Tooth Formation: Are the Hardest Tissues of Human Body Hard to Regenerate?
Abstract
1. Introduction
2. Hard Dental Tissues and Their Genesis
2.1. The Complexity of Dental Tissues
2.2. Signaling Pathways Modulating Hard Dental Tissue Generation
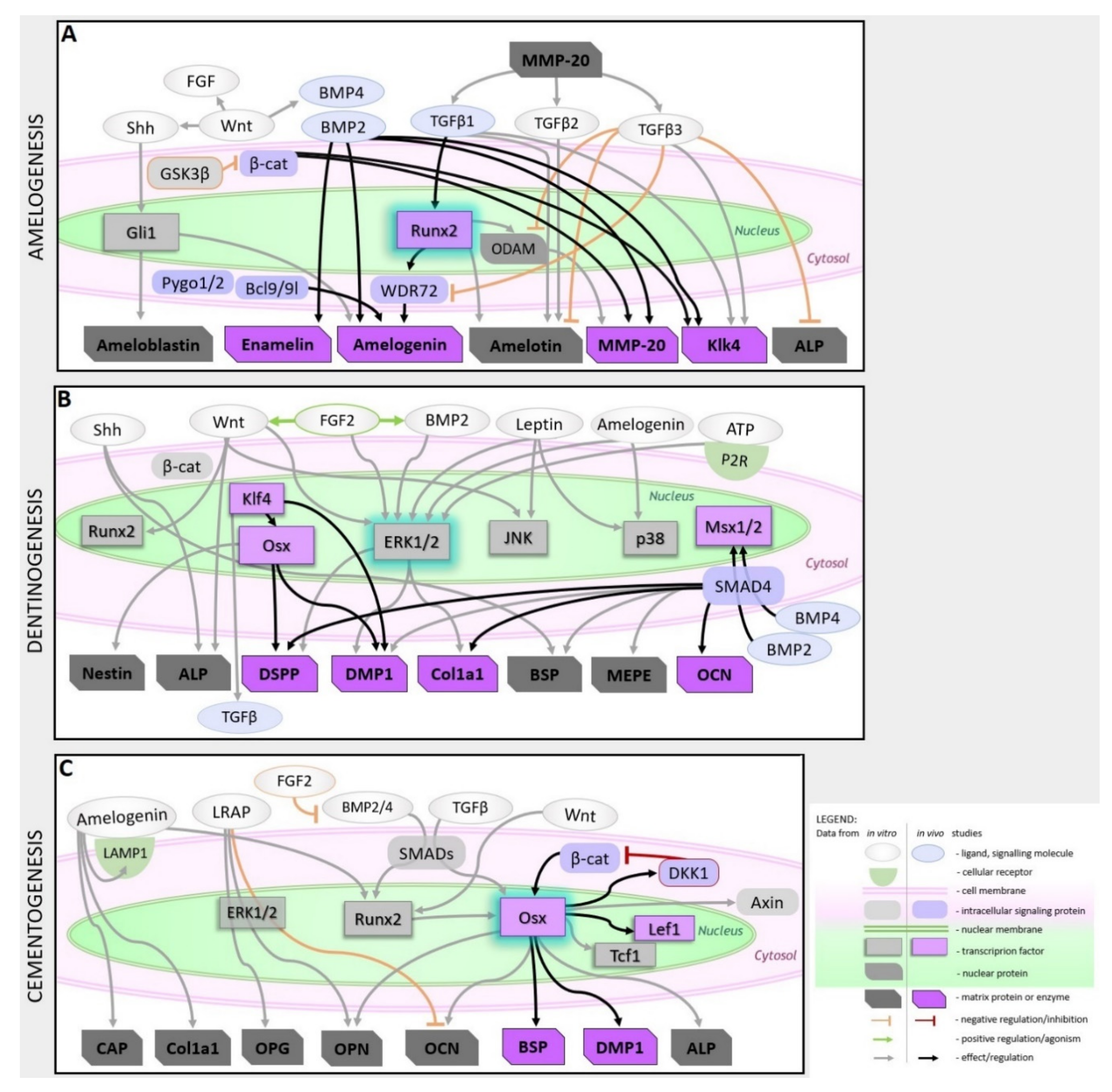
2.2.1. Amelogenesis
2.2.2. Dentinogenesis
2.2.3. Cementogenesis
3. Scaffolds and Drug Release Materials for Tooth Regeneration
3.1. Scaffolds for Enamel, Dentin, and Cementum Regeneration
3.1.1. Enamel Formation
3.1.2. Dentin Formation
3.1.3. Cementum Formation
3.2. Drug Release Systems Useful in Tissue Engineering—To be Adapted to Tooth Engineering
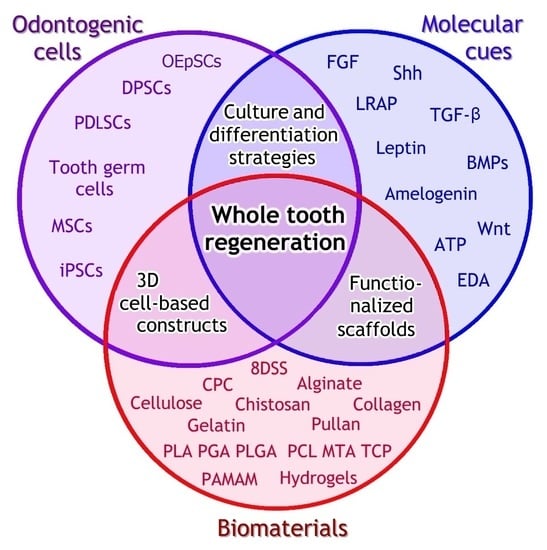
4. Whole Tooth Regeneration
4.1. Reactivation the Odontogenic Potency
4.2. Tissue Recombination Approaches
4.3. Adult Stem Cell Approaches
4.4. Problems in Whole Tooth Regeneration
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Petersen, P.E.; Bourgeois, D.; Ogawa, H.; Estupinan-Day, S.; Ndiaye, C. The global burden of oral diseases and risks to oral health. Bull. World Health Organ. 2005, 83, 661–669. [Google Scholar] [PubMed]
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. (Qassim) 2017, 11, 72–80. [Google Scholar]
- Conrads, G.; About, I. Pathophysiology of Dental Caries. In Monographs in Oral Science; S. Karger AG: Basel, Switzerland, 2018; Volume 27, pp. 1–10. ISBN 0077-0892. [Google Scholar]
- Pan, W.; Wang, Q.; Chen, Q. The cytokine network involved in the host immune response to periodontitis. Int. J. Oral Sci. 2019, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Kreiborg, S.; Jensen, B.L. Tooth formation and eruption—Lessons learnt from cleidocranial dysplasia. Eur. J. Oral Sci. 2018, 126, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Thesleff, I. Current understanding of the process of tooth formation: Transfer from the laboratory to the clinic. Aust. Dent. J. 2014, 59, 48–54. [Google Scholar] [CrossRef]
- Smith, C.E.L.; Poulter, J.A.; Antanaviciute, A.; Kirkham, J.; Brookes, S.J.; Inglehearn, C.F.; Mighell, A.J. Amelogenesis Imperfecta; Genes, Proteins, and Pathways. Front. Physiol. 2017, 8, 435. [Google Scholar] [CrossRef]
- Barron, M.J.; McDonnell, S.T.; Mackie, I.; Dixon, M.J. Hereditary dentine disorders: Dentinogenesis imperfecta and dentine dysplasia. Orphanet J. Rare Dis. 2008, 3, 31. [Google Scholar] [CrossRef]
- Chen, F.-M.; Gao, L.-N.; Tian, B.-M.; Zhang, X.-Y.; Zhang, Y.-J.; Dong, G.-Y.; Lu, H.; Chu, Q.; Xu, J.; Yu, Y.; et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: A randomized clinical trial. Stem Cell Res. Ther. 2016, 7, 33. [Google Scholar] [CrossRef]
- Lacruz, R.S.; Habelitz, S.; Wright, J.T.; Paine, M.L. Dental Enamel Formation and Implications For Oral Health and Disease. Physiol. Rev. 2017, 97, 939–993. [Google Scholar] [CrossRef]
- Tompkins, K. Molecular Mechanisms of Cytodifferentiation in Mammalian Tooth Development. Connect. Tissue Res. 2006, 47, 111–118. [Google Scholar] [CrossRef]
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: Structure, composition and mineralization. Front. Biosci. (Elite Ed.) 2011, 3, 711–735. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Hasegawa, T.; Yamamoto, T.; Hongo, H.; Amizuka, N. Histology of human cementum: Its structure, function, and development. Jpn. Dent. Sci. Rev. 2016, 52, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Nie, H.; Wang, S.; Lee, C.H.; Li, A.; Fu, S.Y.; Zhou, H.; Chen, L.; Mao, J.J. Biomaterial selection for tooth regeneration. Tissue Eng. Part B. Rev. 2011, 17, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Nakata, A.; Kameda, T.; Nagai, H.; Ikegami, K.; Duan, Y.; Terada, K.; Sugiyama, T. Establishment and characterization of a spontaneously immortalized mouse ameloblast-lineage cell line. Biochem. Biophys. Res. Commun. 2003, 308, 834–839. [Google Scholar] [CrossRef]
- Moussa, D.G.; Aparicio, C. Present and future of tissue engineering scaffolds for dentin-pulp complex regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 58–75. [Google Scholar] [CrossRef]
- Jung, C.; Kim, S.; Sun, T.; Cho, Y.-B.; Song, M. Pulp-dentin regeneration: Current approaches and challenges. J. Tissue Eng. 2019, 10. [Google Scholar] [CrossRef]
- Thesleff, I. From understanding tooth development to bioengineering of teeth. Eur. J. Oral Sci. 2018, 126, 67–71. [Google Scholar] [CrossRef]
- Shrestha, S.; Kishen, A. Bioactive Molecule Delivery Systems for Dentin-pulp Tissue Engineering. J. Endod. 2017, 43, 733–744. [Google Scholar] [CrossRef]
- Katsura, K.A.; Horst, J.A.; Chandra, D.; Le, T.Q.; Nakano, Y.; Zhang, Y.; Horst, O.V.; Zhu, L.; Le, M.H.; DenBesten, P.K. WDR72 models of structure and function: A stage-specific regulator of enamel mineralization. Matrix Biol. 2014, 38, 48–58. [Google Scholar] [CrossRef]
- Guo, F.; Feng, J.; Wang, F.; Li, W.; Gao, Q.; Chen, Z.; Shoff, L.; Donly, K.J.; Gluhak-Heinrich, J.; Chun, Y.H.P.; et al. Bmp2 deletion causes an amelogenesis imperfecta phenotype via regulating enamel gene expression. J. Cell. Physiol. 2015, 230, 1871–1882. [Google Scholar] [CrossRef]
- Xie, X.; Liu, C.; Zhang, H.; Jani, P.H.; Lu, Y.; Wang, X.; Zhang, B.; Qin, C. Abrogation of epithelial BMP2 and BMP4 causes Amelogenesis Imperfecta by reducing MMP20 and KLK4 expression. Sci. Rep. 2016, 6, 25364. [Google Scholar] [CrossRef] [PubMed]
- Shimo, T.; Koyama, E.; Kanayama, M.; Kurio, N.; Okui, T.; Yamamoto, D.; Hassan, N.M.M.; Sasaki, A. Sonic Hedgehog Positively Regulates Odontoblast Differentiation by a BMP2/4-dependent Mechanism. J. Oral Tissue Eng. 2009, 7, 26–37. [Google Scholar]
- Li, S.; Shao, J.; Zhou, Y.; Friis, T.; Yao, J.; Shi, B.; Xiao, Y. The impact of Wnt signalling and hypoxia on osteogenic and cementogenic differentiation in human periodontal ligament cells. Mol. Med. Rep. 2016, 14, 4975–4982. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ngo, V.A.; Jung, J.-Y.; Koh, J.-T.; Oh, W.-M.; Hwang, Y.-C.; Lee, B.-N. Leptin Induces Odontogenic Differentiation and Angiogenesis in Human Dental Pulp Cells via Activation of the Mitogen-activated Protein Kinase Signaling Pathway. J. Endod. 2018, 44, 585–591. [Google Scholar] [CrossRef]
- Sagomonyants, K.; Mina, M. Biphasic effects of FGF2 on odontoblast differentiation involve changes in the BMP and Wnt signaling pathways. Connect. Tissue Res. 2014, 55 (Suppl. 1), 53–56. [Google Scholar] [CrossRef]
- Sagomonyants, K.; Kalajzic, I.; Maye, P.; Mina, M. Enhanced Dentinogenesis of Pulp Progenitors by Early Exposure to FGF2. J. Dent. Res. 2015, 94, 1582–1590. [Google Scholar] [CrossRef]
- Malik, Z.; Alexiou, M.; Hallgrimsson, B.; Economides, A.N.; Luder, H.U.; Graf, D. Bone Morphogenetic Protein 2 Coordinates Early Tooth Mineralization. J. Dent. Res. 2018, 97, 835–843. [Google Scholar] [CrossRef]
- Kim, T.-H.; Bae, C.-H.; Lee, J.-Y.; Lee, J.-C.; Ko, S.-O.; Chai, Y.; Cho, E.-S. Temporo-spatial requirement of Smad4 in dentin formation. Biochem. Biophys. Res. Commun. 2015, 459, 706–712. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Nakatomi, M.; Ida-Yonemochi, H.; Ohshima, H. Quiescent adult stem cells in murine teeth are regulated by Shh signaling. Cell Tissue Res. 2017, 369, 497–512. [Google Scholar] [CrossRef]
- Lv, H.; Yang, J.; Wang, C.; Yu, F.; Huang, D.; Ye, L. The WNT7B protein promotes the migration and differentiation of human dental pulp cells partly through WNT/beta-catenin and c-Jun N-terminal kinase signalling pathways. Arch. Oral Biol. 2018, 87, 54–61. [Google Scholar] [CrossRef]
