Advances in DNA Repair—Emerging Players in the Arena of Eukaryotic DNA Repair
Abstract
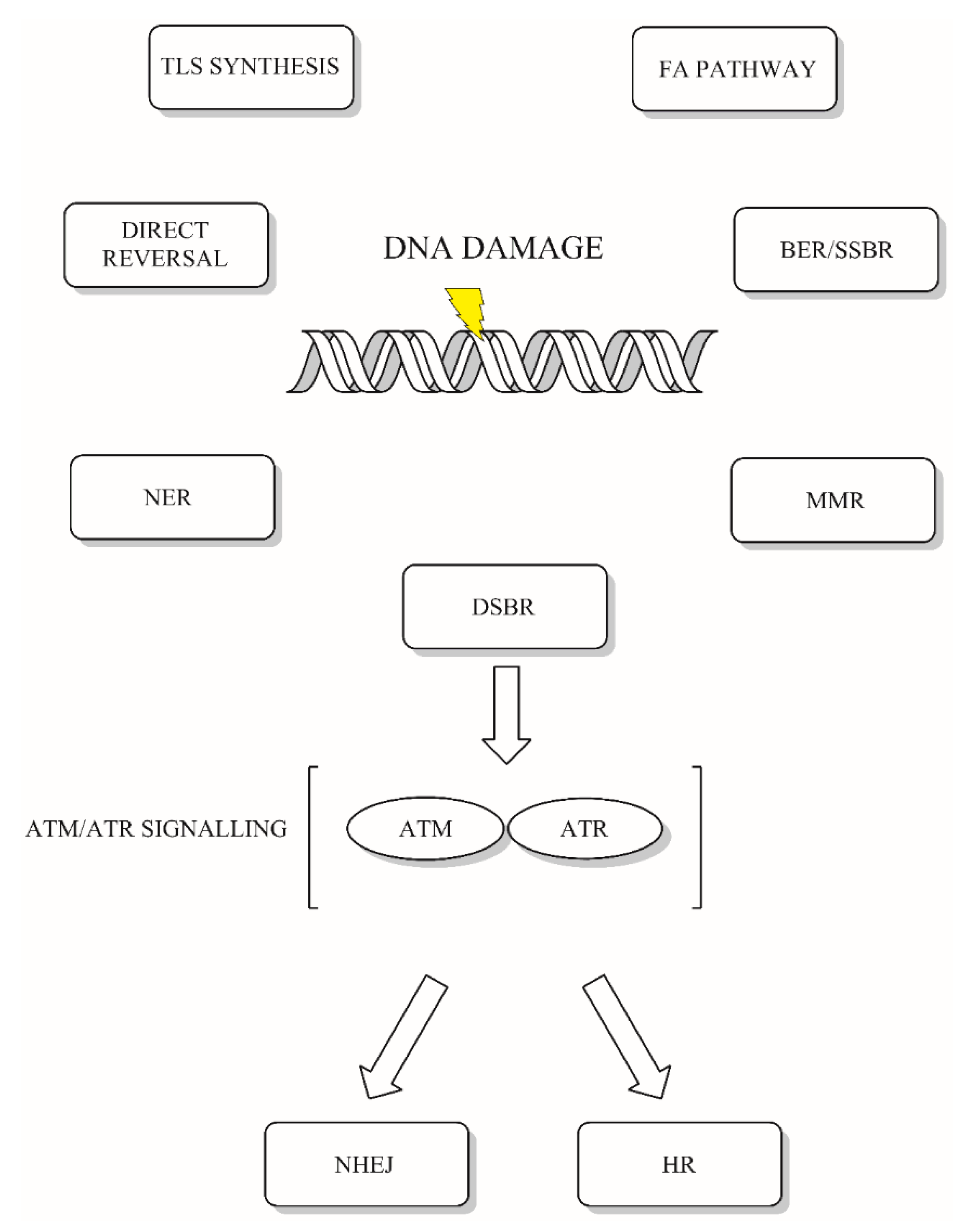
:1. Introduction
2. Sirtuins
3. Long Non-coding RNAs
4. Heat Shock Proteins (HSPs)
5. Circadian Clock
6. Existing Crosstalks
7. Other Players
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| APE1 | AP endonuclease 1 |
| APTX | Aprataxin |
| ATM | Protein kinase ataxia–telangiectasia mutated |
| ATR | Protein kinase ataxia–telangiectasia and Rad3-related |
| ATRIP | ATR interacting protein |
| BER | Base excision repair |
| BIR | Break-induced replication |
| BLM | Bloom syndrome protein |
| BMAL1 | Brain and muscle ARNT-like 1 |
| BRCA1 | Breast cancer type 1 susceptibility protein |
| CCGS | Clock-controlled genes |
| CDK | Cyclin-dependent kinase |
| CHK1/2 | Serine/threonine-protein kinase Chk1/2 |
| CLOCK | Circadian locomotor output cycles protein kaput |
| CPD | Cyclobutane pyrimidine dimers |
| CRY1/2 | Cryptochrome-1 |
| CtIP | CtBP-interacting protein |
| DDB1/2 | DNA damage-binding protein |
| DDR | DNA damage Response |
| DNA2 | DNA replication ATP-dependent helicase/nuclease DNA2 |
| DNA-PKCS | DNA-dependent protein kinase, catalytic subunit |
| DSB | Double-strand break |
| DSS1 | DSS1 protein |
| EXO1 | Exonuclease 1 |
| FANC | Fanconi anaemia pathway |
| FEN1 | Flap endonuclease 1 |
| HLH | Helix–loop–helix motif |
| HMCES | 5-Hydroxymethylcytosine binding, ES-cell-specific |
| hnRNP-K | Ribonucleoprotein-K |
| HR | Homologous recombination |
| HR-23B | UV excision repair protein RAD23 homolog B |
| HSF | Heat shock factor |
| HSP | Heat shock protein |
| ICLs | Inter-/intrastrand crosslinks |
| lncRNA | Long-noncoding RNA |
| MDC1 | Mediator of DNA damage checkpoint protein 1 |
| MGMT | O6-methylguanine methyltransferase |
| MLH1 | MutL homolog 1 |
| MMR | Mismatch repair |
| MMS | Methyl-methanesulfonate |
| MRE11 | MRE11 homolog, double-strand break repair nuclease |
| MRN | MRE11, RAD50 and NBS1 complex |
| MSH2/3/6 | MutS homolog 2/3/6 |
| MYH | MutY homolog |
| NBS1 | Nibrin |
| NER | Nucleotide excision repair |
| NHEJ | Non-homologous end joining |
| OGG1 | 8-oxoguanine DNA glycosylase |
| PARP | Poly(ADP-ribose) polymerase |
| PAS | Per-Arnt-Sim domain |
| PBMCs | Peripheral blood mononuclear cells |
| PCNA | Proliferating cell nuclear antigen |
| PER1/2/3 | Period circadian protein homolog 1 |
| PMS | Mismatch repair endonuclease PMS2 |
| PNK | Polynucleotide kinase |
| RAD51 | RAD41 recombinase |
| RFC | Replication factor C |
| RNF168 | Ring finger protein 168 |
| ROS | Reactive oxygen species |
| RPA | Replication protein A |
| SCN | Suprachiasmatic nucleus |
| SIRT | Sirtuin |
| SNF2h | Sucrose nonfermenting-like 5 |
| SSA | Single-strand annealing |
| SSB | Single-strand break |
| SSBR | Single-strand break repair |
| TDG | Thymine DNA glycosylase |
| TDP1 | Tyrosyl-DNA phosphodiesterase 1 |
| TLS | Trans-lesion synthesis |
| TOPBP1 | DNA topoisomerase 2-binding protein 1 |
| TP53 | Cellular tumor antigen p53 |
| TP53BP1 | Tumor suppressor p53-binding protein 1 |
| TTFL | Transcription–translational feedback loop |
| WRN | Werner syndrome ATP-dependent helicase |
| XP | Xeroderma pigmentosum protein |
| XRCC1 | X-ray repair cross-complementing protein 1 |
| γH2AX | Phosphorylated histone protein H2AX |
References
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagenes. 2017, 58, 235–263. [Google Scholar] [CrossRef] [Green Version]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [Green Version]
- Roos, W.P.; Kaina, B. DNA damage-induced cell death: From specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013, 332, 237–248. [Google Scholar] [CrossRef]
- Harper, J.W.; Elledge, S.J. The DNA damage response: Ten years after. Mol. Cell 2007, 28, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Frydzińska, Z.; Owczarek, A.; Winiarska, K. Sirtuins and their role in metabolism regulation. Postepy Biochem. 2019, 65, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Haigis, M.C.; Guarente, L.P. Mammalian sirtuins—Emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006, 20, 2913–2921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michishita, E.; Park, J.Y.; Burneskis, J.M.; Barrett, J.C.; Horikawa, I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol. Biol. Cell 2005, 16, 4623–4635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, S.; Armstrong, C.M.; Kaeberlein, M.; Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 2000, 403, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.A. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem. Biophys. Res. Commun. 1999, 260, 273–279. [Google Scholar] [CrossRef]
- Rine, J.; Strathern, J.N.; Hicks, J.B.; Herskowitz, I. A Suppressor of Mating-Type Locus Mutations in Saccharomyces Cerevisiae: Evidence for and Identification of Cryptic Mating-Type Loci. Genetics 1979, 93, 877–901. [Google Scholar]
- Gottlieb, S.; Esposito, R.E. A new role for a yeast transcriptional silencer gene, SIR2, in regulation of recombination in ribosomal DNA. Cell 1989, 56, 771–776. [Google Scholar] [CrossRef]
- Braunstein, M.; Rose, A.B.; Holmes, S.G.; Allis, C.D.; Broach, J.R. Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev. 1993, 7, 592–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haigis, M.C.; Sinclair, D.A. Mammalian Sirtuins: Biological Insights and Disease Relevance. Annu. Rev. Pathol. 2010, 5, 253–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulose, N.; Raju, R. Sirtuin regulation in aging and injury. Biochim. Biophys. Acta 2015, 1852, 2442–2455. [Google Scholar] [CrossRef] [Green Version]
- Michan, S.; Sinclair, D. Sirtuins in mammals: Insights into their biological function. Biochem. J. 2007, 404, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, C.-S. SIRT1: Tumor promoter or tumor suppressor? Med. Hypotheses 2006, 67, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-H.; Sengupta, K.; Li, C.; Kim, H.-S.; Cao, L.; Xiao, C.; Kim, S.; Xu, X.; Zheng, Y.; Chilton, B.; et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell 2008, 14, 312–323. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Nikolaev, A.Y.; Imai, S.; Chen, D.; Su, F.; Shiloh, A.; Guarente, L.; Gu, W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 2001, 107, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.-L.; Mostoslavsky, R.; Saito, S.; Manis, J.P.; Gu, Y.; Patel, P.; Bronson, R.; Appella, E.; Alt, F.W.; Chua, K.F. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc. Natl. Acad. Sci. USA 2003, 100, 10794–10799. [Google Scholar] [CrossRef] [Green Version]
