Long Non-Coding RNA HAND2-AS1 Acts as a Tumor Suppressor in High-Grade Serous Ovarian Carcinoma
Abstract
1. Introduction
2. Results
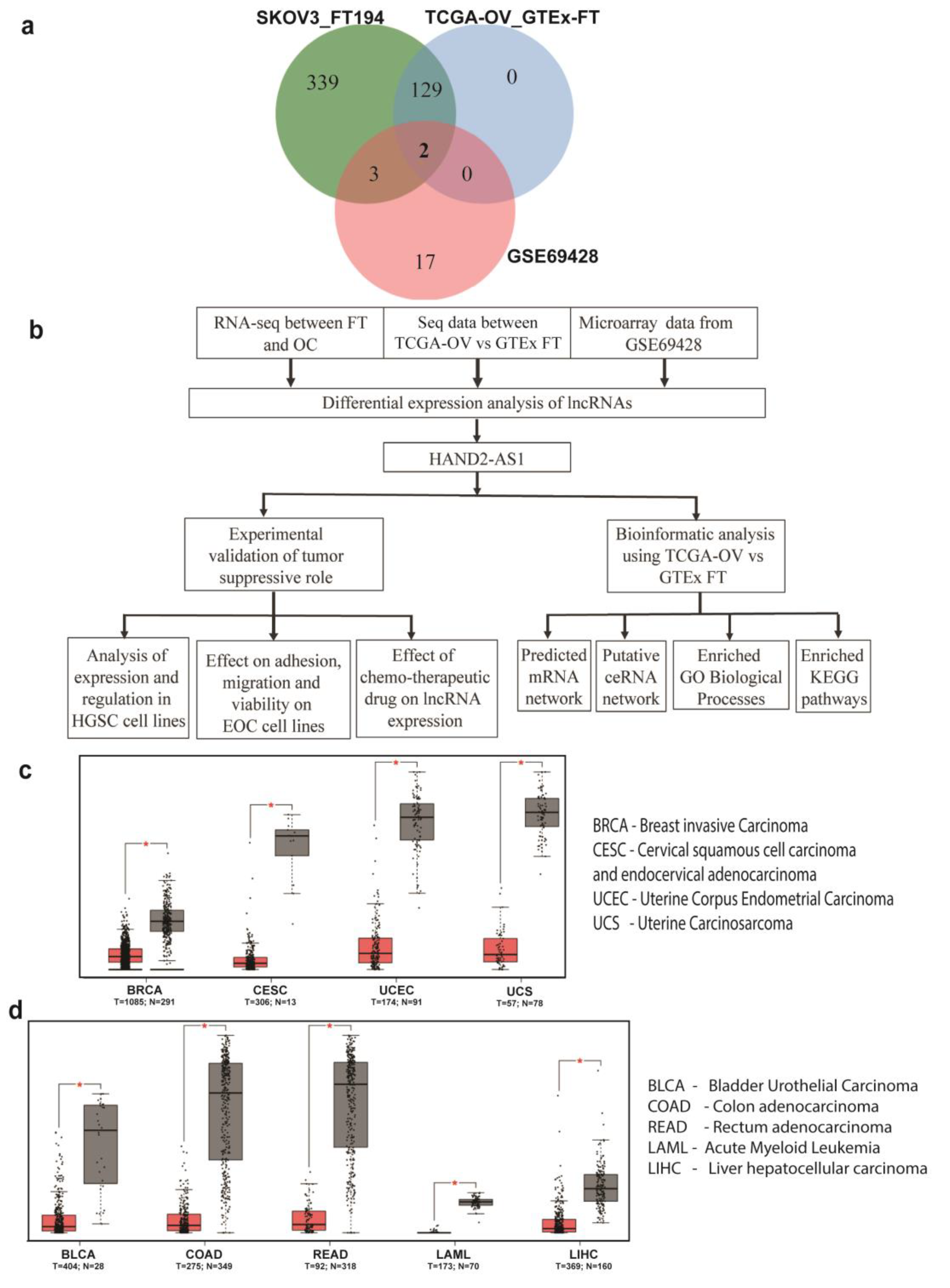
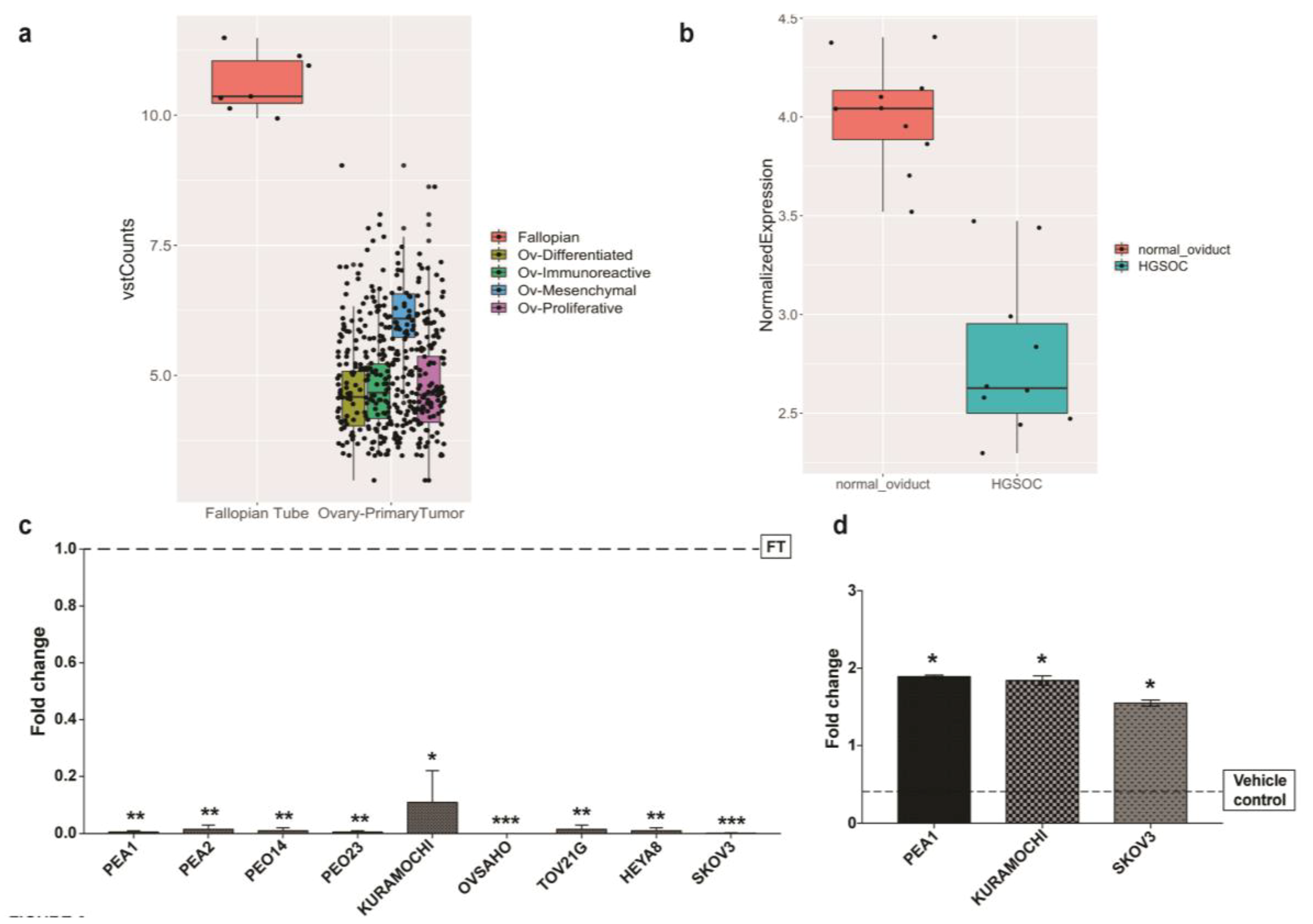
2.1. HAND2-AS1 as an Important Long Non-Coding RNA (lncRNA) in High-Grade Serous Carcinoma (HGSC)
2.2. HAND2-AS1 Expression Is Consistently Downregulated in Ovarian Cancer Cell Lines Possibly Due to Promoter Hypermethylation
2.3. Expression of HAND2-AS1 in Ovarian Cancer Cells Decreases Their Adhesion to Extracellular Matrix, Migration, and Viability
2.4. HAND2-AS1 Expression Is Rescued after Treatment with HDAC Inhibitor
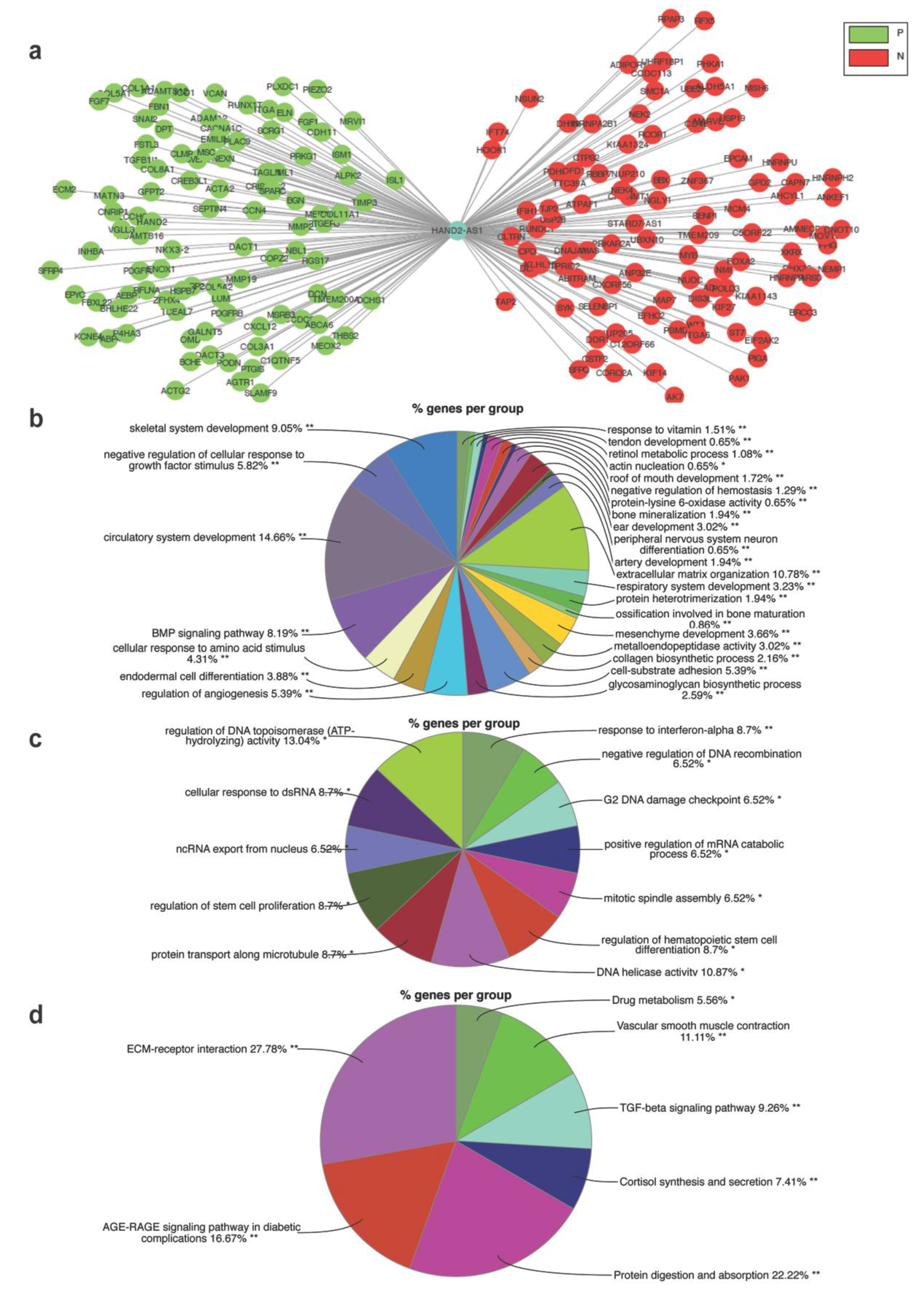
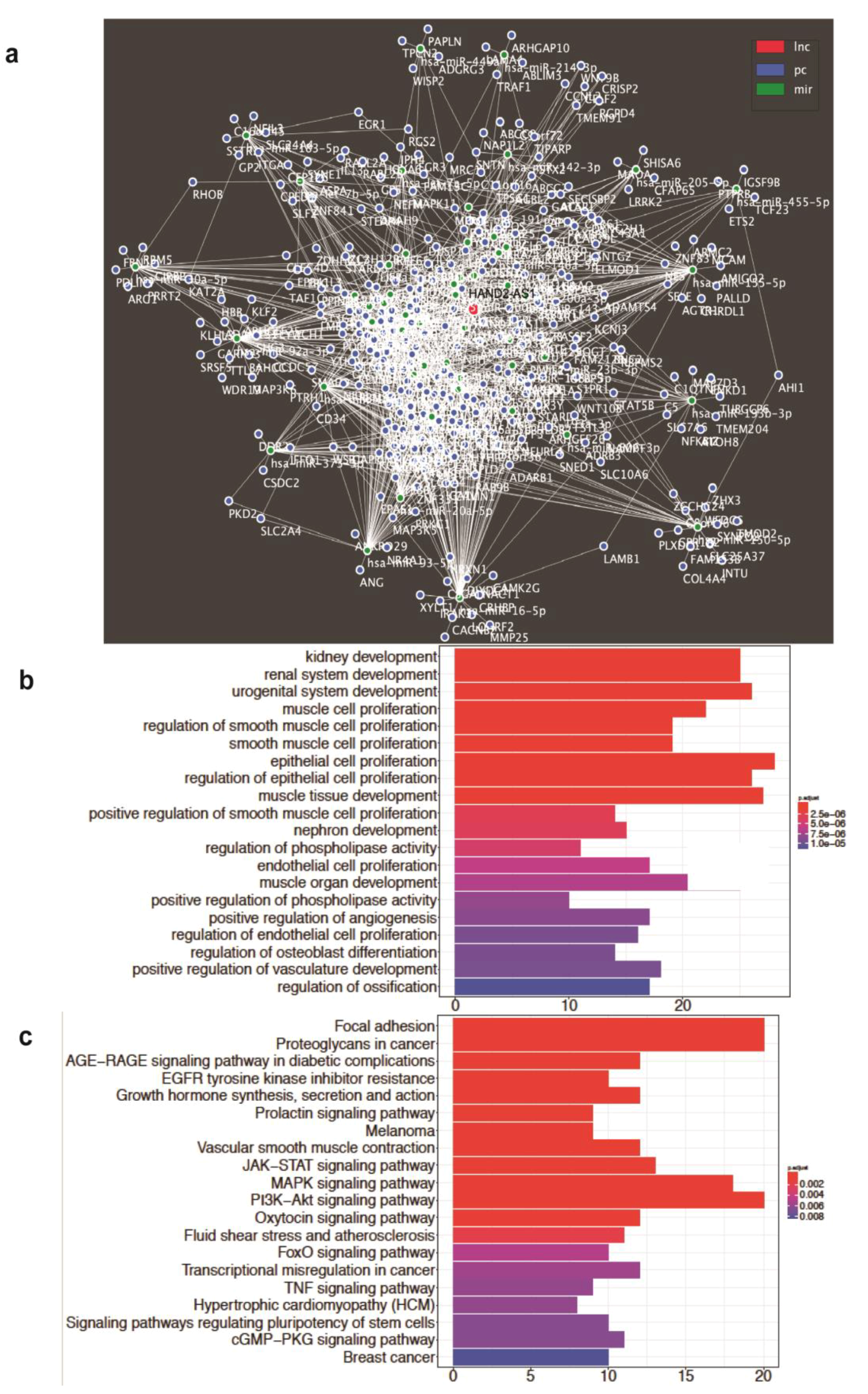
2.5. Identification of the HAND2-AS1-Associated mRNA and ceRNA Network along with Enriched Gene Ontology Biological Processes and Pathways in HGSC
3. Discussion
4. Materials and Methods
4.1. Differential Analysis of Coding and Long Non-Coding RNAs
4.2. Expression of HAND2-AS1 in Various Cancers
4.3. Venn Diagrams
4.4. Cell Lines and Culture Methods
4.5. Treatment with AZA
4.6. Plasmid Preparation
4.7. RNA, cDNA, and qRT-PCR
- ABL fwd 5′-TGGAGATAACACTCTAAGCATAACTAAAGG-3′
- ABL rev 5′-GATGTAGTTGCTTGGGACCCA-3′
- HAND2-AS1 fwd 5′-GTCTACGACTGCCTGTGACT-3′
- HAND2-AS1 rev 5′-TGTCCCATATACAAGAATCGACA-3′
- PAX8 fwd 5′-CCCTTCCAACACGCCACT-3′
- PAX8 rev 5′-CTGCTTTATGGCGAAGGGTG-3′
4.8. Migration Assay
4.9. Adhesion Assay
4.10. Viability Assay
4.11. HDAC Inhibitor Treatment
4.12. mRNA Network
4.13. HAND2-AS1 ceRNA Network Prediction
