Intracellular Cl− Regulation of Ciliary Beating in Ciliated Human Nasal Epithelial Cells: Frequency and Distance of Ciliary Beating Observed by High-Speed Video Microscopy
Abstract
1. Introduction
2. cHNECs
3. Analysis of Ciliary Beating in cHNECs
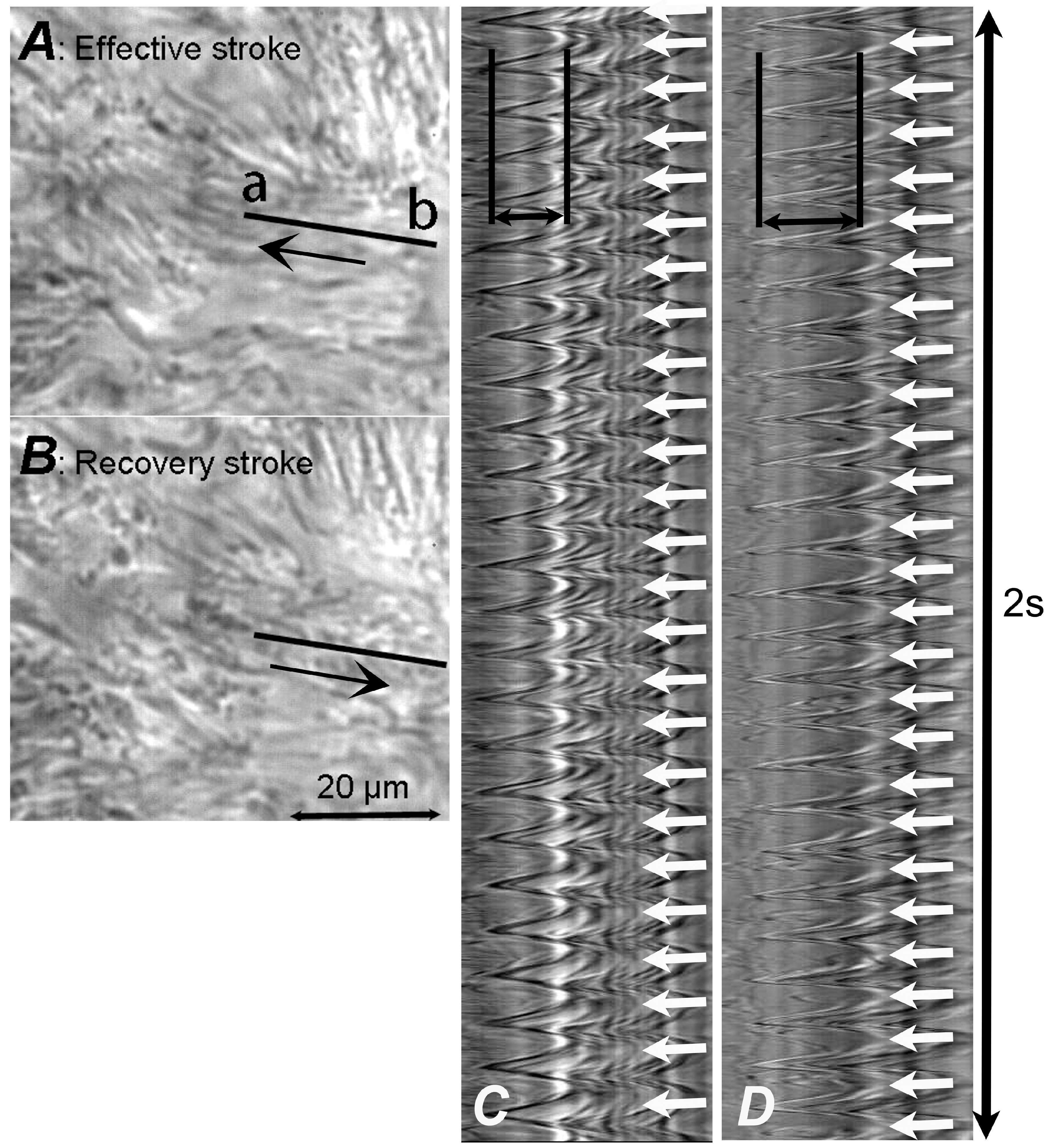
3.1. HSVM
3.2. Digital High-Speed Camera
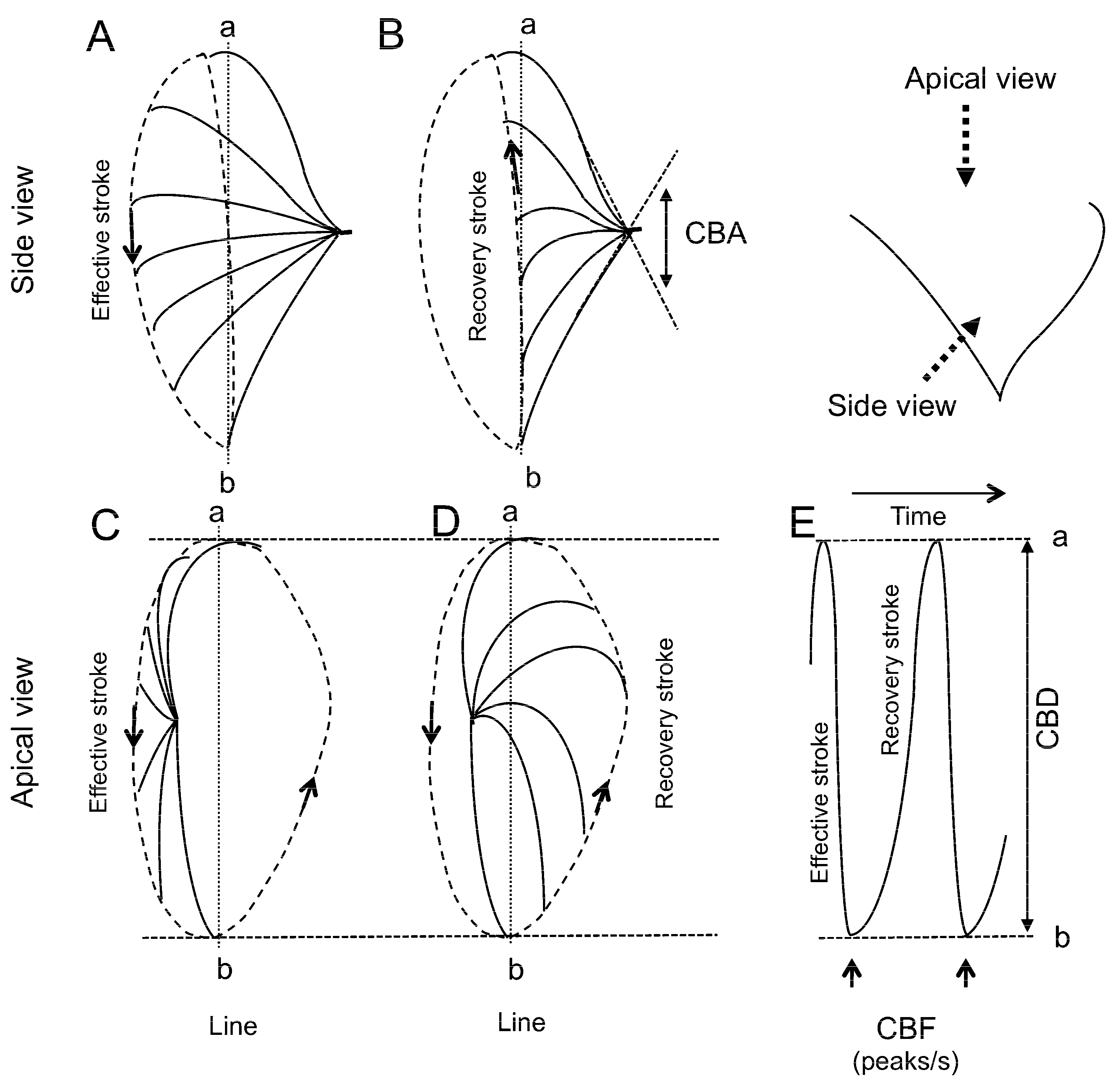
3.3. CBF Measurement
3.4. CBD Measurement
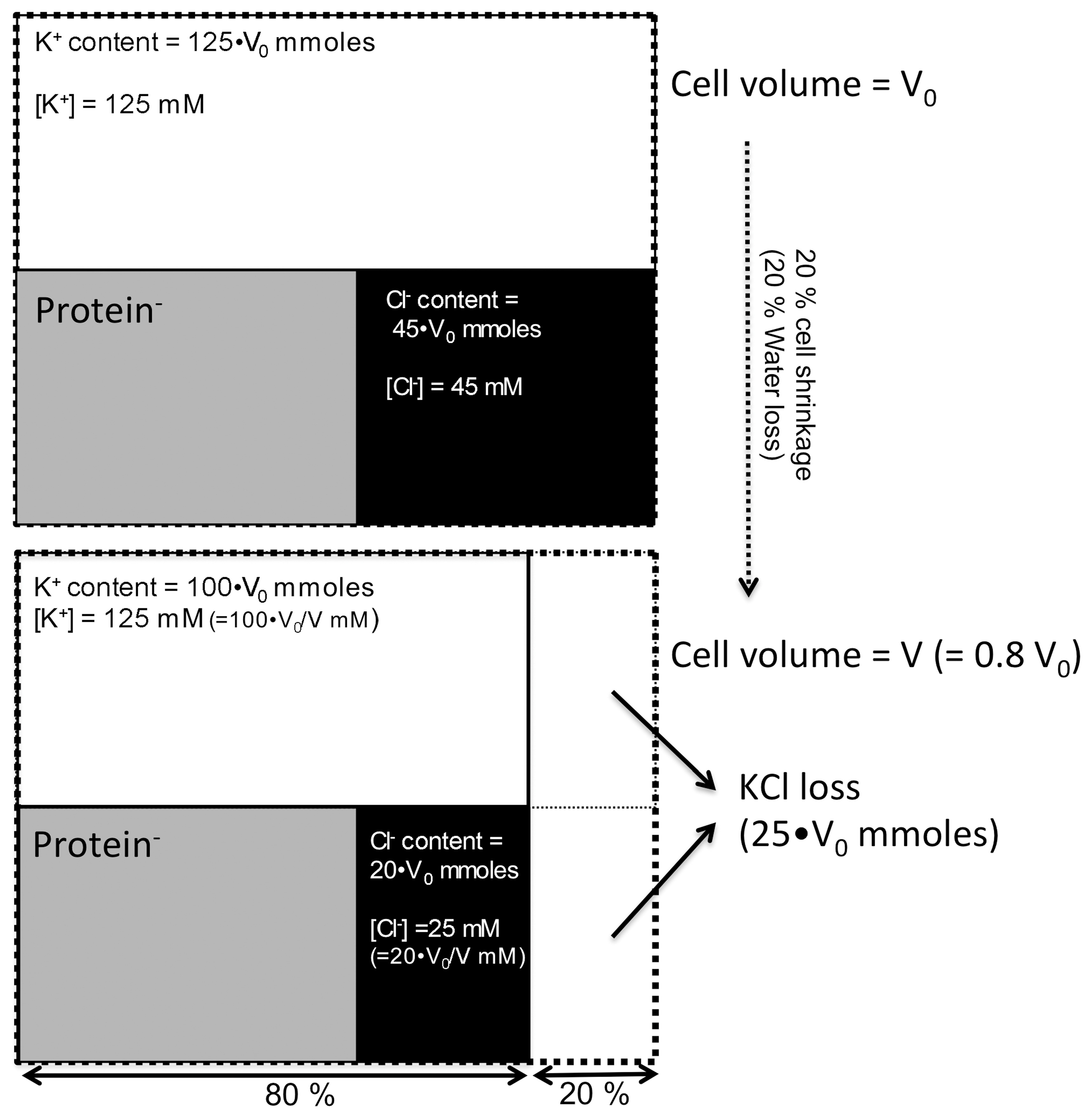
4. Changes in [Cl−]i
5. [Cl−]i Regulation of Ciliary Beating in cHNECs
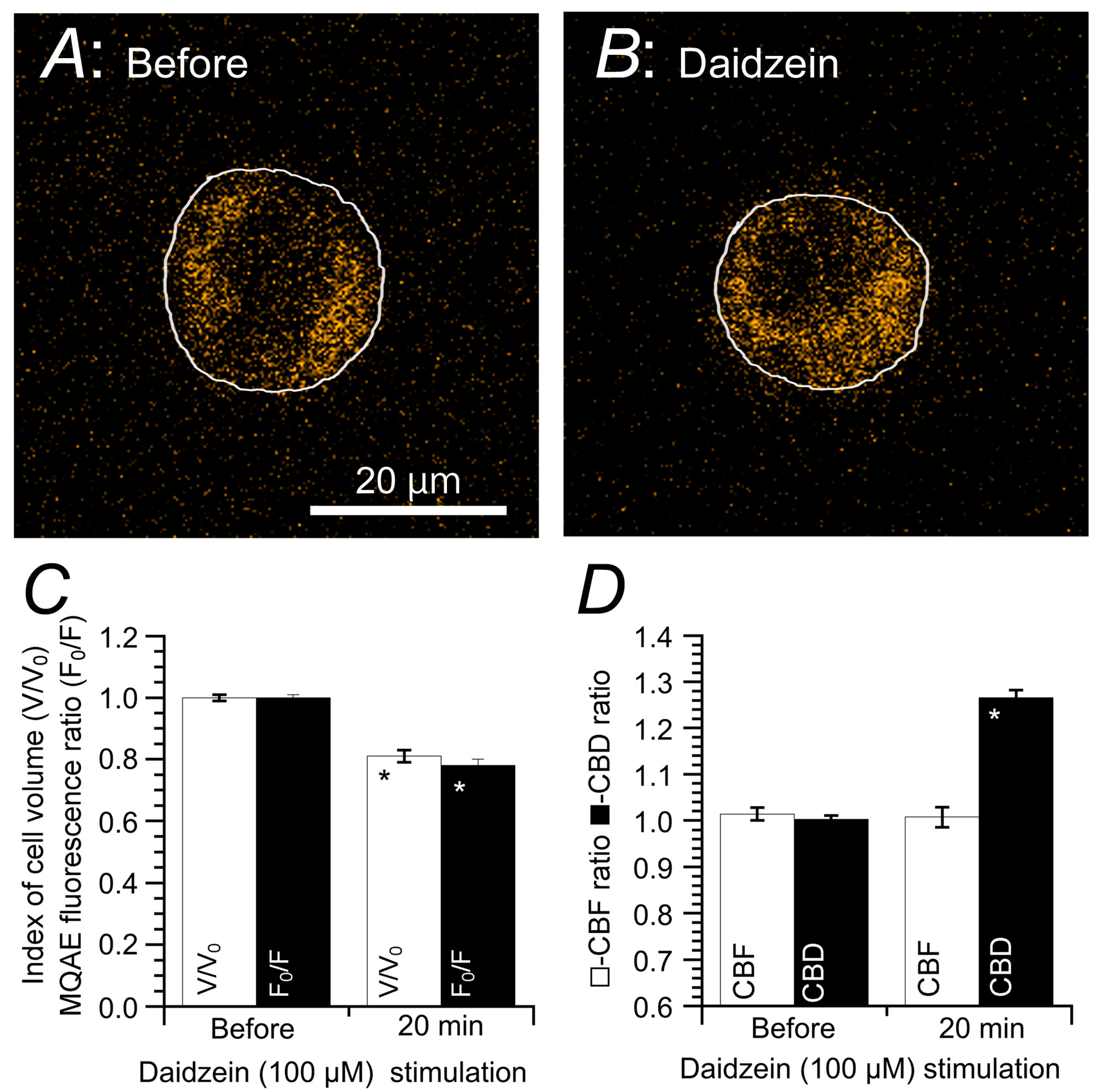
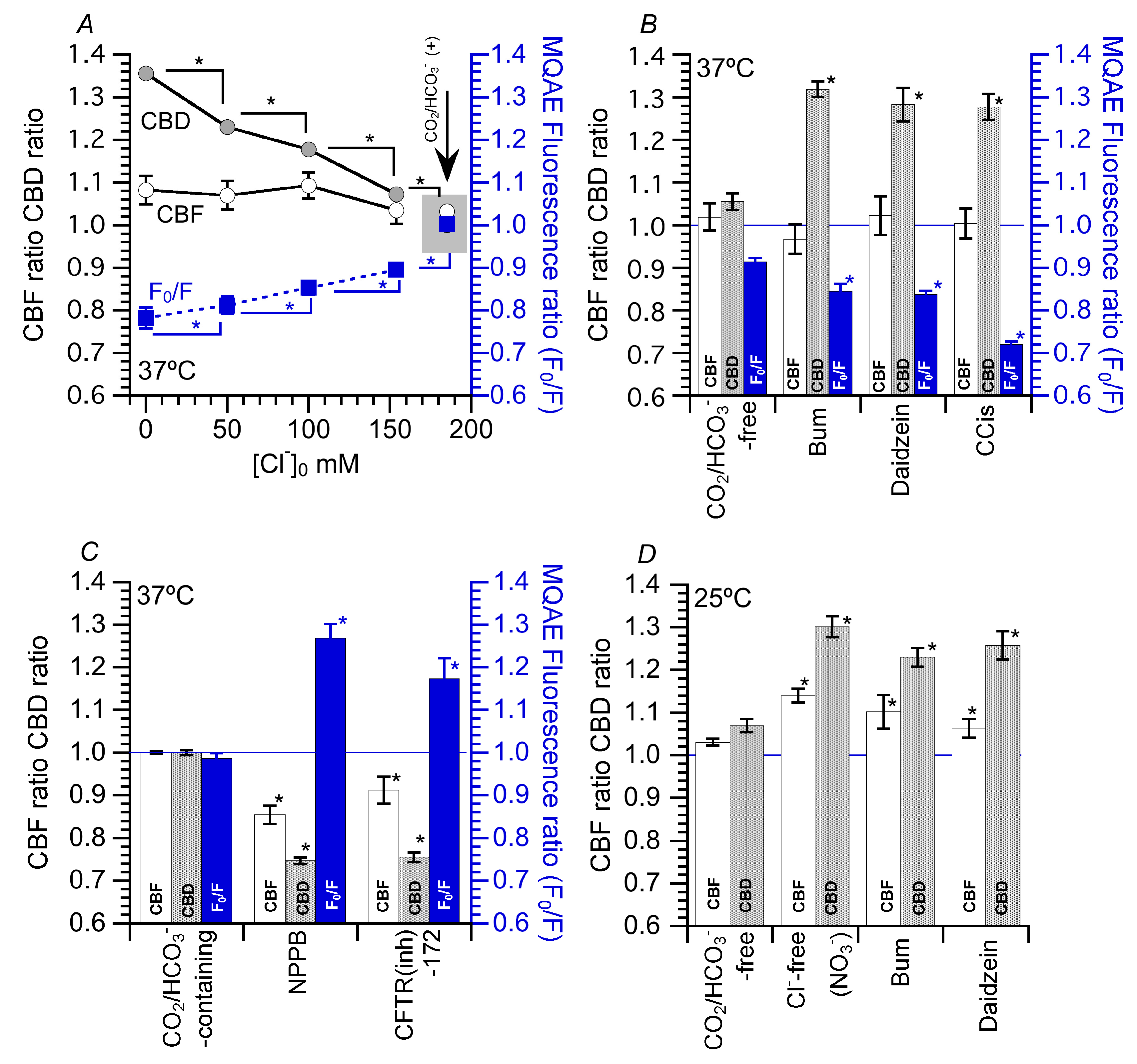
5.1. Effects of Decreased [Cl−]i on CBF and CBD
5.2. Effects of Increase in [Cl−]i on CBF and CBD
5.3. Effects of Decrease in [Cl−]i on CBF and CBD at 25 °C
5.4. Effects of Increased CBD on the Microbead Movement in cHNECs
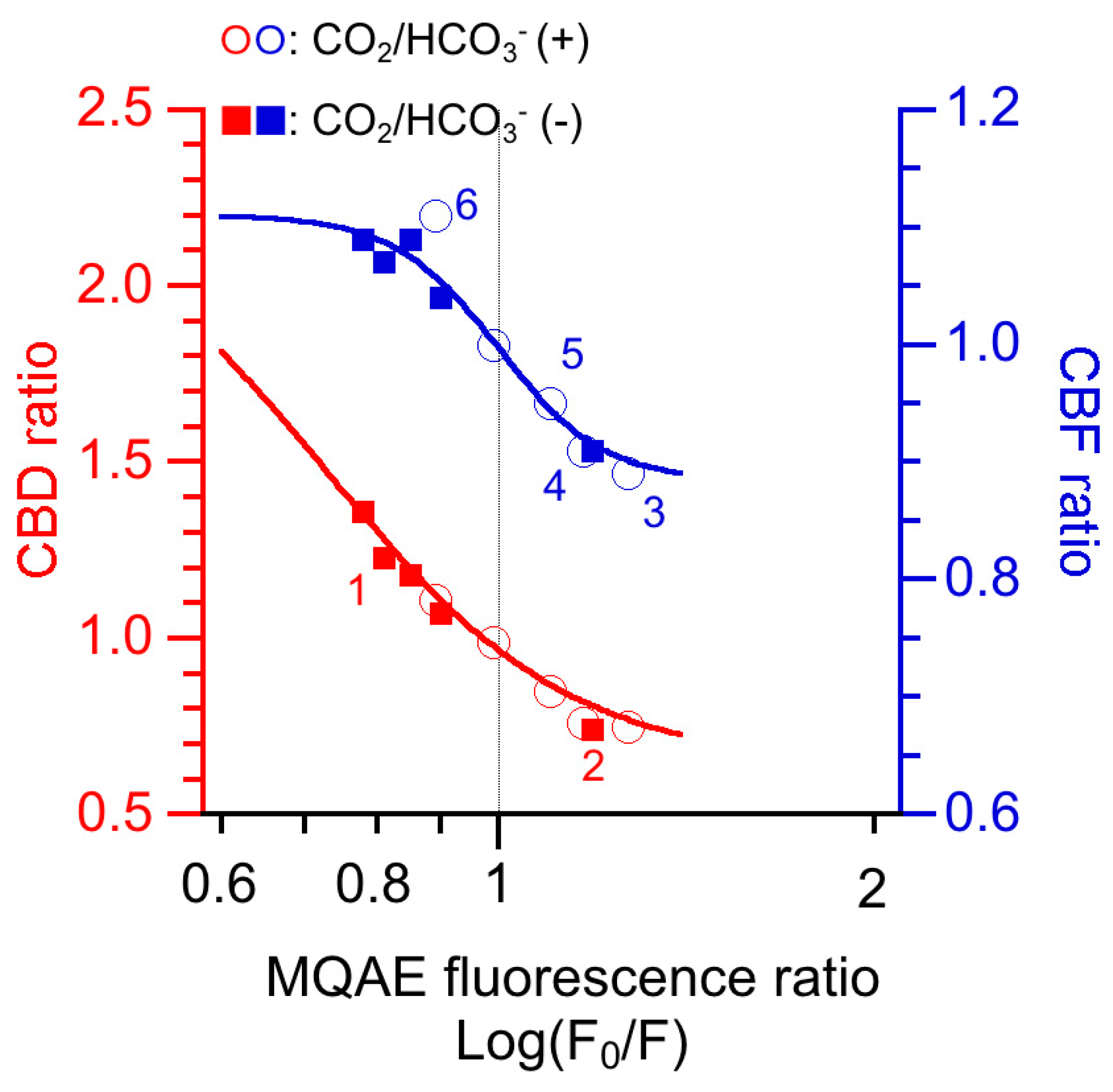
6. [Cl−]i Modulation of CBF and CBD in cHNECs
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Afzelius, B.A. Cilia-related diseases. J. Pathol. 2004, 204, 470–477. [Google Scholar] [CrossRef]
- Wanner, A.; Salathe, M.; O’Riordan, T.G. Mucociliary clearance in the airways. Am. J. Respir. Crit. Care Med. 1996, 154, 1868–1902. [Google Scholar] [CrossRef]
- Satir, P.; Christensen, S.T. Overview of Structure and Function of Mammalian Cilia. Annu. Rev. Physiol. 2007, 69, 377–400. [Google Scholar] [CrossRef] [PubMed]
- Salathe, M. Regulation of mammarian ciliary beating. Annu. Rev. Pysiol. 2007, 69, 401–422. [Google Scholar] [CrossRef] [PubMed]
- Tilley, A.E.; Walters, M.S.; Shaykhiev, R.; Crystal, R.G. Cilia dysfunction in lung disease. Annu. Rev. Physiol. 2015, 77, 379–406. [Google Scholar] [CrossRef] [PubMed]
- Delmotte, P.; Sanderson, M.J. Ciliary Beat Frequency Is Maintained at a Maximal Rate in the Small Airways of Mouse Lung Slices. Am. J. Respir. Cell Mol. Biol. 2006, 35, 110–117. [Google Scholar] [CrossRef]
- Toskala, E.; Nuutinen, J.; Rautiainen, M.; Torkkeli, T. The Correlation of Mucociliary Transport and Scanning Electron Microscopy of Nasal Mucosa. Acta OtoLaryngol. 1995, 115, 61–65. [Google Scholar] [CrossRef]
- Chilvers, M.A.; Rutman, A.; O’Callaghan, C. Ciliary beat pattern is associated with specific ultrastructural defects in primary ciliary dyskinesia. J. Allergy Clin. Immunol. 2003, 112, 518–524. [Google Scholar] [CrossRef]