- Cohen, H.Y.; Lavu, S.; Bitterman, K.J.; Hekking, B.; Imahiyerobo, T.A.; Miller, C.; Frye, R.; Ploegh, H.; Kessler, B.M.; Sinclair, D.A. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol. Cell 2004, 13, 627–638. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, X.; Sengupta, N.; Lane, W.S.; Seto, E. SIRT1 regulates the function of the Nijmegen breakage syndrome protein. Mol. Cell 2007, 27, 149–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Casta, A.; Wang, R.; Lozada, E.; Fan, W.; Kane, S.; Ge, Q.; Gu, W.; Orren, D.; Luo, J. Regulation of WRN protein cellular localization and enzymatic activities by SIRT1-mediated deacetylation. J. Biol. Chem. 2008, 283, 7590–7598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberdoerffer, P.; Michan, S.; McVay, M.; Mostoslavsky, R.; Vann, J.; Park, S.-K.; Hartlerode, A.; Stegmuller, J.; Hafner, A.; Loerch, P.; et al. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell 2008, 135, 907–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, T.; Maier, B.; Koclega, K.D.; Chruszcz, M.; Gluba, W.; Stukenberg, P.T.; Minor, W.; Scrable, H. Phosphorylation regulates SIRT1 function. PLoS ONE 2008, 3, e4020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tennen, R.I.; Chua, K.F. Chromatin regulation and genome maintenance by mammalian SIRT6. Trends Biochem. Sci. 2011, 36, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostoslavsky, R.; Chua, K.F.; Lombard, D.B.; Pang, W.W.; Fischer, M.R.; Gellon, L.; Liu, P.; Mostoslavsky, G.; Franco, S.; Murphy, M.M.; et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 2006, 124, 315–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toiber, D.; Erdel, F.; Bouazoune, K.; Silberman, D.M.; Zhong, L.; Mulligan, P.; Sebastian, C.; Cosentino, C.; Martinez-Pastor, B.; Giacosa, S.; et al. SIRT6 recruits SNF2H to DNA break sites, preventing genomic instability through chromatin remodeling. Mol. Cell 2013, 51, 454–468. [Google Scholar] [CrossRef] [Green Version]
- McCord, R.A.; Michishita, E.; Hong, T.; Berber, E.; Boxer, L.D.; Kusumoto, R.; Guan, S.; Shi, X.; Gozani, O.; Burlingame, A.L.; et al. SIRT6 stabilizes DNA-dependent protein kinase at chromatin for DNA double-strand break repair. Aging 2009, 1, 109–121. [Google Scholar] [CrossRef] [Green Version]
- Mao, Z.; Hine, C.; Tian, X.; Van Meter, M.; Au, M.; Vaidya, A.; Seluanov, A.; Gorbunova, V. SIRT6 promotes DNA repair under stress by activating PARP1. Science 2011, 332, 1443–1446. [Google Scholar] [CrossRef] [Green Version]
- Schreiber, V.; Amé, J.-C.; Dollé, P.; Schultz, I.; Rinaldi, B.; Fraulob, V.; Ménissier-de Murcia, J.; de Murcia, G. Poly(ADP-ribose) polymerase-2 (PARP-2) is required for efficient base excision DNA repair in association with PARP-1 and XRCC1. J. Biol. Chem. 2002, 277, 23028–23036. [Google Scholar] [CrossRef] [Green Version]
- Hwang, B.-J.; Jin, J.; Gao, Y.; Shi, G.; Madabushi, A.; Yan, A.; Guan, X.; Zalzman, M.; Nakajima, S.; Lan, L.; et al. SIRT6 protein deacetylase interacts with MYH DNA glycosylase, APE1 endonuclease, and Rad9-Rad1-Hus1 checkpoint clamp. BMC Mol. Biol. 2015, 16, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamori, T.; DeRicco, J.; Naqvi, A.; Hoffman, T.A.; Mattagajasingh, I.; Kasuno, K.; Jung, S.-B.; Kim, C.-S.; Irani, K. SIRT1 deacetylates APE1 and regulates cellular base excision repair. Nucleic Acids Res. 2010, 38, 832–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madabushi, A.; Hwang, B.-J.; Jin, J.; Lu, A.-L. Histone deacetylase SIRT1 modulates and deacetylates DNA base excision repair enzyme thymine DNA glycosylase. Biochem. J. 2013, 456, 89–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, W.; Luo, J. SIRT1 regulates UV-induced DNA repair through deacetylating XPA. Mol. Cell 2010, 39, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Ming, M.; Shea, C.R.; Guo, X.; Li, X.; Soltani, K.; Han, W.; He, Y.-Y. Regulation of global genome nucleotide excision repair by SIRT1 through xeroderma pigmentosum C. Proc. Natl. Acad. Sci. USA 2010, 107, 22623–22628. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.; Huh, Y.J.; Cho, H.-J.; Lee, B.; Park, J.; Hwang, D.-Y.; Kim, D.-W. SIRT1 Enhances the Survival of Human Embryonic Stem Cells by Promoting DNA Repair. Stem Cell Rep. 2017, 9, 629–641. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Park, S.-H.; Pantazides, B.G.; Karpiuk, O.; Warren, M.D.; Hardy, C.W.; Duong, D.M.; Park, S.-J.; Kim, H.-S.; Vassilopoulos, A.; et al. SIRT2 directs the replication stress response through CDK9 deacetylation. Proc. Natl. Acad. Sci. USA 2013, 110, 13546–13551. [Google Scholar] [CrossRef] [Green Version]
- Serrano, L.; Martínez-Redondo, P.; Marazuela-Duque, A.; Vazquez, B.N.; Dooley, S.J.; Voigt, P.; Beck, D.B.; Kane-Goldsmith, N.; Tong, Q.; Rabanal, R.M.; et al. The tumor suppressor SirT2 regulates cell cycle progression and genome stability by modulating the mitotic deposition of H4K20 methylation. Genes Dev. 2013, 27, 639–653. [Google Scholar] [CrossRef] [Green Version]
- Vakhrusheva, O.; Smolka, C.; Gajawada, P.; Kostin, S.; Boettger, T.; Kubin, T.; Braun, T.; Bober, E. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ. Res. 2008, 102, 703–710. [Google Scholar] [CrossRef] [Green Version]
- Barber, M.F.; Michishita-Kioi, E.; Xi, Y.; Tasselli, L.; Kioi, M.; Moqtaderi, Z.; Tennen, R.I.; Paredes, S.; Young, N.L.; Chen, K.; et al. SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature 2012, 487, 114–118. [Google Scholar] [CrossRef]