4.14. Figure for Graphical Summary
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| lncRNA | Long non-coding RNA |
| ncRNA | Non-coding RNA |
| HGSC | High-Grade Serous Carcinoma |
| FT | Fallopian Tube |
| OC | Ovarian Cancer |
| HAND2-AS1 | HAND2 Anti sense RNA 1 |
| 5-AZA | 5-Aza-2’–deoxycytidine |
| mRNA | Messenger RNA |
| ceRNA | Competing endogenous RNA |
| HDAC | Histone De-Acetylases |
| miRNA | Micro RNA |
| GO BP | Gene Ontology Biological Process |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| TCGA-OV | The Cancer Genome Atlas – Ovarian Cancer |
| GTEx | Genotype Tissue Expression |
| GEPIA | Gene Expression Profiling Interactive Analysis |
References
- Fernandes, J.; Acuña, S.; Aoki, J.; Floeter-Winter, L.; Muxel, S. Long Non-Coding RNAs in the Regulation of Gene Expression: Physiology and Disease. Non Coding RNA 2019, 5, 17. [Google Scholar] [CrossRef]
- Salviano-Silva, A.; Lobo-Alves, S.; Almeida, R.; Malheiros, D.; Petzl-Erler, M. Besides Pathology: Long Non-Coding RNA in Cell and Tissue Homeostasis. Non Coding RNA 2018, 4, 3. [Google Scholar] [CrossRef]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer 2017, 18, 5–18. [Google Scholar] [CrossRef]
- Balas, M.M.; Johnson, A.M. Exploring the mechanisms behind long noncoding RNAs and cancer. Non Coding RNA Res. 2018, 3, 108–117. [Google Scholar] [CrossRef]
- Singh, N.; McCluggage, W.G.; Gilks, C.B. High-grade serous carcinoma of tubo-ovarian origin: Recent developments. Histopathology 2017, 71, 339–356. [Google Scholar] [CrossRef]