- Raidt, J.; Wallmeier, J.; Hjeij, R.; Onnebrink, J.G.; Pennekamp, P.; Loges, N.T.; Olbrich, H.; Häffner, K.; Dougherty, G.W.; Omran, H.; et al. Ciliary beat patternand frequency in genetic variants of primary ciliary dyskinesia. Eur. Respir. J. 2014, 44, 1579–1588. [Google Scholar] [CrossRef]
- Dy, F.J.; Midyat, L.; Wong, W.Y.; Boyer, D. Primary Ciliary Dyskinesia: Ciliary Beat Pattern/Frequency, Role of Molecular Analysis in the Diagnosis, and Neonatal Distress. Am. J. Respir. Crit. Care Med. 2016, 193, 689–691. [Google Scholar] [CrossRef]
- Kempeneers, C.; Seaton, C.; Espinosa, B.G.; Chilvers, M.A. Ciliary functional analysis: Beating a path towards standardization. Pediatr. Pulmonol. 2019, 54, 1627–1638. [Google Scholar] [CrossRef]
- Neesen, J.; Kirschner, R.; Ochs, M.; Schmiedl, A.; Habermann, B.; Mueller, C.; Holstein, A.F.; Nuesslein, T.; Adham, I.; Engel, W. Disruption of an inner arm dynein heavy chain gene results in asthenozoospermia and reduced ciliary beat frequency. Hum. Mol. Genet. 2001, 10, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.R.; Hard, R.; Hennessey, T.M. Targeted gene disruption of dynein heavy chain 7 of Tetrahymena thermophile results in altered ciliary waveform and reduced swim speed. J. Cell Sci. 2007, 120, 3075–3085. [Google Scholar] [CrossRef]
- Komatani-Tamiya, N.; Daikoku, E.; Takemura, Y.; Shimamoto, C.; Nakano, T.; Iwasaki, Y.; Kohda, Y.; Matsumura, H.; Marunaka, Y.; Nakahari, T. Procaterol-stimulated Increases in Ciliary Bend Amplitude and Ciliary Beat Frequency in Mouse Bronchioles. Cell. Physiol. Biochem. 2012, 29, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Inui, T.-A.; Yasuda, M.; Hirano, S.; Ikeuchi, Y.; Kogiso, H.; Inui, T.; Marunaka, Y.; Nakahari, T. Enhancement of ciliary beat amplitude by carbocisteine in ciliated human nasal epithelial cells. Laryngoscope 2020, 130, E289–E297. [Google Scholar] [CrossRef] [PubMed]
- Inui, T.-A.; Yasuda, M.; Hirano, S.; Ikeuchi, Y.; Kogiso, H.; Inui, T.; Marunaka, Y.; Nakahari, T. Daidzein-Stimulated Increase in the Ciliary Beating Amplitude via an [Cl−]i Decrease in Ciliated Human Nasal Epithelial Cells. Int. J. Mol. Sci. 2018, 19, 3754. [Google Scholar] [CrossRef] [PubMed]
- Kogiso, H.; Hosogi, S.; Ikeuchi, Y.; Tanaka, S.; Shimamoto, C.; Matsumura, H.; Nakano, T.; Sano, K.I.; Inui, T.; Marunaka, Y.; et al. A low [Ca2+]i-induced enhancement of cAMP-activated ciliary beating by PDE1Ainhibition in mouse airway cilia. Pflügers Arch. 2017, 469, 1215–1227. [Google Scholar] [CrossRef]