- Vazquez, B.N.; Thackray, J.K.; Simonet, N.G.; Kane-Goldsmith, N.; Martinez-Redondo, P.; Nguyen, T.; Bunting, S.; Vaquero, A.; Tischfield, J.A.; Serrano, L. SIRT7 promotes genome integrity and modulates non-homologous end joining DNA repair. EMBO J. 2016, 35, 1488–1503. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Scheibye-Knudsen, M.; Chua, K.F.; Mattson, M.P.; Croteau, D.L.; Bohr, V.A. Nuclear DNA damage signalling to mitochondria in ageing. Nat. Rev. Mol. Cell Biol. 2016, 17, 308–321. [Google Scholar] [CrossRef] [Green Version]
- Someya, S.; Yu, W.; Hallows, W.C.; Xu, J.; Vann, J.M.; Leeuwenburgh, C.; Tanokura, M.; Denu, J.M.; Prolla, T.A. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 2010, 143, 802–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundaresan, N.R.; Samant, S.A.; Pillai, V.B.; Rajamohan, S.B.; Gupta, M.P. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol. Cell. Biol. 2008, 28, 6384–6401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scher, M.B.; Vaquero, A.; Reinberg, D. SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes Dev. 2007, 21, 920–928. [Google Scholar] [CrossRef] [Green Version]
- Hirschey, M.D.; Shimazu, T.; Jing, E.; Grueter, C.A.; Collins, A.M.; Aouizerat, B.; Stančáková, A.; Goetzman, E.; Lam, M.M.; Schwer, B.; et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol. Cell 2011, 44, 177–190. [Google Scholar] [CrossRef] [Green Version]
- Tchou, J.; Grollman, A.P. Repair of DNA containing the oxidatively-damaged base, 8-oxoguanine. Mutat. Res. 1993, 299, 277–287. [Google Scholar] [CrossRef]
- Haigis, M.C.; Mostoslavsky, R.; Haigis, K.M.; Fahie, K.; Christodoulou, D.C.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; Karow, M.; Blander, G.; et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell 2006, 126, 941–954. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Wang, S.; Gai, J.; Guan, J.; Li, J.; Li, Y.; Zhao, J.; Zhao, C.; Fu, L.; Li, Q. SIRT5 Promotes Cisplatin Resistance in Ovarian Cancer by Suppressing DNA Damage in a ROS-Dependent Manner via Regulation of the Nrf2/HO-1 Pathway. Front. Oncol. 2019, 9, 754. [Google Scholar] [CrossRef] [Green Version]
- Caito, S.; Rajendrasozhan, S.; Cook, S.; Chung, S.; Yao, H.; Friedman, A.E.; Brookes, P.S.; Rahman, I. SIRT1 is a redox-sensitive deacetylase that is post-translationally modified by oxidants and carbonyl stress. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 3145–3159. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.; Jung, J.-W.; Kim, M.K.; Chung, J.H. CK2 is the regulator of SIRT1 substrate-binding affinity, deacetylase activity and cellular response to DNA-damage. PLoS ONE 2009, 4, e6611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Fu, W.; Chen, J.; Olashaw, N.; Zhang, X.; Nicosia, S.V.; Bhalla, K.; Bai, W. SIRT1 sumoylation regulates its deacetylase activity and cellular response to genotoxic stress. Nat. Cell Biol. 2007, 9, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Yamakuchi, M. MicroRNA Regulation of SIRT1. Front. Physiol. 2012, 3, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.-L. Linking Long Noncoding RNA Localization and Function. Trends Biochem. Sci. 2016, 41, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Mathur, R.; Hu, X.; Liu, Y.; Zhang, X.; Peng, G.; Lu, X. Long non-coding RNA ANRIL (CDKN2B-AS) is induced by the ATM-E2F1 signaling pathway. Cell. Signal. 2013, 25, 1086–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhade, V.S.; Pal, D.; Kanduri, C. Long Noncoding RNA: Genome Organization and Mechanism of Action. Adv. Exp. Med. Biol. 2017, 1008, 47–74. [Google Scholar] [CrossRef]
- Thapar, R. Regulation of DNA Double-Strand Break Repair by Non-Coding RNAs. Mol. Basel Switz. 2018, 23, 2789. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, M.T.; Karlseder, J. DNA damage associated with mitosis and cytokinesis failure. Oncogene 2013, 32, 4593–4601. [Google Scholar] [CrossRef] [Green Version]
- Podhorecka, M.; Skladanowski, A.; Bozko, P. H2AX Phosphorylation: Its Role in DNA Damage Response and Cancer Therapy. J. Nucleic Acids 2010, 2010. [Google Scholar] [CrossRef] [Green Version]
- Raleigh, D.R.; Haas-Kogan, D.A. Molecular targets and mechanisms of radiosensitization using DNA damage response pathways. Future Oncol. Lond. Engl. 2013, 9, 219–233. [Google Scholar] [CrossRef] [Green Version]
- Pennisi, R.; Ascenzi, P.; di Masi, A. Hsp90: A New Player in DNA Repair? Biomolecules 2015, 5, 2589–2618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huarte, M.; Guttman, M.; Feldser, D.; Garber, M.; Koziol, M.J.; Kenzelmann-Broz, D.; Khalil, A.M.; Zuk, O.; Amit, I.; Rabani, M.; et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 2010, 142, 409–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, T.; Wang, Y.; Lin, M.F.; Koegel, A.K.; Kotake, Y.; Grant, G.D.; Horlings, H.M.; Shah, N.; Umbricht, C.; Wang, P.; et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat. Genet. 2011, 43, 621–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, A.M.; Garcia, J.T.; Hung, T.; Flynn, R.A.; Shen, Y.; Qu, K.; Payumo, A.Y.; Peres-da-Silva, A.; Broz, D.K.; Baum, R.; et al. An inducible long noncoding RNA amplifies DNA damage signaling. Nat. Genet. 2016, 48, 1370–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoudi, S.; Henriksson, S.; Corcoran, M.; Méndez-Vidal, C.; Wiman, K.G.; Farnebo, M. Wrap53, a natural p53 antisense transcript required for p53 induction upon DNA damage. Mol. Cell 2009, 33, 462–471. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Q.; Hu, Z.; Feng, Y.; Fan, L.; Tang, Z.; Yuan, J.; Shan, W.; Li, C.; Hu, X.; et al. Long noncoding RNA LINP1 regulates repair of DNA double-strand breaks in triple-negative breast cancer. Nat. Struct. Mol. Biol. 2016, 23, 522–530. [Google Scholar] [CrossRef]
- Kim, J.-E.; Chen, J.; Lou, Z. DBC1 is a negative regulator of SIRT1. Nature 2008, 451, 583–586. [Google Scholar] [CrossRef]
- Yi, J.; Luo, J. SIRT1 and p53, effect on cancer, senescence and beyond. Biochim. Biophys. Acta 2010, 1804, 1684–1689. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-H.; Yuan, J.; Pei, H.; Liu, T.; Ann, D.K.; Lou, Z. KAP1 Deacetylation by SIRT1 Promotes Non-Homologous End-Joining Repair. PLoS ONE 2015, 10, e0123935. [Google Scholar] [CrossRef]
