Molecular Regulation in Dopaminergic Neuron Development. Cues to Unveil Molecular Pathogenesis and Pharmacological Targets of Neurodegeneration
Abstract
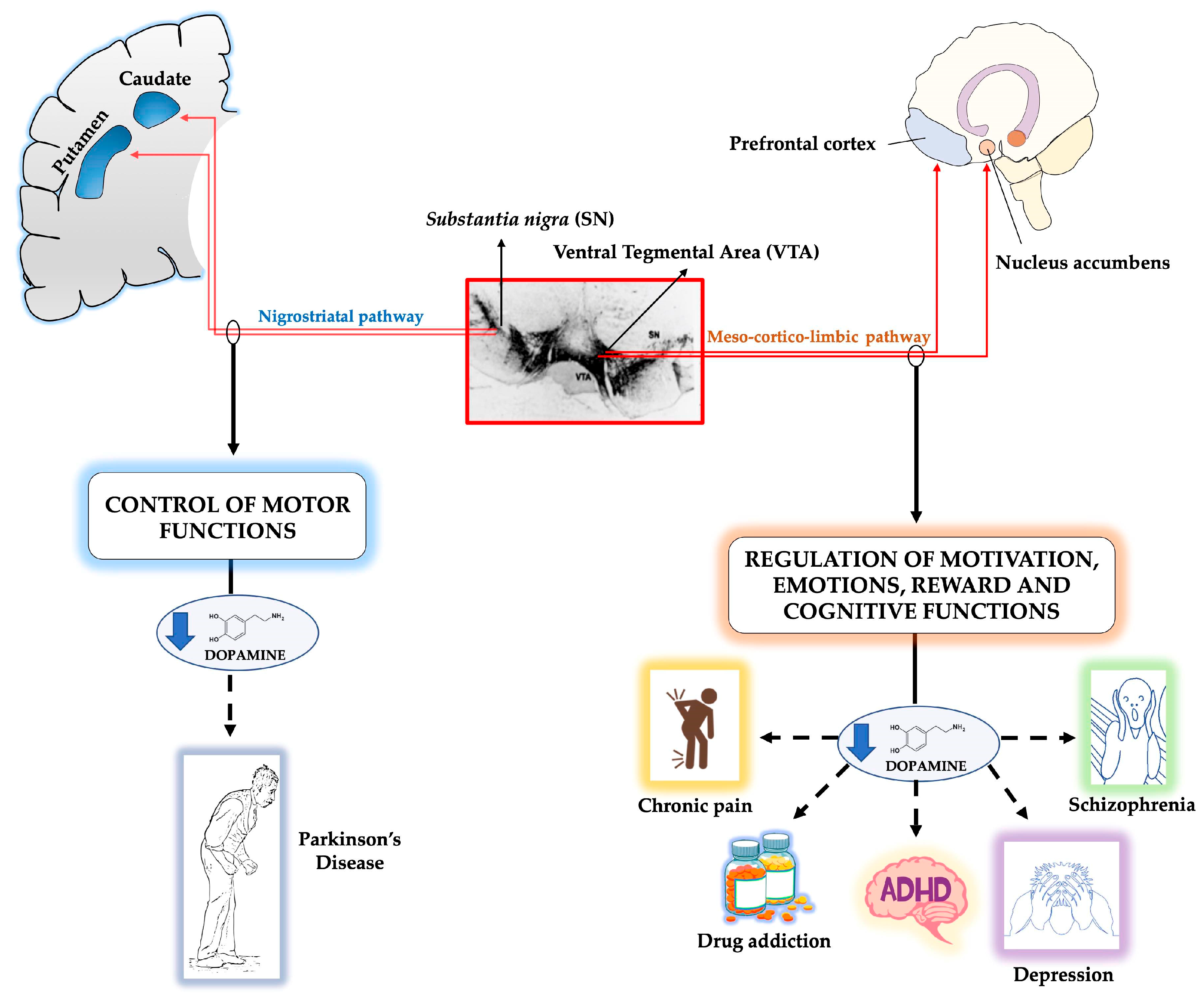
:1. Overview of the Midbrain Dopaminergic System
2. Features of Midbrain Dopaminergic Neurons
2.1. Molecular Characteristics of Dopaminergic Neurons
2.2. The Metabolic Rate and Vulnerability of Dopaminergic Neurons
2.3. ERK Signaling in the Pathophysiology of Dopaminergic Neurons
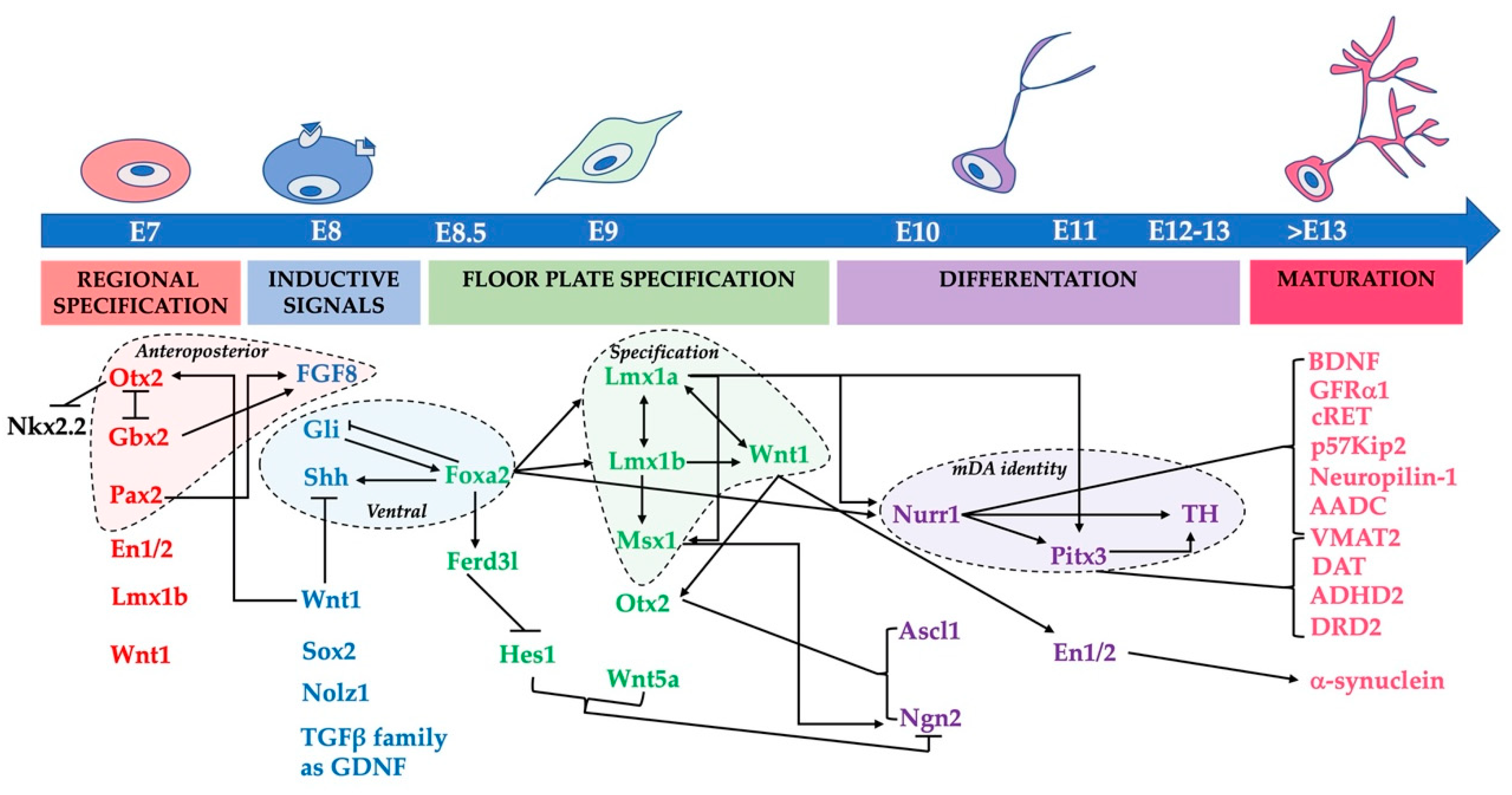
3. Pathways Involved in the Priming Process of the Ventral Midbrain and mDA Precursor Neurogenesis
3.1. The Role of Morphogens and Their Effectors
3.2. Genes Involved in the Acquisition and Stabilization of the Functional mDA Phenotype
4. Interplay between miRNAs, Dopaminergic Neurons Differentiation, Maintenance and Dysfunction
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AADC | Dopa Decarboxylase (Aromatic L-amino acid decarboxylase) |
| ADHD | Attention Deficit Hyperactive Disorder |
| ALDH1A1 | Aldehyde Dehydrogenase 1 family member A1 |
| Ascl1 | Achaete-scute family bHLH transcription factor 1 |
| BDNF | Brain Derived Neurotrophic Factor |
| α-syn | α-synuclein |
| bHLH | basic Helix–Loop–Helix |
| CNS | Central Nervous System |
| DA | Dopamine |
| DAT | Dopamine transporter (SLC6A3) |
| Dbh | ß-hydroxylase |
| Ddc | Gene encoding AADC |
| En1 | Engrailed-1 |
| ENS | Enteric Nervous System |
| ERK | Extracellular-signal regulated kinases 1/2 |
| FGF8 | Fibroblast Growth Factor 8 |
| Foxa1/2 | Forkhead box A1/2 |
| Gbx2 | Gastrulation brain homeobox 2 |
| GDNF | Glial Derived Neurotrophic Factor |
| GFAP | Glial Fibrillary Acid Protein |
| GFRa | GDNF Family Receptor alpha |
| Gli1 | Glioma associated oncogene 1 |
| Glu | Glutamate |
| GRP75 | Glucose Regulated Protein 75 |
| HDAC4 | Histone deacetylase 4 |
| Grp | Gastrin releasing peptide |
| LMX1a/b | LIM homeobox transcription factor 1 alpha/beta |
| Lmo3 | LIM Domain Only 3 |
| MAPK | Mitogen-activated protein kinases |
| mDA | Midbrain dopaminergic |
| mES | Mouse Embryonic Stem cells |
| miRNAs | microRNAs |
| MPTP | 1-Methyl-4-phenyl-1,2,3,6 tetrahydropyridine |
| Msx1 | Msh homeobox 1 |
| mtHsp70 | Mitochondrial heat shock protein 70 |
| mTOR | Mechanistic target of rapamycin (serine/threonine kinase) |
| Neurod6 | Neuronal differentiation 6 |
| Ngn2 | Neurogenin 2 |
| Nkx2.2 | NK2 Homeobox 2 |
| Nurr1 | Nuclear Receptor Subfamily 4 Group A Member 2 (NR4A2) |
| NRLP3 | Nod-like Receptor Protein 3 inflammasome |
| 6-OHDA | 6-hydroxydopamine |
| Otx2 | Orthodenticle homeobox 2 |
| Pax2 | Paired box gene 2 |
| PD | Parkinson’s Disease |
| Pitx3 | Paired-like homeodomain 3 |
| PTC | Patched |
| Ret | Ret protooncogene |
| ROS | Reactive oxygen species |
| RXR | Retinoid X Receptor |
| SHH | Sonic Hedgehog |
| SN | Substantia nigra |
| SNc | Substantia nigra pars compacta |
| SNG1 | Small nucleolar RNA host gene 1 |
| Sox2 | SRY-2 (Sex determining region Y) Box 2 |
| Sox10 | SRY-10 (Sex determining region Y) Box 10 |
| SVZ | Subventricular zone |
| TH | Tyrosine Hydroxylase |
| VEGF | Vascular Endothelial Growth Factor |
| VGLUT2 | Vesicular Glutamate Transporter 2 |
| VMAT2 | Vesicular Monoamine Transporter 2 |
| VTA | Ventral Tegmental Area |
| Wnt1 | Wingless-type MMTV integration site family member 1 |
References
- Bayer, S.A.; Wills, K.V.; Triarhou, L.C.; Ghetti, B. Time of neuron origin and gradients of neurogenesis in midbrain dopaminergic neurons in the mouse. Exp. Brain Res. 1995, 105, 191–199. [Google Scholar] [CrossRef]
- Bonilla, S.; Hall, A.C.; Pinto, L.; Attardo, A.; Götz, M.; Huttner, W.B.; Arenas, E. Identification of midbrain floor plate radial glia-like cells as dopaminergic progenitors. Glia 2008, 56, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Di Porzio, U.; Zuddas, A.; Cosenza-Murphy, D.B.; Barker, J.L. Early appearance of tyrosine hydroxylase immunoreactive cells in the mesencephalon of mouse embryos. Int. J. Dev. Neurosci. 1990, 8, 523–532. [Google Scholar] [CrossRef]
- Kawano, H.; Ohyama, K.; Kawamura, K.; Nagatsu, I. Migration of dopaminergic neurons in the embryonic mesencephalon of mice. Brain Res. Dev. Brain Res. 1995, 86, 101–113. [Google Scholar] [CrossRef]
- Schultz, W.; Dayan, P.; Montague, P.R. A neural substrate of prediction and reward. Science 1997, 275, 1593–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Leo, D.; Sorrentino, E.; Volpicelli, F.; Eyman, M.; Greco, D.; Viggiano, D.; di Porzio, U.; Perrone-Capano, C. Altered midbrain dopaminergic neurotransmission during development in an animal model of ADHD. Neurosci. Biobehav. Rev. 2003, 27, 661–669. [Google Scholar] [CrossRef]
- Purves-Tyson, T.D.; Owens, S.J.; Rothmond, D.A.; Halliday, G.M.; Double, K.L.; Stevens, J.; McCrossin, T.; Shannon Weickert, C. Putative presynaptic dopamine dysregulation in schizophrenia is supported by molecular evidence from post-mortem human midbrain. Transl. Psychiatry 2017, 7, e1003. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Zalesky, A.; Yue, W.; Howes, O.; Yan, H.; Liu, Y.; Fan, L.; Whitaker, K.J.; Xu, K.; Rao, G.; et al. A neuroimaging biomarker for striatal dysfunction in schizophrenia. Nat. Med. 2020, 26, 558–565. [Google Scholar] [CrossRef]