- Kogiso, H.; Hosogi, S.; Ikeuchi, Y.; Tanaka, S.; Inui, T.; Marunaka, Y.; Nakahari, T. [Ca2+ ]i modulation of cAMP-stimulated ciliary beat frequency via PDE1 in airway ciliary cells of mice. Exp. Physiol. 2018, 103, 381–390. [Google Scholar] [CrossRef]
- Kogiso, H.; Ikeuchi, Y.; Sumiya, M.; Hosogi, S.; Tanaka, S.; Shimamoto, C.; Inui, T.; Marunaka, Y.; Nakahari, T. Seihai-to (TJ-90)-Induced Activation of Airway Ciliary Beatings of Mice: Ca2+ Modulation of cAMP-Stimulated Ciliary Beatings via PDE1. Int. J. Mol. Sci. 2018, 19, 658. [Google Scholar] [CrossRef]
- Ikeuchi, Y.; Kogiso, H.; Hosogi, S.; Tanaka, S.; Shimamoto, C.; Matsumura, H.; Inui, T.; Marunaka, Y.; Nakahari, T. Carbocisteine stimulated an increase in ciliary bend angle via a decrease in [Cl−]i in mouse airway cilia. Pflügers Arch. 2018, 471, 365–380. [Google Scholar] [CrossRef]
- Inui, T.-A.; Murakami, K.; Yasuda, M.; Hirano, S.; Ikeuchi, Y.; Kogiso, H.; Hosogi, S.; Inui, T.; Marunaka, Y.; Nakahari, T. Ciliary beating amplitude controlled by intracellular Cl− and a high rate of CO2 production in ciliated human nasal epithelial cells. Pflügers Arch. 2019, 471, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Tohda, H.; Foskett, J.K.; O’Brodovich, H.; Marunaka, Y. Cl− regulation of a Ca2+-activated nonselective cation channel in β-aginist-treated fetal distal lung epithelium. Am. J. Physiol. Cell Physiol. 1994, 266, C104–C109. [Google Scholar] [CrossRef] [PubMed]
- Dinudom, A.; Young, J.A.; Cook, D.I. Na+ and Cl? conductances are controlled by cytosolic Cl? concentration in the intralobular duct cells of mouse mandibular glands. J. Membr. Biol. 1993, 135, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Shimamoto, C.; Umegaki, E.; Katsu, K.-I.; Kato, M.; Fujiwara, S.; Kubota, T.; Nakahari, T. [Cl−]i modulation of Ca2+-regulated exocytosis in ACh-stimulated antral mucous cells of guinea pig. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G824–G837. [Google Scholar] [CrossRef] [PubMed]
- Higashijima, T.; Ferguson, K.M.; Sternweis, P.C. Regulation of hormone-sensitive GTP-dependent regulatory proteins by chloride. J. Biol. Chem. 1987, 262, 3597–3602. [Google Scholar]
- Miyazaki, H.; Shiozaki, A.; Niisato, N.; Ohsawa, R.; Itoi, H.; Ueda, Y.; Otsuji, E.; Yamagishi, H.; Iwasaki, Y.; Nakano, T.; et al. Chloride ions control the G1/S cell-cycle checkpoint by regulating the expression of p21 through a p53-independent pathway in human gastric cancer cells. Biochem. Biophys. Res. Commun. 2008, 366, 506–512. [Google Scholar] [CrossRef]