- Sharma, V.; Khurana, S.; Kubben, N.; Abdelmohsen, K.; Oberdoerffer, P.; Gorospe, M.; Misteli, T. A BRCA1-interacting lncRNA regulates homologous recombination. EMBO Rep. 2015, 16, 1520–1534. [Google Scholar] [CrossRef] [Green Version]
- Polo, S.E.; Blackford, A.N.; Chapman, J.R.; Baskcomb, L.; Gravel, S.; Rusch, A.; Thomas, A.; Blundred, R.; Smith, P.; Kzhyshkowska, J.; et al. Regulation of DNA-end resection by hnRNPU-like proteins promotes DNA double-strand break signaling and repair. Mol. Cell 2012, 45, 505–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betts, J.A.; Moradi Marjaneh, M.; Al-Ejeh, F.; Lim, Y.C.; Shi, W.; Sivakumaran, H.; Tropée, R.; Patch, A.-M.; Clark, M.B.; Bartonicek, N.; et al. Long Noncoding RNAs CUPID1 and CUPID2 Mediate Breast Cancer Risk at 11q13 by Modulating the Response to DNA Damage. Am. J. Hum. Genet. 2017, 101, 255–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.L.; Jin, L.; Xu, A.; Wang, Y.F.; Thorne, R.F.; Zhang, X.D.; Wu, M. GUARDIN is a p53-responsive long non-coding RNA that is essential for genomic stability. Nat. Cell Biol. 2018, 20, 492–502. [Google Scholar] [CrossRef]
- Gazy, I.; Zeevi, D.A.; Renbaum, P.; Zeligson, S.; Eini, L.; Bashari, D.; Smith, Y.; Lahad, A.; Goldberg, M.; Ginsberg, D.; et al. TODRA, a lncRNA at the RAD51 Locus, Is Oppositely Regulated to RAD51, and Enhances RAD51-Dependent DSB (Double Strand Break) Repair. PLoS ONE 2015, 10, e0134120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilyugin, M.; Irminger-Finger, I. Long non-coding RNA and microRNAs might act in regulating the expression of BARD1 mRNAs. Int. J. Biochem. Cell Biol. 2014, 54, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Hollander, M.C.; Alamo, I.; Fornace, A.J. A novel DNA damage-inducible transcript, gadd7, inhibits cell growth, but lacks a protein product. Nucleic Acids Res. 1996, 24, 1589–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Li, D.; Zhang, W.; Guo, M.; Zhan, Q. Long non-coding RNA gadd7 interacts with TDP-43 and regulates Cdk6 mRNA decay. EMBO J. 2012, 31, 4415–4427. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.-C.; Spitale, R.C.; Chang, H.Y. Long intergenic noncoding RNAs: New links in cancer progression. Cancer Res. 2011, 71, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Özeş, A.R.; Miller, D.F.; Özeş, O.N.; Fang, F.; Liu, Y.; Matei, D.; Huang, T.; Nephew, K.P. NF-κB-HOTAIR axis links DNA damage response, chemoresistance and cellular senescence in ovarian cancer. Oncogene 2016, 35, 5350–5361. [Google Scholar] [CrossRef] [Green Version]
- Wan, G.; Hu, X.; Liu, Y.; Han, C.; Sood, A.K.; Calin, G.A.; Zhang, X.; Lu, X. A novel non-coding RNA lncRNA-JADE connects DNA damage signalling to histone H4 acetylation. EMBO J. 2013, 32, 2833–2847. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, M.; Emadi-Baygi, M.; Hoffmann, M.J.; Schulz, W.A.; Nikpour, P. Altered expression of LINC-ROR in cancer cell lines and tissues. Tumor Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2016, 37, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Liu, C.; Cui, F.-M.; Xu, J.-Y.; Tong, J.; Qi, X.-F.; Wang, L.-L.; Zhu, W. Long intergenic non-coding RNA induced by X-ray irradiation regulates DNA damage response signaling in the human bronchial epithelial BEAS-2B cell line. Oncol. Lett. 2015, 9, 169–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Y.; Ma, G.; Zhang, Z.; Hua, Q.; Chu, H.; Tong, N.; Yuan, L.; Qin, C.; Yin, C.; Zhang, Z.; et al. A novel antisense long noncoding RNA regulates the expression of MDC1 in bladder cancer. Oncotarget 2015, 6, 484–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adriaens, C.; Standaert, L.; Barra, J.; Latil, M.; Verfaillie, A.; Kalev, P.; Boeckx, B.; Wijnhoven, P.W.G.; Radaelli, E.; Vermi, W.; et al. p53 induces formation of NEAT1 lncRNA-containing paraspeckles that modulate replication stress response and chemosensitivity. Nat. Med. 2016, 22, 861–868. [Google Scholar] [CrossRef]
- Prensner, J.R.; Iyer, M.K.; Balbin, O.A.; Dhanasekaran, S.M.; Cao, Q.; Brenner, J.C.; Laxman, B.; Asangani, I.A.; Grasso, C.S.; Kominsky, H.D.; et al. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat. Biotechnol. 2011, 29, 742–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prensner, J.R.; Chen, W.; Iyer, M.K.; Cao, Q.; Ma, T.; Han, S.; Sahu, A.; Malik, R.; Wilder-Romans, K.; Navone, N.; et al. PCAT-1, a long noncoding RNA, regulates BRCA2 and controls homologous recombination in cancer. Cancer Res. 2014, 74, 1651–1660. [Google Scholar] [CrossRef] [Green Version]
- Prensner, J.R.; Chen, W.; Han, S.; Iyer, M.K.; Cao, Q.; Kothari, V.; Evans, J.R.; Knudsen, K.E.; Paulsen, M.T.; Ljungman, M.; et al. The long non-coding RNA PCAT-1 promotes prostate cancer cell proliferation through cMyc. Neoplasia 2014, 16, 900–908. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, R.; Gryder, B.; Woods, W.S.; Subramanian, M.; Jones, M.F.; Li, X.L.; Jenkins, L.M.; Shabalina, S.A.; Mo, M.; Dasso, M.; et al. Prosurvival long noncoding RNA PINCR regulates a subset of p53 targets in human colorectal cancer cells by binding to Matrin 3. eLife 2017, 6. [Google Scholar] [CrossRef]
- Marín-Béjar, O.; Marchese, F.P.; Athie, A.; Sánchez, Y.; González, J.; Segura, V.; Huang, L.; Moreno, I.; Navarro, A.; Monzó, M.; et al. Pint lincRNA connects the p53 pathway with epigenetic silencing by the Polycomb repressive complex 2. Genome Biol. 2013, 14, R104. [Google Scholar] [CrossRef] [Green Version]
- Marín-Béjar, O.; Mas, A.M.; González, J.; Martinez, D.; Athie, A.; Morales, X.; Galduroz, M.; Raimondi, I.; Grossi, E.; Guo, S.; et al. The human lncRNA LINC-PINT inhibits tumor cell invasion through a highly conserved sequence element. Genome Biol. 2017, 18, 202. [Google Scholar] [CrossRef]