- Serafini, R.A.; Pryce, K.D.; Zachariou, V. The Mesolimbic Dopamine System in Chronic Pain and Associated Affective Comorbidities. Biol. Psychiatry 2020, 87, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Pakkenberg, B.; Møller, A.; Gundersen, H.J.; Mouritzen Dam, A.; Pakkenberg, H. The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson’s disease estimated with an unbiased stereological method. J. Neurol. Neurosurg. Psychiatry 1991, 54, 30–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morello, F.; Partanen, J. Diversity and development of local inhibitory and excitatory neurons associated with dopaminergic nuclei. FEBS Lett. 2015, 589, 3693–3701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderegg, A.; Poulin, J.F.; Awatramani, R. Molecular heterogeneity of midbrain dopaminergic neurons—Moving toward single cell resolution. FEBS Lett. 2015, 589, 3714–3726. [Google Scholar] [CrossRef] [PubMed]
- Poulin, J.F.; Gaertner, Z.; Moreno-Ramos, O.A.; Awatramani, R. Classification of Midbrain Dopamine Neurons Using Single-Cell Gene Expression Profiling Approaches. Trends Neurosci. 2020, 43, 155–169. [Google Scholar] [CrossRef]
- Björklund, A.; Dunnett, S.B. Dopamine neuron systems in the brain: An update. Trends Neurosci. 2007, 30, 194–202. [Google Scholar] [CrossRef]
- Buttarelli, F.R.; Fanciulli, A.; Pellicano, C.; Pontieri, F.E. The dopaminergic system in peripheral blood lymphocytes: From physiology to pharmacology and potential applications to neuropsychiatric disorders. Curr. Neuropharmacol. 2011, 9, 278–288. [Google Scholar] [CrossRef] [Green Version]
- Perrone-Capano, C.; Tino, A.; di Porzio, U. Target cells modulate dopamine transporter gene expression during brain development. Neuroreport 1994, 5, 1145–1148. [Google Scholar] [CrossRef]
- Lammel, S.; Steinberg, E.E.; Földy, C.; Wall, N.R.; Beier, K.; Luo, L.; Malenka, R.C. Diversity of transgenic mouse models for selective targeting of midbrain dopamine neurons. Neuron 2015, 85, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Pristerà, A.; Lin, W.; Kaufmann, A.K.; Brimblecombe, K.R.; Threlfell, S.; Dodson, P.D.; Magill, P.J.; Fernandes, C.; Cragg, S.J.; Ang, S.L. Transcription factors FOXA1 and FOXA2 maintain dopaminergic neuronal properties and control feeding behavior in adult mice. Proc. Natl. Acad. Sci. USA 2015, 112, E4929–E4938. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.H.; Levesque, M.; Claxton, S.; Johnson, R.L.; Ang, S.L. Lmx1a and lmx1b function cooperatively to regulate proliferation, specification, and differentiation of midbrain dopaminergic progenitors. J. Neurosci. 2011, 31, 12413–12425. [Google Scholar] [CrossRef] [Green Version]
- Nouri, N.; Awatramani, R. A novel floor plate boundary defined by adjacent En1 and Dbx1 microdomains distinguishes midbrain dopamine and hypothalamic neurons. Development 2017, 144, 916–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kee, N.; Volakakis, N.; Kirkeby, A.; Dahl, L.; Storvall, H.; Nolbrant, S.; Lahti, L.; Björklund, Å.; Gillberg, L.; Joodmardi, E.; et al. Single-Cell Analysis Reveals a Close Relationship between Differentiating Dopamine and Subthalamic Nucleus Neuronal Lineages. Cell Stem Cell 2017, 20, 29–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.; Kitai, S.T. Calcium spike underlying rhythmic firing in dopaminergic neurons of the rat substantia nigra. Neurosci. Res. 1993, 18, 195–207. [Google Scholar] [CrossRef]
- Caiazzo, M.; Dell’Anno, M.T.; Dvoretskova, E.; Lazarevic, D.; Taverna, S.; Leo, D.; Sotnikova, T.D.; Menegon, A.; Roncaglia, P.; Colciago, G.; et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 2011, 476, 224–227. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, R.; Pulcrano, S.; De Sanctis, C.; Volpicelli, F.; Guatteo, E.; von Oerthel, L.; Latagliata, E.C.; Esposito, R.; Piscitelli, R.M.; Perrone-Capano, C.; et al. miR-34b/c Regulates Wnt1 and Enhances Mesencephalic Dopaminergic Neuron Differentiation. Stem Cell Reports 2018, 10, 1237–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meiser, J.; Weindl, D.; Hiller, K. Complexity of dopamine metabolism. Cell Commun. Signal. 2013, 11, 34. [Google Scholar] [CrossRef] [Green Version]
- Surmeier, D.J.; Halliday, G.M.; Simuni, T. Calcium, mitochondrial dysfunction and slowing the progression of Parkinson’s disease. Exp. Neurol. 2017, 298, 202–209. [Google Scholar] [CrossRef]
- Lang, C.; Campbell, K.R.; Ryan, B.J.; Carling, P.; Attar, M.; Vowles, J.; Perestenko, O.V.; Bowden, R.; Baig, F.; Kasten, M.; et al. Single-Cell Sequencing of iPSC-Dopamine Neurons Reconstructs Disease Progression and Identifies HDAC4 as a Regulator of Parkinson Cell Phenotypes. Cell Stem Cell 2019, 24, 93–106. [Google Scholar] [CrossRef] [Green Version]