- Wang, W.; Yi, X.; Ren, Y.; Xie, Q. Effects of Adenosine Triphosphate on Proliferation and Odontoblastic Differentiation of Human Dental Pulp Cells. J. Endod. 2016, 42, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Lin, H.; Sun, Z.; Pei, F.; Zhang, J.; Chen, S.; Liu, H.; Chen, Z. Klf4 Promotes Dentinogenesis and Odontoblastic Differentiation via Modulation of TGF-β Signaling Pathway and Interaction With Histone Acetylation. J. Bone Miner. Res. 2019, 34, 1502–1516. [Google Scholar] [CrossRef] [PubMed]
- He, Y.D.; Sui, B.D.; Li, M.; Huang, J.; Chen, S.; Wu, L.A. Site-specific function and regulation of Osterix in tooth root formation. Int. Endod. J. 2016, 49, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Liu, R.; Zhang, H.; Liao, H.; Zhang, Y.; Hinton, R.J.; Feng, J.Q. Osterix controls cementoblast differentiation through downregulation of Wnt-signaling via enhancing DKK1 expression. Int. J. Biol. Sci. 2015, 11, 335–344. [Google Scholar] [CrossRef]
- Choi, H.; Kim, T.-H.; Yang, S.; Lee, J.-C.; You, H.-K.; Cho, E.-S. A Reciprocal Interaction between β-Catenin and Osterix in Cementogenesis. Sci. Rep. 2017, 7, 8160. [Google Scholar] [CrossRef]
- Choi, H.; Ahn, Y.-H.; Kim, T.-H.; Bae, C.-H.; Lee, J.-C.; You, H.-K.; Cho, E.-S. TGF-β Signaling Regulates Cementum Formation through Osterix Expression. Sci. Rep. 2016, 6, 26046. [Google Scholar] [CrossRef]
- Zhang, H.; Tompkins, K.; Garrigues, J.; Snead, M.L.; Gibson, C.W.; Somerman, M.J. Full length amelogenin binds to cell surface LAMP-1 on tooth root/periodontium associated cells. Arch. Oral Biol. 2010, 55, 417–425. [Google Scholar] [CrossRef]
- Hakki, S.S.; Bozkurt, S.B.; Türkay, E.; Dard, M.; Purali, N.; Götz, W. Recombinant amelogenin regulates the bioactivity of mouse cementoblasts in vitro. Int. J. Oral Sci. 2018, 10, 15. [Google Scholar] [CrossRef]
- Olley, R.; Xavier, G.M.; Seppala, M.; Volponi, A.A.; Geoghegan, F.; Sharpe, P.T.; Cobourne, M.T. Expression analysis of candidate genes regulating successional tooth formation in the human embryo. Front. Physiol. 2014, 5, 445. [Google Scholar] [CrossRef]
- Foster, B.L.; Nagatomo, K.J.; Nociti, F.H., Jr.; Fong, H.; Dunn, D.; Tran, A.B.; Wang, W.; Narisawa, S.; Millán, J.L.; Somerman, M.J. Central role of pyrophosphate in acellular cementum formation. PLoS ONE 2012, 7, e38393. [Google Scholar] [CrossRef]
- Takahashi, S.; Kawashima, N.; Sakamoto, K.; Nakata, A.; Kameda, T.; Sugiyama, T.; Katsube, K.; Suda, H. Differentiation of an ameloblast-lineage cell line (ALC) is induced by Sonic hedgehog signaling. Biochem. Biophys. Res. Commun. 2007, 353, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-K.; Lee, D.-S.; Ryoo, H.-M.; Park, J.-T.; Park, S.-J.; Bae, H.-S.; Cho, M.-I.; Park, J.-C. The odontogenic ameloblast-associated protein (ODAM) cooperates with RUNX2 and modulates enamel mineralization via regulation of MMP-20. J. Cell. Biochem. 2010, 111, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, C.; Tian, Y.; Sun, Y.; Zhang, J.; Bai, J.; Pan, Z.; Feng, W.; Xu, M.; Li, C.; et al. RUNX2 contributes to TGF-β1-induced expression of Wdr72 in ameloblasts during enamel mineralization. Biomed. Pharmacother. 2019, 118, 109235. [Google Scholar] [CrossRef] [PubMed]
- Aurrekoetxea, M.; Irastorza, I.; García-Gallastegui, P.; Jiménez-Rojo, L.; Nakamura, T.; Yamada, Y.; Ibarretxe, G.; Unda, F.J. Wnt/β-Catenin Regulates the Activity of Epiprofin/Sp6, SHH, FGF, and BMP to Coordinate the Stages of Odontogenesis. Front. Cell Dev. Biol. 2016, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Shijian, D.; Xin, S.; Mengmeng, L.; Cheng, S.; Wang, Y.; Gao, Y.; Chu, C.-H.; Zhan, Q. Constitutive activation of β-catenin in ameloblasts leads to incisor enamel hypomineralization. J. Mol. Histol. 2018, 49, 499–507. [Google Scholar] [CrossRef]
- Okubo, M.; Chiba, R.; Karakida, T.; Yamazaki, H.; Yamamoto, R.; Kobayashi, S.; Niwa, T.; Margolis, H.C.; Nagano, T.; Yamakoshi, Y.; et al. Potential function of TGF-β isoforms in maturation-stage ameloblasts. J. Oral Biosci. 2019, 61, 43–54. [Google Scholar] [CrossRef]
- Yelick, P.C.; Sharpe, P.T. Tooth Bioengineering and Regenerative Dentistry. J. Dent. Res. 2019, 98, 1173–1182. [Google Scholar] [CrossRef]
- Dosedělová, H.; Dumková, J.; Lesot, H.; Glocová, K.; Kunová, M.; Tucker, A.S.; Veselá, I.; Krejčí, P.; Tichý, F.; Hampl, A.; et al. Fate of the molar dental lamina in the monophyodont mouse. PLoS ONE 2015, 10, e0127543. [Google Scholar] [CrossRef]
- Ikeda, E.; Morita, R.; Nakao, K.; Ishida, K.; Nakamura, T.; Takano-Yamamoto, T.; Ogawa, M.; Mizuno, M.; Kasugai, S.; Tsuji, T. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 13475–13480. [Google Scholar] [CrossRef]
- Ono, M.; Oshima, M.; Ogawa, M.; Sonoyama, W.; Hara, E.S.; Oida, Y.; Shinkawa, S.; Nakajima, R.; Mine, A.; Hayano, S.; et al. Practical whole-tooth restoration utilizing autologous bioengineered tooth germ transplantation in a postnatal canine model. Sci. Rep. 2017, 7, 44522. [Google Scholar] [CrossRef]
- Wang, F.; Wu, Z.; Fan, Z.; Wu, T.; Wang, J.; Zhang, C.; Wang, S. The cell re-association-based whole-tooth regeneration strategies in large animal, Sus scrofa. Cell Prolif. 2018, 51, e12479. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Seo, S.-J. Biomedical Application of Dental Tissue-Derived Induced Pluripotent Stem Cells. Stem Cells Int. 2016, 2016, 9762465. [Google Scholar] [PubMed]
- Morsczeck, C.; Reichert, T.E. Dental stem cells in tooth regeneration and repair in the future. Expert Opin. Biol. Ther. 2018, 18, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Vazquez, B.; Oreadi, D.; Yelick, P.C. Decellularized Tooth Bud Scaffolds for Tooth Regeneration. J. Dent. Res. 2017, 96, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.E.; Angstadt, S.; Monteiro, N.; Zhang, W.; Khademhosseini, A.; Yelick, P.C. Bioengineered Tooth Buds Exhibit Features of Natural Tooth Buds. J. Dent. Res. 2018, 97, 1144–1151. [Google Scholar] [CrossRef]
- Yang, L.; Angelova Volponi, A.; Pang, Y.; Sharpe, P.T. Mesenchymal Cell Community Effect in Whole Tooth Bioengineering. J. Dent. Res. 2016, 96, 186–191. [Google Scholar] [CrossRef]
- Smith, E.E.; Yelick, P.C. Progress in Bioengineered Whole Tooth Research: From Bench to Dental Patient Chair. Curr. Oral Health Rep. 2016, 3, 302–308. [Google Scholar] [CrossRef]
- Schulze, M.; Tobiasch, E. Artificial Scaffolds and Mesenchymal Stem Cells for Hard Tissues. In Tissue Engineering III: Cell—Surface Interactions for Tissue Culture; Kasper, C., Witte, F., Pörtner, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 153–194. [Google Scholar]
- Leiendecker, A.; Witzleben, S.; Tobiasch, M.S. and E. Template-Mediated Biomineralization for Bone Tissue Engineering. Curr. Stem Cell Res. Ther. 2017, 12, 103–123. [Google Scholar] [CrossRef]
- El Khaldi-Hansen, B.; El-Sayed, F.; Tobiasch, E.; Witzleben, S.; Schulze, M. Functionalized 3D Scaffolds for Template- Mediated Biomineralization in Bone Regeneration. Front. Stem Cell Regen. Med. Res. 2017, 4, 3–58. [Google Scholar] [CrossRef]
- Götz, W.; Tobiasch, E.; Witzleben, S.; Schulze, M. Effects of Silicon Compounds on Biomineralization, Osteogenesis, and Hard Tissue Formation. Pharmaceutics 2019, 11, 117. [Google Scholar] [CrossRef]
- Witzler, M.; Alzagameem, A.; Bergs, M.; El Khaldi-Hansen, B.; Klein, S.; Hielscher, D.; Kamm, B.; Kreyenschmidt, J.; Tobiasch, E.; Schulze, M. Lignin-Derived Biomaterials for Drug Release and Tissue Engineering. Molecules 2018, 23, 1885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, S.; Zhou, C.; Cheng, L.; Gao, X.; Xie, X.; Sun, J.; Wang, H.; Weir, M.D.; Reynolds, M.A.; et al. Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res. 2018, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ren, J.; Li, R.; Guan, C.; Feng, Z.; Bao, B.; Wang, W.; Zhou, C. Tooth Regeneration: Insights from Tooth Development and Spatial-Temporal Control of Bioactive Drug Release. Stem Cell Rev. Rep. 2020, 16, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Patel, E.; Pradeep, P.; Kumar, P.; Choonara, Y.E.; Pillay, V. Oroactive dental biomaterials and their use in endodontic therapy. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 201–212. [Google Scholar] [CrossRef]
