SYMMETRIC PETALS 1 Encodes an ALOG Domain Protein that Controls Floral Organ Internal Asymmetry in Pea (Pisum sativum L.)
Abstract
1. Introduction
2. Results
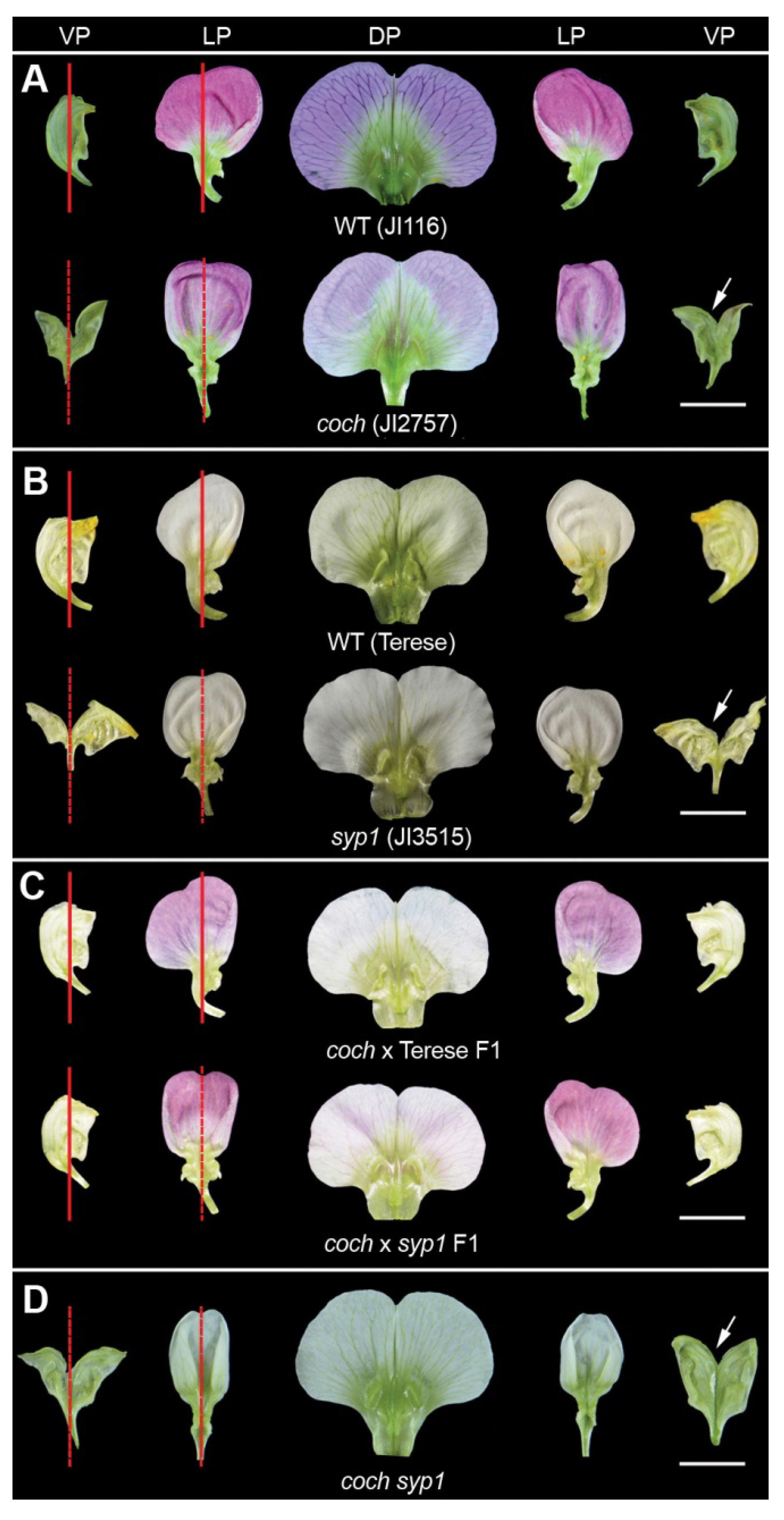
2.1. Phenotypes of Coch and syp1 Mutants with Defects in Organ Internal Asymmetry
2.2. Molecular Cloning of SYP1 in Pea
2.3. ALOG Family Transcription Factors in Pea
2.4. Expression and Protein Localization of COCH and SYP1
2.5. Physical Interaction between SYP1 and COCH
2.6. COCH Represses the Decrease in SYP1 Levels Mediated by the 26S Proteasome
3. Discussion
3.1. COCH and SYP1 Are Involved in the IN Asymmetry of Floral Organs
3.2. SYP1 Encodes an ALOG Family Protein
3.3. COCH Interacts with and Promotes SYP1 Stability
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Comparative Mapping and Molecular Cloning
4.3. RNA Extraction and Reverse Transcription Quantitative PCR (RT-qPCR)
4.4. RNA In Situ Hybridization
4.5. Subcellular Localization Assays
4.6. Yeast Two-Hybrid (Y2H) Assays
4.7. Co-immunoprecipitation (CoIP) Assays
4.8. Expression of Proteins in Escherichia coli
4.9. Protein Extraction and Cell-Free Degradation Assay
4.10. Virus-Induced Gene Silencing (VIGS) Assays
4.11. Phylogenetic Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ingram, G.C.; Waites, R. Keeping it together: Co-ordinating plant growth. Curr. Opin. Plant Biol. 2006, 9, 12–20. [Google Scholar] [CrossRef]
- Tucker, S.C. Floral development in legumes. Plant Physiol. 2006, 131, 911–926. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, Y.; Li, X.; Wang, L.; Xu, S.; Yang, J.; Weng, L.; Sato, S.; Tabata, S.; Ambrose, M.; et al. Genetic control of floral zygomorphy in pea (Pisum sativum L.). Proc. Natl. Acad. Sci. USA 2008, 105, 10414–10419. [Google Scholar] [CrossRef]
- Feng, X.; Zhao, Z.; Tian, Z.; Xu, S.; Luo, Y.; Cai, Z.; Wang, Y.; Yang, J.; Wang, Z.; Weng, L.; et al. Control of petal shape and floral zygomorphy in Lotus japonicus. Proc. Natl. Acad. Sci. USA 2006, 103, 4970–4975. [Google Scholar] [CrossRef]
- Li, X.; Zhuang, L.L.; Ambrose, M.; Rameau, C.; Hu, X.H.; Yang, J.; Luo, D. Genetic analysis of ele mutants and comparative mapping of ele1 locus in the control of organ internal asymmetry in garden pea. J. Integr. Plant Biol. 2010, 52, 528–535. [Google Scholar] [CrossRef]
- Li, X.; Liu, W.; Zhuang, L.; Zhu, Y.; Wang, F.; Chen, T.; Yang, J.; Ambrose, M.; Hu, Z.; Weller, J.L.; et al. BIGGER ORGANS and ELEPHANT EAR-LIKE LEAF1 control organ size and floral organ internal asymmetry in pea. J. Exp. Bot. 2019, 70, 179–191. [Google Scholar] [CrossRef]