- Tran, H.T.; Chung, C.H.; Iba, M.; Zhang, B.; Trojanowski, J.Q.; Luk, K.C.; Lee, V.M. A-synuclein immunotherapy blocks uptake and templated propagation of misfolded α-synuclein and neurodegeneration. Cell Rep. 2014, 7, 2054–2065. [Google Scholar] [CrossRef] [Green Version]
- Mehra, S.; Sahay, S.; Maji, S.K. α-Synuclein misfolding and aggregation: Implications in Parkinson’s disease pathogenesis. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 890–908. [Google Scholar] [CrossRef]
- Rocha, E.M.; De Miranda, B.; Sanders, L.H. Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol. Dis. 2018, 109, 249–257. [Google Scholar] [CrossRef]
- Colucci-D’Amato, L.; Farina, A.; Vissers, J.P.; Chambery, A. Quantitative neuroproteomics: Classical and novel tools for studying neural differentiation and function. Stem Cell Rev. Rep. 2011, 7, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Colucci-D’Amato, L.; Cicatiello, A.E.; Reccia, M.G.; Volpicelli, F.; Severino, V.; Russo, R.; Sandomenico, A.; Doti, N.; D’Esposito, V.; Formisano, P.; et al. A targeted secretome profiling by multiplexed immunoassay revealed that secreted chemokine ligand 2 (MCP-1/CCL2) affects neural differentiation in mesencephalic neural progenitor cells. Proteomics 2015, 15, 714–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Severino, V.; Farina, A.; Colucci-D’Amato, L.; Reccia, M.G.; Volpicelli, F.; Parente, A.; Chambery, A. Secretome profiling of differentiated neural mes-c-myc A1 cell line endowed with stem cell properties. Biochim. Biophys. Acta 2013, 1834, 2385–2395. [Google Scholar] [CrossRef] [PubMed]
- Willis, S.D.; Hanley, S.E.; Beishke, T.; Tati, P.D.; Cooper, K.F. Ubiquitin-proteasome-mediated cyclin C degradation promotes cell survival following nitrogen starvation. Mol. Biol. Cell 2020, 31, 1015–1031. [Google Scholar] [CrossRef]
- Jin, J.; Hulette, C.; Wang, Y.; Zhang, T.; Pan, C.; Wadhwa, R.; Zhang, J. Proteomic identification of a stress protein, mortalin/mthsp70/GRP75: Relevance to Parkinson disease. Mol. Cell. Proteomics 2006, 5, 1193–1204. [Google Scholar] [CrossRef] [Green Version]
- Burbulla, L.F.; Fitzgerald, J.C.; Stegen, K.; Westermeier, J.; Thost, A.K.; Kato, H.; Mokranjac, D.; Sauerwald, J.; Martins, L.M.; Woitalla, D.; et al. Mitochondrial proteolytic stress induced by loss of mortalin function is rescued by Parkin and PINK1. Cell Death Dis. 2014, 5, e1180. [Google Scholar] [CrossRef] [Green Version]
- Breen, D.P.; Halliday, G.M.; Lang, A.E. Gut-brain axis and the spread of α-synuclein pathology: Vagal highway or dead end? Mov. Disord. 2019, 34, 307–316. [Google Scholar] [CrossRef]
- Scheperjans, F.; Derkinderen, P.; Borghammer, P. The Gut and Parkinson’s Disease: Hype or Hope? J. Parkinsons Dis. 2018, 8, S31–S39. [Google Scholar] [CrossRef] [Green Version]
- Lubomski, M.; Tan, A.H.; Lim, S.Y.; Holmes, A.J.; Davis, R.L.; Sue, C.M. Parkinson’s disease and the gastrointestinal microbiome. J. Neurol. 2019. [Google Scholar] [CrossRef]
- Colucci-D’Amato, L.; Perrone-Capano, C.; di Porzio, U. Chronic activation of ERK and neurodegenerative diseases. Bioessays 2003, 25, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Fusco, F.R.; Anzilotti, S.; Giampà, C.; Dato, C.; Laurenti, D.; Leuti, A.; Colucci D’Amato, L.; Perrone, L.; Bernardi, G.; Melone, M.A. Changes in the expression of extracellular regulated kinase (ERK 1/2) in the R6/2 mouse model of Huntington’s disease after phosphodiesterase IV inhibition. Neurobiol. Dis. 2012, 46, 225–233. [Google Scholar] [CrossRef]
- Zhu, J.H.; Kulich, S.M.; Oury, T.D.; Chu, C.T. Cytoplasmic aggregates of phosphorylated extracellular signal-regulated protein kinases in Lewy body diseases. Am. J. Pathol. 2002, 161, 2087–2098. [Google Scholar] [CrossRef] [Green Version]
- Andrew, R.; Watson, D.G.; Best, S.A.; Midgley, J.M.; Wenlong, H.; Petty, R.K. The determination of hydroxydopamines and other trace amines in the urine of parkinsonian patients and normal controls. Neurochem. Res. 1993, 18, 1175–1177. [Google Scholar] [CrossRef]
- Kulich, S.M.; Chu, C.T. Sustained extracellular signal-regulated kinase activation by 6-hydroxydopamine: Implications for Parkinson’s disease. J. Neurochem. 2001, 77, 1058–1066. [Google Scholar] [CrossRef] [Green Version]
- Vaudry, D.; Stork, P.J.; Lazarovici, P.; Eiden, L.E. Signaling pathways for PC12 cell differentiation: Making the right connections. Science 2002, 296, 1648–1649. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.T.; Levinthal, D.J.; Kulich, S.M.; Chalovich, E.M.; DeFranco, D.B. Oxidative neuronal injury. The dark side of ERK1/2. Eur. J. Biochem. 2004, 271, 2060–2066. [Google Scholar] [CrossRef] [Green Version]
- Subramaniam, S.; Unsicker, K. Extracellular signal-regulated kinase as an inducer of non-apoptotic neuronal death. Neuroscience 2006, 138, 1055–1065. [Google Scholar] [CrossRef]