- Sa, Y.; Gao, Y.; Wang, M.; Wang, T.; Feng, X.; Wang, Z.; Wang, Y.; Jiang, T. Bioactive calcium phosphate cement with excellent injectability, mineralization capacity and drug-delivery properties for dental biomimetic reconstruction and minimum intervention therapy. RSC Adv. 2016, 6, 27349–27359. [Google Scholar] [CrossRef]
- Nosrati, H.; Pourmotabed, S.; Sharifi, E. A Review on Some Natural Biopolymers and Their Applications in Angiogenesis and Tissue Engineering. J. Appl. Biotechnol. Rep. 2018, 5, 81–91. [Google Scholar] [CrossRef][Green Version]
- Hu, L.; Liu, Y.; Wang, S. Stem cell-based tooth and periodontal regeneration. Oral Dis. 2018, 24, 696–705. [Google Scholar] [CrossRef]
- Takeo, M.; Tsuji, T. Organ regeneration based on developmental biology: Past and future. Curr. Opin. Genet. Dev. 2018, 52, 42–47. [Google Scholar] [CrossRef]
- Yu, T.; Klein, O.D. Molecular and cellular mechanisms of tooth development, homeostasis and repair. Development 2020, 147, dev184754. [Google Scholar] [CrossRef]
- Calamari, Z.T.; Hu, J.K.-H.; Klein, O.D. Tissue Mechanical Forces and Evolutionary Developmental Changes Act Through Space and Time to Shape Tooth Morphology and Function. Bioessays 2018, 40, e1800140. [Google Scholar] [CrossRef]
- Yuan, Y.; Chai, Y. Regulatory mechanisms of jaw bone and tooth development. Curr. Top. Dev. Biol. 2019, 133, 91–118. [Google Scholar] [PubMed]
- Orchardson, R.; Cadden, S.W. An Update on the Physiology of the Dentine–Pulp Complex. Dent. Update 2001, 28, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.-Y.; Lee, J.-H.; Kang, K.-J.; Jang, Y.-J. Effect of FGF-2, TGF-β-1, and BMPs on Teno/Ligamentogenesis and Osteo/Cementogenesis of Human Periodontal Ligament Stem Cells. Mol. Cells 2017, 40, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, N.; Okiji, T. Odontoblasts: Specialized hard-tissue-forming cells in the dentin-pulp complex. Congenit. Anom. (Kyoto) 2016, 56, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Seppala, M.; Fraser, G.J.; Birjandi, A.A.; Xavier, G.M.; Cobourne, M.T. Sonic Hedgehog Signaling and Development of the Dentition. J. Dev. Biol. 2017, 5, 6. [Google Scholar] [CrossRef]
- Saygin, N.E.; Tokiyasu, Y.; Giannobile, W.V.; Somerman, M.J. Growth factors regulate expression of mineral associated genes in cementoblasts. J. Periodontol. 2000, 71, 1591–1600. [Google Scholar] [CrossRef]
- Boabaid, F.; Gibson, C.W.; Kuehl, M.A.; Berry, J.E.; Snead, M.L.; Nociti, F.H., Jr.; Katchburian, E.; Somerman, M.J. Leucine-Rich Amelogenin Peptide: A Candidate Signaling Molecule During Cementogenesis. J. Periodontol. 2004, 75, 1126–1136. [Google Scholar] [CrossRef]
- Casagrande, L.; Demarco, F.F.; Zhang, Z.; Araujo, F.B.; Shi, S.; Nör, J.E. Dentin-derived BMP-2 and Odontoblast Differentiation. J. Dent. Res. 2010, 89, 603–608. [Google Scholar] [CrossRef]
- Yi, X.; Wang, W.; Xie, Q. Adenosine receptors enhance the ATP-induced odontoblastic differentiation of human dental pulp cells. Biochem. Biophys. Res. Commun. 2018, 497, 850–856. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, D.; Ma, L.; Ren, Y.; Dirksen, R.T.; Liu, X. Purinergic Signaling Modulates Survival/Proliferation of Human Dental Pulp Stem Cells. J. Dent. Res. 2019, 98, 242–249. [Google Scholar] [CrossRef]
- Chen, D.; Yu, F.; Wu, F.; Bai, M.; Lou, F.; Liao, X.; Wang, C.; Ye, L. The role of Wnt7B in the mediation of dentinogenesis via the ERK1/2 pathway. Arch. Oral Biol. 2019, 104, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.-Y.; Yamazaki, H.; Beniash, E.; Yang, X.; Margolis, S.; Pugach, M.; Simmer, J.; Margolis, H. Amelogenin phosphorylation regulates tooth enamel formation by stabilizing a transient amorphous mineral precursor. J. Biol. Chem. 2020, 295, jbc.RA119.010506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Zhang, W.; Putnis, C. V Phosphorylated/Nonphosphorylated Motifs in Amelotin Turn Off/On the Acidic Amorphous Calcium Phosphate-to-Apatite Phase Transformation. Langmuir 2020, 36, 2102–2109. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Schulte, F.; Lee, K.-H.; Pugach, M.K.; Hardt, M.; Bidlack, F.B. Mapping the Tooth Enamel Proteome and Amelogenin Phosphorylation Onto Mineralizing Porcine Tooth Crowns. Front. Physiol. 2019, 10, 925. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Lee, J.-W.; Zheng, X.; Zhang, J.; Lin, X.; Song, Y.; Wang, B.; Hu, X.; Chang, H.-H.; Chen, Y.; et al. Efficient induction of functional ameloblasts from human keratinocyte stem cells. Stem Cell Res. Ther. 2018, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Shinmura, Y.; Tsuchiya, S.; Hata, K.; Honda, M.J. Quiescent epithelial cell rests of Malassez can differentiate into ameloblast-like cells. J. Cell. Physiol. 2008, 217, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Padma Priya, S.; Higuchi, A.; Abu Fanas, S.; Pooi Ling, M.; Kumari Neela, V.; Sunil, P.M.; Saraswathi, T.R.; Murugan, K.; Alarfaj, A.A.; Munusamy, M.A.; et al. Odontogenic epithelial stem cells: Hidden sources. Lab. Investig. 2015, 95, 1344–1352. [Google Scholar] [CrossRef]
- Ferro, F.; Spelat, R.; Falini, G.; Gallelli, A.; D’Aurizio, F.; Puppato, E.; Pandolfi, M.; Beltrami, A.P.; Cesselli, D.; Beltrami, C.A.; et al. Adipose tissue-derived stem cell in vitro differentiation in a three-dimensional dental bud structure. Am. J. Pathol. 2011, 178, 2299–2310. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, Y.; Liu, P.; Chen, S.; Wu, X.; Sun, Y.; Li, A.; Huang, K.; Luo, R.; Wang, L.; et al. Generation of tooth-like structures from integration-free human urine induced pluripotent stem cells. Cell Regen. (Lond. Engl.) 2013, 2, 6. [Google Scholar] [CrossRef]
- Kim, E.-J.; Yoon, K.-S.; Arakaki, M.; Otsu, K.; Fukumoto, S.; Harada, H.; Green, D.W.; Lee, J.-M.; Jung, H.-S. Effective Differentiation of Induced Pluripotent Stem Cells Into Dental Cells. Dev. Dyn. 2019, 248, 129–139. [Google Scholar] [CrossRef]
- Hosoya, A.; Shalehin, N.; Takebe, H.; Shimo, T.; Irie, K. Sonic Hedgehog Signaling and Tooth Development. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Mao, J.; Liu, Y. Concise review: Pulp stem cells derived from human permanent and deciduous teeth: Biological characteristics and therapeutic applications. Stem Cells Transl. Med. 2020, 9, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, Y. Runx2 is involved in regulating amelotin promoter activity and gene expression in ameloblasts. In Proceedings of the 2013 ICME International Conference on Complex Medical Engineering, Beijing, China, 25–28 May 2013; pp. 91–96. [Google Scholar]
- Cantù, C.; Pagella, P.; Shajiei, T.D.; Zimmerli, D.; Valenta, T.; Hausmann, G.; Basler, K.; Mitsiadis, T.A. A cytoplasmic role of Wnt/β-catenin transcriptional cofactors Bcl9, Bcl9l, and Pygopus in tooth enamel formation. Sci. Signal. 2017, 10, eaah4598. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Xu, M.; Millar, S.E.; Bartlett, J.D. Beta-catenin is essential for ameloblast movement during enamel development. Eur. J. Oral Sci. 2016, 124, 221–227. [Google Scholar] [CrossRef]
- Järvinen, E.; Shimomura-Kuroki, J.; Balic, A.; Jussila, M.; Thesleff, I. Mesenchymal Wnt/β-catenin signaling limits tooth number. Development 2018, 145, dev158048. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Z.; Chen, G.; Li, J.; Li, H.; Yu, M.; Zhang, W.; Guo, W.; Tian, W. GSK3β regulates ameloblast differentiation via Wnt and TGF-β pathways. J. Cell. Physiol. 2018, 233, 5322–5333. [Google Scholar] [CrossRef]