- Li, X.L.; Subramanian, M.; Jones, M.F.; Chaudhary, R.; Singh, D.K.; Zong, X.; Gryder, B.; Sindri, S.; Mo, M.; Schetter, A.; et al. Long Noncoding RNA PURPL Suppresses Basal p53 Levels and Promotes Tumorigenicity in Colorectal Cancer. Cell Rep. 2017, 20, 2408–2423. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, Y.; Segura, V.; Marín-Béjar, O.; Athie, A.; Marchese, F.P.; González, J.; Bujanda, L.; Guo, S.; Matheu, A.; Huarte, M. Genome-wide analysis of the human p53 transcriptional network unveils a lncRNA tumour suppressor signature. Nat. Commun. 2014, 5, 5812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maringele, L.; Lydall, D. EXO1-dependent single-stranded DNA at telomeres activates subsets of DNA damage and spindle checkpoint pathways in budding yeast yku70Delta mutants. Genes Dev. 2002, 16, 1919–1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porro, A.; Feuerhahn, S.; Lingner, J. TERRA-reinforced association of LSD1 with MRE11 promotes processing of uncapped telomeres. Cell Rep. 2014, 6, 765–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.; Shan, B.; He, D.; Cheng, Y.; Li, B.; Zhang, C.; Duan, C. Recent Progress in Characterizing Long Noncoding RNAs in Cancer Drug Resistance. J. Cancer 2019, 10, 6693–6702. [Google Scholar] [CrossRef]
- Ritossa, F. Discovery of the heat shock response. Cell Stress Chaperones 1996, 1, 97–98. [Google Scholar] [CrossRef] [Green Version]
- De Maio, A.; Santoro, M.G.; Tanguay, R.M.; Hightower, L.E. Ferruccio Ritossa’s scientific legacy 50 years after his discovery of the heat shock response: A new view of biology, a new society, and a new journal. Cell Stress Chaperones 2012, 17, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Roh, H.-T.; Cho, S.-Y.; So, W.-Y.; Paik, I.-Y.; Suh, S.-H. Effects of different fluid replacements on serum HSP70 and lymphocyte DNA damage in college athletes during exercise at high ambient temperatures. J. Sport Health Sci. 2016, 5, 448–455. [Google Scholar] [CrossRef] [Green Version]
- Dubrez, L.; Causse, S.; Borges Bonan, N.; Dumétier, B.; Garrido, C. Heat-shock proteins: Chaperoning DNA repair. Oncogene 2019. [Google Scholar] [CrossRef]
- Macario, A.J.L.; Conway de Macario, E. Molecular chaperones: Multiple functions, pathologies, and potential applications. Front. Biosci. 2007, 12, 2588–2600. [Google Scholar] [CrossRef] [Green Version]
- Akerfelt, M.; Morimoto, R.I.; Sistonen, L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010, 11, 545–555. [Google Scholar] [CrossRef]
- Slimen, I.B.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperth. 2014, 30, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Konishi, T.; Hirano, T.; Kasai, H.; Shimizu, K.; Kashimura, M.; Higashi, K. Possible correlation between DNA damage induced by hydrogen peroxide and translocation of heat shock 70 protein into the nucleus. Biochem. Biophys. Res. Commun. 1995, 206, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Martínez de Toda, I.; De la Fuente, M. The role of Hsp70 in oxi-inflamm-aging and its use as a potential biomarker of lifespan. Biogerontology 2015, 16, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Nadin, S.B.; Vargas-Roig, L.M.; Cuello-Carrión, F.D.; Ciocca, D.R. Deoxyribonucleic acid damage induced by doxorubicin in peripheral blood mononuclear cells: Possible roles for the stress response and the deoxyribonucleic acid repair process. Cell Stress Chaperones 2003, 8, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Nadin, S.B.; Vargas-Roig, L.M.; Drago, G.; Ibarra, J.; Ciocca, D.R. DNA damage and repair in peripheral blood lymphocytes from healthy individuals and cancer patients: A pilot study on the implications in the clinical response to chemotherapy. Cancer Lett. 2006, 239, 84–97. [Google Scholar] [CrossRef]
- Nadin, S.B.; Vargas-Roig, L.M.; Drago, G.; Ibarra, J.; Ciocca, D.R. Hsp27, Hsp70 and mismatch repair proteins hMLH1 and hMSH2 expression in peripheral blood lymphocytes from healthy subjects and cancer patients. Cancer Lett. 2007, 252, 131–146. [Google Scholar] [CrossRef]
- Nadin, S.B.; Cuello-Carrión, F.D.; Sottile, M.L.; Ciocca, D.R.; Vargas-Roig, L.M. Effects of hyperthermia on Hsp27 (HSPB1), Hsp72 (HSPA1A) and DNA repair proteins hMLH1 and hMSH2 in human colorectal cancer hMLH1-deficient and hMLH1-proficient cell lines. Int. J. Hyperth. 2012, 28, 191–201. [Google Scholar] [CrossRef]
- Sottile, M.L.; Losinno, A.D.; Fanelli, M.A.; Cuello-Carrión, F.D.; Montt-Guevara, M.M.; Vargas-Roig, L.M.; Nadin, S.B. Hyperthermia effects on Hsp27 and Hsp72 associations with mismatch repair (MMR) proteins and cisplatin toxicity in MMR-deficient/proficient colon cancer cell lines. Int. J. Hyperth. 2015, 31, 464–475. [Google Scholar] [CrossRef]
- Tung, C.-L.; Chiu, H.-C.; Jian, Y.-J.; Jian, Y.-T.; Chen, C.-Y.; Syu, J.-J.; Wo, T.-Y.; Huang, Y.-J.; Tseng, S.-C.; Lin, Y.-W. Down-regulation of MSH2 expression by an Hsp90 inhibitor enhances pemetrexed-induced cytotoxicity in human non-small-cell lung cancer cells. Exp. Cell Res. 2014, 322, 345–354. [Google Scholar] [CrossRef]
- Fedier, A.; Stuedli, A.; Fink, D. Presence of MLH1 protein aggravates the potential of the HSP90 inhibitor radicicol to sensitize tumor cells to cisplatin. Int. J. Oncol. 2005, 27, 1697–1705. [Google Scholar] [PubMed]
- Park, S.H.; Lee, S.J.; Chung, H.Y.; Kim, T.H.; Cho, C.K.; Yoo, S.Y.; Lee, Y.S. Inducible heat-shock protein 70 is involved in the radioadaptive response. Radiat. Res. 2000, 153, 318–326. [Google Scholar] [CrossRef]
- Bases, R. Heat shock protein 70 enhanced deoxyribonucleic acid base excision repair in human leukemic cells after ionizing radiation. Cell Stress Chaperones 2006, 11, 240–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenny, M.K.; Mendez, F.; Sandigursky, M.; Kureekattil, R.P.; Goldman, J.D.; Franklin, W.A.; Bases, R. Heat shock protein 70 binds to human apurinic/apyrimidinic endonuclease and stimulates endonuclease activity at abasic sites. J. Biol. Chem. 2001, 276, 9532–9536. [Google Scholar] [CrossRef] [Green Version]
- Fang, Q.; Inanc, B.; Schamus, S.; Wang, X.; Wei, L.; Brown, A.R.; Svilar, D.; Sugrue, K.F.; Goellner, E.M.; Zeng, X.; et al. HSP90 regulates DNA repair via the interaction between XRCC1 and DNA polymerase β. Nat. Commun. 2014, 5, 5513. [Google Scholar] [CrossRef] [Green Version]
- Mendez, F.; Sandigursky, M.; Franklin, W.A.; Kenny, M.K.; Kureekattil, R.; Bases, R. Heat-shock proteins associated with base excision repair enzymes in HeLa cells. Radiat. Res. 2000, 153, 186–195. [Google Scholar] [CrossRef]