- Lin, E.; Cavanaugh, J.E.; Leak, R.K.; Perez, R.G.; Zigmond, M.J. Rapid activation of ERK by 6-hydroxydopamine promotes survival of dopaminergic cells. J. Neurosci. Res. 2008, 86, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Colucci-D’Amato, G.L.; D’Alessio, A.; Califano, D.; Cali, G.; Rizzo, C.; Nitsch, L.; Santelli, G.; de Franciscis, V. Abrogation of nerve growth factor-induced terminal differentiation by ret oncogene involves perturbation of nuclear translocation of ERK. J. Biol. Chem. 2000, 275, 19306–19314. [Google Scholar] [CrossRef] [Green Version]
- Wainstein, E.; Seger, R. The dynamic subcellular localization of ERK: Mechanisms of translocation and role in various organelles. Curr. Opin. Cell Biol. 2016, 39, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.Y.; Kim, W.; Nam, S.M.; Kim, D.W.; Chung, J.Y.; Choi, S.Y.; Yoon, Y.S.; Won, M.H.; Hwang, I.K. Synergistic effects of sodium butyrate, a histone deacetylase inhibitor, on increase of neurogenesis induced by pyridoxine and increase of neural proliferation in the mouse dentate gyrus. Neurochem. Res. 2011, 36, 1850–1857. [Google Scholar] [CrossRef]
- Colucci-D’Amato, L.; di Porzio, U. Neurogenesis in adult CNS: From denial to opportunities and challenges for therapy. Bioessays 2008, 30, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Font-Nieves, M.; Van den Haute, C.; Baekelandt, V.; Planas, A.M.; Pozas, E. IL-10 regulates adult neurogenesis by modulating ERK and STAT3 activity. Front. Cell. Neurosci. 2015, 9, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrone-Capano, C.; Di Porzio, U. Genetic and epigenetic control of midbrain dopaminergic neuron development. Int. J. Dev. Biol. 2000, 44, 679–687. [Google Scholar] [PubMed]
- Puelles, E.; Annino, A.; Tuorto, F.; Usiello, A.; Acampora, D.; Czerny, T.; Brodski, C.; Ang, S.L.; Wurst, W.; Simeone, A. Otx2 regulates the extent, identity and fate of neuronal progenitor domains in the ventral midbrain. Development 2004, 131, 2037–2048. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Song, J.J.; Puspita, L.; Valiulahi, P.; Shim, J.W.; Lee, S.H. In vitro generation of mature midbrain-type dopamine neurons by adjusting exogenous Nurr1 and Foxa2 expressions to their physiologic patterns. Exp. Mol. Med. 2017, 49, e300. [Google Scholar] [CrossRef]
- Domanskyi, A.; Alter, H.; Vogt, M.A.; Gass, P.; Vinnikov, I.A. Transcription factors Foxa1 and Foxa2 are required for adult dopamine neurons maintenance. Front. Cell. Neurosci. 2014, 8, 275. [Google Scholar] [CrossRef] [Green Version]
- Hynes, M.; Porter, J.A.; Chiang, C.; Chang, D.; Tessier-Lavigne, M.; Beachy, P.A.; Rosenthal, A. Induction of midbrain dopaminergic neurons by Sonic hedgehog. Neuron 1995, 15, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Ye, W.; Shimamura, K.; Rubenstein, J.L.; Hynes, M.A.; Rosenthal, A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell 1998, 93, 755–766. [Google Scholar] [CrossRef] [Green Version]
- Volpicelli, F.; Consales, C.; Caiazzo, M.; Colucci-D’Amato, L.; Perrone-Capano, C.; di Porzio, U. Enhancement of dopaminergic differentiation in proliferating midbrain neuroblasts by sonic hedgehog and ascorbic acid. Neural Plast. 2004, 11, 45–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, Y.; Nakatani, T.; Minaki, Y.; Kumai, M. The basic helix-loop-helix transcription factor Nato3 controls neurogenic activity in mesencephalic floor plate cells. Development 2010, 137, 1897–1906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kameda, Y.; Saitoh, T.; Fujimura, T. Hes1 regulates the number and anterior-posterior patterning of mesencephalic dopaminergic neurons at the mid/hindbrain boundary (isthmus). Dev. Biol. 2011, 358, 91–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegarty, S.V.; Sullivan, A.M.; O’Keeffe, G.W. Midbrain dopaminergic neurons: A review of the molecular circuitry that regulates their development. Dev. Biol. 2013, 379, 123–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattugini, N.; Bocchi, R.; Scheuss, V.; Russo, G.L.; Torper, O.; Lao, C.L.; Götz, M. Inducing Different Neuronal Subtypes from Astrocytes in the Injured Mouse Cerebral Cortex. Neuron 2019, 103, 1086–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, J.; Donaldson, A.; Yang, M.; German, M.S.; Enikolopov, G.; Iacovitti, L. The role of Lmx1a in the differentiation of human embryonic stem cells into midbrain dopamine neurons in culture and after transplantation into a Parkinson’s disease model. Stem Cells 2009, 27, 220–229. [Google Scholar] [CrossRef]
- Rivetti di Val Cervo, P.; Romanov, R.A.; Spigolon, G.; Masini, D.; Martín-Montañez, E.; Toledo, E.M.; La Manno, G.; Feyder, M.; Pifl, C.; Ng, Y.H.; et al. Induction of functional dopamine neurons from human astrocytes in vitro and mouse astrocytes in a Parkinson’s disease model. Nat. Biotechnol. 2017, 35, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Mesman, S.; von Oerthel, L.; Smidt, M.P. Mesodiencephalic dopaminergic neuronal differentiation does not involve GLI2A-mediated SHH-signaling and is under the direct influence of canonical WNT signaling. PLoS ONE 2014, 9, e97926. [Google Scholar] [CrossRef] [Green Version]