- Kobayashi-Kinoshita, S.; Yamakoshi, Y.; Onuma, K.; Yamamoto, R.; Asada, Y. TGF-β1 autocrine signalling and enamel matrix components. Sci. Rep. 2016, 6, 33644. [Google Scholar] [CrossRef]
- Yang, J.; Ye, L.; Hui, T.-Q.; Yang, D.-M.; Huang, D.-M.; Zhou, X.-D.; Mao, J.J.; Wang, C.-L. Bone morphogenetic protein 2-induced human dental pulp cell differentiation involves p38 mitogen-activated protein kinase-activated canonical WNT pathway. Int. J. Oral Sci. 2015, 7, 95–102. [Google Scholar] [CrossRef]
- Davies, O.G.; Cooper, P.R.; Shelton, R.M.; Smith, A.J.; Scheven, B.A. A comparison of the in vitro mineralisation and dentinogenic potential of mesenchymal stem cells derived from adipose tissue, bone marrow and dental pulp. J. Bone Miner. Metab. 2015, 33, 371–382. [Google Scholar] [CrossRef]
- Xie, H.; Dubey, N.; Shim, W.; Ramachandra, C.J.A.; Min, K.S.; Cao, T.; Rosa, V. Functional Odontoblastic-Like Cells Derived from Human iPSCs. J. Dent. Res. 2017, 97, 77–83. [Google Scholar] [CrossRef]
- Naihui, Y.; Shiting, L.; Yong, J.; Songbo, Q.; Yinghui, T. Amelogenin promotes odontoblast-like MDPC-23 cell differentiation via activation of ERK1/2 and p38 MAPK. Mol. Cell. Biochem. 2011, 355, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Martín-González, J.; Pérez-Pérez, A.; Cabanillas-Balsera, D.; Vilariño-García, T.; Sánchez-Margalet, V.; Segura-Egea, J.J. Leptin stimulates DMP-1 and DSPP expression in human dental pulp via MAPK 1/3 and PI3K signaling pathways. Arch. Oral Biol. 2019, 98, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H.; Jang, J.-H.; Koh, J.-T.; Chang, H.-S.; Hwang, Y.-C.; Hwang, I.-N.; Lee, B.-N.; Oh, W.-M. Effect of Leptin on Odontoblastic Differentiation and Angiogenesis: An In Vivo Study. J. Endod. 2019, 45, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Åberg, T.; Wozney, J.; Thesleff, I. Expression patterns of bone morphogenetic proteins (Bmps) in the developing mouse tooth suggest roles in morphogenesis and cell differentiation. Dev. Dyn. 1997, 210, 383–396. [Google Scholar] [CrossRef]
- Jani, P.; Liu, C.; Zhang, H.; Younes, K.; Benson, M.D.; Qin, C. The role of bone morphogenetic proteins 2 and 4 in mouse dentinogenesis. Arch. Oral Biol. 2018, 90, 33–39. [Google Scholar] [CrossRef]
- Tucker, A.S.; Khamis, A.A.; Sharpe, P.T. Interactions between Bmp-4 and Msx-1 act to restrict gene expression to odontogenic mesenchyme. Dev. Dyn. 1998, 212, 533–539. [Google Scholar] [CrossRef]
- Lu, X.; Yang, J.; Zhao, S.; Liu, S. Advances of Wnt signalling pathway in dental development and potential clinical application. Organogenesis 2019, 15, 101–110. [Google Scholar] [CrossRef]
- Neves, V.C.M.; Babb, R.; Chandrasekaran, D.; Sharpe, P.T. Promotion of natural tooth repair by small molecule GSK3 antagonists. Sci. Rep. 2017, 7, 39654. [Google Scholar] [CrossRef]
- Foster, B.L. Methods for studying tooth root cementum by light microscopy. Int. J. Oral Sci. 2012, 4, 119–128. [Google Scholar] [CrossRef]
- Sowmya, S.; Chennazhi, K.P.; Arzate, H.; Jayachandran, P.; Nair, S.V.; Jayakumar, R. Periodontal Specific Differentiation of Dental Follicle Stem Cells into Osteoblast, Fibroblast, and Cementoblast. Tissue Eng. Part C Methods 2015, 21, 1044–1058. [Google Scholar] [CrossRef]
- Duan, X.; Tu, Q.; Zhang, J.; Ye, J.; Sommer, C.; Mostoslavsky, G.; Kaplan, D.; Yang, P.; Chen, J. Application of induced pluripotent stem (iPS) cells in periodontal tissue regeneration. J. Cell. Physiol. 2011, 226, 150–157. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, H.; Zhou, X.; Han, X.; Ren, Y.; Gao, T.; Xiao, Y.; de Crombrugghe, B.; Somerman, M.J.; Feng, J.Q. Genetic evidence for the vital function of Osterix in cementogenesis. J. Bone Miner. Res. 2012, 27, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- Nagayasu-Tanaka, T.; Anzai, J.; Takaki, S.; Shiraishi, N.; Terashima, A.; Asano, T.; Nozaki, T.; Kitamura, M.; Murakami, S. Action Mechanism of Fibroblast Growth Factor-2 (FGF-2) in the Promotion of Periodontal Regeneration in Beagle Dogs. PLoS ONE 2015, 10, e0131870. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.-F.; Zhang, J.; Duan, Y.-Z.; Jin, Y. Ameloblasts serum-free conditioned medium: Bone morphogenic protein 4-induced odontogenic differentiation of mouse induced pluripotent stem cells. J. Tissue Eng. Regen. Med. 2016, 10, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chatzeli, L.; Panousopoulou, E.; Tucker, A.S.; Green, J.B.A. Epithelial stratification and placode invagination are separable functions in early morphogenesis of the molar tooth. Development 2016, 143, 670–681. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, T.; Bai, D.; Tian, W.; Chen, Y. Smad7 Regulates Dental Epithelial Proliferation during Tooth Development. J. Dent. Res. 2019, 98, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Liu, M.; Cao, X.; Yang, S. Ciliary IFT80 regulates dental pulp stem cells differentiation by FGF/FGFR1 and Hh/BMP2 signaling. Int. J. Biol. Sci. 2019, 15, 2087–2099. [Google Scholar] [CrossRef]
- Zaugg, L.K.; Banu, A.; Walther, A.R.; Chandrasekaran, D.; Babb, R.C.; Salzlechner, C.; Hedegaard, M.A.B.; Gentleman, E.; Sharpe, P.T. Translation Approach for Dentine Regeneration Using GSK-3 Antagonists. J. Dent. Res. 2020, 0022034520908593. [Google Scholar] [CrossRef]
- Zippel, N.; Schulze, M.; Tobiasch, E. Biomaterials and Mesenchymal Stem Cells for Regenerative Medicine. Recent Pat. Biotechnol. 2010, 4, 1–22. [Google Scholar] [CrossRef]
- Tonk, C.; Witzler, M.; Schulze, M.; Tobiasch, E. Mesenchymal Stem Cells. In Essential Current Concepts in Stem Cell Biology; Brand-Saberi, B., Ed.; Springer: Berlin, Germany, 2020; pp. 21–39. ISBN 978-3-030-33922-7. [Google Scholar]
- Ottensmeyer, P.F.; Witzler, M.; Schulze, M.; Tobiasch, E. Small Molecules Enhance Scaffold-Based Bone Grafts via Purinergic Receptor Signaling in Stem Cells. Int. J. Mol. Sci. 2018, 19, 3601. [Google Scholar] [CrossRef]
- Zabrovsky, A.; Beyth, N.; Pietrokovski, Y.; Ben-Gal, G.; Houri-Haddad, Y. 5-Biocompatibility and functionality of dental restorative materials. In Woodhead Publishing Series in Biomaterials; Shelton, R., Ed.; Woodhead Publishing: Sawston/Cambridge, UK, 2017; pp. 63–75. ISBN 978-0-08-100884-3. [Google Scholar]
- El Gezawi, M.; Wölfle, U.C.; Haridy, R.; Fliefel, R.; Kaisarly, D. Remineralization, Regeneration, and Repair of Natural Tooth Structure: Influences on the Future of Restorative Dentistry Practice. ACS Biomater. Sci. Eng. 2019, 5, 4899–4919. [Google Scholar] [CrossRef]
- Jazayeri, H.E.; Lee, S.-M.; Kuhn, L.; Fahimipour, F.; Tahriri, M.; Tayebi, L. Polymeric scaffolds for dental pulp tissue engineering: A review. Dental Mater. 2020, 36, e47–e58. [Google Scholar] [CrossRef] [PubMed]
- Haugen, J.H.; Basu, P.; Sukul, M.; Mano, F.J.; Reseland, E.J. Injectable Biomaterials for Dental Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 3442. [Google Scholar] [CrossRef] [PubMed]
- Pandya, M.; Diekwisch, T. Enamel biomimetics—Fiction or future of dentistry. Int. J. Oral Sci. 2019, 11, 1–9. [Google Scholar] [CrossRef]
- Yu, H.-P.; Zhu, Y.-J.; Lu, B.-Q. Dental Enamel-Mimetic Large-Sized Multi-Scale Ordered Architecture Built by a Well Controlled Bottom-Up Strategy. Chem. Eng. J. 2018, 360, 1633–1645. [Google Scholar] [CrossRef]