- Zhao, L.; Nakazawa, M.; Takase, T.; Manabe, K.; Kobayashi, M.; Seki, M.; Shinozaki, K.; Matsui, M. Overexpression of LSH1, a member of an uncharacterised gene family, causes enhanced light regulation of seedling development. Plant J. 2004, 37, 694–706. [Google Scholar] [CrossRef]
- Yoshida, A.; Suzaki, T.; Tanaka, W.; Hirano, H.Y. The homeotic gene long sterile lemma (G1) specifies sterile lemma identity in the rice spikelet. Proc. Natl. Acad. Sci. USA 2009, 106, 20103–20108. [Google Scholar] [CrossRef]
- Cho, E.; Zambryski, P.C. ORGAN BOUNDARY1 defines a gene expressed at the junction between the shoot apical meristem and lateral organs. Proc. Natl. Acad. Sci. USA 2011, 108, 2154–2159. [Google Scholar] [CrossRef]
- Yoshida, A.; Sasao, M.; Yasuno, N.; Takagi, K.; Daimon, Y.; Chen, R.; Yamazaki, R.; Tokunaga, H.; Kitaguchi, Y.; Sato, Y.; et al. TAWAWA1, a regulator of rice inflorescence architecture, functions through the suppression of meristem phase transition. Proc. Natl. Acad. Sci. USA 2013, 110, 767–772. [Google Scholar] [CrossRef]
- MacAlister, C.A.; Park, S.J.; Jiang, K.; Marcel, F.; Bendahmane, A.; Izkovich, Y.; Eshed, Y.; Lippman, Z.B. Synchronization of the flowering transition by the tomato TERMINATING FLOWER gene. Nat. Genet. 2012, 44, 1393–1398. [Google Scholar] [CrossRef]
- Yan, D.; Zhou, Y.; Ye, S.; Zeng, L.; Zhang, X.; He, Z. Beak-shaped grain 1/TRIANGULAR HULL 1, a DUF640 gene, is associated with grain shape, size and weight in rice. Sci. China Life Sci. 2013, 56, 275–283. [Google Scholar] [CrossRef]
- Peng, P.; Liu, L.; Fang, J.; Zhao, J.; Yuan, S.; Li, X. The rice TRIANGULAR HULL1 protein acts as a transcriptional repressor in regulating lateral development of spikelet. Sci. Rep. 2017, 7, 13712. [Google Scholar] [CrossRef]
- Xiao, W.; Ye, Z.; Yao, X.; He, L.; Lei, Y.; Luo, D.; Su, S. Evolution of ALOG gene family suggests various roles in establishing plant architecture of Torenia fournieri. BMC Plant Biol. 2018, 18. [Google Scholar] [CrossRef]
- Lei, Y.; Su, S.; He, L.; Hu, X.; Luo, D. A member of the ALOG gene family has a novel role in regulating nodulation in Lotus japonicus. J. Integr. Plant Biol. 2019, 61, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Hanano, K.; Kariya, A.; Shimizu, S.; Zhao, L.; Matsui, M.; Tasaka, M.; Aida, M. CUP-SHAPED COTYLEDON1 transcription factor activates the expression of LSH4 and LSH3, two members of the ALOG gene family, in shoot organ boundary cells. Plant J. 2011, 66, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Park, S.J.; Van Eck, J.; Lippman, Z.B. Control of inflorescence architecture in tomato by BTB/POZ transcriptional regulators. Genes Dev. 2016, 30, 2048–2061. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tang, L.; Yu, Y.; Dalrymple, J.; Lippman, Z.B.; Xu, C. Control of flowering and inflorescence architecture in tomato by synergistic interactions between ALOG transcription factors. J. Genet. Genomics 2018, 45, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Su, S.; Higashiyama, T.; Luo, D. A homolog of the ALOG family controls corolla tube differentiation in Torenia fournieri. Development 2019, 146, dev177410. [Google Scholar] [CrossRef] [PubMed]
- Yaxley, J.L.; Jablonski, W.; Reid, J.B. Leaf and flower development in pea (Pisum sativum L.): Mutants cochleata and unifoliata. Ann. Bot. 2001, 88, 225–234. [Google Scholar]
- Couzigou, J.M.; Zhukov, V.; Mondy, S.; Abu el Heba, G.; Cosson, V.; Ellis, T.H.; Ambrose, M.; Wen, J.; Tadege, M.; Tikhonovich, I.; et al. NODULE ROOT and COCHLEATA maintain nodule development and are legume orthologs of Arabidopsis BLADE-ON-PETIOLE genes. Plant Cell 2012, 24, 4498–4510. [Google Scholar] [CrossRef] [PubMed]
- Hofer, J.; Turner, L.; Moreau, C.; Ambrose, M.; Isaac, P.; Butcher, S.; Weller, J.; Dupin, A.; Dalmais, M.; Le Signor, C.; et al. Tendril-less regulates tendril formation in pea leaves. Plant Cell 2009, 21, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Constantin, G.D.; Krath, B.N.; MacFarlane, S.A.; Nicolaisen, M.; Johansen, I.E.; Lund, O.S. Virus-induced gene silencing as a tool for functional genomics in a legume species. Plant J. 2004, 40, 622–631. [Google Scholar] [CrossRef]
- Liu, Y.C.; Lei, Y.W.; Weng, L.; Lei, M.J.; Hu, X.H.; Dong, Z.C.; Luo, D.; Yang, J. LjCOCH interplays with LjAPP1 to maintain the nodule development in Lotus japonicus. Plant Growth Regul. 2018, 85, 267–279. [Google Scholar] [CrossRef]
- Kulaeva, O.A.; Zhernakov, A.I.; Afonin, A.M.; Boikov, S.S.; Sulima, A.S.; Tikhonovich, I.A.; Zhukov, V.A. Pea Marker Database (PMD)-A new online database combining known pea (Pisum sativum L.) gene-based markers. PLoS ONE 2017, 12, e0186713. [Google Scholar]
