Abstract
Serotonin transporter (SerT) in the brain is an important neurotransmitter transporter involved in mental health. However, its role in peripheral organs is poorly understood. In this study, we investigated the function of SerT in the development of the compound eye in Drosophila melanogaster. We found that SerT knockdown led to excessive cell death and an increased number of cells in S-phase in the posterior eye imaginal disc. Furthermore, the knockdown of SerT in the eye disc suppressed the activation of Akt, and the introduction of PI3K effectively rescued this phenotype. These results suggested that SerT plays a role in the healthy eye development of D. melanogaster by controlling cell death through the regulation of the PI3K/Akt pathway.
1. Introduction
The serotonergic system is conserved from insects to mammals, and it plays a pivotal role in the mental health of organisms [1,2]. The serotonergic system exists in both the brain and peripheral organs. In the brain, serotonin (5-hydroxytryptamine, 5-HT), the core molecule of the system, acts as a neurotransmitter and is associated with the feeling of wellness and happiness [3]. Altered regulation of serotonin concentration in the brain has been recorded not only in a vast range of behavioral functions, such as wake/sleep states, appetite, and sexual behavior, but also in the causation of multiple severe psychotic disorders, such as depression, bipolar disorder, and schizophrenia [4,5]. Although the roles of serotonin in the brain have been intensively investigated, the serotonergic function in peripheral organs, where serotonin acts as a hormonal molecule, is mostly uninvestigated, even though 95% of the serotonin in the whole body is produced by the gastrointestinal tract [6]. One of the essential components in the regulation of serotonin is serotonin transporter (SerT) or 5-hydroxytryptamine transporter (5-HTT), which is a monoamine transporter protein responsible for the reuptake of serotonin into neurons after synaptic transmission in mammals [7]. SerT is a transmembrane transporter that is highly conserved in various organisms from insects to mammals. SerT in the central nervous system is associated with anxiety and depression-related psychological conditions, thereby making it a target for a large number of antidepressant drugs called selective serotonin reuptake inhibitors (SSRIs) [8]. On the other hand, peripheral SerT has been reported to play significant roles in cardiovascular processes, appetite regulation, endocrine mediation, and reproduction [9,10,11,12]. Still, the function of SerT in developmental processes is poorly understood.
Drosophila melanogaster is a versatile model organism for researching various biological and physiological mechanisms [13]. The compound eye of an adult fly is composed of approximately 750 unit eyes, known as ommatidia. Each ommatidium consists of a core of eight photoreceptor neurons, R1–R8. Drosophila compound eyes are derived from the eye-antennal disc. In the eye disc of Drosophila third-instar larva, the morphogenetic furrow (MF) represents the mitotic wave of cells, which sweeps from posterior to anterior. The cells exiting just ahead of MF in the anterior area and within the MF will undergo cell cycle arrest in the synchronized G1 phase, waiting for their fate to be determined, and form a progenitor cell pool. As MF propagates, the progenitor cells are recruited into photoreceptor pre-clusters and specified as several types of photoreceptor neurons (R8, R2, R5, R3, or R4). In cells just at the back of MF in the posterior area, which have not started to differentiate, one more round of division called the second mitotic wave (SMW) occurs to recruit cells into other types of photoreceptor neurons (R1, R6, or R7) [14,15,16]. In the posterior region, after the SMW passes, no additional cell cycles occur within the larval disc. Because there are more cells than needed in the eye disc to form ~750 ommatidia, undifferentiated cells will either later differentiate into photoreceptors or accessory cells, or be removed via apoptosis during pupation [17]. Since the core of photoreceptor neurons is arrayed in a structure that is accurately repetitive in healthy individuals [18], small abnormalities in the compound eyes can be easily observed, which make compound eye tissues ideal for examining cell fate via signaling transduction pathways. Thus, we chose the Drosophila compound eye as target tissues for investigating the role of SerT in the eye development.