- Mendez, F.; Kozin, E.; Bases, R. Heat shock protein 70 stimulation of the deoxyribonucleic acid base excision repair enzyme polymerase beta. Cell Stress Chaperones 2003, 8, 153–161. [Google Scholar] [CrossRef]
- Kotoglou, P.; Kalaitzakis, A.; Vezyraki, P.; Tzavaras, T.; Michalis, L.K.; Dantzer, F.; Jung, J.U.; Angelidis, C. Hsp70 translocates to the nuclei and nucleoli, binds to XRCC1 and PARP-1, and protects HeLa cells from single-strand DNA breaks. Cell Stress Chaperones 2009, 14, 391–406. [Google Scholar] [CrossRef] [Green Version]
- Wano, C.; Kita, K.; Takahashi, S.; Sugaya, S.; Hino, M.; Hosoya, H.; Suzuki, N. Protective role of HSP27 against UVC-induced cell death in human cells. Exp. Cell Res. 2004, 298, 584–592. [Google Scholar] [CrossRef]
- Xiao, C.; Chen, S.; Li, J.; Hai, T.; Lu, Q.; Sun, E.; Wang, R.; Tanguay, R.M.; Wu, T. Association of HSP70 and genotoxic damage in lymphocytes of workers exposed to coke-oven emission. Cell Stress Chaperones 2002, 7, 396–402. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.-J.; Xiao, C.-F.; Chen, S.; Wang, R.-B.; He, H.-Z.; Tanguay, R.M.; Wu, T.-C. In vitro study on role of Hsp70 expression in DNA damage of human embryonic lung cells exposed to Benzo [a] pyrene. Biomed. Environ. Sci. BES 2004, 17, 144–152. [Google Scholar]
- Niu, P.; Liu, L.; Gong, Z.; Tan, H.; Wang, F.; Yuan, J.; Feng, Y.; Wei, Q.; Tanguay, R.M.; Wu, T. Overexpressed heat shock protein 70 protects cells against DNA damage caused by ultraviolet C in a dose-dependent manner. Cell Stress Chaperones 2006, 11, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, X.; Niu, P.; Zou, Y.; Duan, Y. Correlations and co-localizations of Hsp70 with XPA, XPG in human bronchial epithelia cells exposed to benzo [a] pyrene. Toxicology 2009, 265, 10–14. [Google Scholar] [CrossRef]
- Sottile, M.L.; Nadin, S.B. Heat shock proteins and DNA repair mechanisms: An updated overview. Cell Stress Chaperones 2018, 23, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, M.; Kuroda, M.; Urano, M.; Nishimura, Y.; Kawasaki, S.; Kato, H.; Okumura, Y.; Akaki, S.; Kanazawa, S.; Asaumi, J.; et al. The effect of various chemotherapeutic agents given with mild hyperthermia on different types of tumours. Int. J. Hyperth. 2003, 19, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Urano, M.; Ling, C.C. Thermal enhancement of melphalan and oxaliplatin cytotoxicity in vitro. Int. J. Hyperth. 2002, 18, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.-C.; Zips, D.; Heinrich, V.; Lamprecht, U.; Voigt, O.; Burock, S.; Budach, V.; Wust, P.; Ghadjar, P. Regional hyperthermia and moderately dose-escalated salvage radiotherapy for recurrent prostate cancer. Protocol of a phase II trial. Radiat. Oncol. Lond. Engl. 2015, 10, 138. [Google Scholar] [CrossRef] [Green Version]
- Seifert, G.; Budach, V.; Keilholz, U.; Wust, P.; Eggert, A.; Ghadjar, P. Regional hyperthermia combined with chemotherapy in paediatric, adolescent and young adult patients: Current and future perspectives. Radiat. Oncol. Lond. Engl. 2016, 11, 65. [Google Scholar] [CrossRef] [Green Version]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wessalowski, R.; Reichardt, P.; Wust, P.; Ghadjar, P.; Hohenberger, P.; Angele, M.; Salat, C.; et al. Effect of Neoadjuvant Chemotherapy Plus Regional Hyperthermia on Long-term Outcomes Among Patients With Localized High-Risk Soft Tissue Sarcoma: The EORTC 62961-ESHO 95 Randomized Clinical Trial. JAMA Oncol. 2018, 4, 483–492. [Google Scholar] [CrossRef]
- Niture, S.K.; Doneanu, C.E.; Velu, C.S.; Bailey, N.I.; Srivenugopal, K.S. Proteomic analysis of human O6-methylguanine-DNA methyltransferase by affinity chromatography and tandem mass spectrometry. Biochem. Biophys. Res. Commun. 2005, 337, 1176–1184. [Google Scholar] [CrossRef]
- Sakamoto, A.N. Translesion Synthesis in Plants: Ultraviolet Resistance and Beyond. Front. Plant Sci. 2019, 10, 1208. [Google Scholar] [CrossRef]
- Katsogiannou, M.; Andrieu, C.; Baylot, V.; Baudot, A.; Dusetti, N.J.; Gayet, O.; Finetti, P.; Garrido, C.; Birnbaum, D.; Bertucci, F.; et al. The functional landscape of Hsp27 reveals new cellular processes such as DNA repair and alternative splicing and proposes novel anticancer targets. Mol. Cell. Proteom. MCP 2014, 13, 3585–3601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solier, S.; Kohn, K.W.; Scroggins, B.; Xu, W.; Trepel, J.; Neckers, L.; Pommier, Y. Heat shock protein 90α (HSP90α), a substrate and chaperone of DNA-PK necessary for the apoptotic response. Proc. Natl. Acad. Sci. USA 2012, 109, 12866–12872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Causse, S.Z.; Marcion, G.; Chanteloup, G.; Uyanik, B.; Boudesco, C.; Grigorash, B.B.; Douhard, R.; Dias, A.M.M.; Dumetier, B.; Dondaine, L.; et al. HSP110 translocates to the nucleus upon genotoxic chemotherapy and promotes DNA repair in colorectal cancer cells. Oncogene 2019, 38, 2767–2777. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Yu, D.; Hirayama, R.; Ninomiya, Y.; Sekine, E.; Kubota, N.; Ando, K.; Okayasu, R. Inhibition of homologous recombination repair in irradiated tumor cells pretreated with Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Biochem. Biophys. Res. Commun. 2006, 351, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Dote, H.; Burgan, W.E.; Camphausen, K.; Tofilon, P.J. Inhibition of hsp90 compromises the DNA damage response to radiation. Cancer Res. 2006, 66, 9211–9220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oda, T.; Hayano, T.; Miyaso, H.; Takahashi, N.; Yamashita, T. Hsp90 regulates the Fanconi anemia DNA damage response pathway. Blood 2007, 109, 5016–5026. [Google Scholar] [CrossRef]
- Ha, K.; Fiskus, W.; Rao, R.; Balusu, R.; Venkannagari, S.; Nalabothula, N.R.; Bhalla, K.N. Hsp90 inhibitor-mediated disruption of chaperone association of ATR with hsp90 sensitizes cancer cells to DNA damage. Mol. Cancer Ther. 2011, 10, 1194–1206. [Google Scholar] [CrossRef] [Green Version]
- Guttmann, D.M.; Hart, L.; Du, K.; Seletsky, A.; Koumenis, C. Inhibition of Hsp27 radiosensitizes head-and-neck cancer by modulating deoxyribonucleic acid repair. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 168–175. [Google Scholar] [CrossRef]