- Zheng, W.; Ding, L.; Wang, Y.; Han, S.; Zheng, S.; Guo, Q.; Li, W.; Zhou, X.; Zhang, L. The effects of 8DSS peptide on remineralization in a rat model of enamel caries evaluated by two nondestructive techniques. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019827798. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Y.; Gao, L.; Wu, C.; Chang, J. Synthesis of Artificial Dental Enamel by Elastin-like Polypeptide Assisted Biomimetic Approach. J. Mater. Chem. B 2018, 6, 844–853. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, M.; Xu, H.H.K.; Tao, S.; Yu, Z.; Yang, J.; Yuan, H.; Zhou, X.; Liang, K.; Li, J. Remineralization effectiveness of the PAMAM dendrimer with different terminal groups on artificial initial enamel caries in vitro. Dent. Mater. 2020, 36, 210–220. [Google Scholar] [CrossRef]
- Gao, Y.; Liang, K.; Weir, M.D.; Gao, J.; Imazato, S.; Tay, F.R.; Lynch, C.D.; Oates, T.W.; Li, J.; Xu, H.H.K. Enamel remineralization via poly(amido amine) and adhesive resin containing calcium phosphate nanoparticles. J. Dent. 2020, 92, 103262. [Google Scholar] [CrossRef]
- Raddall, G.; Mello, I.; Leung, B.M. Biomaterials and Scaffold Design Strategies for Regenerative Endodontic Therapy. Front. Bioeng. Biotechnol. 2019, 7, 317. [Google Scholar] [CrossRef]
- Metlerska, J.; Fagogeni, I.; Nowicka, A. Efficacy of Autologous Platelet Concentrates in Regenerative Endodontic Treatment: A Systematic Review of Human Studies. J. Endod. 2019, 45, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, E.; Ricucci, D.; Albert, J.; Alobaid, A.S.; Gibbs, J.L.; Huang, G.T.-J.; Lin, L.M. Clinical, Radiographic, and Histological Observation of a Human Immature Permanent Tooth with Chronic Apical Abscess after Revitalization Treatment. J. Endod. 2013, 39, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Mandakhbayar, N.; El-Fiqi, A.; Lee, J.-H.; Kim, H.-W. Evaluation of Strontium-Doped Nanobioactive Glass Cement for Dentin–Pulp Complex Regeneration Therapy. ACS Biomater. Sci. Eng. 2019, 5, 6117–6126. [Google Scholar] [CrossRef]
- Moonesi Rad, R.; Atila, D.; Akgün, E.E.; Evis, Z.; Keskin, D.; Tezcaner, A. Evaluation of human dental pulp stem cells behavior on a novel nanobiocomposite scaffold prepared for regenerative endodontics. Mater. Sci. Eng. C 2019, 100, 928–948. [Google Scholar] [CrossRef]
- Pankajakshan, D.; Voytik-Harbin, S.L.; Nör, J.E.; Bottino, M.C. Injectable Highly Tunable Oligomeric Collagen Matrices for Dental Tissue Regeneration. ACS Appl. Bio Mater. 2020, 3, 859–868. [Google Scholar] [CrossRef]
- Goudouri, O.-M.; Kontonasaki, E.; Boccaccini, A.R. 17-Layered scaffolds for periodontal regeneration. In Biomaterials for Oral and Dental Tissue Engineering; Tayebi, L., Moharamzadeh, K., Eds.; Woodhead Publishing: Sawston/Cambridge, UK, 2017; pp. 279–295. ISBN 978-0-08-100961-1. [Google Scholar]
- Iwasaki, K.; Washio, K.; Meinzer, W.; Tsumanuma, Y.; Yano, K.; Ishikawa, I. Application of cell-sheet engineering for new formation of cementum around dental implants. Heliyon 2019, 5, e01991. [Google Scholar] [CrossRef]
- Fakheran, O.; Birang, R.; Schmidlin, P.R.; Razavi, S.M.; Behfarnia, P. Retro MTA and tricalcium phosphate/retro MTA for guided tissue regeneration of periodontal dehiscence defects in a dog model: A pilot study. Biomater. Res. 2019, 23, 14. [Google Scholar] [CrossRef]
- Wei, L.; Teng, F.; Deng, L.; Liu, G.; Luan, M.; Jiang, J.; Liu, Z.; Liu, Y. Periodontal regeneration using bone morphogenetic protein 2 incorporated biomimetic calcium phosphate in conjunction with barrier membrane: A pre-clinical study in dogs. J. Clin. Periodontol. 2019, 46, 1254–1263. [Google Scholar] [CrossRef]
- Wang, B.; Mastrogiacomo, S.; Yang, F.; Shao, J.; Ong, M.; Chanchareonsook, N.; Jansen, J.; Walboomers, X.; Yu, N. Application of BMP-Bone Cement and FGF-Gel on Periodontal Tissue Regeneration in Nonhuman Primates. Tissue Eng. Part C Methods 2019, 25, 748–756. [Google Scholar] [CrossRef]
- Oortgiesen, D.A.W.; Walboomers, X.F.; Bronckers, A.L.J.J.; Meijer, G.J.; Jansen, J.A. Periodontal regeneration using an injectable bone cement combined with BMP-2 or FGF-2. J. Tissue Eng. Regen. Med. 2014, 8, 202–209. [Google Scholar] [CrossRef]
- Vaquette, C.; Saifzadeh, S.; Farag, A.; Hutmacher, D.W.; Ivanovski, S. Periodontal Tissue Engineering with a Multiphasic Construct and Cell Sheets. J. Dent. Res. 2019, 98, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Witzler, M.; Büchner, D.; Shoushrah, S.H.; Babczyk, P.; Baranova, J.; Witzleben, S.; Tobiasch, E.; Schulze, M. Polysaccharide-Based Systems for Targeted Stem Cell Differentiation and Bone Regeneration. Biomolecules 2019, 9, 840. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.A.R.; Cuttaz, E.A.; Goding, J.A.; Green, R.A. Actively controlled local drug delivery using conductive polymer-based devices. Appl. Phys. Lett. 2020, 116, 10501. [Google Scholar] [CrossRef]
- Kikuchi, N.; Kitamura, C.; Morotomi, T.; Inuyama, Y.; Ishimatsu, H.; Tabata, Y.; Nishihara, T.; Terashita, M. Formation of Dentin-like Particles in Dentin Defects above Exposed Pulp by Controlled Release of Fibroblast Growth Factor 2 from Gelatin Hydrogels. J. Endod. 2007, 33, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Moioli, E.K.; Clark, P.A.; Xin, X.; Lal, S.; Mao, J.J. Matrices and scaffolds for drug delivery in dental, oral and craniofacial tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, C.; Nishihara, T.; Terashita, M.; Tabata, Y.; Washio, A. Local regeneration of dentin-pulp complex using controlled release of fgf-2 and naturally derived sponge-like scaffolds. Int. J. Dent. 2012, 2012, 190561. [Google Scholar] [CrossRef]
- Piva, E.; Silva, A.F.; Nör, J.E. Functionalized scaffolds to control dental pulp stem cell fate. J. Endod. 2014, 40, S33–S40. [Google Scholar] [CrossRef]
- Zhang, N.; Weir, M.D.; Chen, C.; Melo, M.A.S.; Bai, Y.; Xu, H.H.K. Orthodontic cement with protein-repellent and antibacterial properties and the release of calcium and phosphate ions. J. Dent. 2016, 50, 51–59. [Google Scholar] [CrossRef]
- Monteiro, N.; Yelick, P.C. Advances and perspectives in tooth tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 2443–2461. [Google Scholar] [CrossRef]
- Tabatabaei, F.; Torres, R.; Tayebi, L. Biomedical Materials in Dentistry. In Applications of Biomedical Engineering in Dentistry; Tayebi, L., Ed.; Springer: Cham, Switzerland, 2020; pp. 3–20. ISBN 978-3-030-21582-8. [Google Scholar]
- Moon, C.-Y.; Nam, O.H.; Kim, M.; Lee, H.-S.; Kaushik, S.N.; Cruz Walma, D.A.; Jun, H.-W.; Cheon, K.; Choi, S.C. Effects of the nitric oxide releasing biomimetic nanomatrix gel on pulp-dentin regeneration: Pilot study. PLoS ONE 2018, 13, e0205534. [Google Scholar] [CrossRef] [PubMed]
- Ishimatsu, H.; Kitamura, C.; Morotomi, T.; Tabata, Y.; Nishihara, T.; Chen, K.-K.; Terashita, M. Formation of Dentinal Bridge on Surface of Regenerated Dental Pulp in Dentin Defects by Controlled Release of Fibroblast Growth Factor–2 From Gelatin Hydrogels. J. Endod. 2009, 35, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Tutar, R.; Motealleh, A.; Khademhosseini, A.; Kehr, N.S. Functional Nanomaterials on 2D Surfaces and in 3D Nanocomposite Hydrogels for Biomedical Applications. Adv. Funct. Mater. 2019, 29, 1904344. [Google Scholar] [CrossRef]
- Inoue, B.S.; Streit, S.; dos Santos Schneider, A.L.; Meier, M.M. Bioactive bacterial cellulose membrane with prolonged release of chlorhexidine for dental medical application. Int. J. Biol. Macromol. 2020, 148, 1098–1108. [Google Scholar] [CrossRef]
- Gericke, M.; Heinze, T. Homogeneous tosylation of agarose as an approach toward novel functional polysaccharide materials. Carbohydr. Polym. 2015, 127, 236–245. [Google Scholar] [CrossRef]
