKT1;1, as the Arabidopsis camta6 mutant showed restricted expression of HKT1;1 in the radicles. This mutant also showed enhanced salt adaptation during germination, but older seedlings were salt-sensitive [67]. These together point to the importance of the Ca2+ signature in harmonizing Na+ distribution on both cellular (SOS pathway) and systematic (HKT pathway) levels (Figure 1 [2C→6&9]) [60].
3. Decoding Signals to Understandable Cellular Language
3.1. Ca2+ Ion: a Cellular Central "Signaling-Maker”
Calcium plays a central role in all kingdoms of life, and is involved in nearly all biological processes. Despite such universality, signals of Ca2+ gain specificity from their signature patterns that are shaped by a powerful Ca2+-buffering capacity [68]. These Ca2+ signals can be perceived by adaptor/ Ca2+-modulated proteins, and are further propagated by releasing them from membrane-enclosed organelles, especially vacuoles [69]. In terms of plant–water relations, Ca2+ has a dual but paradoxical function. Submicromolar Ca2+ concentrations can phosphorylate AQP SoPIP2;1 at Ser274 by a PM-associated protein kinase, resulting in fluxing water under nonstressful conditions [8,70]; the resting cells exhibit nanomolar concentrations of Ca2+ in the cytosol (~100–200 nM) [71]. In contrast, the opposite occurs under water stress as active AQPs lead to cell turgor loss and plasmolysis. Therefore, preventing, or at least decreasing, water loss is the top priority of plants. Intriguingly, Ca2+ signals seem to be in/directly involved in AQP regulating mechanisms. As mentioned above, effluxing water out of the cell via PM AQPs might generate an endogenous turgor-driven mechanical stimulus that leads to cytoskeletal reorganization and/or affects PM dynamicity, which in turn activates the PM MSCCs-mediated Ca2+ influx. Indeed, under a different type of mechanical stimuli, for example, Ca2+cyt peaks within seconds, showing stimulus-specific signature patterns in Arabidopsis roots [72], and in mannitol-, sorbitol-, or salinity-stressed seedlings (Figure 1 [1I, 2O, 4O & 3N→1→1]) [25,73]. These studies show that pretreatment with a bona fide Ca2+ channel blocker inhibits not only Ca2+cyt induction, but also pH changes and extracellular ROS. A PM fluxing system thus first initiates Ca2+cyt increases where its signal governs ROS and H+ accumulation [37,72]. Indeed, Ca2+ spikes are accompanied by changes in cytosolic pH, and both Ca2+ and pH signatures act in harmony [74]. Furthermore, Ca2+ application and cytoplasmic acidification strongly reduce the hydraulic conductivity of isolated vesicles from Beta vulgaris in a dose-dependent manner [75]. Similar results were obtained when micromolar Ca2+ concentrations and low pH reduced PM AQPs in Arabidopsis suspension cells (Figure 1 [2C→1]) [76]. It remains unclear whether the enhancement of Ca2+ under water-related stress works directly on AQPs by promoting their dephosphorylation, as shown in isolated vesicles, or indirectly by increasing cytosolic transient changes in pH and/or extracellular ROS (Figure 1 [2C→2&3]). Further research is required to advance our knowledge about the exact role of water-induced Ca2+ on AQP gating.
Under increased salinity, however, a plant is further affected by Na+ ions. Therefore, it has to deploy parallel mechanisms to (1) arrest the additional Na+ influx, (2) remove it from the place of action (cytosol), as well as (3) reduce its side effects. The first task can be partially accomplished via cAMP or cGMP, while the Ca2+-dependent SOS pathway seems to be a central hub for Na+ exclusion and sequestration [77]. To this end, the rapidly salt-induced Ca2+cyt promotes the kinase activity of the Ser/Thr protein kinase SOS2 by two nonredundant Ca2+ binding proteins, SOS3 and SOS3 homolog SCaBP8 (also known as calcineurin B-like protein 10 CBL10; Figure 1 [2C→6&10]; for more details about Ca2+-dependent protein kinases, [78,79]). The SOS3 interacts with SOS2 at the FISL/NAF motif in the C-terminus, and because of the SOS3 myristoylation motif at the N terminus, the resultant SOS3/SOS2 complex is PM-localized [80,81,82,83]. SOS3/SOS2 subsequently phosphorylates the C-terminal autoinhibitory domain of the PM Na+/H+ antiporter SOS1, relieving its autoinhibition and hence unleashing its Na+-efflux activity (Figure 1 [2C→6-Root]) [84,85]. On the other hand, SCaBP8 interacts with, and recruits, SOS2 to activate an unknown Na+-sequestrating target at the tonoplast (Figure 1 [2C→10→1]) [86]. In fact, the vacuolar Na+, K+/H+ antiporter (NHX)-type exchangers NHX1 and 2 demonstrate equivalent Na+/H+ and K+/H+ exchange, which are essential for maintaining intracellular K+ and pH homeostasis, turgor regulation, and stomatal function [87,88]. Thus, it is evident that the tonoplast-localized NHXs are plausible candidates for SCaBP8/SOS2 phosphorylation [89], but this needs confirmation. The SCaBP8/SOS2 complex is also involved in fine-tuning Ca2+ signals upon salinity by initially interacting with and activating AtANN4 (a member of AtANNEXINs Ca2+-dependent membrane binding proteins) resulting in further Ca2+ influx. However, AtANN4 is lately phosphorylated and deactivated by SCaBP8/SOS2 [90]. Moreover, the vacuolar-localized AtCaM15 interacts with the AtNHX1 at the C-terminal hydrophilic region in a Ca2+- and pH-dependent manner, resulting in it modifying its cation selectivity [91].
Interestingly, as the SOS3 functions mainly in roots, plants deploy the preferentially-shoot expressed SCaBP8 to activate SOS1 in the shoot via recruiting the SOS2 to the PM (Figure 1 [2C→6-Shoot]). However, as the N-terminal myristoylation domain is missing, SCaBP8 localizes the PM by its N-terminal hydrophobic domain [92]. Moreover, the PM-bound SCaBP8/SOS2 complex is further stabilized, and the activity of SOS1 is enhanced by SOS2-dependent phosphorylation to its interacting SCaBP8 partner at the C-terminal Ser237 in a NaCl-dependent manner (Figure 1 [3N→4-Shoot]) [93]. Recent phosphoproteomic approaches in Arabidopsis roots revealed that the phosphorylation of SOS1 and NHX1 takes place within 45–120 min of salinity increase [11]. However, salt-stressed Arabidopsis suspension cells showed rapid changes within 5–15 min of phosphorylation activity of SOS1 compared to NHX2 (and to a lesser extend NHX1), which started after 15–60 min [10]. The fast response activity of SOS1 is further confirmed as the Na+ content inside salt-stressed V. rupestris cells is halted from 2–10 min, followed by a significant reduction [47]. These results highlight the activation priority of the SOS3/SOS2 pathway under increased salinity in root tissues before SCaBP8/SOS2 occurs in the shoots. Intriguingly, neither SOS1 nor NHX1 can bestow a salinity adaptation when genetically engineered, and only a few successful results have been obtained [45,94]. In other words, although restricting the cellular Na+ strategy via the SOS pathway is essential, it must be integrated with other resilience pathways at the same time to eliminate NaCl-related disturbances. For instance, a plant must restore the electrochemical potential across the PM and the tonoplast, controlling the powerful second messenger H+ (Figure 1 [2C→2 & 2C→10→3]). Interestingly, 14-3-3 proteins, the positive regulators of PM H+-ATPases, can also bind to SOS2 at the Ser294 residue under nonstressful conditions, resulting in a repression of its basal kinase activity. However, NaCl reduces this interaction, thereby activating the SOS pathway for salt adaptation [95]. The involvement of Ca2+-promoted pathways and their crosstalk with H+ will be discussed later in this article, along with the cellular levels of NaCl-promoted ROS that have to be fine-tuned and correctly integrated in the salinity-adaptive machinery.