Drosophila and mammals share a highly conserved phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway, which plays a vital role in regulating cellular growth and energy metabolism [19,20]. Moreover, multiple pieces of evidence showed the relation between this pathway and the development of the Drosophila compound eye. Palomero et al. (2007) showed the interaction of PTEN or Akt with Notch results in abnormal eye growth and tumorigenesis [21]. The target of rapamycin (TOR) is also involved in eye development, as TOR directly couples with the adaptor protein Unk. Bateman, J.M. [22] showed that together, TOR and Unk mediate the differentiation fate of photoreceptors R1, R6 and R7. Moreover, the activity of the TOR complexes are required for hyperplasia upon elevated PI3K signaling in the compound eye [23]. Slade and Staveley (2015) showed that numerous novel Akt1 mutants exhibit fewer ommatidia with reduced size [24]. We hypothesized that the PI3K/Akt pathway mediates the role of SerT in the development of the compound eye in Drosophila melanogaster.
2. Results
2.1. SerT Knockdown Disrupts Healthy Eye Development
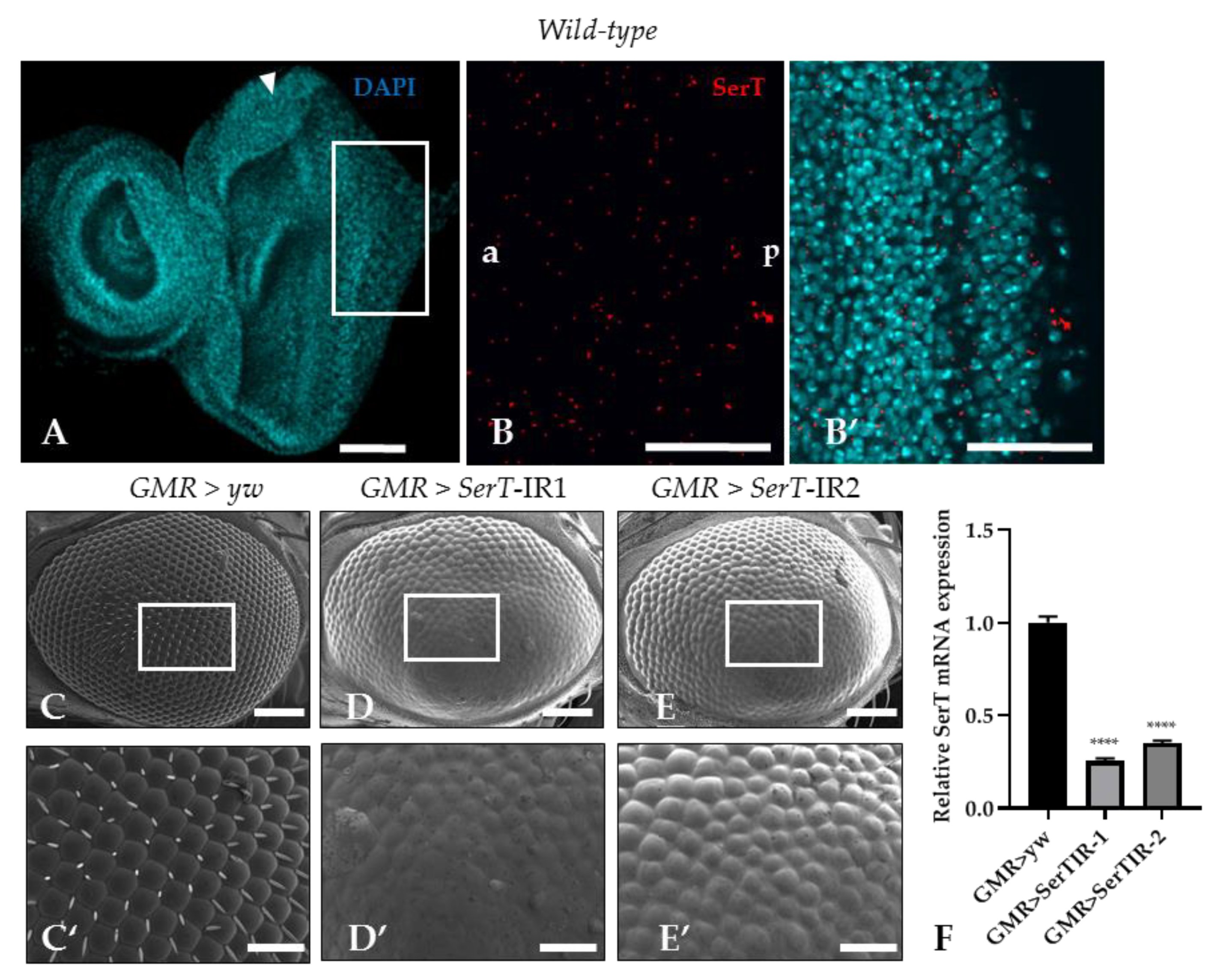
Despite multiple studies that explore SerT expression in the central nervous system of Drosophila [25,26], there is no evidence of SerT expression in the imaginal eye discs. In this research, SerT expression was visualized by a specific antibody for Drosophila SerT. Anti-SerT signals were distributed outside of the nucleus throughout the eye imaginal disc, showing high intensity (Figure 1B,B′).