- Kreplak, J.; Madoui, M.A.; Cápal, P.; Novák, P.; Labadie, K.; Aubert, G.; Bayer, P.E.; Gali, K.K.; Syme, R.A.; Main, D.; et al. A reference genome for pea provides insight into legume genome evolution. Nat. Genet. 2019, 51, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Jiao, K.; Li, X.; Guo, W.; Su, S.; Luo, D. High-Throughput RNA-Seq Data Analysis of the Single Nucleotide Polymorphisms (SNPs) and Zygomorphic Flower Development in Pea (Pisum sativum L.). Int. J. Mol. Sci. 2017, 18, 2710. [Google Scholar] [CrossRef] [PubMed]
- Alves-Carvalho, S.; Aubert, G.; Carrère, S.; Cruaud, C.; Brochot, A.L.; Jacquin, F.; Klein, A.; Martin, C.; Boucherot, K.; Kreplak, J.; et al. Full-length de novo assembly of RNA-seq data in pea (Pisum sativum L.) provides a gene expression atlas and gives insights into root nodulation in this species. Plant J. 2015, 84, 1–19. [Google Scholar] [PubMed]
- Wang, J.; Sun, P.; Li, Y.; Liu, Y.; Yu, J.; Ma, X.; Sun, S.; Yang, N.; Xia, R.; Lei, T.; et al. Hierarchically aligning 10 legume genomes establishes a family-level genomics platform. Plant Physiol. 2017, 174, 284–300. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, S.; Bai, Y.; Wu, Y.; Zhang, S.; Chen, M.; Guilfoyle, T.J.; Wu, P.; Qi, Y. Functional analysis of the structural domain of ARF proteins in rice (Oryza sativa L.). J. Exp. Bot. 2010, 61, 3971–3981. [Google Scholar] [CrossRef]
- Zhang, B.; Holmlund, M.; Lorrain, S.; Norberg, M.; Bakó, L.; Fankhauser, C.; Nilsson, O. BLADE-ON-PETIOLE proteins act in an E3 ubiquitin ligase complex to regulate PHYTOCHROME INTERACTING FACTOR 4 abundance. Elife 2017, 6, e26759. [Google Scholar] [CrossRef] [PubMed]
- Chahtane, H.; Zhang, B.; Norberg, M.; LeMasson, M.; Thévenon, E.; Bakó, L.; Benlloch, R.; Holmlund, M.; Parcy, F.; Nilsson, O.; et al. LEAFY activity is post-transcriptionally regulated by BLADE ON PETIOLE2 and CULLIN3 in Arabidopsis. New Phytol. 2018, 220, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Radivojac, P.; Vacic, V.; Haynes, C.; Cocklin, R.R.; Mohan, A.; Heyen, J.W.; Goebl, M.G.; Iakoucheva, L.M. Identification, analysis, and prediction of protein ubiquitination sites. Proteins 2010, 78, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Aubert, G.; Morin, J.; Jacquin, F.; Loridon, K.; Quillet, M.C.; Petit, A.; Rameau, C.; Lejeune-Hénaut, I.; Huguet, T.; Burstin, J. Functional mapping in pea, as an aid to the candidate gene selection and for investigating synteny with the model legume Medicago truncatula. Theor. Appl. Genet. 2006, 112, 1024–1041. [Google Scholar] [CrossRef] [PubMed]
- Deulvot, C.; Charrel, H.; Marty, A.; Jacquin, F.; Donnadieu, C.; Lejeune-Hénaut, I.; Burstin, J.; Aubert, G. Highly-multiplexed SNP genotyping for genetic mapping and germplasm diversity studies in pea. BMC Genomics 2010, 11, 468. [Google Scholar] [CrossRef]