- Kostag, M.; Gericke, M.; Heinze, T.; El Seoud, O.A. Twenty-five years of cellulose chemistry: Innovations in the dissolution of the biopolymer and its transformation into esters and ethers. Cellulose 2019, 26, 139–184. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Zippel, N.; Limbach, C.A.; Ratajski, N.; Urban, C.; Luparello, C.; Pansky, A.; Kassack, M.U.; Tobiasch, E. Purinergic Receptors Influence the Differentiation of Human Mesenchymal Stem Cells. Stem Cells Dev. 2011, 21, 884–900. [Google Scholar] [CrossRef]
- Kaebisch, C.; Schipper, D.; Babczyk, P.; Tobiasch, E. The role of purinergic receptors in stem cell differentiation. Comput. Struct. Biotechnol. J. 2014, 13, 75–84. [Google Scholar] [CrossRef]
- Schipper, D.; Babczyk, P.; Elsayed, F.; Klein, S.E.; Schulze, M.; Tobiasch, E. The effect of nanostructured surfaces on stem cell fate. In Nanostructures for Novel Therapy. Synthesis, Characterization and Applications; Grumezescu, F., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 567–589. ISBN 978-0-323-46142-9. [Google Scholar]
- Zhang, Y.; Lau, P.; Pansky, A.; Kassack, M.; Hemmersbach, R.; Tobiasch, E. The Influence of Simulated Microgravity on Purinergic Signaling Is Different between Individual Culture and Endothelial and Smooth Muscle Cell Coculture. Biomed Res. Int. 2014, 2014, 413708. [Google Scholar] [CrossRef]
- Babczyk, P.; Conzendorf, C.; Klose, J.; Schulze, M.; Harre, K.; Tobiasch, E. Stem Cells on Biomaterials for Synthetic Grafts to Promote Vascular Healing. J. Clin. Med. 2014, 3, 39–87. [Google Scholar] [CrossRef] [PubMed]
- Grotheer, V.; Schulze, M.; Tobiasch, E. Trends in Bone Tissue Engineering: Proteins for Osteogenic Differentiation and the Respective Scaffolding. In Protein Purification: Principles and Trends; iConcept Press: Hong Kong, China, 2014; ISBN 978-1-922227-40-9. [Google Scholar]
- Witzler, M.; Ottensmeyer, P.F.; Gericke, M.; Heinze, T.; Tobiasch, E.; Schulze, M. Non-Cytotoxic Agarose/Hydroxyapatite Composite Scaffolds for Drug Release. Int. J. Mol. Sci. 2019, 20, 3565. [Google Scholar] [CrossRef] [PubMed]
- Kolanthai, E.; Dikeshwar Colon, V.S.; Sindu, P.A.; Chandra, V.S.; Karthikeyan, K.R.; Babu, M.S.; Sundaram, S.M.; Palanichamy, M.; Kalkura, S.N. Effect of solvent; enhancing the wettability and engineering the porous structure of a calcium phosphate/agarose composite for drug delivery. RSC Adv. 2015, 5, 18301–18311. [Google Scholar] [CrossRef]
- Izadi, Z.; Derakhshankhah, H.; Alaei, L.; Karkazis, E.; Jafari, S.; Tayebi, L. Recent Advances in Nanodentistry. In Applications of Biomedical Engineering in Dentistry; Tayebi, L., Ed.; Springer: Cham, Switzerland, 2020; pp. 263–287. [Google Scholar]
- Fan, Y.; Wen, Z.; Liao, S.; Lallier, T.; Hagan, J.; Twomley, J.; Zhang, J.-F.; Sun, Z.; Xu, X. Novel amelogenin-releasing hydrogel for remineralization of enamel artificial caries. J. Bioact. Compat. Polym. 2012, 27, 585–603. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, L.A.J.; Vonwil, D.; Christensen, J.; Shastri, V.P. Gelatin device for the delivery of growth factors involved in endochondral ossification. PLoS ONE 2017, 12, e0175095. [Google Scholar] [CrossRef] [PubMed]
- Gellynck, K.; Abou Neel, E.A.; Li, H.; Mardas, N.; Donos, N.; Buxton, P.; Young, A.M. Cell attachment and response to photocured, degradable bone adhesives containing tricalcium phosphate and purmorphamine. Acta Biomater. 2011, 7, 2672–2677. [Google Scholar] [CrossRef]
- De la Vega, L.; Karmirian, K.; Willerth, S.M. Engineering Neural Tissue from Human Pluripotent Stem Cells Using Novel Small Molecule Releasing Microspheres. Adv. Biosyst. 2018, 2, 1800133. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Y.; Zhou, A.; Chen, X.; Li, K.; Chen, S.; Qiao, B.; Jiang, D. Controlled release of basic fibroblast growth factor from a peptide biomaterial for bone regeneration. R. Soc. Open Sci. 2020, 7, 191830. [Google Scholar] [CrossRef]
- Tachibana, A.; Yasuma, D.; Takahashi, R.; Tanabe, T. Chitin degradation enzyme-responsive system for controlled release of fibroblast growth factor-2. J. Biosci. Bioeng. 2020, 129, 116–120. [Google Scholar] [CrossRef]
- Karahaliloğlu, Z.; Yalçın, E.; Demirbilek, M.; Denkbaş, E.B. Magnetic silk fibroin e-gel scaffolds for bone tissue engineering applications. J. Bioact. Compat. Polym. 2017, 32, 596–614. [Google Scholar] [CrossRef]
- Scarpa, E.; Janeczek, A.A.; Hailes, A.; de Andrés, M.C.; De Grazia, A.; Oreffo, R.O.C.; Newman, T.A.; Evans, N.D. Polymersome nanoparticles for delivery of Wnt-activating small molecules. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, R.A.; Mahmoud, N.; Soliman, S.; Nouh, S.R.; Cunningham, L.; El-Ghannam, A. Acceleration of Alveolar Ridge Augmentation Using a Low Dose of Recombinant Human Bone Morphogenetic Protein-2 Loaded on a Resorbable Bioactive Ceramic. J. Oral Maxillofac. Surg. 2015, 73, 2257–2272. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Q.; Wang, M. Cryogenic 3D printing for producing hierarchical porous and rhBMP-2-loaded Ca-P/PLLA nanocomposite scaffolds for bone tissue engineering. Biofabrication 2017, 9, 25031. [Google Scholar] [CrossRef] [PubMed]
- Minardi, S.; Fernandez-Moure, S.J.; Fan, D.; Murphy, B.M.; Yazdi, K.I.; Liu, X.; Weiner, K.B.; Tasciotti, E. Biocompatible PLGA-Mesoporous Silicon Microspheres for the Controlled Release of BMP-2 for Bone Augmentation. Pharmaceutics 2020, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Damanik, F.F.R.; Brunelli, M.; Pastorino, L.; Ruggiero, C.; van Blitterswijk, C.; Rotmans, J.; Moroni, L. Sustained delivery of growth factors with high loading efficiency in a layer by layer assembly. Biomater. Sci. 2020, 8, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Ardeshirylajimi, A.; Ghaderian, S.M.-H.; Omrani, M.D.; Moradi, S.L. Biomimetic scaffold containing PVDF nanofibers with sustained TGF-β release in combination with AT-MSCs for bladder tissue engineering. Gene 2018, 676, 195–201. [Google Scholar] [CrossRef]
- Mahmoudi, Z.; Mohammadnejad, J.; Razavi Bazaz, S.; Abouei Mehrizi, A.; Saidijam, M.; Dinarvand, R.; Ebrahimi Warkiani, M.; Soleimani, M. Promoted chondrogenesis of hMCSs with controlled release of TGF-β3 via microfluidics synthesized alginate nanogels. Carbohydr. Polym. 2020, 229, 115551. [Google Scholar] [CrossRef]
- Díaz-Saldívar, P.; Huidobro-Toro, J.P. ATP-loaded biomimetic nanoparticles as controlled release system for extracellular drugs in cancer applications. Int. J. Nanomed. 2019, 14, 2433–2447. [Google Scholar] [CrossRef]
- Mohammadi Amirabad, L.; Zarrintaj, P.; Lindemuth, A.; Tayebi, L. Whole Tooth Engineering. In Applications of Biomedical Engineering in Dentistry; Tayebi, L., Ed.; Springer: Cham, Switzerland, 2020; pp. 443–462. ISBN 978-3-030-21582-8. [Google Scholar]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.-M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.-Y.; Wang, S.; et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef]
- Oshima, M.; Inoue, K.; Nakajima, K.; Tachikawa, T.; Yamazaki, H.; Isobe, T.; Sugawara, A.; Ogawa, M.; Tanaka, C.; Saito, M.; et al. Functional tooth restoration by next-generation bio-hybrid implant as a bio-hybrid artificial organ replacement therapy. Sci. Rep. 2014, 4, 6044. [Google Scholar] [CrossRef]
- Gao, Z.H.; Hu, L.; Liu, G.L.; Wei, F.L.; Liu, Y.; Liu, Z.H.; Fan, Z.P.; Zhang, C.M.; Wang, J.S.; Wang, S.L. Bio-Root and Implant-Based Restoration as a Tooth Replacement Alternative. J. Dent. Res. 2016, 95, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Oshima, M.; Tsuji, T. Whole Tooth Regeneration as a Future Dental Treatment. Adv. Exp. Med. Biol. 2015, 881, 255–269. [Google Scholar] [PubMed]