- Ohsawa, R.; Miyazaki, H.; Niisato, N.; Shiozaki, A.; Iwasaki, Y.; Otsuji, E.; Marunaka, Y. Intracellular chloride regulates cell proliferation through the activation of stress-activated protein kinases in MKN28 human gastric cancer cells. J. Cell. Physiol. 2010, 223, 764–770. [Google Scholar] [CrossRef]
- Nakajima, K.-I.; Marunaka, Y. Intracellular chloride ion concentration in differentiating neuronal cell and its role in growing neurite. Biochem. Biophys. Res. Commun. 2016, 479, 338–342. [Google Scholar] [CrossRef]
- Nakajima, K.-I.; Niisato, N.; Marunaka, Y. Enhancement of tubulin polymerization by Cl−-induced blockade of intrinsic GTPase. Biochem. Biophys. Res. Commun. 2012, 425, 225–229. [Google Scholar] [CrossRef]
- Nakajima, K.-I.; Miyazaki, H.; Niisato, N.; Marunaka, Y. Essential role of NKCC1 in NGF-induced neurite outgrowth. Biochem. Biophys. Res. Commun. 2007, 359, 604–610. [Google Scholar] [CrossRef]
- Niisato, N.; Eaton, D.C.; Marunaka, Y. Involvement of cytosolic Cl− in osmoregulation of α-ENaC gene expression. Am. J. Physiol. Renal Physiol. 2004, 287, F932–F939. [Google Scholar] [CrossRef] [PubMed]
- Shiima-Kinoshita, C.; Min, K.-Y.; Hanafusa, T.; Mori, H.; Nakahari, T. β2-adrenergic regulation of ciliary beat frequency in rat bronchiolar epithelium: Potentiation by isosmotic cell shrinkage. J. Physiol. 2003, 554, 403–416. [Google Scholar] [CrossRef]
- Lee, D.D.H.; Petris, A.; Hynds, R.E.; O’Callaghan, C. Ciliated Epithelial Cell Differentiation at Air-Liquid Interface Using Commercially Available Culture Media. Methods Mol. Biol. 2020, 2109, 275–291. [Google Scholar] [CrossRef]
- Hirst, R.A.; Rutman, A.; Williams, G.; O’Callaghan, C. Ciliated air-liquid culturesas an aid to diagnostic testing of primary ciliary dyskinesia. Chest 2010, 138, 1441–1447. [Google Scholar] [CrossRef]
- Hirst, R.A.; Jackson, C.L.; Coles, J.L.; Williams, G.; Rutman, A.; Goggin, P.M.; Adam, E.C.; Page, A.; Evans, H.J.; Lackie, P.M.; et al. Culture of primary ciliary dyskinesia epithelial cells at air-liquid interface can alter cilaiary phenotype but remains a robust and informative diagnostic aid. PLoS ONE 2014, 9, e89675. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Brighton, L.E.; Carson, J.L.; Fischer, W.A.; Jaspers, I. Culturing of Human Nasal Epithelial Cells at the Air Liquid Interface. J. Vis. Exp. 2013, 80, 50646. [Google Scholar] [CrossRef]