- Testa, U.; Petrucci, E.; Pasquini, L.; Castelli, G.; Pelosi, E. Ovarian Cancers: Genetic Abnormalities, Tumor Heterogeneity and Progression, Clonal Evolution and Cancer Stem Cells. Medicines 2018, 5, 16. [Google Scholar] [CrossRef]
- Kroeger, P.T.; Drapkin, R. Pathogenesis and heterogeneity of ovarian cancer. Curr. Opin. Obstet. Gynecol. 2017, 29, 26–34. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Doxtater, K.; Keramatnia, F.; Zacheaus, C.; Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Role of lncRNAs in ovarian cancer: Defining new biomarkers for therapeutic purposes. Drug Discov. Today 2018, 23, 1635–1643. [Google Scholar] [CrossRef]
- Anderson, K.M.; Anderson, D.M.; McAnally, J.R.; Shelton, J.M.; Bassel-Duby, R.; Olson, E.N. Transcription of the non-coding RNA upperhand controls Hand2 expression and heart development. Nature 2016, 539, 433–436. [Google Scholar] [CrossRef]
- Cheng, X.; Jiang, H. Long non-coding RNA HAND2-AS1 downregulation predicts poor survival of patients with end-stage dilated cardiomyopathy. J. Int. Med. Res. 2019, 47, 3690–3698. [Google Scholar] [CrossRef]
- Voth, H.; Oberthuer, A.; Simon, T.; Kahlert, Y.; Berthold, F.; Fischer, M. Co-regulated expression of HAND2 and DEIN by a bidirectional promoter with asymmetrical activity in neuroblastoma. BMC Mol. Biol. 2009, 10. [Google Scholar] [CrossRef]