Moreover, Ca2+ signals are also self-regulated, whether by further releasing Ca2+ from or sequestrating Ca2+ into intracellular organelles and the apoplast [96]. Of particular interest are the large central vacuoles that harbor different types of Ca2+-channels (e.g., TPC1), Ca2+/H+ exchangers (CAXs), and the Ca2+ pumps (autoinhibited Ca2+-ATPases; ACA4 and ACA11), which represent the major internal Ca2+ stores in mature plant cells [97]. The Arabidopsis TPC1/SV channel is a perplexing player in Ca2+ signaling [69]. This TPC1/SV transmembrane comprises two Shaker-like six-transmembrane (6-TM) domains connected by a cytosolic loop that contains two EF-hand Ca2+ binding domains, in addition to a voltage sensor [98,99,100]. Thus, TPC1/SV is Ca2+cyt-activated in a voltage-dependent manner, but inhibited by increased luminal Ca2+ (Figure 1 [2C→7]). In contrast, the fou2 mutant that carries a point mutation D454N of the TPC1 gene remains active under 100-fold accumulation of vacuolar Ca2+ [101,102]. Interestingly, fou2 exhibits a higher jasmonic acid (JA background that strongly increases upon wounding, as well as increased resistance to the fungus Botrytis cinerea [103]. This pronounced JA was suggested as unlikely to be attributed to TPC1/SV-mediated Ca2+ in fuo2 mesophyll cells, but rather due to its insensitivity to higher levels of luminal Ca2+ (via CAXs pathway, as discussed later) that enables K+ fluxes and cellular K+ homeostasis [101]. Furthermore, aequorin-expressing wild type, tpc1-2 knockout, and TPC1-overexpressing plants exhibit no difference in their Ca2+ signature pattern, or a Ca2+-dependent response under different stress types, including NaCl and mannitol-derived osmotic stress [104]. Thus, it is speculated that other vacuolar transporters, but not TPC1, mediate wounding-induced Ca2+ release through the tonoplast, finally resulting in JA overaccumulation [101]. However, using Arabidopsis plants expressing the cytoplasmic Ca2+ sensor YCNano-65, Choi et al. [105] showed that only localized NaCl application to Arabidopsis roots was able to generate Ca2+ waves at rates up to ~400 μm/s, which decreased by ∼25-fold in speed in the tpc1-2 mutant. The improvement in salinity adaptation in TPC1-overexpressing plants compared to wild types and tpc1-2 mutants suggest that the Ca2+wave/(TPC1/SV) elicits a rapid and systemic root-to-shoot signaling response in plants [105]. It is worth noting that although AtTPC1 is ubiquitously expressed, these salt-specific Ca2+ waves are primarily channeled in the cortical and endodermal layers, and require ROS-supporting signals that are generated via AtRBOHD in a Ca2+-dependent manner [105,106]. In contrast, SOS1 is preferentially expressed in xylem parenchyma cells of the roots and shoots, where AtHKT1;1 and OsHKT1;5 are also expressed [59,84]. Hence, Na+ routes are largely confined to the xylem vessels, unless partially unloaded and replaced by K+ ions via AtHKT1;1 and OsHKT1;5 [60]. Therefore, it seems that plants retain a Ca2+-discrete root-to-shoot route, thereby giving priority to the Ca2+ signal over Na+ and K+ ions arriving at distal parts under increased salinity [107]. On the other hand, it might help prevent the Na+ signal within the waves of Ca2+.
Plants have evolved different mechanisms of Ca2+cyt-sequestration/buffering that interplay with Ca2+cyt-propagation strategies to guarantee fine-tuning (on/off) of stimulus-induced Ca2+ signaling, thereby preventing cellular damage [108]. For example, elevated Ca2+cyt can deactivate the PM CNGCs via its interaction with Ca2+/CaM in a negative feedback loop [109]. Of paramount importance are the low affinity, high capacity vacuolar Ca2+ exchangers (CAXs) that effectively sequestrate Ca2+ into the vacuole subsequently after the Ca2+cyt burst. In Arabidopsis thaliana, CAX transporters comprise six members that are further subgrouped into type 1A (CAX1, 3, and 4) and type 1B (CAX2, 5, and 6) [97]. Of interest are CAX1 and its closest homolog CAX3, which differ in their regulation and transport capacity; here, CAX1 is the main Ca2+-transporter compared to the weak CAX3 transporter [110,111]. The starting point of the NaCl-induced Ca2+ wave at the roots draws attention to the pivotal roles of the CAXs in fine-tuning Ca2+-signal transduction and ion homeostasis under increased salinity [112]. In particular, CAX3 is predominantly expressed in the roots, and the cax3 mutant is saline-sensitive [108,113]. It seems that, under increasing salinity, CAX3 accumulates less Ca2+ inside the root vacuoles, which might not be able to efficiently impair TPC1 activity. It is important to note that the SOS1 is also preferentially expressed in the epidermal cells at the root tip where SOS3 is very strongly expressed, while generally also detected in the roots [84,92]. In contrast, AtNHX1 showed the expression pattern in nearly all tissues throughout plant development [114], but was not expressed at the root tip [84]. One can assume that much less Na+ is also accumulated inside root vacuoles, as high levels of luminal Na+, similar to luminal Ca2+, impair TPC1 activity [101,104]. Therefore, the simultaneous effects of SOS1, SOS3, and CAX3 allows Ca2+-activated root TPC1 to generate salt-specific Ca2+ waves from root to shoot (Figure 1 [2C→9→ root]). Once arriving via the cortical/endodermal route, Ca2+ signals prime the expressions of different NaCl-related genes in leaves, including JA-related genes [105]. However, JA is a dangerous switch, and sustained Ca2+ signals lead to harmful consequences [45,115]. Therefore, at this time, CAX1, an efficient Ca2+-transporter, responds. The CAX1 is the only member of the CAX genes that is highly expressed in Arabidopsis leaves, and, within the leaf, CAX1 accumulates at a 6-fold rate of the Ca2+ level in the mesophyll cell vacuoles compared to the epidermal cell vacuoles [110]. Interestingly, CAX1 and CAX3 can physically interact, forming a heteromeric CAX1–CAX3 protein complex with distinct functions when expressed in yeast and plant cells [116]. For instance, coexpression of both CAX1 and CAX3 in the yeast wild type enhances salt accumulation, while suppressing the salinity sensitivity of nhx1 yeast strains, compared to their single expressions. The authors speculated that CAX3 might occasionally act as a cofactor when interacting with CAX1, modifying transport activity under stress or hormonal treatment [116]. Intriguingly, this could be the case when ABA upregulates the expression of CAX3 in guard cells, where the expression of CAX1 is also detected [117]. Notably, CAX1 can also be activated via SOS2, which interacts with its hydrophilic N-terminal tail, although it remains unclear whether this interaction requires Ca2+ or not [118]. The high levels of Ca2+ at the luminal side are enough to inhibit the activity of TPC1 in the mesophyll cells (Figure 1 [ 2C→10→2→ mesophyll]) [98,99]. In addition, the arrival of Na+ ions to the shoot results in the accumulation of more Na+ ions inside the vacuoles of mature leaves via the SOS2/NHXs pathway, which further blocks TPC1 (Figure 1 [ 2C→10→1→ mesophyll]) [101]. This might explain why mesophyll cells or leaf discs derived from Arabidopsis wild type, tpc1-2 knockout, or TPC1-overexpressing plants fail to exhibit differences in their Ca2+ signature patterns under NaCl stress [101,104]. Furthermore, when reaching intolerable levels, a plant drops its high Na+-containing old leaves to protect young leaves, thereby rescuing the whole plant from salt toxicity [49]. For complexity, the pattern of Ca2+ storage in cereal monocots (barley, wheat, and Sorghum bicolor) is shifted into leaf epidermal cells instead of mesophyll cells [119].
In summary, Ca2+ is a central signal maker at the cellular level across the whole plant. Ca2+-signals are highly intricate and are involved not only in regulating Na+, but also with other crucial secondary messengers (H+ and ROS), as well as ABA and JA. In addition, Ca2+-signals are self-regulated to guarantee the specificity of action, and to prevent Ca2+-induced cell death. Future research is required to explain more about this master signal element in both dicots and monocots.
3.2. Proton (H+): the Power of Cellular Buffering
The proton is a powerful, multifaceted cellular plant component involved in growth and developmental processes [120,121]. Moreover, rapid (within seconds) H+ entry takes place downstream of Ca2+ signals, probably through PM nonselective cation/anion channels, and is suggested to act as a second messenger under stress (Figure 1 [2C→2]) [45,72,122,123]. Therefore, plants have evolved different strategies to utilize it in a timely manner, and at the same time prevent its undesirable influences. For instance, the proton-consuming system is a metabolic-based fine-tuning mechanism that counteracts protonogenic reactions to strictly control the intracellular pH [124]. Furthermore, plant membranes are armed by different types of proton pumps that generate proton-derived pH gradients and, as a result, energize membranes with the required driving force for ion and metabolite transport [123]. Of particular interest are the PM electrogenic proton pumps (P-type H+-ATPases) that act coordinately with the vacuolar V-type H+-ATPases (V-ATPases) and V-pyrophosphatases (V-PPases) to extrude H+ out of the cytosol [125,126]. Under nonstressful conditions, PM H+-ATPases (12 members of the Arabidopsis PM H+-ATPases, AHAs) acidify the apoplast, promoting cell growth [127]. However, the opposite occurs under stress conditions, during which plants undergo cellular disturbances. For example, the biosynthetic capacity of the cellular currency, ATP, via oxidative phosphorylation becomes limited under flooding-triggered hypoxia/anoxia. Subsequently, PM H+-ATPases turn inactive and the resultant intracellularly accumulated H+ acidifies the cytosol [128]. Moreover, hypoxia-induced intracellular H+ acts as a second messenger and is sensed by the highly-conserved histidine residue in loop D of PIPs (corresponding to His193 in spinach SoPIP2;1), resulting in protonation-induced PIP gating (Figure 1 [5H→1]) [7,129]. Indeed, cytosol acidosis strongly reduces the hydraulic conductivity of isolated vesicles from Beta vulgaris and Arabidopsis suspension cells [75,76]. It is worth noting that, while PM H+-ATPases can also be deactivated by different types of stress, the H+-promoting PIP protonation is flooding-exclusive, and there are no reports regarding other stresses.