- Schögler, A.; Blank, F.; Brügger, M.; Beyeler, S.; Tschanz, S.A.; Regamey, N.; Casaulta, C.; Geiser, T.; Alves, M.P. Characterization of pediatric cystic fibrosis airway epithelial cell cultures at the air-liquid interface obtained by non-invasive nasal cytology brush sampling. Respir. Res. 2017, 18, 215. [Google Scholar] [CrossRef] [PubMed]
- Sutto, Z.; Conner, G.E.; Salathe, M. Regulation of human airway ciliary beat frequency by intracellular pH. J. Physiol. 2004, 560, 519–532. [Google Scholar] [CrossRef]
- Tokuda, S.; Shimamoto, C.; Yoshida, H.; Murao, H.; Kishima, G.; Ito, S.; Kubota, T.; Hanafusa, T.; Sugimoto, T.; Niisato, N.; et al. HCO3−-dependent pHi recovery and overacidification induced by NH4+ pulse in rat lung alveolar typeII cells: HCO3−-dependent NH3 excretion from lungs? Pflügers Arch. 2007, 455, 223–239. [Google Scholar] [CrossRef]
- Kuremoto, T.; Kogiso, H.; Yasuda, M.; Inui, T.-A.; Murakami, K.; Hirano, S.; Ikeuchi, Y.; Hosogi, S.; Inui, T.; Marunaka, Y.; et al. Spontaneous oscillation of the ciliary beat frequency regulated by release of Ca2+ from intracellular stores in mouse nasal epithelia. Biochem. Biophys. Res. Commun. 2018, 507, 211–216. [Google Scholar] [CrossRef]
- Rubbo, B.; Shoemark, A.; Jackson, C.L.; Hirst, R.; Thompson, J.; Hayes, J.; Frost, E.; Copeland, F.; Hogg, C.; O’Callaghan, C.; et al. Accuracy of high-speed video analysis to diagnose primary ciliary dyskineasia. Chest 2019, 155, 1008–1017. [Google Scholar] [CrossRef]
- Yaghi, A.; Dolovich, M.B. Airway Epithelial Cell Cilia and Obstructive Lung Disease. Cells 2016, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Foskett, J.K.; Melvin, J.E. Activation of salivary secretion: Coupling of cell volume and [Ca2+]i in single cells. Science 1989, 244, 1582–1585. [Google Scholar] [CrossRef] [PubMed]
- Nakahari, T.; Murakami, M.; Yoshida, H.; Miyamoto, M.; Sohma, Y.; Imai, Y. Decrease in rat submandibular acinar cell volume during ACh stimulation. Am. J. Physiol. Liver Physiol. 1990, 258, G878–G886. [Google Scholar] [CrossRef]
- Fujiwara, S.; Shimamoto, C.; Katsu, K.-I.; Imai, Y.; Nakahari, T. Isosmotic modulation of Ca2+-regulated exocytosis in guinea-pig antral mucous cells: Role of cell volume. J. Physiol. 1999, 516, 85–100. [Google Scholar] [CrossRef]
- Marunaka, Y. Hormonal and Osmotic Regulation of NaCl Transport in Renal Distal Nephron Epithelium. Jpn. J. Physiol. 1997, 47, 499–511. [Google Scholar] [CrossRef]