- Angelova Volponi, A.; Zaugg, L.K.; Neves, V.; Liu, Y.; Sharpe, P.T. Tooth Repair and Regeneration. Curr. Oral Health Rep. 2018, 5, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tang, Q.; Wang, A.; Chen, Y. Regrowing a tooth: In vitro and in vivo approaches. Curr. Opin. Cell Biol. 2019, 61, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Popa, E.M.; Buchtova, M.; Tucker, A.S. Revitalising the rudimentary replacement dentition in the mouse. Development 2019, 146, dev171363. [Google Scholar] [CrossRef] [PubMed]
- Balic, A. Biology Explaining Tooth Repair and Regeneration: A Mini-Review. Gerontology 2018, 64, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Mina, M.; Kollar, E.J. The induction of odontogenesis in non-dental mesenchyme combined with early murine mandibular arch epithelium. Arch. Oral Biol. 1987, 32, 123–127. [Google Scholar] [CrossRef]
- Young, C.S.; Terada, S.; Vacanti, J.P.; Honda, M.; Bartlett, J.D.; Yelick, P.C. Tissue Engineering of Complex Tooth Structures on Biodegradable Polymer Scaffolds. J. Dent. Res. 2002, 81, 695–700. [Google Scholar] [CrossRef]
- Duailibi, M.T.; Duailibi, S.E.; Young, C.S.; Bartlett, J.D.; Vacanti, J.P.; Yelick, P.C. Bioengineered Teeth from Cultured Rat Tooth Bud Cells. J. Dent. Res. 2004, 83, 523–528. [Google Scholar] [CrossRef]
- Nakao, K.; Morita, R.; Saji, Y.; Ishida, K.; Tomita, Y.; Ogawa, M.; Saitoh, M.; Tomooka, Y.; Tsuji, T. The development of a bioengineered organ germ method. Nat. Methods 2007, 4, 227–230. [Google Scholar] [CrossRef]
- Oshima, M.; Mizuno, M.; Imamura, A.; Ogawa, M.; Yasukawa, M.; Yamazaki, H.; Morita, R.; Ikeda, E.; Nakao, K.; Takano-Yamamoto, T.; et al. Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy. PLoS ONE 2011, 6, e21531. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, F.; Fan, Z.; Wu, T.; He, J.; Wang, J.; Zhang, C.; Wang, S. Whole-Tooth Regeneration by Allogeneic Cell Reassociation in Pig Jawbone. Tissue Eng. Part A 2019, 25, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Young, C.S.; Abukawa, H.; Asrican, R.; Ravens, M.; Troulis, M.J.; Kaban, L.B.; Vacanti, J.P.; Yelick, P.C. Tissue-Engineered Hybrid Tooth and Bone. Tissue Eng. 2005, 11, 1599–1610. [Google Scholar] [CrossRef] [PubMed]
- Young, C.S.; Kim, S.-W.; Qin, C.; Baba, O.; Butler, W.T.; Taylor, R.R.; Bartlett, J.D.; Vacanti, J.P.; Yelick, P.C. Developmental analysis and computer modelling of bioengineered teeth. Arch. Oral Biol. 2005, 50, 259–265. [Google Scholar] [CrossRef]
- Ohazama, A.; Modino, S.A.C.; Miletich, I.; Sharpe, P.T. Stem-cell-based Tissue Engineering of Murine Teeth. J. Dent. Res. 2004, 83, 518–522. [Google Scholar] [CrossRef]
- Angelova Volponi, A.; Kawasaki, M.; Sharpe, P.T. Adult human gingival epithelial cells as a source for whole-tooth bioengineering. J. Dent. Res. 2013, 92, 329–334. [Google Scholar] [CrossRef]
- Hu, B.; Nadiri, A.; Kuchler-Bopp, S.; perrin-schmitt, F.; Peters, H.; Lesot, H. Tissue Engineering of Tooth Crown, Root, and Periodontium. Tissue Eng. 2006, 12, 2069–2075. [Google Scholar] [CrossRef]
- Zheng, Y.; Cai, J.; Hutchins, A.P.; Jia, L.; Liu, P.; Yang, D.; Chen, S.; Ge, L.; Pei, D.; Wei, S. Remission for Loss of Odontogenic Potential in a New Micromilieu In Vitro. PLoS ONE 2016, 11, e0152893. [Google Scholar] [CrossRef]
- Kuchler-Bopp, S.; Bécavin, T.; Kökten, T.; Weickert, J.L.; Keller, L.; Lesot, H.; Deveaux, E.; Benkirane-Jessel, N. Three-dimensional Micro-culture System for Tooth Tissue Engineering. J. Dent. Res. 2016, 95, 657–664. [Google Scholar] [CrossRef]
- Zhang, W.; Vázquez, B.; Yelick, P.C. Bioengineered post-natal recombinant tooth bud models. J. Tissue Eng. Regen. Med. 2017, 11, 658–668. [Google Scholar] [CrossRef]
- Yang, K.-C.; Kitamura, Y.; Wu, C.-C.; Chang, H.-H.; Ling, T.-Y.; Kuo, T.-F. Tooth Germ-Like Construct Transplantation for Whole-Tooth Regeneration: An In Vivo Study in the Miniature Pig. Artif. Organs 2016, 40, E39–E50. [Google Scholar] [CrossRef] [PubMed]
- Nait Lechguer, A.; Kuchler-Bopp, S.; Hu, B.; Haïkel, Y.; Lesot, H. Vascularization of Engineered Teeth. J. Dent. Res. 2008, 87, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Strub, M.; Keller, L.; Idoux-Gillet, Y.; Lesot, H.; Clauss, F.; Benkirane-Jessel, N.; Kuchler-Bopp, S. Bone Marrow Stromal Cells Promote Innervation of Bioengineered Teeth. J. Dent. Res. 2018, 97, 1152–1159. [Google Scholar] [CrossRef] [PubMed]


| Tissue | Plausible Cell Sources | Signaling Pathway/Node | Interfering Molecule(s) | |
|---|---|---|---|---|
| Stimulatory | Inhibitory | |||
| Enamel | Keratinocyte stem cells [87]; ERM from periodontal ligament [88]; OEpSCs [89]; AT-MSCs [90]; iPSCs [91,92,117] | Hh | Shh [42]; purmorphamine [118] a | cyclopamine [118] a |
| FGF | FGF8 [87], FGF10 [118] a | pan-FGF receptor inhibitor SU5402 [118] a | ||
| Wnt/β-catenin | 6-Bromoindirubin-3′-oxime (BIO) (GSK3βi) [45] | GSK3β [99], ICG-001 [97] | ||
| BMP | BMP2/4 [21,22] b | Noggin (BMP4i) [117] | ||
| TGFβ | TGF-β1,2,3 [47,100] | SMAD7 [119] a | ||
| Dentin | DPSCs [25,81,101,102]; SHEDs [80]; AT-MSCS [102]; iPSCs [103] | Hh | Shh [23], purmorphamine [120] | _ |
| FGF | FGF2 [26,27,120] | PD173074 (FGFR1i) [120] | ||
| Wnt/β-catenin | BIO, CHIR, Tideglusib (GSK3bi) [111,121] b, Wnt7b [83]; | XAV939 (tankyrasei) [31,101], rhDKK1 [101] | ||
| BMP | BMP2 [28,108] b, BMP4 [108] b | Noggin, LDN193189 [101] | ||
| P2Rs | ATP, ARL 67156 (ATPasei) [32] | Suramin [32], iso-PPADS tetrasodium salt [82] | ||
| ERK1/2 | Leptin [105] | PD98059 (ERK1/2i) [105] | ||
| ERK1/2 | Amelogenin [104] | U0126 (ERK1/2i) [104] | ||
| Cementum | PDLSCs [75]; DFSCs [113]; iPSCs [114] | Wnt/β-catenin | LiCl, Wnt3a [35] | DKK1 [35] |
| FGF | FGF2 [116] b | _ | ||
| BMP | BMP2/4 [75] | FGF2 [75] | ||
| TGFβ | rhTGFβ-1 [78] | SIS3 (Smad3i) [37] | ||
| ERK1/2 | Amelogenin [39], LRAP [79] | U0126 (ERK1/2i) [79] | ||
| Tissue | Scaffold Material | Study Model | Results | Ref. |
|---|---|---|---|---|
| Enamel | 8DSS: Oligopeptide of eight repetitive sequences of aspartate-serine-serine | In vivo model using Sprague-Dawley rats with induced caries. | Increased remineralization by 8DSS due to inhibited enamel demineralization and promoted remineralization. | [131] |
| Elastin-like polypeptide functionalized with glutamic acid residues | In vitro remineralization of bovine enamel specimens by pH cycling after immersion in biomaterial solution. | Formation of a dense layer of highly orientated apatite nanorods with mechanical properties close to natural enamel and high chemical stability against acidic impacts. | [132] | |
| PAMAM-dendrimers with varying terminal groups: -NH2, -COOH, -OH | In vitro remineralization of bovine enamel specimens by pH cycling. | Remineralization is affected by electrostatic interactions between scaffold and enamel surface. PAMAM-NH2 shows the best results, followed by PAMAM-COOH. | [133] | |
| ACP-loaded PAMAM dendrimers functionalized with SN15 peptide sequence. | In vitro enamel remineralization by cycling immersion in artificial saliva and demineralization solution. | Evaluated biomaterial achieves 90% higher remineralization compared to control. | [134] | |
| Dentin | Nanobioactive glass cements with or without Sr | In vitro evaluation of biocompatibility and differentiation of DPSCs. In vivo evaluation using an ectopic odontogenesis model and a tooth defect model in rats. | Fast release of bioactive Ca-, Sr- and Si-ions. Promotion of the odontogenic differentiation of DPSCs in vitro. More new dentin formation by Sr-containing biomaterial in vivo. | [138] |
| The organic matrix of cellulose acetate, oxidized pullulan and gelatin loaded with boron-modified bioactive glass nanoparticles. | In vitro evaluation of biomineralization, biocompatibility, proliferation, and differentiation with hDPSCs. | Boron-modified bioactive glass nanoparticles exhibit promotive effects on the deposition of a CaP as well as on adhesion, migration, and differentiation of hDPSCs. | [139] | |