Under high external pH values, the SOS2-like protein kinase PKS5 (CIPK11; SnRK3.22) was shown to negatively regulate the activity of AHA2 in a Ca2+ (SCaBP1/CBL2)-dependent manner. In contrast to Thr947 phosphorylation in the C-terminal regulatory domain of AHA2 that enhances interaction with, and subsequent activation by, 14-3-3 proteins, PKS5-induced phosphorylation of the Ser931 residue prevents interaction with 14-3-3 proteins [130,131]. Therefore, adaptation to high external pH was improved in the pks5 mutant, and the membrane potential was not affected compared to the Arabidopsis wild type. Intriguingly, neither NaCl, which promotes membrane depolarization (similar to alkali stress), nor drought, which slowly steeps down the membrane potential, resulted in a phenotypic response when applied to the pks5 mutant [130]. The upregulation of PKS5 transcripts by NaCl, drought, or mannitol/glucose-induced osmotic stress suggest the involvement of other functionally redundant proteins with PKS5 under such conditions [130]. However, these stresses were applied at very high doses, especially NaCl, and it is possible that this induction of PKS5 expression occurs in the cell death program, rather than the adaptation process. Undoubtedly, cellular accumulation of NaCl inhibits PM ATPase via membrane depolarization (Figure 1 [3N→1→2]). However, elevated salinity tolerance in halophyte quinoa, saltbush, and salt-tolerant barley genotypes were attributed to prompt activation of PM H+-ATPase, rather than higher transcription of AHAs, as observed in salt-sensitive Arabidopsis under increased salinity [132,133]. Here, Ca2+ signals activate PM H+-ATPase, as well as SOS2, by binding to the 14-3-3 proteins promoting its interaction with PKS5, and subsequently repressing its kinase activity (Figure 1 [2C→4]) [134]. Moreover, overexpressing the mutated version of PM H+-ATPase4 that lacks the autoinhibitory domain (∆PMA4) improved the salinity tolerance of transgenic tobacco [54,135]. Additionally, kinase activity of PKS5 can be repressed by a physical interaction with the chaperone J3, unleashing the PM H+-ATPase activity compared to j3 mutants that also exhibit hypersensitivity to alkali and salt stress [136]. On the other hand, ABA enhances PKS5 kinase activity, promoting its interaction with, and phosphorylation of, ABA-insensitive5 (ABI5) at the Ser42 residue [137]. Interestingly, intracellular pH homeostasis has been shown to play a critical role in regulating the expression of ABA-biosynthetic genes, in general, and under PEG-induced osmotic stress, in particular [138]. Therefore, other possible mechanisms need to be investigated.
Notably, post-translation regulation of PM H+-ATPases seems to be very intricate, since the regulatory/autoinhibitory C-terminal domain can be phosphorylated at different sites, resulting in inactive/active forms of AHAs [131]. Phosphoproteomic approaches have shown that both AHA1 (AT2G18960.1; known also as open stomata2 OST2) and AHA2 (AT4G30190.1) were rapidly phosphorylated within 5 min and 15 min, respectively, in Arabidopsis suspension cells under saline conditions [10]. However, the phosphorylation of AHA1 and AHA2 was shown to occur at Thr948 (corresponding to Thr947 in AHA2) and Ser899, respectively, resulting in the activation of the predominantly shoot expressed AHA1, but the inactivation of the mainly root-expressed AHA2 [10]. It seems that there is an interaction within AHAs, and with other PM transporters, such as SOS1, that become gradually phosphorylated in roots [11], but that peak at 15 min in suspension cells under exposure to salinity [10]. In fact, V. rupestris suspension cells exhibited a reduction of extracellular alkalinization (H+ efflux) concomitant with decreasing cellular levels of Na+ after 10–15 min of salinity exposure [45,47]. In addition, constitutively active AHA1 in Arabidopsis (ost2 mutant) results in stomatal closure failure and an ABA-insensitive phenotype [139,140,141]. However, phosphorylation of AHA1 and AHA2 in the Ser2 residue opens new questions [11]; further research is required to enhance our understanding in this regard.
The activity of important transporters, such as NHXs and CAXs, on the tonoplast are affected by the efficiency of V-ATPases and V-PPases, and vice versa [126,142]. For example, cax1 and cax3 mutations decrease V-ATPase activity, maybe due to the accumulation of luminal H+ [113,143]. In addition, Ca2+ signaling is also interconnected to V-ATPase via the SOS pathway. Here, the Ca2+-dependent SCaBP8/SOS2 can activate V-ATPase by interacting with its regulatory subunits VHA-B1 and 2, resulting in fine-tuning of Ca2+ signaling and ion homeostasis under salinity [69,118,144]. In addition, in salt-stressed Solanum lycopersicum, the SCaBP8/SOS2 complex is also proposed to activate the tonoplastic H+-pyrophosphatase (SlAVP1) and SlV-ATPase, as well as SlTPC1, resulting in the regulation of Na+ and Ca2+ fluxes in the leaf vacuole, and hence improving salt adaptation (Figure 1 [2C→10→4]) [145]. Subsequently, stimulated V-ATPases reduce the luminal pH, which, in turn, promotes the interaction of AtCaM15 with the C-terminal hydrophilic region of AtNHX1, resulting in decreasing Na+/H+ exchange activity in yeast vacuoles [91]. Although the AtCaM15/AtNHX1 interaction was shown to take place in plants, modification of NHX activity has not been proven yet. However, if this was the case, it might be a mechanism to initially prevent Na+ accumulation in leaf vacuoles, thereby favoring Na+ redistribution. Interestingly, 14-3-3 proteins, the positive regulators of PM H+-ATPases, can also bind to the SOS2 junction domain where a critical Ser294 phosphorylation site exists, resulting in kinase activity repression under nonstressful conditions, but not under salinity exposure [95]. Therefore, it seems that plants pay critical attention to the Ca2+-activated SOS pathway to further fine tune Ca2+-signals.
3.3. ROS: Do Not Completely Unleash the Fire
ROS are inevitable byproducts of plant aerobic metabolism that turn toxic, unless ameliorating measures are taken. Interestingly, plants have not only evolved indispensable ROS-scavenging strategies, but also deliberately generate ROS as pivotal cellular second messengers [146]. Therefore, plants master a delicate fine-tuning to channel ROS in desirable processes. As discussed earlier, the within-second stimulus-induced elevations of Ca2+cyt triggers the accumulation of H+ in the cytosol, as well as ROS [72,73]. The latter signal is launched when Ca2+cyt activates the PM NADPH oxidase ROBHD via CPK5, resulting in superoxide O2•− production in the apoplast, which is converted to H2O2 by superoxide dismutase [147]. These ROS signals act locally and systemically under different stress types, including salinity, as well as in cooperation with other cellular signals, especially, Ca2+ [148]. For instance, ROS can positively feedback on the Ca2+ signal, activating the hyperpolarization NSCCs [149]. Many excellent reviews on ROS signaling have been published recently [150,151,152]. One of the intriguing questions that often rises in that regard is how such elevated extracellular ROS are sensed on the cell surface. Recently, by utilizing forward genetic screens in combination with Ca2+ imaging, Wu et al. [153] identified an Arabidopsis mutant that exhibits low Ca2+ influx upon H2O2 treatment; hence came its name hydrogen-peroxide-induced Ca2+ increases (hpca). HPCA1 belongs to the leucine-rich-repeat (LRR) receptor kinases, and was shown to mediate stomatal closure via the activation of Ca2+ channels in guard cells under H2O2 application [153]. Further research is required to elucidate the role of HPCA1 in response to developmental and environmental stimuli.
Under water-related stress, reserving cellular water is among the top priorities of the cell and is achieved via controlling AQP (PIP) function. The Ca2+-triggered H2O2 on the apoplast can rapidly diffuse into the cell via PM PIPs [154], and thereby might feed negatively on PIPs by promoting their internalization. In Arabidopsis roots, H2O2-induced PIP internalization under salinity stress, as well as H2O2 per se, can be removed by catalase application [155]. Moreover, H2O2 has been shown to dephosphorylate AtPIP2;1 at the Ser283 residue, similar to salinity exposure, but within a shorter application time, and hence, the nonphosphorylated forms of AtPIP2;1 accumulate intracellularly (Figure 1 [6R→1]) [12]. Therefore, downregulation of root hydraulic conductivity under salinity is attributed to the ROS-activated cell signal chain where Ca2+-signaling is partially involved, rather than direct oxidative action [155]. Interestingly, H2O2 stress resulted in phosphorylating AHA1 and AHA2 at the same sites, Thr948 and Ser899, respectively, similar to salinity stress, but with a different kinetic pattern [10]. These results indicate the interconnection of ROS and H+ signals, adding additional levels of complexity to cellular signals. In addition, the role of ROS in activating ABA and JA signals adds further layers of complexity (Figure 1 [6R→2&3]) [151].
On the other hand, overaccumulating ROS under pathogen stress [156] acts as a fire that burns the local infected site with a PCD strategy to restrict the spread of invaders. In the context of abiotic stress, PCD is unlikely to be a suitable response in plants [47,52]. Therefore, plants have evolved ROS-homeostatic strategies for the timely scavenging of ROS. To do so, gene expression of the ROS-scavenging enzymes peroxidase (POD), Glutathione S-transferase (GST), and catalase (CAT) were enhanced in cotton Gossypium davidsonii leaves and roots (Figure 1 [6R→antioxidant system]) [157]. Interesting, Ca2+ and its related signals seem to play a crucial role in preventing ROS-induced cellular damage. For example, Oryza sativa OsCPK4 expression was promoted in rice roots under salt and drought stresses, as well as ABA treatment, and OsCPK4 transgenic rice plants are salt- and drought-tolerant. The upregulation of redox-finetuning transcripts, such as POD, GST, and Laccase in the OsCPK4 transgenic lines, is evidence that OsCPK4 is involved in protecting cellular membranes from ROS-induced oxidative damage [78,158,159]. Future research shall further unravel additional layers of the intricate networks among ROS and other signals, such as Ca2+ and H+.