- Yang, X.; Wang, C.C.; Lee, W.Y.W.; Trovik, J.; Chung, T.K.H.; Kwong, J. Long non-coding RNA HAND2-AS1 inhibits invasion and metastasis in endometrioid endometrial carcinoma through inactivating neuromedin U. Cancer Lett. 2018, 413, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Zhu, X.; Xu, Y.; Tang, Q.; Huang, Z.; Zhao, Z.; Lu, J.; Song, G.; Xu, H.; Deng, C.; et al. Energy stress-induced lncRNA HAND2-AS1 represses HIF1α-mediated energy metabolism and inhibits osteosarcoma progression. Am. J. Cancer Res. 2018, 8, 526–537. [Google Scholar] [PubMed]
- Zhou, J.; Lin, J.; Zhang, H.; Zhu, F.; Xie, R. LncRNA HAND2-AS1 sponging miR-1275 suppresses colorectal cancer progression by upregulating KLF14. Biochem. Biophys. Res. Commun. 2018, 503, 1848–1853. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Chen, J.; Shi, M.; Song, Y.; Chen, Z.; Pang, L. LncRNA HAND2-AS1 inhibits non-small cell lung cancer migration, invasion and maintains cell stemness through the interactions with TGF-β1. Biosci. Rep. 2019, 39, BSR20181525. [Google Scholar] [CrossRef]
- Yang, J.-R.; Shi, M.-X.; Zeng, Y. LncRNA HAND2-AS1 inhibits proliferation and promotes apoptosis of chronic myeloid leukemia cells by sponging with micRNA-1275. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2103–2111. [Google Scholar]
- Yan, Y.; Li, S.; Wang, S.; Rubegni, P.; Tognetti, L.; Zhang, J.; Yan, L. Long noncoding RNA HAND2-AS1 inhibits cancer cell proliferation, migration, and invasion in esophagus squamous cell carcinoma by regulating microRNA-21. J. Cell. Biochem. 2019, 120, 9564–9571. [Google Scholar] [CrossRef]
- Chen, J.; Lin, Y.; Jia, Y.; Xu, T.; Wu, F.; Jin, Y. LncRNA HAND2-AS1 exerts anti-oncogenic effects on ovarian cancer via restoration of BCL2L11 as a sponge of microRNA-340-5p. J. Cell. Physiol. 2019. [Google Scholar] [CrossRef]