- Elaimy, A.L.; Ahsan, A.; Marsh, K.; Pratt, W.B.; Ray, D.; Lawrence, T.S.; Nyati, M.K. ATM is the primary kinase responsible for phosphorylation of Hsp90α after ionizing radiation. Oncotarget 2016, 7, 82450–82457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell-Pedersen, D.; Cassone, V.M.; Earnest, D.J.; Golden, S.S.; Hardin, P.E.; Thomas, T.L.; Zoran, M.J. Circadian rhythms from multiple oscillators: Lessons from diverse organisms. Nat. Rev. Genet. 2005, 6, 544–556. [Google Scholar] [CrossRef]
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef]
- Hastings, M.H.; Reddy, A.B.; Maywood, E.S. A clockwork web: Circadian timing in brain and periphery, in health and disease. Nat. Rev. Neurosci. 2003, 4, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Murre, C. Helix-loop-helix proteins and the advent of cellular diversity: 30 years of discovery. Genes Dev. 2019, 33, 6–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streuli, C.H.; Meng, Q.-J. Influence of the extracellular matrix on cell-intrinsic circadian clocks. J. Cell Sci. 2019, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef] [Green Version]
- Rahman, S.; Kraljević Pavelić, S.; Markova-Car, E. Circadian (De)regulation in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 2662. [Google Scholar] [CrossRef] [Green Version]
- Pacheco-Bernal, I.; Becerril-Pérez, F.; Aguilar-Arnal, L. Circadian rhythms in the three-dimensional genome: Implications of chromatin interactions for cyclic transcription. Clin. Epigenetics 2019, 11, 79. [Google Scholar] [CrossRef]
- Sancar, A.; Lindsey-Boltz, L.A.; Kang, T.-H.; Reardon, J.T.; Lee, J.H.; Ozturk, N. Circadian Clock Control of the Cellular Response to DNA Damage. FEBS Lett. 2010, 584, 2618–2625. [Google Scholar] [CrossRef] [Green Version]
- Li, H.-X. The role of circadian clock genes in tumors. OncoTargets Ther. 2019, 12, 3645–3660. [Google Scholar] [CrossRef] [Green Version]
- Nakahata, Y.; Kaluzova, M.; Grimaldi, B.; Sahar, S.; Hirayama, J.; Chen, D.; Guarente, L.P.; Sassone-Corsi, P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 2008, 134, 329–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F.W.; Schibler, U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 2008, 134, 317–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, S.-A.; Fang, M.Z.; Rustgi, V.; Zarbl, H.; Androulakis, I.P. At the Interface of Lifestyle, Behavior, and Circadian Rhythms: Metabolic Implications. Front. Nutr. 2019, 6, 132. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Rigor, P.; Cervantes, M.; Ceglia, N.; Sebastian, C.; Xiao, C.; Roqueta-Rivera, M.; Deng, C.; Osborne, T.F.; Mostoslavsky, R.; et al. Partitioning circadian transcription by SIRT6 leads to segregated control of cellular metabolism. Cell 2014, 158, 659–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Hu, Y.; Li, X.; Jin, G.; Chen, X.; Chen, G.; Chen, Y.; Huang, S.; Liao, W.; Liao, Y.; et al. Sirt1 Antisense Long Noncoding RNA Promotes Cardiomyocyte Proliferation by Enhancing the Stability of Sirt1. J. Am. Heart Assoc. 2018, 7, e009700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Xu, D.-Y.; Sha, W.-G.; Shen, L.; Lu, G.-Y. Long non-coding RNA MALAT1 interacts with transcription factor Foxo1 to regulate SIRT1 transcription in high glucose-induced HK-2 cells injury. Biochem. Biophys. Res. Commun. 2018, 503, 849–855. [Google Scholar] [CrossRef]
- Xu, Y.; Deng, W.; Zhang, W. Long non-coding RNA TUG1 protects renal tubular epithelial cells against injury induced by lipopolysaccharide via regulating microRNA-223. Biomed. Pharmacother. 2018, 104, 509–519. [Google Scholar] [CrossRef]
- Westerheide, S.D.; Anckar, J.; Stevens, S.M.; Sistonen, L.; Morimoto, R.I. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science 2009, 323, 1063–1066. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Chen, Y.; Aponte, A.M.; Battaglia, V.; Gucek, M.; Sack, M.N. Prolonged fasting identifies heat shock protein 10 as a Sirtuin 3 substrate: Elucidating a new mechanism linking mitochondrial protein acetylation to fatty acid oxidation enzyme folding and function. J. Biol. Chem. 2015, 290, 2466–2476. [Google Scholar] [CrossRef] [Green Version]
- Lellahi, S.M.; Rosenlund, I.A.; Hedberg, A.; Kiær, L.T.; Mikkola, I.; Knutsen, E.; Perander, M. The long noncoding RNA NEAT1 and nuclear paraspeckles are up-regulated by the transcription factor HSF1 in the heat shock response. J. Biol. Chem. 2018, 293, 18965–18976. [Google Scholar] [CrossRef] [Green Version]
- Place, R.F.; Noonan, E.J. Non-coding RNAs turn up the heat: An emerging layer of novel regulators in the mammalian heat shock response. Cell Stress Chaperones 2014, 19, 159–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, M.; Zheng, M.; Sun, B.; Wang, Y.; Ye, L.; Zhang, X. A long noncoding RNA perturbs the circadian rhythm of hepatoma cells to facilitate hepatocarcinogenesis. Neoplasia 2015, 17, 79–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Z.; Zhao, M.; Joshi, P.D.; Li, P.; Zhang, Y.; Guo, W.; Xu, Y.; Wang, H.; Zhao, Z.; Yan, J. A class of circadian long non-coding RNAs mark enhancers modulating long-range circadian gene regulation. Nucleic Acids Res. 2017, 45, 5720–5738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinke, H.; Saini, C.; Fleury-Olela, F.; Dibner, C.; Benjamin, I.J.; Schibler, U. Differential display of DNA-binding proteins reveals heat-shock factor 1 as a circadian transcription factor. Genes Dev. 2008, 22, 331–345. [Google Scholar] [CrossRef]
- Schneider, R.; Linka, R.M.; Reinke, H. HSP90 affects the stability of BMAL1 and circadian gene expression. J. Biol. Rhythm. 2014, 29, 87–96. [Google Scholar] [CrossRef]
- Mohni, K.N.; Wessel, S.R.; Zhao, R.; Wojciechowski, A.C.; Luzwick, J.W.; Layden, H.; Eichman, B.F.; Thompson, P.S.; Mehta, K.P.M.; Cortez, D. HMCES Maintains Genome Integrity by Shielding Abasic Sites in Single-Strand DNA. Cell 2019, 176, 144–153.e13. [Google Scholar] [CrossRef] [Green Version]