4. Conclusions
To understand the underlying mechanisms of water-related stress, it is important to contextualize cellular events. The current review summarizes new advances in the field that might help in formulating an integrative perspective of cellular events under salt-related stress. It is also of paramount interest that future research focuses on the puzzling points, in addition to running parallel studies that investigate the phenomenon at different plant levels. In addition, comprehensive parallel studies on tolerant and sensitive cultivars will aid in correlating signals/events to the right cellular context.
Author Contributions
A.I. contributed to the writing—original draft preparation and preparing the final draft; I.E.S. and S.S contributed to the writing—review, editing, and preparing the final draft. All authors have read and agreed to the published version of the manuscript.
Funding
This works was supported by Marie Skłodowska-Curie Individual Fellowships (EU project# 735105) to AI; National Academy of Sciences (NAS) (Project #2000009143) to I.E.; and Virginia Catalyst (Project# 460380) to S.S.
Conflicts of Interest
The authors declare that no conflict of interest exists regarding the completion and publication of this research.
Abbreviations
| AQPs | Aquaporins |
| PIPs | Plasma membrane intrinsic proteins |
| TIPs | Tonoplast intrinsic proteins |
| PM | Plasma membrane |
| MSCCs | Mechanosensitive – NSCCs |
| OST1 | Open stomata 1 |
| ABA | Abscisic acid |
| NSCCs | Nonselective cation channels |
| KORs | K+ outward rectifiers |
| Ψs | Osmotic potential |
| MCA | Mid1-Complementing Activity |
| OSCAs | reduced hyperosmolality-induced [Ca2+]i increase |
| RLKs | receptor-like kinases |
| MTs | microtubules |
| FER | the malectin-like-domain RK FERONIA |
| RALF | rapid alkalinization factor |
| MOCA1 | a glucuronosyltransferase for glycosyl inositol phosphorylceramide (GIPC) sphingolipids |
| GIPC | Glycosyl Inositol Phospho Ceramides |
| DA-NSCCs | Depolarization-activated-NSCCs |
| VI-NSCCs | Voltage-insensitive- NSCCs |
| HA-NSCCs | hyperpolarization-activated NSCCs |
| ROS | Reactive oxygen species |
| PCD | Programed Cell Death |
| cAMP | cyclic adenosine monophosphate |
| cGMP | Cyclic guanosine monophosphate |
| cNMP | ionic-induced cyclic nucleotide monophosphate |
| CNGCs | cyclic nucleotide gated NSCCs |
| SOS | Salt overly sensitive |
| PLC | phospholipase C |
| HKTs | high affinity K+ transporters |
| CAMTA6 | Ca2+-dependent calmodulin-binding transcription activator 6 |
| SCaBP8 | SOS3-like calcium binding protein 8 |
| (also known as calcineurin B-like protein 10, CBL10) | |
| NHXs | Vacuolar Na+/H+ exchangers |
| AtANN4 | a member of AtANNEXINs calcium-dependent membrane binding proteins |
| TPC1 | Two pore channel 1 |
| CAXs | Ca2+/H+ exchangers |
| JA | Jasmonic acid |
| JA-Il | Jasmonyl isoleucine |
| RBOHD | Respiratory burst oxidase homolog D |
| H+-ATPases | PM electrogenic proton pumps |
| V-ATPases | vacuolar V-type H+-ATPases |
| V-PPases | vacuolar V-pyrophosphatases |
| PKS5 | SOS2-like protein kinase |
| ABI5 | ABA-insensitive5 |
| CPKs | calcium-dependent protein kinases |
| CAT | Catalase |
| POD | Peroxidase |
| GST | Glutathione S-transferase |
References
- Majumder, A.L.; Sengupta, S.; Goswami, L. Osmolyte Regulation in Abiotic Stress. In Abiotic Stress Adaptation in Plants; Pareek, A., Sopory, S., Bohnert, H., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 349–370. [Google Scholar]
- Murata, K.; Mitsuoka, K.; Hirai, T.; Walz, T.; Agre, P.; Heymann, J.B.; Engel, A.; Fujiyoshi, Y. Structural determinants of water permeation through aquaporin-1. Nature 2000, 407, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, F.; Tyerman, S.D. Aquaporins: Highly regulated channels controlling plant water relations. Plant Physiol. 2014, 164, 1600–1618. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Verdoucq, L.; Rodrigues, O. Aquaporins and plant transpiration. Plant Cell Environ. 2016, 39, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- MacRobbie, E.A. Osmotic effects on vacuolar ion release in guard cells. Proc. Natl. Acad. Sci. USA 2006, 103, 1135–1140. [Google Scholar] [CrossRef]
- Johansson, I.; Karlsson, M.; Shukla, V.P.; Chrispeels, M.J.; Larsson, C.; Kjellbom, P. Water transport activity of the plasma membrane aquaporin PM28A is regulated by phosphorylation. Plant Cell 1998, 10, 451–459. [Google Scholar] [CrossRef]
- Törnroth-Horsefield, S.; Wang, Y.; Hedfalk, K.; Johanson, U.; Karlsson, M.; Tajkhorshid, E.; Neutze, R.; Kjellbom, P. Structural mechanism of plant aquaporin gating. Nature 2006, 439, 688–694. [Google Scholar] [CrossRef]
- Baral, A.; Irani, N.G.; Fujimoto, M.; Nakano, A.; Mayor, S.; Mathew, M.K. Salt-induced remodeling of spatially restricted clathrin-independent endocytic pathways in Arabidopsis root. Plant Cell 2015, 27, 1297–1315. [Google Scholar] [CrossRef]
- Boursiac, Y.; Chen, S.; Luu, D.T.; Sorieul, M.; van den Dries, N.; Maurel, C. Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiol. 2005, 139, 790–805. [Google Scholar] [CrossRef]
- Chen, Y.; Hoehenwarter, W. Changes in the phosphoproteome and metabolome link early signaling events to rearrangement of photosynthesis and central metabolism in salinity and oxidative stress response in Arabidopsis. Plant Physiol. 2015, 169, 3021–3033. [Google Scholar] [CrossRef]
- Vialaret, J.; Di Pietro, M.; Hem, S.; Maurel, C.; Rossignol, M.; Santoni, V. Phosphorylation dynamics of membrane proteins from Arabidopsis roots submitted to salt stress. Proteomics 2014, 14, 1058–1070. [Google Scholar] [CrossRef]
- Prak, S.; Hem, S.; Boudet, J.; Viennois, G.; Sommerer, N.; Rossignol, M.; Maurel, C.; Santoni, V. Multiple phosphorylations in the C-terminal tail of plant plasma membrane aquaporins: Role in subcellular trafficking of AtPIP2;1 in response to salt stress. Mol. Cell Proteom. 2008, 7, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.N.; Lew, R.R. Turgor regulation in osmotically stressed Arabidopsis epidermal root cells. Direct support for the role of inorganic ion uptake as revealed by concurrent flux and cell turgor measurements. Plant Physiol. 2002, 129, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Harada, A.; Sakai, T.; Takagi, S. Ca2+ transient induced by extracellular changes in osmotic pressure in Arabidopsis leaves: Differential involvement of cell wall-plasma membrane adhesion. Plant Cell Env. 2006, 29, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.S.; Schlegel, A.M.; Haswell, E.S. United in diversity: Mechanosensitive ion channels in plants. Annu. Rev. Plant Biol. 2015, 66, 113–137. [Google Scholar] [CrossRef] [PubMed]
- Grondin, A.; Rodrigues, O.; Verdoucq, L.; Merlot, S.; Leonhardt, N.; Maurel, C. Aquaporins contribute to ABA-triggered stomatal closure through OST1-mediated phosphorylation. Plant Cell 2015, 27, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hu, H.; Qin, X.; Zeise, B.; Xu, D.; Rappel, W.J.; Boron, W.F.; Schroeder, J.I. Reconstitution of CO2 Regulation of SLAC1 Anion Channel and Function of CO2-Permeable PIP2;1 Aquaporin as CARBONIC ANHYDRASE4 Interactor. Plant Cell 2016, 28, 568–582. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.I.; Kwak, J.M.; Allen, G.J. Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature 2001, 410, 327–330. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A.; Szarejko, I. Open or close the gate—Stomata action under the control of phytohormones in drought stress conditions. Front. Plant Sci. 2013, 4, 138. [Google Scholar] [CrossRef]
- Brodribb, T.J.; McAdam, S.A.M. Passive origins of stomatal control in vascular plants. Science 2011, 331, 582–585. [Google Scholar] [CrossRef]