- Gokulnath, P.; De Cristofaro, T.; Manipur, I.; Di Palma, T.; Soriano, A.A.; Guarracino, M.R.; Zannini, M. Long Non-Coding RNA MAGI2-AS3 is a New Player with a Tumor Suppressive Role in High Grade Serous Ovarian Carcinoma. Cancers 2019, 11, 2008. [Google Scholar] [CrossRef]
- Liu, P.; Du, R.; Yu, X. LncRNA HAND2-AS1 overexpression inhibits cancer cell proliferation in melanoma by downregulating ROCK1. Oncol. Lett. 2019, 1–6. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ning, G.; Howitt, B.E.; Mehra, K.; Wu, L.; Wang, X.; Hong, Y.; Kern, F.; Wei, T.S.; Zhang, T.; et al. In vitro and in vivo correlates of physiological and neoplastic human Fallopian tube stem cells. J. Pathol. 2016, 284, 519–530. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]
- Michaelson-Cohen, R.; Keshet, I.; Straussman, R.; Hecht, M.; Cedar, H.; Beller, U. Genome-wide de novo methylation in epithelial ovarian cancer. Int. J. Gynecol. Cancer 2011, 21, 269–279. [Google Scholar] [CrossRef]
- Xiong, Y.; Wei, Y.; Gu, Y.; Zhang, S.; Lyu, J.; Zhang, B.; Chen, C.; Zhu, J.; Wang, Y.; Liu, H.; et al. DiseaseMeth version 2.0: A major expansion and update of the human disease methylation database. Nucleic Acids Res. 2016, 45, D888–D895. [Google Scholar] [CrossRef]