- Allen, B.L.; Song, J.Y.; Izzi, L.; Althaus, I.W.; Kang, J.S.; Charron, F.; Krauss, R.S.; McMahon, A.P. Overlapping roles and collective requirement for the coreceptors GAS1, CDO, and BOC in SHH pathway function. Dev. Cell 2011, 20, 775–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, E.; Tryggvason, U.; Deng, Q.; Friling, S.; Alekseenko, Z.; Robert, B.; Perlmann, T.; Ericson, J. Identification of intrinsic determinants of midbrain dopamine neurons. Cell 2006, 124, 393–405. [Google Scholar] [CrossRef] [Green Version]
- Wurst, W.; Prakash, N. Wnt1-regulated genetic networks in midbrain dopaminergic neuron development. J. Mol. Cell Biol. 2014, 6, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, B. Wnt/β-Catenin Signaling Pathway Governs a Full Program for Dopaminergic Neuron Survival, Neurorescue and Regeneration in the MPTP Mouse Model of Parkinson’s Disease. Int. J. Mol. Sci. 2018, 19, 3743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferri, A.L.; Lin, W.; Mavromatakis, Y.E.; Wang, J.C.; Sasaki, H.; Whitsett, J.A.; Ang, S.L. Foxa1 and Foxa2 regulate multiple phases of midbrain dopaminergic neuron development in a dosage-dependent manner. Development 2007, 134, 2761–2769. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Fischer, D.; Fuchs, J.; Castagner, F.; Stettler, O.; Massiani-Beaudoin, O.; Moya, K.L.; Bouillot, C.; Oertel, W.H.; Lombès, A.; Faigle, W.; et al. Engrailed protects mouse midbrain dopaminergic neurons against mitochondrial complex I insults. Nat. Neurosci. 2011, 14, 1260–1266. [Google Scholar] [CrossRef]
- Brown, A.; Machan, J.T.; Hayes, L.; Zervas, M. Molecular organization and timing of Wnt1 expression define cohorts of midbrain dopamine neuron progenitors in vivo. J. Comp. Neurol. 2011, 519, 2978–3000. [Google Scholar] [CrossRef] [Green Version]
- Arenas, E.; Denham, M.; Villaescusa, J.C. How to make a midbrain dopaminergic neuron. Development 2015, 142, 1918–1936. [Google Scholar] [CrossRef] [Green Version]
- Doi, D.; Samata, B.; Katsukawa, M.; Kikuchi, T.; Morizane, A.; Ono, Y.; Sekiguchi, K.; Nakagawa, M.; Parmar, M.; Takahashi, J. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Reports 2014, 2, 337–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkeby, A.; Grealish, S.; Wolf, D.A.; Nelander, J.; Wood, J.; Lundblad, M.; Lindvall, O.; Parmar, M. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 2012, 1, 703–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kriks, S.; Shim, J.W.; Piao, J.; Ganat, Y.M.; Wakeman, D.R.; Xie, Z.; Carrillo-Reid, L.; Auyeung, G.; Antonacci, C.; Buch, A.; et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 2011, 480, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Kirkeby, A.; Nolbrant, S.; Tiklova, K.; Heuer, A.; Kee, N.; Cardoso, T.; Ottosson, D.R.; Lelos, M.J.; Rifes, P.; Dunnett, S.B.; et al. Predictive Markers Guide Differentiation to Improve Graft Outcome in Clinical Translation of hESC-Based Therapy for Parkinson’s Disease. Cell Stem Cell 2017, 20, 135–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ang, S.L. Transcriptional control of midbrain dopaminergic neuron development. Development 2006, 133, 3499–3506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panman, L.; Papathanou, M.; Laguna, A.; Oosterveen, T.; Volakakis, N.; Acampora, D.; Kurtsdotter, I.; Yoshitake, T.; Kehr, J.; Joodmardi, E.; et al. Sox6 and Otx2 control the specification of substantia nigra and ventral tegmental area dopamine neurons. Cell Rep. 2014, 8, 1018–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendez, J.A.; Bourque, M.J.; Dal Bo, G.; Bourdeau, M.L.; Danik, M.; Williams, S.; Lacaille, J.C.; Trudeau, L.E. Developmental and target-dependent regulation of vesicular glutamate transporter expression by dopamine neurons. J. Neurosci. 2008, 28, 6309–6318. [Google Scholar] [CrossRef]
- Descarries, L.; Bérubé-Carrière, N.; Riad, M.; Bo, G.D.; Mendez, J.A.; Trudeau, L.E. Glutamate in dopamine neurons: Synaptic versus diffuse transmission. Brain Res. Rev. 2008, 58, 290–302. [Google Scholar] [CrossRef]
- Bérubé-Carrière, N.; Riad, M.; Dal Bo, G.; Lévesque, D.; Trudeau, L.E.; Descarries, L. The dual dopamine-glutamate phenotype of growing mesencephalic neurons regresses in mature rat brain. J. Comp. Neurol. 2009, 517, 873–891. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Qi, J.; Wang, H.L.; Zhang, S.; Morales, M. Glutamatergic and dopaminergic neurons in the mouse ventral tegmental area. Eur. J. Neurosci. 2015, 41, 760–772. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.H.; Zell, V.; Gutierrez-Reed, N.; Wu, J.; Ressler, R.; Shenasa, M.A.; Johnson, A.B.; Fife, K.H.; Faget, L.; Hnasko, T.S. Ventral tegmental area glutamate neurons co-release GABA and promote positive reinforcement. Nat. Commun. 2016, 7, 13697. [Google Scholar] [CrossRef]
- Bodea, G.O.; Blaess, S. Establishing diversity in the dopaminergic system. FEBS Lett. 2015, 589, 3773–3785. [Google Scholar] [CrossRef] [Green Version]