- Tombesi, S.; Nardini, A.; Frioni, T.; Soccolini, M.; Zadra, C.; Farinelli, D.; Poni, S.; Palliotti, A. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Sci. Rep. 2015, 5, 12449. [Google Scholar] [CrossRef]
- Demidchik, V.; Maathuis, F.J.M. Physiological roles of nonselective cation channels in plants: From salt stress to signalling and development. New Phytol. 2007, 175, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Katagiri, T.; Shinozaki, K.; Qi, Z.; Tatsumi, H.; Furuichi, T.; Kishigami, A.; Sokabe, M.; Kojima, I.; Sato, S.; et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc. Natl. Acad. Sci. USA 2007, 104, 3639–3644. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, T.; Nakagawa, Y.; Mori, K.; Nakano, M.; Imamura, T.; Kataoka, H.; Terashima, A.; Iida, K.; Kojima, I.; Katagiri, T.; et al. MCA1 and MCA2 that mediate Ca2+ uptake have distinct and overlapping roles in Arabidopsis. Plant Physiol. 2010, 152, 1284–1296. [Google Scholar] [CrossRef] [PubMed]
- Stephan, A.B.; Kunz, H.H.; Yang, E.; Schroeder, J.I. Rapid hyperosmotic-induced Ca2+ responses in Arabidopsis thaliana exhibit sensory potentiation and involvement of plastidial KEA transporters. Proc. Natl. Acad. Sci. USA 2016, 113, E5242–E5249. [Google Scholar] [CrossRef]
- Haswell, E.S.; Peyronnet, R.; Barbier-Brygoo, H.; Meyerowitz, E.M.; Frachisse, J.M. Two MscS homologs provide mechanosensitive channel activities in the Arabidopsis root. Curr. Biol. 2008, 18, 730–734. [Google Scholar] [CrossRef]
- Yuan, F.; Yang, H.; Xue, Y.; Kong, D.; Ye, R.; Li, C.; Zhang, J.; Theprungsirikul, L.; Shrift, T.; Krichilsky, B.; et al. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 2014, 514, 367–371. [Google Scholar] [CrossRef]
- Hou, C.; Tian, W.; Kleist, T.; He, K.; Garcia, V.; Bai, F.; Hao, Y.; Luan, S.; Li, L. DUF221 proteins are a family of osmosensitive calcium-permeable cation channels conserved across eukaryotes. Cell Res. 2014, 24, 632–635. [Google Scholar] [CrossRef]
- Edel, K.H.; Marchadier, E.; Brownlee, C.; Kudla, J.; Hetherington, A.M. The evolution of calcium-based signalling in plants. Curr. Biol. 2017, 27, 667–679. [Google Scholar] [CrossRef]
- Liu, Z.; Persson, S.; Sánchez-Rodríguez, C. At the border: The plasma membrane-cell wall continuum. J Exp. Bot. 2015, 66, 1553–1563. [Google Scholar] [CrossRef]
- Liu, Q.; Qiao, F.; Ismail, A.; Chang, X.; Nick, P. The plant cytoskeleton controls regulatory volume increase. Biochim. Biophys. Acta 2013, 1828, 2111–2120. [Google Scholar] [CrossRef]
- Komis, G.; Apostolakos, P.; Galatis, B. Hyperosmotic-stress induced actin filament reorganization in leaf cells of Chlorophytum comosum. J. Exp. Bot. 2002, 53, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Buchholz, G.; Nick, P. Tubulin marker line of grapevine suspension cells as a tool to follow early stress responses. J. Plant Physiol. 2015, 176, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, W.G.; Zauber, H.; Erban, A.; Gorka, M.; Wu, X.N.; Schulze, W.X. Cytoskeletal components define protein location to membrane microdomains. Mol. Cell. Proteom. 2015, 14, 2493–2509. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kurepa, J.; Hashimoto, T.; Smalle, J.A. Salt stress-induced disassembly of Arabidopsis cortical microtubule arrays involves 26S proteasome-dependent degradation of SPIRAL1. Plant Cell 2011, 23, 3412–3427. [Google Scholar] [CrossRef]
- Endler, A.; Kesten, C.; Schneider, R.; Zhang, Y.; Ivakov, A.; Froehlich, A.; Funke, N.; Persson, S. A mechanism for sustained cellulose synthesis during salt stress. Cell 2015, 162, 1353–1364. [Google Scholar] [CrossRef]
- Kesten, C.; Wallmann, A.; Schneider, R.; McFarlane, H.E.; Diehl, A.; Khan, G.A.; van Rossum, B.J.; Lampugnani, E.R.; Szymanski, W.G.; Cremer, N.; et al. The companion of cellulose synthase 1 confers salt tolerance through a Tau-like mechanism in plants. Nat. Commun. 2019, 10, 857. [Google Scholar] [CrossRef]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef]
- Feng, W.; Kita, D.; Peaucelle, A.; Cartwright, H.N.; Doan, V.; Duan, Q.; Liu, M.C.; Maman, J.; Steinhorst, L.; Schmitz-Thom, I.; et al. The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr. Biol. 2018, 28, 666–675. [Google Scholar] [CrossRef]
- Ismail, A.; Riemann, M.; Nick, P. The jasmonate pathway mediates salt tolerance in grapevines. J. Exp. Bot. 2012, 63, 2127–2139. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Lilley, C.J.; Urwin, P.E. Identification of genes involved in the response of Arabidopsis to simultaneous biotic and abiotic stresses. Plant Physiol. 2013, 162, 2028–2041. [Google Scholar] [CrossRef]
- Haruta, M.; Sabat, G.; Stecker, K.; Minkoff, B.B.; Sussman, M.R. A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 2014, 343, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhou, X.; Tao, M.; Yuan, F.; Liu, L.; Wu, F.; Wu, X.; Xiang, Y.; Niu, Y.; Liu, F.; et al. Plant cell-surface GIPC sphingolipids sense salt to trigger Ca2+ influx. Nature 2019, 572, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.P.; Pickard, B.G. Mechanosensory calcium-selective cation channels in epidermal cells. Plant J. 1993, 3, 83–110. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, F.J.M. Sodium in plants: Perception, signalling, and regulation of sodium fluxes. J. Exp. Bot. 2014, 65, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Takeda, S.; Nick, P. Life and death under salt stress: Same players, different timing? J. Exp. Bot. 2014, 65, 2963–2979. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Seo, M.; Takebayashi, Y.; Kamiya, Y.; Eiche, E.; Nick, P. Salt adaptation requires fine-tuning of jasmonate signaling. Protoplasma 2014, 251, 881–898. [Google Scholar] [CrossRef]
- Shabala, S.; Demidchik, V.; Shabala, L.; Cuin, T.A.; Smith, S.J.; Miller, A.J.; Davies, J.M.; Newman, I.A. Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol. 2006, 141, 1653–1665. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Essah, P.A.; Davenport, R.; Tester, M. Sodium influx and accumulation in Arabidopsis. Plant Physiol. 2003, 133, 307–318. [Google Scholar] [CrossRef]
- Demidchik, V.; Cuin, T.A.; Svistunenko, D.; Smith, S.J.; Miller, A.J.; Shabala, S.; Sokolik, A.; Yurin, V. Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: Single-channel properties, genetic basis and involvement in stress-induced cell death. J. Cell Sci. 2010, 123, 1468–1479. [Google Scholar] [CrossRef]
- Monetti, E.; Kadono, T.; Tran, D.; Azzarello, E.; Arbelet-Bonnin, D.; Biligui, B.; Briand, J.; Kawano, T.; Mancuso, S.; Bouteau, F. Deciphering early events involved in hyperosmotic stress-induced programmed cell death in tobacco BY-2 cells. J. Exp. Bot. 2014, 65, 1361–1375. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, V.A.; Beilby, M.J.; Al Khazaaly, S.; Shimmen, T. Mechano-perception in Chara cells: The influence of salinity and calcium on touch-activated receptor potentials, action potentials and ion transport. Plant Cell Environ. 2008, 31, 1575–1591. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Bose, J.; Fuglsang, A.T.; Pottosin, I. On a quest for stress tolerance genes: Membrane transporters in sensing and adapting to hostile soils. J. Exp. Bot. 2016, 67, 1015–1031. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, F.J.M.; Sanders, D. Sodium uptake in Arabidopsis roots is regulated by cyclic nucleotides. Plant Physiol. 2001, 127, 1617–1625. [Google Scholar] [CrossRef]
- Donaldson, L.; Ludidi, N.; Knight, M.R.; Gehring, C.; Denby, K. Salt and osmotic stress cause rapid increases in Arabidopsis thaliana cGMP levels. FEBS Lett. 2004, 569, 317–320. [Google Scholar] [CrossRef]