- Ikeuchi, Y.; Kogiso, H.; Hosogi, S.; Tanaka, S.; Shimamoto, C.; Inui, T.; Nakahari, T.; Marunaka, Y. Measurement of [Cl−]i unaffected by the cell volume change using MQAE-based two-photon microscopy in airway ciliary cells of mice. J. Physiol. Sci. 2018, 68, 191–199. [Google Scholar] [CrossRef]
- Zhang, S.; Smith, N.; Schuster, D.; Azbell, C.; Sorscher, E.J.; Rowe, S.M.; Woodworth, B.A. Quercetin increases cystic fibrosis transmembrane conductance regulator-mediated chloride transport and ciliary beat frequency: Therapeutic implications for chronic rhinosinusitis. Am. J. Rhinol. Allergy 2011, 25, 307–312. [Google Scholar] [CrossRef]
- Zhang, S.; Skinner, D.; Hicks, S.B.; Bevensee, M.O.; Sorscher, E.J.; Lazrak, A.; Matalon, S.; McNicholas, C.M.; Woodworth, B.A. Sinupret Activates CFTR and TMEM16A-Dependent Transepithelial Chloride Transport and Improves Indicators of Mucociliary Clearance. PLoS ONE 2014, 9, e104090. [Google Scholar] [CrossRef]
- Azbell, C.; Zhang, S.; Skinner, D.; Fortenberry, J.; Sorscher, E.J.; Woodworth, B.A.; Zhang, S.; Sorscher, E.J.; Woodworth, B.A. Hesperidin stimulates cystic fibrosis transmembrane conductance regulator-mediated chloride secretion and ciliary beat frequency in sinonasal epithelium. Otolaryngol. Neck Surg. 2010, 143, 397–404. [Google Scholar] [CrossRef]
- Gibbons, I.R.; Rowe, A.J. Dynein: A protein with adenosine triohosphatase activity from cilia. Science 1965, 149, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Brokaw, C.J. Control of flagellar bending: A new agenda based on dynein diversity. Cell Motil. Cytoskelet. 1994, 28, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Brokaw, C.J.; Kamiya, R. Bending patterns of Chlamydomonas flagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil. Cytoskelet. 1987, 8, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; McBrayer, D.; Lytle, C. [Cl-]i-dependent Phosphorylation of the Na-K-Cl Cotransport Protein of Dog Tracheal Epithelial Cells. J. Biol. Chem. 1995, 270, 28955–28961. [Google Scholar] [CrossRef] [PubMed]
- Piala, A.T.; Moon, T.M.; Akella, R.; He, H.; Cobb, M.H.; Goldsmith, E.J. Chloride Sensing by WNK1 Involves Inhibition of Autophosphorylation. Sci. Signal. 2014, 7, ra41. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.-E.; English, J.M.; Wilsbacher, J.L.; Stippec, S.; Goldsmith, E.J.; Cobb, M.H. WNK1, a Novel Mammalian Serine/Threonine Protein Kinase Lacking the Catalytic Lysine in Subdomain II. J. Biol. Chem. 2000, 275, 16795–16801. [Google Scholar] [CrossRef] [PubMed]