- Stuber, G.D.; Hnasko, T.S.; Britt, J.P.; Edwards, R.H.; Bonci, A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J. Neurosci. 2010, 30, 8229–8233. [Google Scholar] [CrossRef]
- Hnasko, T.S.; Hjelmstad, G.O.; Fields, H.L.; Edwards, R.H. Ventral tegmental area glutamate neurons: Electrophysiological properties and projections. J. Neurosci. 2012, 32, 15076–15085. [Google Scholar] [CrossRef] [Green Version]
- Steinkellner, T.; Zell, V.; Farino, Z.J.; Sonders, M.S.; Villeneuve, M.; Freyberg, R.J.; Przedborski, S.; Lu, W.; Freyberg, Z.; Hnasko, T.S. Role for VGLUT2 in selective vulnerability of midbrain dopamine neurons. J. Clin. Investig. 2018, 128, 774–788. [Google Scholar] [CrossRef] [Green Version]
- Perlmann, T.; Wallén-Mackenzie, A. Nurr1, an orphan nuclear receptor with essential functions in developing dopamine cells. Cell Tissue Res. 2004, 318, 45–52. [Google Scholar] [CrossRef]
- Volpicelli, F.; Perrone-Capano, C.; Da Pozzo, P.; Colucci-D’Amato, L.; di Porzio, U. Modulation of nurr1 gene expression in mesencephalic dopaminergic neurones. J. Neurochem. 2004, 88, 1283–1294. [Google Scholar] [CrossRef] [PubMed]
- Saucedo-Cardenas, O.; Quintana-Hau, J.D.; Le, W.D.; Smidt, M.P.; Cox, J.J.; De Mayo, F.; Burbach, J.P.; Conneely, O.M. Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc. Natl. Acad. Sci. USA 1998, 95, 4013–4018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smits, S.M.; Ponnio, T.; Conneely, O.M.; Burbach, J.P.; Smidt, M.P. Involvement of Nurr1 in specifying the neurotransmitter identity of ventral midbrain dopaminergic neurons. Eur. J. Neurosci. 2003, 18, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, E.; Joseph, B.; Castro, D.; Lindqvist, E.; Aarnisalo, P.; Wallén, A.; Benoit, G.; Hengerer, B.; Olson, L.; Perlmann, T. Nurr1 regulates dopamine synthesis and storage in MN9D dopamine cells. Exp. Cell Res. 2003, 288, 324–334. [Google Scholar] [CrossRef]
- Sacchetti, P.; Brownschidle, L.A.; Granneman, J.G.; Bannon, M.J. Characterization of the 5’-flanking region of the human dopamine transporter gene. Brain Res. Mol. Brain Res. 1999, 74, 167–174. [Google Scholar] [CrossRef]
- Joseph, B.; Wallén-Mackenzie, A.; Benoit, G.; Murata, T.; Joodmardi, E.; Okret, S.; Perlmann, T. p57(Kip2) cooperates with Nurr1 in developing dopamine cells. Proc. Natl. Acad. Sci. USA 2003, 100, 15619–15624. [Google Scholar] [CrossRef] [Green Version]
- Wallén A, A.; Castro, D.S.; Zetterström, R.H.; Karlén, M.; Olson, L.; Ericson, J.; Perlmann, T. Orphan nuclear receptor Nurr1 is essential for Ret expression in midbrain dopamine neurons and in the brain stem. Mol. Cell Neurosci. 2001, 18, 649–663. [Google Scholar] [CrossRef]
- Galleguillos, D.; Fuentealba, J.A.; Gómez, L.M.; Saver, M.; Gómez, A.; Nash, K.; Burger, C.; Gysling, K.; Andrés, M.E. Nurr1 regulates RET expression in dopamine neurons of adult rat midbrain. J. Neurochem. 2010, 114, 1158–1167. [Google Scholar] [CrossRef]
- Volpicelli, F.; Caiazzo, M.; Greco, D.; Consales, C.; Leone, L.; Perrone-Capano, C.; Colucci D’Amato, L.; di Porzio, U. Bdnf gene is a downstream target of Nurr1 transcription factor in rat midbrain neurons in vitro. J. Neurochem. 2007, 102, 441–453. [Google Scholar] [CrossRef]
- Oh, S.M.; Chang, M.Y.; Song, J.J.; Rhee, Y.H.; Joe, E.H.; Lee, H.S.; Yi, S.H.; Lee, S.H. Combined Nurr1 and Foxa2 roles in the therapy of Parkinson’s disease. EMBO Mol. Med. 2015, 7, 510–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Xiang, M. Subtype specification of GABAergic amacrine cells by the orphan nuclear receptor Nr4a2/Nurr1. J. Neurosci. 2009, 29, 10449–10459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarakad, A.; Jankovic, J. Anosmia and Ageusia in Parkinson’s Disease. Int. Rev. Neurobiol. 2017, 133, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Spathis, A.D.; Asvos, X.; Ziavra, D.; Karampelas, T.; Topouzis, S.; Cournia, Z.; Qing, X.; Alexakos, P.; Smits, L.M.; Dalla, C.; et al. Nurr1:RXRα heterodimer activation as monotherapy for Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, 3999–4004. [Google Scholar] [CrossRef] [Green Version]
- Volpicelli, F.; De Gregorio, R.; Pulcrano, S.; Perrone-Capano, C.; di Porzio, U.; Bellenchi, G.C. Direct regulation of Pitx3 expression by Nurr1 in culture and in developing mouse midbrain. PLoS ONE 2012, 7, e30661. [Google Scholar] [CrossRef] [Green Version]
- Hwang, D.Y.; Ardayfio, P.; Kang, U.J.; Semina, E.V.; Kim, K.S. Selective loss of dopaminergic neurons in the substantia nigra of Pitx3-deficient aphakia mice. Brain Res. Mol. Brain Res. 2003, 114, 123–131. [Google Scholar] [CrossRef]
- Frim, D.M.; Uhler, T.A.; Galpern, W.R.; Beal, M.F.; Breakefield, X.O.; Isacson, O. Implanted fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevent 1-methyl-4-phenylpyridinium toxicity to dopaminergic neurons in the rat. Proc. Natl. Acad. Sci. USA 1994, 91, 5104–5108. [Google Scholar] [CrossRef] [Green Version]
- Hyman, C.; Hofer, M.; Barde, Y.A.; Juhasz, M.; Yancopoulos, G.D.; Squinto, S.P.; Lindsay, R.M. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature 1991, 350, 230–232. [Google Scholar] [CrossRef]