- Li, L.; Wang, F.; Yan, P.; Jing, W.; Zhang, C.; Kudla, J.; Zhang, W. A phosphoinositide-specific phospholipase C pathway elicits stress-induced Ca2+ signals and confers salt tolerance to rice. New Phytol. 2017, 214, 1172–1187. [Google Scholar] [CrossRef]
- Uozumi, N.; Kim, E.J.; Rubio, F.; Yamaguchi, T.; Muto, S.; Tsuboi, A.; Bakker, E.P.; Nakamura, T.; Schroeder, J.I. The Arabidopsis HKT1 gene homologue mediates inward Na+ currents in Xenopus oocytes and Na+ uptake in Saccharomyces cerevisiae. Plant Physiol. 2000, 121, 1249–1259. [Google Scholar] [CrossRef]
- Horie, T.; Motoda, J.; Kubo, M.; Yang, H.; Yoda, K.; Horie, R.; Chan, W.Y.; Leung, H.Y.; Hattori., K.; Konomi, M., M.; et al. Enhanced Salt Tolerance Mediated by AtHKT1 Transporter-Induced Na Unloading From Xylem Vessels to Xylem Parenchyma Cells. Plant J. 2005, 44, 928–938. [Google Scholar] [CrossRef]
- Horie, T.; Hauser, F.; Schroeder, J.I. HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends Plant Sci. 2009, 14, 660–668. [Google Scholar] [CrossRef]
- Møller, I.S.; Gilliham, M.; Jha, D.; Mayo, G.M.; Roy, S.J.; Coates, J.C.; Haseloff, J.; Tester, M. Shoot Na+ exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na+ transport in Arabidopsis. Plant Cell 2009, 21, 2163–2178. [Google Scholar] [CrossRef]
- Plett, D.; Safwat, G.; Gilliham, M.; Skrumsager Møller, I.; Roy, S.; Shirley, N.; Jacobs, A.; Johnson, A.; Tester, M. Improved salinity tolerance of rice through cell type-specific expression of AtHKT1;1. PLoS ONE 2010, 5, e12571. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; James, RA.; Xu, B.; Athman, A.; Conn, S.J.; Jordans, C.; Byrt, C.S.; Hare, R.A.; Tyerman, S.D.; Tester, M.; et al. Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nature Biotech. 2012, 30, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Mäser, P.; Eckelman, B.; Vaidyanathan, R.; Horie, T.; Fairbairn, D.J.; Kubo, M.; Yamagami, M.; Yamaguchi, K.; Nishimura, M.; Uozumi, N.; et al. Altered shoot/root Na+ distribution and bifurcating salt sensitivity in Arabidopsis by genetic disruption of the Na+ transporter AtHKT1. FEBS Lett. 2002, 531, 157–161. [Google Scholar] [CrossRef]
- Horie, T.; Horie, R.; Chan, W.Y.; Leung, H.Y.; Schroeder, J.I. Calcium regulation of sodium hypersensitivities of sos3 and athkt1 mutants. Plant Cell Physiol. 2006, 47, 622–633. [Google Scholar] [CrossRef]
- Rus, A.; Yokoi, S.; Sharkhuu, A.; Reddy, M.; Lee, B.H.; Matsumoto, T.K.; Koiwa, H.; Zhu, J.K.; Bressan, R.A.; Hasegawa, P.M. AtHKT1 is a salt tolerance determinant that controls Na+ entry into plant roots. Proc. Natl. Acad. Sci. USA 2001, 98, 14150–14155. [Google Scholar] [CrossRef]
- Shkolnik, D.; Finkler, A.; Pasmanik-Chor, M.; Fromm, H. CALMODULIN-BINDING TRANSCRIPTION ACTIVATOR 6: A key regulator of Na+ homeostasis during germination. Plant Physiol. 2019, 180, 1101–1118. [Google Scholar] [CrossRef]
- Dodd, A.N.; Kudla, J.; Sanders, D. The language of calcium signaling. Annu. Rev. Plant Biol. 2010, 61, 593–620. [Google Scholar] [CrossRef]
- Peiter, E. The plant vacuole: Emitter and receiver of calcium signals. Cell Calcium 2011, 50, 120–128. [Google Scholar] [CrossRef]
- Johansson, I.; Larsson, C.; Ek, B.; Kjellbom, P. The major integral proteins of spinach leaf plasma membranes are putative aquaporins and are phosphorylated in response to Ca2+ and apoplastic water potential. Plant Cell 1996, 8, 1181–1191. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef]
- Monshausen, G.B.; Bibikova, T.N.; Weisenseel, M.H.; Gilroy, S. Ca2+ regulates reactive oxygen species production and pH during mechanosensing in Arabidopsis roots. Plant Cell 2009, 21, 2341–2356. [Google Scholar] [CrossRef] [PubMed]
- Knight, H.; Trewavas, A.J.; Knight, M.R. Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J. 1997, 12, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.; Zhaolong, X.; Luoni, L.; Bonza, M.C.; Doccula, F.G.; De Michelis, M.I.; Morris, R.J.; Schwarzländer, M.; Costa, A. cellular Ca2+ signals generate defined pH signatures in plants. Plant Cell 2018, 30, 2704–2719. [Google Scholar] [CrossRef] [PubMed]
- Alleva, K.; Niemietz, C.M.; Sutka, M.; Maurel, C.; Parisi, M.; Tyerman, S.D.; Amodeo, G. Plasma membrane of Beta vulgaris storage root shows high water channel activity regulated by cytoplasmic pH and a dual range of calcium concentrations. J. Exp. Bot. 2006, 57, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Gerbeau, P.; Amodeo, G.; Henzler, T.; Santoni, V.; Ripoche, P.; Maurel, C. The water permeability of Arabidopsis plasma membrane is regulated by divalent cations and pH. Plant J. 2002, 30, 71–81. [Google Scholar] [CrossRef]
- Ji, H.; Pardo, J.M.; Batelli, G.; Van Oosten, M.J.; Bressan, R.A.; Li, X. The Salt Overly Sensitive (SOS) pathway: Established and emerging roles. Mol. Plant 2013, 6, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Boudsocq, M.; Sheen, J. CDPKs in immune and stress signaling. Trends Plant Sci. 2013, 18, 30–40. [Google Scholar] [CrossRef]
- Schulz, P.; Herde, M.; Romeis, T. Calcium-Dependent Protein Kinases: Hubs in Plant Stress Signaling and Development. Plant Physiol. 2013, 163, 523–530. [Google Scholar] [CrossRef]
- Batistic, O.; Sorek, N.; Schultke, S.; Yalovsky, S.; Kudla, J. Dual fatty acyl modification determines the localization and plasma membrane targeting of CBL/CIPK Ca2+ signaling complexes in Arabidopsis. Plant Cell 2008, 20, 1346–1362. [Google Scholar] [CrossRef]
- Chaves-Sanjuan, A.; Sanchez-Barrena, M.J.; Gonzalez-Rubio, J.M.; Moreno, M.; Ragel, P.; Jimenez, M.; Pardo, J.M.; Martinez-Ripoll, M.; Quintero, F.J.; Albert, A. Structural basis of the regulatory mechanism of the plant CIPK family of protein kinases controlling ion homeostasis and abiotic stress. Proc. Natl. Acad. Sci. USA 2014, 111, 4532–4541. [Google Scholar] [CrossRef]
- Guo, Y.; Halfter, U.; Ishitani, M.; Zhu, J.-K. Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance. Plant Cell 2001, 13, 1383–1400. [Google Scholar] [CrossRef] [PubMed]
- Halfter, U.; Ishitani, M.; Zhu, J.K. The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc. Natl. Acad. Sci. USA 2000, 97, 3735–3740. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Quintero, F.J.; Pardo, J.M.; Zhu, J.K. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 2002, 14, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Quintero, F.J.; Martinez-Atienza, J.; Villalta, I.; Jiang, X.; Kim, W.Y.; Ali, Z.; Fujii, H.; Mendoza, I.; Yun, D.J.; Zhu, J.K.; et al. Activation of the plasma membrane Na/H antiporter Salt-Overly-Sensitive 1 (SOS1) by phosphorylation of an auto-inhibitory C-terminal domain. Proc. Natl. Acad. Sci. USA 2011, 108, 2611–2616. [Google Scholar] [CrossRef]
- Kim, B.G.; Waadt, R.; Cheong, Y.H.; Pandey, G.K.; Dominguez-Solis, J.R.; Schultke, S.; Lee, S.C.; Kudla, J.; Luan, S. The calcium sensor CBL10 mediates salt tolerance by regulating ion homeostasis in Arabidopsis. Plant J. 2007, 52, 473–484. [Google Scholar] [CrossRef]
- Barragán, V.; Leidi, E.O.; Andrés, Z.; Rubio, L.; De Luca, A.; Fernández, J.A.; Cubero, B.; Pardo, J.M. Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 2012, 24, 1127–1142. [Google Scholar] [CrossRef]
- Bassil, E.; Tajima, H.; Liang, Y.C.; Ohto, M.A.; Ushijima, K.; Nakano, R.; Esumi, T.; Coku, A.; Belmonte, M.; Blumwald, E. The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell 2011, 23, 3482–3497. [Google Scholar] [CrossRef]
- Qiu, Q.S.; Guo, Y.; Quintero, F.J.; Pardo, J.M.; Schumaker, K.S.; Zhu, J.K. Regulation of vacuolar Na+/H+ exchange in Arabidopsis thaliana by the salt-overly-sensitive (SOS) pathway. J. Biol. Chem. 2004, 279, 207–215. [Google Scholar] [CrossRef]