- Terker, A.S.; Zhang, C.; Erspamer, K.J.; Gamba, G.; Yang, C.-L.; Ellison, D.H. Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int. 2016, 89, 127–134. [Google Scholar] [CrossRef]
- Murthy, M.; Kurz, T.; O’Shaughnessy, K.M. WNK signalling pathways in blood pressure regulation. Cell. Mol. Life Sci. 2016, 74, 1261–1280. [Google Scholar] [CrossRef]
- Yang, C.-L.; Liu, X.; Paliege, A.; Zhu, X.; Bachmann, S.; Dawson, D.C.; Ellison, D.H. WNK1 and WNK4 modulate CFTR activity. Biochem. Biophys. Res. Commun. 2007, 353, 535–540. [Google Scholar] [CrossRef]
- Hengl, T.; Kaneko, H.; Dauner, K.; Vocke, K.; Frings, S.; Möhrlen, F. Molecular components of signal amplification in olfactory sensory cilia. Proc. Natl. Acad. Sci. USA 2010, 107, 6052–6057. [Google Scholar] [CrossRef]
- Ring, A.M.; Cheng, S.X.; Leng, Q.; Kahle, K.T.; Rinehart, J.; Lalioti, M.D.; Volkman, H.M.; Wilson, F.H.; Hebert, S.C.; Lifton, R.P. WNK4 regulates activity of the epithelial Na+ channel in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 4020–4024. [Google Scholar] [CrossRef] [PubMed]
- Farfel, Z.; Mayan, H.; Yaacov, Y.; Mouallem, M.; Shaharabany, M.; Pauzner, R.; Kerem, E.; Wilschanski, M. WNK4 regulates airway Na+ transport: Study of familial hyperkalaemia and hypertension. Eur. J. Clin. Invest. 2005, 35, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Niisato, N.; Miyazaki, H.; Iwasaki, Y.; Hama, T.; Dejima, K.; Hisa, Y.; Marunaka, Y. Epithelial Na+ channel and ion transport in human nasal polyp and paranasal sinus mucosa. Biochem. Biophys. Res. Commun. 2007, 362, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-C.; Lo, Y.-F.; Lin, Y.-W.; Lin, S.-H.; Huang, C.-L.; Cheng, C.-J. WNK4 kinase is a physiological intracellular chloride sensor. Proc. Natl. Acad. Sci. USA 2019, 116, 4502–4507. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.N.; Eamest, S.; Chen, W.; Juang, Y.C.; Kim, S.C.; Zhao, Y.M.; Cobb, M.H. WNK1 and OSR1 regulate the Na+, K+, 2Cl- cotransporter in Hela cells. Proc. Natl. Acad. Sci. USA 2006, 103, 10883–10888. [Google Scholar] [CrossRef]
- Hadchouel, J.; Ellison, D.H.; Gamba, G. Regulation of Renal Electrolyte Transport by WNK and SPAK-OSR1 Kinases. Annu. Rev. Physiol. 2016, 78, 367–389. [Google Scholar] [CrossRef]
- Bazua-Valenti, S.; Gamba, G. Revisiting the NaCl cotransporter regulation by with-no-lysine kinases. Am. J. Physiol. Cell Physiol. 2015, 308, C779–C791. [Google Scholar] [CrossRef]