| Biphasic collagen matrix: Inner section of lower stiffness loaded with VEGF covered by an outer section of higher stiffness loaded with BMP2. | In vitro evaluation using hDPSCs regarding biocompatibility, proliferation, and differentiation. | The direction of DPSCs differentiation is regulated by material stiffness and amplified by the respective growth factor. | [140] | |
| Cementum | retroMTA + tricalcium phosphate | In vivo test using dehiscence periodontal defects in dogs. | Significantly increased the new bone and cementum formation. The biodegradability of retroMTA is enhanced by adding TCP. | [143] |
| Calcium phosphate loaded with BMP2 | In vivo periodontitis model using critical-sized supra-alveolar defects in dogs. | Significant increase in regeneration of mineralized tissues. Loading with BMP2 leads to a further 2–3-fold increase. | [144] | |
| Bilayered material: FGF2-propyleneglycol alginate gel covered by BMP2-PLGA/CaP cement. | In vivo test using three wall periodontal defects in non-human primates. | Significantly enhanced regeneration of cementum and periodontal ligament. Newly formed PDL is highly vascularized. | [145] | |
| PCL-based bilayered material: a flexible porous membrane delivers cell sheets and is covered by a fibrous and porous 3D compartment. | In vivo test using dehiscence periodontal defects in sheep to evaluate the potential of different cell types forming the cell sheets: Gingival cells (GCs), PDLCs, and hBM-MSCs. | Scaffolds containing BM-MSCs and PDLCs show superior new bone and cementum formation compared to scaffolds containing gingival cells. | [147] |
| Signaling Molecule | Material for Drug Loading/Encapsulation and Release | Application | Release Efficiency/Kinetics Tested in | Reference |
|---|---|---|---|---|
| Amelogenin (EKR1/2 activator) | Self-assembled nanogels of cholesterol-bearing mannan as templates for hierarchical hybrid nanostructures | Amelogenin-releasing hydrogel for remineralization of enamel damage (artificial caries) | Cytotoxicity—in PDL fibroblasts; ex vivo enamel caries models of human molars | [174] |
| Purmorphamine (Hh activator/Smo agonist) | Glutaraldehyde (GA)-crosslinked gelatin type B matrix (for small molecules and proteins release) | In vitro delivery system for Wnt, Hh agonists and growth factors (e.g., FGF2, VEGF) beneficial for endochondral ossification | Release kinetics (burst vs. sustained release) studied without using cell culture; released molecules bioactivity verified in cell culture/biological assays | [175] |
| Poly(propylene glycol–co-lactide) dimethacrylate (PPLM) adhesives for incorporating purmorphamine and TCP | Cell attachment and response to photocured, degradable bone adhesives containing TCP and purmorphamine | MC3T3-E1 (mouse pre-osteoblast cell line) | [176] | |
| PCL microspheres for encapsulating small molecules using a single emulsion oil-in-water method | Purmorphamine and retinoic acid-loaded microspheres for prolonged release during neural differentiation | Human iPSC aggregates differentiating into motor neurons | [177] | |
| FGF | D-RADA16 peptide hydrogels coated on artificial bone composed of nanohydroxy-apatite/polyamide 66 (nHA/PA66) (for basic FGF release) | Porous growth factor-releasing structure for treating large bone defects | Female SD rat BM-MSCs; female SD rats with induced large bone defects | [178] |
| Acetyl chitosan (chitin) gel (for binding and release of chitin binding peptide-FGF2 fusion protein) | Lysozyme-responsive (dose-dependent or activity-dependent) release of CBP-FGF2 | Studies without using cell culture/biological assays | [179] | |
| Silk fibroin e-gel scaffolds (loaded with albumin = Fe3O4-bFGF conjugate) | Enhancing alkaline phosphatase, calcium deposition, and collagen synthesis during osteogenic differentiation | SaOS-2, osteogenic differentiation | [180] | |
| BIO (Wnt/β-catenin activator) | Polymersomes (PMs) consisting of PEG-PCL block copolymer (approved for clinical use) loaded with BIO | BIO-loaded PMs for controlled activation of Wnt signaling and Runx2 during osteogenesis | Murine 3T3 Wnt reporter cells; Human BM-MSCs, osteogenic differentiation | [181] |
| None | Local application of Wnt pathway modulators (BIO, CHIR, and Tegusib) to promote dentine regeneration | Wistar rats and CD1 mice molar damage | [121] | |
| BMP2 | Porous silica–calcium phosphate composite (SCPC50) (loaded with rhBMP2) | Sustained release of fhBMP2 for alveolar ridge augmentation in saddle-type defect | Mongrel dog with induced mandible defect | [182] |
| Calcium phosphate (Ca-P)/poly(L-lactic acid) (PLLA) nanocomposites loaded with rhBMP2 | 3D Ca-P-PLLA scaffold sustainably releasing Ca2+ and rhBMP2 for enhanced osteogenesis | Human BM-MSCs, osteogenic differentiation | [183] | |
| Poly(lactic-co-glycolic acid)-multistage vector composite microspheres (PLGA-MSV) (for BMP2 release) | Controlled prolonged release of BMP2 for osteoinduction of rat BM-MSCs | Male SD rat BM-MSCs, osteogenic differentiation | [184] | |
| TGF-β 1, 3 | Poly(ethylene oxide terephthalate)/ poly(butylene terephthalate) (PEOT/PBT) fibrous resins for loading the growth factors | Sustained delivery of growth factors (TGF-β1, PDGF-ββ, IGF-1) using a layer by layer assembly for supporting fibroblast attachment and proliferation | TK173 (human renal fibroblast cell line), neonatal rat dermal fibroblasts (nRDFs) | [185] |
| Poly(vinylidene fluoride) (PVDF) nanofibers fabricated via electro-spinning method with/without chitosan nanoparticles (loaded with TGF-β1) | PVDF-TGF-β1 as a bio-functionalscaffold for enhancing smooth muscle cells (SMC) differentiation | AT-MSCs, SMC differentiation | [186] | |
| Alginate nanogel with cross-junction microchannels (encapsulating TGF-β3) | Controlled release of TGF-β3 from polymeric nanogel for enhanced chondrogenesis | Human MSCs, chondrogenic differentiation | [187] | |
| ATP, suramin (P2XR activators) | Albumin nanoparticles (aNPs) of low polydispersity loaded with ATP and coated with erythrocyte membrane (EM) | EM-aNPs developed as a delivery vehicle for ATP to be used as an anticancer agent | HeLa, HEK-293 cell lines | [188] |
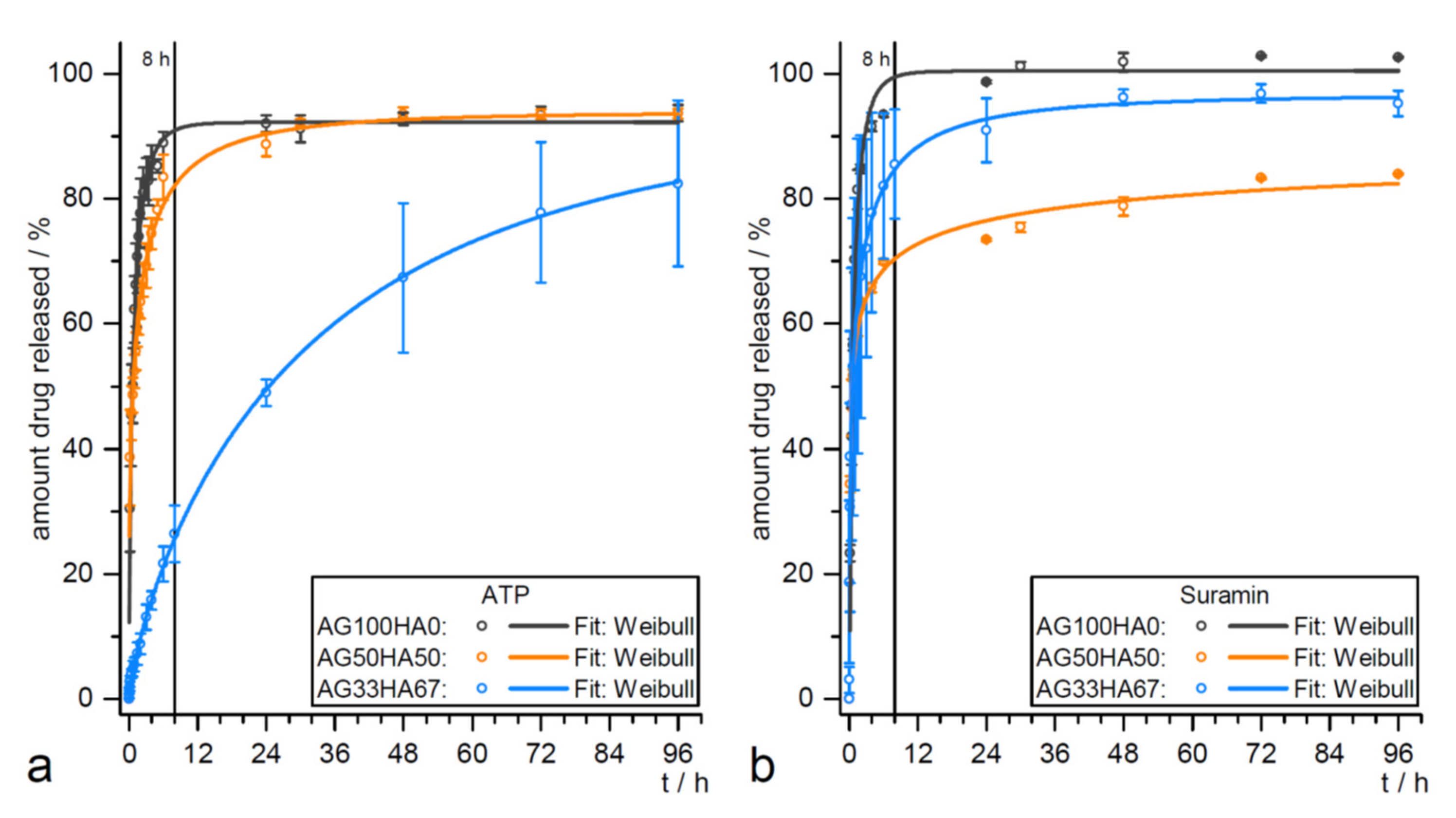
| Hydroxyapatite (HA)/agarose hybrids for ATP and suramin release | ATP and suramin release for hard tissue formation | Release kinetic studies without cells (see Figure 4); biocompatibility test using AT-MSCs and MG-63 cell line | [171] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranova, J.; Büchner, D.; Götz, W.; Schulze, M.; Tobiasch, E. Tooth Formation: Are the Hardest Tissues of Human Body Hard to Regenerate? Int. J. Mol. Sci. 2020, 21, 4031. https://doi.org/10.3390/ijms21114031
Baranova J, Büchner D, Götz W, Schulze M, Tobiasch E. Tooth Formation: Are the Hardest Tissues of Human Body Hard to Regenerate? International Journal of Molecular Sciences. 2020; 21(11):4031. https://doi.org/10.3390/ijms21114031
Chicago/Turabian StyleBaranova, Juliana, Dominik Büchner, Werner Götz, Margit Schulze, and Edda Tobiasch. 2020. "Tooth Formation: Are the Hardest Tissues of Human Body Hard to Regenerate?" International Journal of Molecular Sciences 21, no. 11: 4031. https://doi.org/10.3390/ijms21114031
APA StyleBaranova, J., Büchner, D., Götz, W., Schulze, M., & Tobiasch, E. (2020). Tooth Formation: Are the Hardest Tissues of Human Body Hard to Regenerate? International Journal of Molecular Sciences, 21(11), 4031. https://doi.org/10.3390/ijms21114031