- Arenas, E.; Trupp, M.; Akerud, P.; Ibáñez, C.F. GDNF prevents degeneration and promotes the phenotype of brain noradrenergic neurons in vivo. Neuron 1995, 15, 1465–1473. [Google Scholar] [CrossRef] [Green Version]
- Akerud, P.; Canals, J.M.; Snyder, E.Y.; Arenas, E. Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson’s disease. J. Neurosci. 2001, 21, 8108–8118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascual, A.; Hidalgo-Figueroa, M.; Piruat, J.I.; Pintado, C.O.; Gómez-Díaz, R.; López-Barneo, J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat. Neurosci 2008, 11, 755–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, C.; Aron, L.; Klein, R.; Li, M.; Wurst, W.; Prakash, N.; Le, W. Pitx3 is a critical mediator of GDNF-induced BDNF expression in nigrostriatal dopaminergic neurons. J. Neurosci. 2011, 31, 12802–12815. [Google Scholar] [CrossRef]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef] [Green Version]
- Man, J.H.K.; Groenink, L.; Caiazzo, M. Cell reprogramming approaches in gene- and cell-based therapies for Parkinson’s disease. J. Control. Release 2018, 286, 114–124. [Google Scholar] [CrossRef]
- Valdés, P.; Schneider, B.L. Gene Therapy: A Promising Approach for Neuroprotection in Parkinson’s Disease? Front. Neuroanat. 2016, 10, 123. [Google Scholar] [CrossRef] [Green Version]
- Grondin, R.; Littrell, O.M.; Zhang, Z.; Ai, Y.; Huettl, P.; Pomerleau, F.; Quintero, J.E.; Andersen, A.H.; Stenslik, M.J.; Bradley, L.H.; et al. GDNF revisited: A novel mammalian cell-derived variant form of GDNF increases dopamine turnover and improves brain biodistribution. Neuropharmacology 2019, 147, 28–36. [Google Scholar] [CrossRef]
- Ecker, J.R.; Geschwind, D.H.; Kriegstein, A.R.; Ngai, J.; Osten, P.; Polioudakis, D.; Regev, A.; Sestan, N.; Wickersham, I.R.; Zeng, H. The BRAIN Initiative Cell Census Consortium: Lessons Learned toward Generating a Comprehensive Brain Cell Atlas. Neuron 2017, 96, 542–557. [Google Scholar] [CrossRef]
- La Manno, G.; Gyllborg, D.; Codeluppi, S.; Nishimura, K.; Salto, C.; Zeisel, A.; Borm, L.E.; Stott, S.R.W.; Toledo, E.M.; Villaescusa, J.C.; et al. Molecular Diversity of Midbrain Development in Mouse, Human, and Stem Cells. Cell 2016, 167, 566–580.e519. [Google Scholar] [CrossRef] [Green Version]
- Hook, P.W.; McClymont, S.A.; Cannon, G.H.; Law, W.D.; Morton, A.J.; Goff, L.A.; McCallion, A.S. Single-Cell RNA-Seq of Mouse Dopaminergic Neurons Informs Candidate Gene Selection for Sporadic Parkinson Disease. Am. J. Hum. Genet. 2018, 102, 427–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiklová, K.; Björklund, Å.; Lahti, L.; Fiorenzano, A.; Nolbrant, S.; Gillberg, L.; Volakakis, N.; Yokota, C.; Hilscher, M.M.; Hauling, T.; et al. Single-cell RNA sequencing reveals midbrain dopamine neuron diversity emerging during mouse brain development. Nat. Commun. 2019, 10, 581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, D.J.; Risso, D.; Kosillo, P.; Ngai, J.; Bateup, H.S. Combinatorial Expression of Grp and Neurod6 Defines Dopamine Neuron Populations with Distinct Projection Patterns and Disease Vulnerability. eNeuro 2018, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartel, D.P.; Chen, C.Z. Micromanagers of gene expression: The potentially widespread influence of metazoan microRNAs. Nat. Rev. Genet. 2004, 5, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Lagos-Quintana, M.; Rauhut, R.; Yalcin, A.; Meyer, J.; Lendeckel, W.; Tuschl, T. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 2002, 12, 735–739. [Google Scholar] [CrossRef] [Green Version]
- Kapsimali, M.; Kloosterman, W.P.; de Bruijn, E.; Rosa, F.; Plasterk, R.H.; Wilson, S.W. MicroRNAs show a wide diversity of expression profiles in the developing and mature central nervous system. Genome Biol. 2007, 8, R173. [Google Scholar] [CrossRef] [Green Version]
- McNeill, E.; Van Vactor, D. MicroRNAs shape the neuronal landscape. Neuron 2012, 75, 363–379. [Google Scholar] [CrossRef] [Green Version]
- Volpicelli, F.; Speranza, L.; Pulcrano, S.; De Gregorio, R.; Crispino, M.; De Sanctis, C.; Leopoldo, M.; Lacivita, E.; di Porzio, U.; Bellenchi, G.C.; et al. The microRNA-29a Modulates Serotonin 5-HT7 Receptor Expression and Its Effects on Hippocampal Neuronal Morphology. Mol. Neurobiol. 2019, 56, 8617–8627. [Google Scholar] [CrossRef]
- Rosas-Hernandez, H.; Chigurupati, S.; Raymick, J.; Robinson, B.; Cuevas, E.; Hanig, J.; Sarkar, S. Identification of altered microRNAs in serum of a mouse model of Parkinson’s disease. Neurosci. Lett. 2018, 687, 1–9. [Google Scholar] [CrossRef]
- Cuellar, T.L.; Davis, T.H.; Nelson, P.T.; Loeb, G.B.; Harfe, B.D.; Ullian, E.; McManus, M.T. Dicer loss in striatal neurons produces behavioral and neuroanatomical phenotypes in the absence of neurodegeneration. Proc. Natl. Acad. Sci. USA 2008, 105, 5614–5619. [Google Scholar] [CrossRef] [Green Version]