- Halabelian, L.; Ravichandran, M.; Li, Y.; Zeng, H.; Rao, A.; Aravind, L.; Arrowsmith, C.H. Structural basis of HMCES interactions with abasic DNA and multivalent substrate recognition. Nat. Struct. Mol. Biol. 2019, 26, 607–612. [Google Scholar] [CrossRef]
- Thompson, P.S.; Amidon, K.M.; Mohni, K.N.; Cortez, D.; Eichman, B.F. Protection of abasic sites during DNA replication by a stable thiazolidine protein-DNA cross-link. Nat. Struct. Mol. Biol. 2019, 26, 613–618. [Google Scholar] [CrossRef]
- Shukla, V.; Halabelian, L.; Balagere, S.; Samaniego-Castruita, D.; Feldman, D.E.; Arrowsmith, C.H.; Rao, A.; Aravind, L. HMCES Functions in the Alternative End-Joining Pathway of the DNA DSB Repair during Class Switch Recombination in B Cells. Mol. Cell 2020, 77, 384–394.e4. [Google Scholar] [CrossRef]
- Pawłowska, E.; Szczepanska, J.; Blasiak, J. DNA2-An Important Player in DNA Damage Response or Just Another DNA Maintenance Protein? Int. J. Mol. Sci. 2017, 18, 1562. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Meng, Y.; Campbell, J.L.; Shen, B. Multiple roles of DNA2 nuclease/helicase in DNA metabolism, genome stability and human diseases. Nucleic Acids Res. 2020, 48, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Stefanovie, B.; Hengel, S.R.; Mlcouskova, J.; Prochazkova, J.; Spirek, M.; Nikulenkov, F.; Nemecek, D.; Koch, B.G.; Bain, F.E.; Yu, L.; et al. DSS1 interacts with and stimulates RAD52 to promote the repair of DSBs. Nucleic Acids Res. 2020, 48, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Jalan, M.; Olsen, K.S.; Powell, S.N. Emerging Roles of RAD52 in Genome Maintenance. Cancers 2019, 11, 1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisemann, T.; Pascal, J.M. Poly(ADP-ribose) polymerase enzymes and the maintenance of genome integrity. Cell. Mol. Life Sci. CMLS 2020, 77, 19–33. [Google Scholar] [CrossRef] [PubMed]


| DDR Mechanisms | Type of DNA Lesion | Key Components | HSP | Partner | Effect on DNA Repair | Reference |
|---|---|---|---|---|---|---|
| Direct DNA-lesion reversal | - O6 alkylguanine | O6-methylguanine methyltransferase (MGMT) | HSPC2 (Hsp90α), HSPC3 (Hsp90β) | MGMT | Not clear | [130] |
| Base excision repair (BER) and single-strand break repair (SSBR) | Chemically modified DNA bases (DNA adducts; oxidized bases; alkylated bases; single-strand breaks) | DNA glycosylases, APE1 endonuclease, DNA polymerases (β, δ, ε), flap endonuclease FEN1, ligase I or ligase III, XRCC1, PARP enzymes (PARP-1, PARP-2), DNA ends- modifying enzymes polynucleotide kinase (PNK), aprataxin (APTX), tyrosyl-DNA phosphodiesterase 1 (TDP1) | HSP70 | APE1 | Stimulation of DNA repair | [114] |
| Polβ | Stimulation of DNA repair | [116,117] | ||||
| HSP90 | XRCC1 | Choice between DNA repair mechanism (polymerase-β-dependent or -independent) | [115] | |||
| HSP70 | PARP1, XRCC1 | Stimulation of SSBR repair | [118] | |||
| Nucleotide excision repair (NER) | Lesions that significantly disrupt the DNA double-helix (massive DNA adducts; 6’-4’ photoproducts; cyclobutane pyrimidine dimers (CPDs)) | XP proteins, RNA polymerase, XPC-HR23B DDB1/2 | HSP27 | Not identified | Stimulation of NER | [119] |
| HSP70 | XPA and XPG | Not identified | [123] | |||
| Mismatch repair (MMR) | - DNA mismatches - insertion/deletion loops | protein complexes (MSH2-MSH6, MSH2-MSH3 MLH1-PMS2 MLH1-PMS1, PLH1-MLH3), EXO1, polymerases δ and ε, PCNA, RFC, RPA, ligase I | HSP27/HSP70 | MSH2/MLH1 | Not identified | [107] |
| HSP90 | MSH2 | Stabilization of the interacting partner | [110] | |||
| Trans-lesion synthesis (TLS) | - damaged bases that prevent replication fork progression | “Error-prone” DNA polymerases | HSP90 | TLS polymerases | Promotes TLS activity in plants | [131] |
| Non-homologous end- joining (NHEJ) | - double-strand breaks (DSBs) | Ku 70/80, DNA-PKcs, XRCC4, XLF/cernunnos, ligase IV, Artemis nuclease, PNK, Aprataxin and polymerases μ and λ | HSP27 | Ku80 | Prevention of Ku80-DNA-PKcs interactions | [132] |
| HSP90 | DNA-PKcs | Activation and stabilization of DNA-PKcs for efficient repair | [133] | |||
| HSP110 | Ku70/Ku80 | Recruitment of NHEJ proteins (Ku70/80, DNA-PKCS) for efficient repair | [134] | |||
| Homologous recombination (HR) | - double-strand breaks (DSBs) - inter- and intrastrand crosslinks (ICLs) - stalled replication forks - abortive topoisomerase II action | RAD51 and RAD51-related protein, RAD52, BRCA2, RPA, FEN1, DNA polymerases, MRN, CtIP, BRCA1 | HSP90 | BRCA2 | RAD51 foci formation and effective DSB repair | [135] |
| MRN | MRN/ATM/ATR complex stabilization | [136] | ||||
| Fanconi anemia (FANC) pathway | - inter-strand DNA cross-links | FA-proteins | HSP90 | FANCA | Stabilization of FANCA | [137] |
| ATR mediated DDR signaling | - single-strand breaks (SSBs) | RPA, ATRIP, RAD9-RAD1-HUS1 (911) complex, ATR, MRN, CtIP, TOPBP1, Claspin | HSP90 | ATR | ATR is a direct client of HSP90, exact function remains to be elucidated | [138] |
| ATM mediated DDR signaling | - double-strand breaks (DSBs) | MDC1, 53BP1, RNF8 | HSP27 | ATM | Required for ATM-mediated DSBR repair upon radiation | [139] |
| RNF168, BRCA1, ATM, MRN, CHK2 | HSP90 | ATM | Required for ATM/ATR mediated HR repair upon radiation and replicative stress | [140] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kciuk, M.; Bukowski, K.; Marciniak, B.; Kontek, R. Advances in DNA Repair—Emerging Players in the Arena of Eukaryotic DNA Repair. Int. J. Mol. Sci. 2020, 21, 3934. https://doi.org/10.3390/ijms21113934
Kciuk M, Bukowski K, Marciniak B, Kontek R. Advances in DNA Repair—Emerging Players in the Arena of Eukaryotic DNA Repair. International Journal of Molecular Sciences. 2020; 21(11):3934. https://doi.org/10.3390/ijms21113934
Chicago/Turabian StyleKciuk, Mateusz, Karol Bukowski, Beata Marciniak, and Renata Kontek. 2020. "Advances in DNA Repair—Emerging Players in the Arena of Eukaryotic DNA Repair" International Journal of Molecular Sciences 21, no. 11: 3934. https://doi.org/10.3390/ijms21113934
APA StyleKciuk, M., Bukowski, K., Marciniak, B., & Kontek, R. (2020). Advances in DNA Repair—Emerging Players in the Arena of Eukaryotic DNA Repair. International Journal of Molecular Sciences, 21(11), 3934. https://doi.org/10.3390/ijms21113934