- Hull, E.E.; Montgomery, M.R.; Leyva, K.J. HDAC Inhibitors as Epigenetic Regulators of the Immune System: Impacts on Cancer Therapy and Inflammatory Diseases. Biomed Res. Int. 2016, 2016. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Lin, H.; Moh, J.S.; Den Chen, K.; Wang, I.W.; Ou, Y.C.; You, Y.S.; Lung, C.C. Low-dose LBH589 increases the sensitivity of cisplatin to cisplatin-resistant ovarian cancer cells. Taiwan J. Obstet. Gynecol. 2011, 50, 165–171. [Google Scholar] [CrossRef][Green Version]
- Garrett, L.A.; Growdon, W.B.; Rueda, B.R.; Foster, R. Influence of a novel histone deacetylase inhibitor panobinostat (LBH589) on the growth of ovarian cancer. J. Ovarian Res. 2016, 9, 1–8. [Google Scholar] [CrossRef]
- Shi, K.; Yin, X.; Cai, M.C.; Yan, Y.; Jia, C.; Ma, P.; Zhang, S.; Zhang, Z.; Gu, Z.; Zhang, M.; et al. Pax8 regulon in human ovarian cancer links lineage dependency with epigenetic vulnerability to hdac inhibitors. Elife 2019, 8. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci Signal 2014, 6, 11. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Bindea, G.; Galon, J.; Mlecnik, B. CluePedia Cytoscape plugin: Pathway insights using integrated experimental and in silico data. Bioinformatics 2013, 29, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Qu, H.; Wang, S.; We, J.; Zhang, L.; Ma, R.; Lu, J.; Zhu, J.; De Zhong, W.; Jia, Z. GDCRNATools: An R/Bioconductor package for integrative analysis of lncRNA, miRNA and mRNA data in GDC. Bioinformatics 2018, 34, 2515–2517. [Google Scholar] [CrossRef] [PubMed]
- Corzo, C.; Iniesta, M.D.; Patrono, M.G.; Lu, K.H.; Ramirez, P.T. Role of Fallopian Tubes in the Development of Ovarian Cancer. J. Minim. Invasive Gynecol. 2017, 24, 230–234. [Google Scholar] [CrossRef] [PubMed]