- Kawase-Koga, Y.; Otaegi, G.; Sun, T. Different timings of Dicer deletion affect neurogenesis and gliogenesis in the developing mouse central nervous system. Dev. Dyn. 2009, 238, 2800–2812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.S.; Nakahara, K.; Pham, J.W.; Kim, K.; He, Z.; Sontheimer, E.J.; Carthew, R.W. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell 2004, 117, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Inoue, K.; Ishii, J.; Vanti, W.B.; Voronov, S.V.; Murchison, E.; Hannon, G.; Abeliovich, A. A MicroRNA feedback circuit in midbrain dopamine neurons. Science 2007, 317, 1220–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Li, T.; Wang, Y.; Tang, Y.; Cui, H.; Zhang, X.; Chen, D.; Shen, N.; Le, W. miR-132 regulates the differentiation of dopamine neurons by directly targeting Nurr1 expression. J. Cell Sci. 2012, 125, 1673–1682. [Google Scholar] [CrossRef] [Green Version]
- Miñones-Moyano, E.; Porta, S.; Escaramís, G.; Rabionet, R.; Iraola, S.; Kagerbauer, B.; Espinosa-Parrilla, Y.; Ferrer, I.; Estivill, X.; Martí, E. MicroRNA profiling of Parkinson’s disease brains identifies early downregulation of miR-34b/c which modulate mitochondrial function. Hum. Mol. Genet. 2011, 20, 3067–3078. [Google Scholar] [CrossRef]
- Shanesazzade, Z.; Peymani, M.; Ghaedi, K.; Nasr Esfahani, M.H. miR-34a/BCL-2 signaling axis contributes to apoptosis in MPP. Mol. Genet. Genomic Med. 2018, 6, 975–981. [Google Scholar] [CrossRef]
- de Chevigny, A.; Coré, N.; Follert, P.; Gaudin, M.; Barbry, P.; Béclin, C.; Cremer, H. miR-7a regulation of Pax6 controls spatial origin of forebrain dopaminergic neurons. Nat. Neurosci. 2012, 15, 1120–1126. [Google Scholar] [CrossRef]
- Qian, Y.; Song, J.; Ouyang, Y.; Han, Q.; Chen, W.; Zhao, X.; Xie, Y.; Chen, Y.; Yuan, W.; Fan, C. Advances in Roles of miR-132 in the Nervous System. Front. Pharmacol. 2017, 8, 770. [Google Scholar] [CrossRef]
- Gillardon, F.; Mack, M.; Rist, W.; Schnack, C.; Lenter, M.; Hildebrandt, T.; Hengerer, B. MicroRNA and proteome expression profiling in early-symptomatic α-synuclein(A30P)-transgenic mice. Proteom. Clin. Appl. 2008, 2, 697–705. [Google Scholar] [CrossRef]
- Lungu, G.; Stoica, G.; Ambrus, A. MicroRNA profiling and the role of microRNA-132 in neurodegeneration using a rat model. Neurosci. Lett. 2013, 553, 153–158. [Google Scholar] [CrossRef]
- Alieva, A.K.h.; Filatova, E.V.; Karabanov, A.V.; Illarioshkin, S.N.; Limborska, S.A.; Shadrina, M.I.; Slominsky, P.A. miRNA expression is highly sensitive to a drug therapy in Parkinson’s disease. Parkinsonism Relat. Disord. 2015, 21, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Di Rita, A.; Maiorino, T.; Bruqi, K.; Volpicelli, F.; Bellenchi, G.C.; Strappazzon, F. miR-218 Inhibits Mitochondrial Clearance by Targeting PRKN E3 Ubiquitin Ligase. Int. J. Mol. Sci. 2020, 21, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, X.; Wang, H.; Ye, Y.; Shu, Y.; Deng, Y.; He, X.; Lu, G.; Zhang, S. miR-124 regulates cell apoptosis and autophagy in dopaminergic neurons and protects them by regulating AMPK/mTOR pathway in Parkinson’s disease. Am. J. Transl. Res. 2016, 8, 2127–2137. [Google Scholar]
- Wang, H.; Ye, Y.; Zhu, Z.; Mo, L.; Lin, C.; Wang, Q.; Gong, X.; He, X.; Lu, G.; Lu, F.; et al. MiR-124 Regulates Apoptosis and Autophagy Process in MPTP Model of Parkinson’s Disease by Targeting to Bim. Brain Pathol. 2016, 26, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Yan, Z.; Zhu, H.; Zhao, H. MiR-410 exerts neuroprotective effects in a cellular model of Parkinson’s disease induced by 6-hydroxydopamine via inhibiting the PTEN/AKT/mTOR signaling pathway. Exp. Mol. Pathol. 2019, 109, 16–24. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volpicelli, F.; Perrone-Capano, C.; Bellenchi, G.C.; Colucci-D’Amato, L.; di Porzio, U. Molecular Regulation in Dopaminergic Neuron Development. Cues to Unveil Molecular Pathogenesis and Pharmacological Targets of Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 3995. https://doi.org/10.3390/ijms21113995
Volpicelli F, Perrone-Capano C, Bellenchi GC, Colucci-D’Amato L, di Porzio U. Molecular Regulation in Dopaminergic Neuron Development. Cues to Unveil Molecular Pathogenesis and Pharmacological Targets of Neurodegeneration. International Journal of Molecular Sciences. 2020; 21(11):3995. https://doi.org/10.3390/ijms21113995
Chicago/Turabian StyleVolpicelli, Floriana, Carla Perrone-Capano, Gian Carlo Bellenchi, Luca Colucci-D’Amato, and Umberto di Porzio. 2020. "Molecular Regulation in Dopaminergic Neuron Development. Cues to Unveil Molecular Pathogenesis and Pharmacological Targets of Neurodegeneration" International Journal of Molecular Sciences 21, no. 11: 3995. https://doi.org/10.3390/ijms21113995
APA StyleVolpicelli, F., Perrone-Capano, C., Bellenchi, G. C., Colucci-D’Amato, L., & di Porzio, U. (2020). Molecular Regulation in Dopaminergic Neuron Development. Cues to Unveil Molecular Pathogenesis and Pharmacological Targets of Neurodegeneration. International Journal of Molecular Sciences, 21(11), 3995. https://doi.org/10.3390/ijms21113995