- Zhuang, L.L.; Ambrose, M.; Rameau, C.; Weng, L.; Yang, J.; Hu, X.H.; Luo, D.; Li, X. LATHYROIDES, encoding a WUSCHEL-related Homeobox1 transcription factor, controls organ lateral growth, and regulates tendril and dorsal petal identities in garden pea (Pisum sativum L.). Mol. Plant 2012, 5, 1333–1345. [Google Scholar] [CrossRef]
- Coen, E.S.; Romero, J.M.; Doyle, S.; Elliott, R.; Murphy, G.; Carpenter, R. Floricaula: A homeotic gene required for flower development in Antirrhinum majus. Cell 1990, 63, 1311–1322. [Google Scholar] [CrossRef]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef]
- Chen, T.; Zhu, H.; Ke, D.; Cai, K.; Wang, C.; Gou, H.; Hong, Z.; Zhang, Z. A MAP kinase kinase interacts with SymRK and regulates nodule organogenesis in Lotus japonicus. Plant Cell 2012, 24, 823–838. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, H.; Huang, X.; Xia, R.; Zhao, Q.; Lai, J.; Teng, K.; Li, Y.; Liang, L.; Du, Q.; et al. BSCTV C2 attenuates the degradation of SAMDC1 to suppress DNA methylation-mediated gene silencing in Arabidopsis. Plant Cell 2011, 23, 273–288. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Lei, Y.; Li, X.; Peng, Q.; Liu, W.; Jiao, K.; Su, S.; Hu, Z.; Shen, Z.; Luo, D. SYMMETRIC PETALS 1 Encodes an ALOG Domain Protein that Controls Floral Organ Internal Asymmetry in Pea (Pisum sativum L.). Int. J. Mol. Sci. 2020, 21, 4060. https://doi.org/10.3390/ijms21114060
He L, Lei Y, Li X, Peng Q, Liu W, Jiao K, Su S, Hu Z, Shen Z, Luo D. SYMMETRIC PETALS 1 Encodes an ALOG Domain Protein that Controls Floral Organ Internal Asymmetry in Pea (Pisum sativum L.). International Journal of Molecular Sciences. 2020; 21(11):4060. https://doi.org/10.3390/ijms21114060
Chicago/Turabian StyleHe, Liang, Yawen Lei, Xin Li, Qincheng Peng, Wei Liu, Keyuan Jiao, Shihao Su, Zhubing Hu, Zhenguo Shen, and Da Luo. 2020. "SYMMETRIC PETALS 1 Encodes an ALOG Domain Protein that Controls Floral Organ Internal Asymmetry in Pea (Pisum sativum L.)" International Journal of Molecular Sciences 21, no. 11: 4060. https://doi.org/10.3390/ijms21114060
APA StyleHe, L., Lei, Y., Li, X., Peng, Q., Liu, W., Jiao, K., Su, S., Hu, Z., Shen, Z., & Luo, D. (2020). SYMMETRIC PETALS 1 Encodes an ALOG Domain Protein that Controls Floral Organ Internal Asymmetry in Pea (Pisum sativum L.). International Journal of Molecular Sciences, 21(11), 4060. https://doi.org/10.3390/ijms21114060