- McKnight, R.; Cohen, C.; Siddiqui, M.T. Utility of paired box gene 8 (PAX8) expression in fluid and fine-needle aspiration cytology: An immunohistochemical study of metastatic ovarian serous carcinoma. Cancer Cytopathol. 2010, 118, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, X.; Lu, S.; Hu, B. Long non-coding RNA HAND2-AS1 targets glucose metabolism and inhibits cancer cell proliferation in osteosarcoma. Oncol. Lett. 2019, 18, 1323–1329. [Google Scholar] [CrossRef]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. Jvenn: An interactive Venn diagram viewer. BMC Bioinf. Softw. 2014, 15, 1–7. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Francavilla, C.; Lupia, M.; Tsafou, K.; Villa, A.; Kowalczyk, K.; Rakownikow Jersie-Christensen, R.; Bertalot, G.; Confalonieri, S.; Brunak, S.; Jensen, L.J.; et al. Phosphoproteomics of Primary Cells Reveals Druggable Kinase Signatures in Ovarian Cancer. Cell Rep. 2017, 18, 3242–3256. [Google Scholar] [CrossRef]
- Paraskevopoulou, M.D.; Vlachos, I.S.; Karagkouni, D.; Georgakilas, G.; Kanellos, I.; Vergoulis, T.; Zagganas, K.; Tsanakas, P.; Floros, E.; Dalamagas, T.; et al. DIANA-LncBase v2: Indexing microRNA targets on non-coding transcripts. Nucleic Acids Res. 2016, 44, D231–D238. [Google Scholar] [CrossRef]
- Chou, C.H.; Shrestha, S.; Yang, C.D.; Chang, N.W.; Lin, Y.L.; Liao, K.W.; Huang, W.C.; Sun, T.H.; Tu, S.J.; Lee, W.H.; et al. MiRTarBase update 2018: A resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 2018, 46, D296–D302. [Google Scholar] [CrossRef] [PubMed]