- Ma, L.; Ye, J.; Yang, Y.; Lin, H.; Yue, L.; Luo, J.; Long, Y.; Fu, H.; Liu, X.; Zhang, Y.; et al. The SOS2-SCaBP8 complex generates and fine-tunes an AtANN4-dependent calcium signature under salt stress. Dev. Cell 2019, 48, 697–709. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Aharon, G.S.; Sottosanto, J.B.; Blumwald, E. Vacuolar Na+/H+ antiporter cation selectivity is regulated by calmodulin from within the vacuole in a Ca2+- and pH-dependent manner. Proc. Natl. Acad. Sci. USA 2005, 102, 16107–16112. [Google Scholar] [CrossRef]
- Quan, R.; Lin, H.; Mendoza, I.; Zhang, Y.; Cao, W.; Yang, Y.; Shang, M.; Chen, S.; Pardo, J.M.; Guo, Y. SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell 2007, 19, 1415–1431. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Yang, Y.; Quan, R.; Mendoza, I.; Wu, Y.; Du, W.; Zhao, S.; Schumaker, K.S.; Pardo, J.M.; Guo, Y. Phosphorylation of SOS3-LIKE CALCIUM BINDING PROTEIN8 by SOS2 protein kinase stabilizes their protein complex and regulates salt tolerance in Arabidopsis. Plant Cell 2009, 21, 1607–1619. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Britto, D.T. Sodium transport in plants: A critical review. New Phytol. 2011, 189, 54–81. [Google Scholar] [CrossRef]
- Zhou, H.; Lin, H.; Chen, S.; Becker, K.; Yang, Y.; Zhao, J.; Kudla, J.; Schumaker, KS.; Guo, Y. Inhibition of the Arabidopsis salt overly sensitive pathway by 14-3-3 proteins. Plant Cell 2014, 26, 1166–1182. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.S.N.; Ali, G.S.; Celesnik, H.; Day, I.S. Coping with stresses: Roles of calcium- and calcium/calmodulin-regulated gene expression. Plant Cell 2011, 23, 2010–2032. [Google Scholar] [CrossRef] [PubMed]
- Pittman, J.K. Vacuolar Ca2+ uptake. Cell Calcium 2011, 50, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zeng, W.; Chen, Q.; Lee, C.; Chen, L.; Yang, Y.; Cang, C.; Ren, D.; Jiang, Y. Structure of the voltage-gated two-pore channel TPC1 from Arabidopsis thaliana. Nature 2016, 531, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Kintzer, A.F.; Stroud, R.M. Structure, inhibition and regulation of two pore channel TPC1 from Arabidopsis thaliana. Nature 2016, 531, 258–262. [Google Scholar] [CrossRef]
- Peiter, E.; Maathuis, F.J.M.; Mills, L.N.; Knight, H.; Pelloux, J.; Hetherington, A.M.; Sanders, D. The vacuolar Ca2+- activated channel TPC1 regulates germination and stomatal movement. Nature 2005, 434, 404–408. [Google Scholar] [CrossRef]
- Beyhl, D.; Hörtensteiner, S.; Martinoia, E.; Farmer, E.E.; Fromm, J.; Marten, I.; Hedrich, R. The fou2 mutation in the major vacuolar cation channel TPC1 confers tolerance to inhibitory luminal calcium. Plant J. 2009, 58, 715–723. [Google Scholar] [CrossRef]
- Dadacz-Narloch, B.; Beyhl, D.; Larisch, C.; López-Sanjurjo, E.J.; Reski, R.; Kuchitsu, K.; Müller, T.D.; Becker, D.; Schönknecht, G.; Hedrich, R. A novel calcium binding site in the slow vacuolar cation channel TPC1 senses luminal calcium levels. Plant Cell 2011, 23, 2696–2707. [Google Scholar] [CrossRef] [PubMed]
- Bonaventure, G.; Gfeller, A.; Proebsting, W.M.; Hortensteiner, S.; Chetelat, A.; Martinoia, E.; Farmer, E.E. A gain-of-function allele of TPC1 activates oxylipin biogenesis after leaf wounding in Arabidopsis. Plant J. 2007, 49, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Ranf, S.; Wünnenberg, P.; Lee, J.; Becker, D.; Dunkel, M.; Hedrich, R.; Scheel, D.; Dietrich, P. Loss of the vacuolar cation channel, AtTPC1, does not impair Ca2+ signals induced by abiotic and biotic stresses. Plant J. 2008, 53, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.-G.; Toyota, M.; Kim, S.-H.; Hilleary, R.; Gilroy, S. Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc. Natl. Acad. Sci. USA 2014, 111, 6497–6502. [Google Scholar] [CrossRef]
- Evans, M.J.; Choi, W.G.; Gilroy, S.; Morris, R.J. A ROS-assisted calcium wave dependent on the AtRBOHD NADPH oxidase and TPC1 cation channel propagates the systemic response to salt stress. Plant Physiol. 2016, 171, 1771–1784. [Google Scholar] [CrossRef]
- Choi, W.-G.; Hilleary, R.; Swanson, S.J.; Kim, S.-H.; Gilroy, S. Rapid, long-distance electrical and calcium signaling in plants. Annu. Rev. Plant Biol. 2016, 67, 287–307. [Google Scholar] [CrossRef]
- Bose, J.; Pottosin, I.I.; Shabala, S.S.; Palmgren, M.G.; Shabala, S. Calcium efflux systems in stress signaling and adaptation in plants. Front. Plant Sci. 2011, 2, 85. [Google Scholar] [CrossRef]
- Talke, I.N.; Blaudez, D.; Maathuis, F.J.M.; Sanders, D. CNGCs: Prime targets of plant cyclic nucleotide signalling? Trends Plant Sci. 2003, 8, 286–293. [Google Scholar] [CrossRef]
- Conn, S.J.; Gillham, M.; Athman, A.; Schreiber, A.W.; Baumann, U.; Moller, I.; Cheng, N.H.; Stancombe, M.A.; Hirschi, K.D.; Webb, A.R.; et al. Cell specific vacuolar calcium compartmentation regulates apoplastic calcium concentration, gas exchange and plant productivity. Plant Cell 2011, 23, 240–257. [Google Scholar] [CrossRef]
- Manohar, M.; Shigaki, T.; Mei, H.; Park, S.; Marshall, J.; Aguilar, J.; Hirschi, K.D. Characterization of Arabidopsis Ca2+/H+ exchanger CAX3. Biochemistry 2011, 50, 6189–6195. [Google Scholar] [CrossRef]
- Cheng, N.H.; Pittman, J.K.; Barkla, B.J.; Shigaki, T.; Hirschi, K.D. The Arabidopsis cax1 mutant exhibits impaired ion homeostasis, development, and hormonal responses and reveals interplay among vacuolar transporters. Plant Cell 2003, 15, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.H.; Pittman, J.K.; Shigaki, T.; Lachmansingh, J.; LeClere, S.; Lahner, B.; Salt, DE.; Hirschi, K.D. Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol. 2005, 138, 2048–2060. [Google Scholar] [CrossRef] [PubMed]
- Apse, M.P.; Sottosanto, J.B.; Blumwald, E. Vacuolar cation/H+ exchange, ion homeostasis, and leaf development are altered in a T-DNA insertional mutant of AtNHX1, the Arabidopsis vacuolar Na+/H+ antiporter. Plant J. 2003, 36, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Case, R.M.; Eisner, D.; Gurney, A.; Jones, O.; Muallem, S.; Verkhratsky, A. Evolution of calcium homeostasis: From birth of the first cell to an omnipresent signalling system. Cell Calcium 2007, 42, 345–350. [Google Scholar] [CrossRef]
- Zhao, J.; Shigaki, T.; Mei, H.; Guo, Y.Q.; Cheng, NH.; Hirschi, K.D. Interaction between Arabidopsis Ca2+/H+ exchangers CAX1 and CAX3. J. Biol. Chem. 2009, 284, 4605–4615. [Google Scholar] [CrossRef]
- Leonhardt, N.; Kwak, J.M.; Robert, N.; Waner, D.; Leonhardt, G.; Schroeder, J.I. Microarray expression analyses of Arabidopsis guard cells and isolation of a recessive abscisic acid hypersensitive protein phosphatase 2C mutant. Plant Cell 2004, 16, 596–615. [Google Scholar] [CrossRef]
- Cheng, N.-H.; Pittman, J.K.; Zhu, J.-K.; Hirschi, K.D. The protein kinase SOS2 activates the Arabidopsis H+ ⁄Ca2+antiporter/CAX1 to integrate calcium transport and salt tolerance. J. Biol. Chem. 2004, 279, 2922–2926. [Google Scholar] [CrossRef]
- Conn, S.; Gilliham, M. Comparative physiology of elemental distributions in plants. Ann. Bot. 2010, 105, 1081–1102. [Google Scholar] [CrossRef]
- Falhof, J.; Pedersen, J.T.; Fuglsang, A.T.; Palmgren, M. Plasma Membrane H+-ATPase regulation in the center of plant physiology. Mol. Plant 2016, 9, 323–337. [Google Scholar] [CrossRef]
- Palmgren, M.G. Plant plasma membrane H+-ATPases: Powerhouses for nutrient uptake. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 817–845. [Google Scholar] [CrossRef]
- Gao, D.; Knight, M.R.; Trewavas, A.J.; Sattelmacher, B.; Plieth, C. Self-Reporting arabidopsis expressing pH and [Ca2+] indicators unveil ion dynamics in the cytoplasm and in the apoplast under abiotic stress. Plant Physiol. 2004, 134, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Geilfus, C.M.; Mühling, K.H. Ratiometric monitoring of transient apoplastic alkalinizations in the leaf apoplast of living Vicia faba plants: Chloride primes and PM–H+-ATPase shapes NaCl-induced systemic alkalinizations. New Phytol. 2013, 197, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Sakano, K. Revision of biochemical pH-stat: Involvement of alternative pathway metabolisms. Plant Cell Physiol. 1998, 39, 467–473. [Google Scholar] [CrossRef]