- Shpetner, H.S.; Paschal, B.M.; Vallee, R.B. Characterization of the microtubule-activated ATPase of brain cytoplasmic dynein (MAP 1C). J. Cell Biol. 1988, 107, 1001–1009. [Google Scholar] [CrossRef]
- Treharne, K.J.; Marshall, L.J.; Mehta, A. A novel chloride-dependent GTP-utilizing protein kinase in plasma membranes from human respiratory epithelium. Am. J. Physiol. Cell. Mol. Physiol. 1994, 267, L592–L601. [Google Scholar] [CrossRef]
- Hirosue, S.; Senn, K.; Clement, N.; Nonnenmacher, M.; Gigout, L.; Linden, R.M.; Weber, T. Effect of inhibition of dynein function and microtubule-altering drugs on AAV2 transduction. Virology 2007, 367, 10–18. [Google Scholar] [CrossRef]
- Yasuda, M.; Niisato, N.; Miyazaki, H.; Hama, T.; Dejima, K.; Hisa, Y.; Marunaka, Y. Epithelial Ion Transport of Human Nasal Polyp and Paranasal Sinus Mucosa. Am. J. Respir. Cell Mol. Biol. 2007, 36, 466–472. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasuda, M.; Inui, T.-a.; Hirano, S.; Asano, S.; Okazaki, T.; Inui, T.; Marunaka, Y.; Nakahari, T. Intracellular Cl− Regulation of Ciliary Beating in Ciliated Human Nasal Epithelial Cells: Frequency and Distance of Ciliary Beating Observed by High-Speed Video Microscopy. Int. J. Mol. Sci. 2020, 21, 4052. https://doi.org/10.3390/ijms21114052
Yasuda M, Inui T-a, Hirano S, Asano S, Okazaki T, Inui T, Marunaka Y, Nakahari T. Intracellular Cl− Regulation of Ciliary Beating in Ciliated Human Nasal Epithelial Cells: Frequency and Distance of Ciliary Beating Observed by High-Speed Video Microscopy. International Journal of Molecular Sciences. 2020; 21(11):4052. https://doi.org/10.3390/ijms21114052
Chicago/Turabian StyleYasuda, Makoto, Taka-aki Inui, Shigeru Hirano, Shinji Asano, Tomonori Okazaki, Toshio Inui, Yoshinori Marunaka, and Takashi Nakahari. 2020. "Intracellular Cl− Regulation of Ciliary Beating in Ciliated Human Nasal Epithelial Cells: Frequency and Distance of Ciliary Beating Observed by High-Speed Video Microscopy" International Journal of Molecular Sciences 21, no. 11: 4052. https://doi.org/10.3390/ijms21114052
APA StyleYasuda, M., Inui, T.-a., Hirano, S., Asano, S., Okazaki, T., Inui, T., Marunaka, Y., & Nakahari, T. (2020). Intracellular Cl− Regulation of Ciliary Beating in Ciliated Human Nasal Epithelial Cells: Frequency and Distance of Ciliary Beating Observed by High-Speed Video Microscopy. International Journal of Molecular Sciences, 21(11), 4052. https://doi.org/10.3390/ijms21114052