 —denotes downregulation,
—denotes downregulation,  —denotes upregulation.
—denotes upregulation.
 —denotes downregulation,
—denotes downregulation,  —denotes upregulation.
—denotes upregulation.
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gokulnath, P.; de Cristofaro, T.; Manipur, I.; Di Palma, T.; Soriano, A.A.; Guarracino, M.R.; Zannini, M. Long Non-Coding RNA HAND2-AS1 Acts as a Tumor Suppressor in High-Grade Serous Ovarian Carcinoma. Int. J. Mol. Sci. 2020, 21, 4059. https://doi.org/10.3390/ijms21114059
Gokulnath P, de Cristofaro T, Manipur I, Di Palma T, Soriano AA, Guarracino MR, Zannini M. Long Non-Coding RNA HAND2-AS1 Acts as a Tumor Suppressor in High-Grade Serous Ovarian Carcinoma. International Journal of Molecular Sciences. 2020; 21(11):4059. https://doi.org/10.3390/ijms21114059
Chicago/Turabian StyleGokulnath, Priyanka, Tiziana de Cristofaro, Ichcha Manipur, Tina Di Palma, Amata Amy Soriano, Mario Rosario Guarracino, and Mariastella Zannini. 2020. "Long Non-Coding RNA HAND2-AS1 Acts as a Tumor Suppressor in High-Grade Serous Ovarian Carcinoma" International Journal of Molecular Sciences 21, no. 11: 4059. https://doi.org/10.3390/ijms21114059
APA StyleGokulnath, P., de Cristofaro, T., Manipur, I., Di Palma, T., Soriano, A. A., Guarracino, M. R., & Zannini, M. (2020). Long Non-Coding RNA HAND2-AS1 Acts as a Tumor Suppressor in High-Grade Serous Ovarian Carcinoma. International Journal of Molecular Sciences, 21(11), 4059. https://doi.org/10.3390/ijms21114059