- Kriegel, A.; Andrés, Z.; Medzihradszky, A.; Krüger, F.; Scholl, S.; Delang, S.; Patir-Nebioglu, M.G.; Gute, G.; Yang, H.; Murphy, A.S.; et al. Job sharing in the endomembrane system: Vacuolar acidification requires the combined activity of V-ATPase and V-PPase. Plant Cell 2015, 27, 3383–3396. [Google Scholar] [CrossRef]
- Hedrich, R. Ion channels in plants. Physiol. Rev. 2012, 92, 1777–1811. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Hématy, K.; Höfte, H. Growth control and cell wall signaling in plants. Annu. Rev. Plant Biol. 2012, 63, 381–407. [Google Scholar] [CrossRef]
- van Dongen, J.T.; Licausi, F. Oxygen sensing and signaling. Annu. Rev. Plant. Biol. 2015, 66, 345–367. [Google Scholar] [CrossRef]
- Tournaire-Roux, C.; Sutka, M.; Javot, H.; Gout, E.; Gerbeau, P.; Luu, D.T.; Bligny, R.; Maurel, C. Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 2003, 425, 393–397. [Google Scholar] [CrossRef]
- Fuglsang, A.T.; Guo, Y.; Cuin, T.A.; Qiu, Q.; Song, C.P.; Kristiansen, K.A.; Bych, K.; Schulz, A.; Shabala, S.; Schumaker, K.S.; et al. Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+-ATPase by preventing interaction with 14-3-3 protein. Plant Cell 2007, 19, 1617–1634. [Google Scholar] [CrossRef]
- Haruta, M.; Gray, W.M.; Sussman, M.R. Regulation of the plasma membrane proton pump (H+-ATPase) by phosphorylation. Curr. Opin. Plant Biol. 2015, 28, 68–75. [Google Scholar] [CrossRef]
- Bose, J.; Rodrigo-Moreno, A.; Lai, D.; Xie, Y.; Shen, W.; Shabala, S. Rapid regulation of the plasma membrane H+-ATPase activity is essential to salinity tolerance in two halophyte species, Atriplex lentiformis and Chenopodium quinoa. Ann. Bot. 2015, 115, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Pottosin, I.I.; Cuin, T.A.; Fuglsang, A.T.; Tester, M.; Jha, D.; Zepeda-Jazo, I.; Zhou, M.; Palmgren, M.G.; Newman, I.A.; et al. Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiol. 2007, 145, 1714–1725. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, C.; Xue, Y.; Liu, X.; Chen, S.; Song, C.; Yang, Y.; Guo, Y. Calcium-activated 14-3-3 proteins as a molecular switch in salt stress tolerance. Nat. Commun. 2019, 10, 1199. [Google Scholar] [CrossRef] [PubMed]
- Swarbreck, S.M.; Colaço, R.; Davies, J.M. Plant calcium-permeable channels. Plant Physiol. 2013, 163, 514–522. [Google Scholar] [CrossRef]
- Yang, Y.; Qin, Y.; Xie, C.; Zhao, F.; Zhao, J.; Liu, D.; Chen, S.; Fuglsang, A.T.; Palmgren, MG.; Schumaker, K.S.; et al. The Arabidopsis chaperone J3 regulates the plasma membrane H+-ATPase through interaction with the PKS5 kinase. Plant Cell 2010, 22, 1313–1332. [Google Scholar] [CrossRef]
- Zhou, X.; Hao, H.; Zhang, Y.; Bai, Y.; Zhu, W.; Qin, Y.; Yuan, F.; Zhao, F.; Wang, M.; Hu, J.; et al. SOS2-LIKE PROTEIN KINASE5, an SNF1-RELATED PROTEIN KINASE3-type protein kinase, is important for abscisic acid responses in Arabidopsis through phosphorylation of ABSCISIC ACID-INSENSITIVE5. Plant Physiol. 2015, 168, 659–676. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Gehring, C.; Zhu, J.; Li, F.M.; Zhu, J.K.; Xiong, L. The Arabidopsis Vacuolar Sorting Receptor1 is required for osmotic stress-induced abscisic acid biosynthesis. Plant Physiol. 2015, 167, 137–152. [Google Scholar] [CrossRef]
- Merlot, S.; Leonhardt, N.; Fenzi, F.; Valon, C.; Costa, M.; Piette, L.; Vavasseur, A.; Genty, B.; Boivin, K.; Muller, A.; et al. Constitutive activation of a plasma membrane H+- ATPase prevents abscisic acid-mediated stomatal closure. EMBO J. 2007, 26, 3216–3226. [Google Scholar] [CrossRef]
- Kim, T.H.; Böhmer, M.; Hu, H.; Nishimura, N.; Schroeder, J. Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 2010, 61, 561–591. [Google Scholar] [CrossRef]
- Murata, Y.; Mori, I.C.; Munemasa, S. Diverse stomatal signaling and the signal integration mechanism. Annu. Rev. Plant Biol. 2015, 66, 369–392. [Google Scholar] [CrossRef]
- Pittman, JK.; Shigaki, T.; Hirschi, K.D. Evidence of differential pH regulation of the Arabidopsis vacuolar Ca2+/H+ antiporters CAX1 and CAX2. FEBS Lett. 2005, 579, 2648–2656. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Barkla, B.; Marshall, J.; Pittman, J.; Hirschi, K. The Arabidopsis cax3 mutants display altered salt tolerance, pH sensitivity and reduced plasma membrane H+-ATPase activity. Planta 2008, 227, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Batelli, G.; Verslues, P.E.; Agius, F.; Qiu, Q.; Fujii, H.; Pan, S.; Schumaker, K.S.; Grillo, S.; Zhu, J.K. SOS2 promotes salt tolerance in part by interacting with the vacuolar H+-ATPase and upregulating its transport activity. Mol. Cell. Biol. 2007, 27, 7781–7790. [Google Scholar] [CrossRef] [PubMed]
- Egea, I.; Pineda, B.; Ortíz-Atienza, A.; Plasencia, F.A.; Drevensek, S.; García-Sogo, B.; Yuste-Lisbona, F.J.; Barrero-Gil, J.; Atarés, A.; Flores, F.B.; et al. The SlCBL10 calcineurin B-like protein ensures plant growth under salt stress by regulating Na+ and Ca2+ homeostasis. Plant Physiol. 2018, 176, 1676–1693. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Env. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Dubiella, U.; Seybold, H.; Durian, G.; Komander, E.; Lassig, R.; Witte, C.P.; Schulze, W.X.; Romeis, T. Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc. Natl. Acad. Sci. USA 2013, 110, 8744–8749. [Google Scholar] [CrossRef]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaev, V.; Dangl, J.L.; Mittler, R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2009, 2, ra45. [Google Scholar] [CrossRef]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D.; et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Gilroy, S.; Suzuki, N.; Miller, G.; Choi, W.G.; Toyota, M.; Devireddy, A.R.; Mittler, R. A tidal wave of signals: Calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci. 2014, 19, 623–630. [Google Scholar] [CrossRef]
- Kimura, S.; Waszczak, C.; Hunter, K.; Wrzaczek, M. Bound by fate: The role of reactive oxygen species in receptor-like kinase signaling. Plant Cell 2017, 29, 638–654. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Chi, Y.; Jiang, Z.; Xu, Y.; Xie, L.; Huang, F.; Wan, D.; Ni, J.; Yuan, F.; Wu, X.; et al. Hydrogen peroxide sensor HPCA1 is an LRR receptor kinase in Arabidopsis. Nature 2020, 578, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Wang, X.; Li, P.; Wang, H.; Ji, H.; Xie, J.; Qiu, Q.; Shen, D.; Dong, H. Plant aquaporin AtPIP1;4 links apoplastic H2O2 induction to disease immunity pathways. Plant Physiol. 2016, 171, 1635–1650. [Google Scholar] [CrossRef] [PubMed]
- Boursiac, Y.; Boudet, J.; Postaire, O.; Luu, D.T.; Tournaire-Roux, C.; Maurel, C. Stimulus-induced downregulation of root water transport involves reactive oxygen species-activated cell signalling and plasma membrane intrinsic protein internalization. Plant J. 2008, 56, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, G.; Du, L.; Shang, X.; Cheng, C.; Yang, B.; Hu, Y.; Cai, C.; Guo, W. Genetic regulation of salt stress tolerance revealed by RNA-Seq in cotton diploid wild species, Gossypium davidsonii. Sci. Rep. 2016, 6, 20582. [Google Scholar] [CrossRef]
- Campo, S.; Baldrich, P.; Messeguer, J.; Lalanne, E.; Coca, M.; San Segundo, B. Overexpression of a calcium-dependent protein kinase confers salt and drought tolerance in rice by preventing membrane lipid peroxidation. Plant Physiol. 2014, 165, 688–704. [Google Scholar] [CrossRef]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2014, 65, 1241–1257. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).