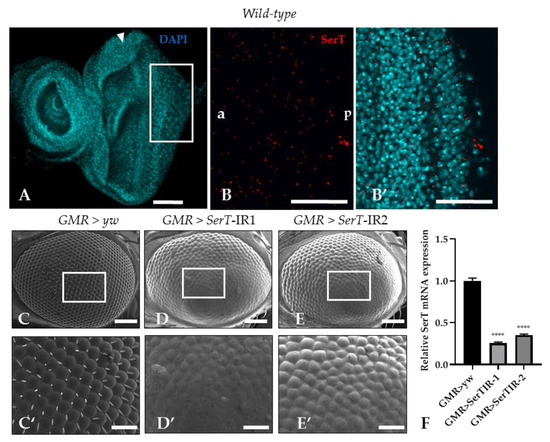
Figure 1.
SerT knockdown induces a rough phenotype in the adult compound eye. The eye imaginal discs of wild-type third instar larvae were stained with 4′,6-diamidino-2-phenylindole (DAPI) to visualize the nucleus (A). The posterior region in the box stained with rabbit anti-Drosophila SerT antibody followed by anti-rabbit IgG Alexa FluorTM 594 antibody was used for detecting SerT (B), and the merged image is shown (B′). The images are representative of 10 eye imaginal discs. Scanning electron micrographs of the compound eyes of flies carrying GMR-Gal4/yw; +; + (C), GMR-Gal4/Y; UAS-SerT-IR1/+; +, (D), GMR-Gal4/Y; and UAS-SerT-IR2/+; + (E). Larger images of the boxed regions are also shown (C′–E′). Phenotypes were observed independently in at least three individuals of each fly lines, and no significant change was found in three individuals of the same line. Relative mRNA expression of SerT gene in eye discs of flies contains GMR > yw, GMR > SerTIR-1, and GMR > SerTIR-2, n = 5 (F). Scale bars indicate 100 μm (A,B,B′,C–E) and 30 μm (C′–E′). The triangles indicate MF. a, anterior; p, posterior; **** p < 0.0001
Then, to determine the role of SerT in eye development, we investigated the effect of the reduction of SerT protein in the compound eye by using a GAL4/Upstream activation sequence (GAL4/UAS) system. Two UAS-SerT RNAi strains that have different inverted repeat sequences downstream of the UAS sequence with no overlap were used to exclude the possibility of off-target knockdown. The UAS-SerT RNAi strains were crossed with the GMR-GAL4 driver that expresses GAL4 in the eye under the control of glass enhancer; therefore, the offspring strongly expressed SerT RNAi in all the cells behind the MF, causing its specific knockdown in the eye. The knockdown strains, GMR > SerT-inverted repeat (IR)1 (Figure 1D,D′) and GMR > SerT-IR2 (Figure 1E,E′), showed a rough phenotype in 100% of the adult compound eyes, in which the ommatidia were oddly shaped, and the bristles were lost. As the GMR > SerT-IR1 strain expressed a more severe phenotype, we chose this strain for further experiments. These data suggested that SerT played an important role in the development of the compound eye.
2.2. SerT Knockdown Induces Cell Death via a Caspase-Dependent Pathway
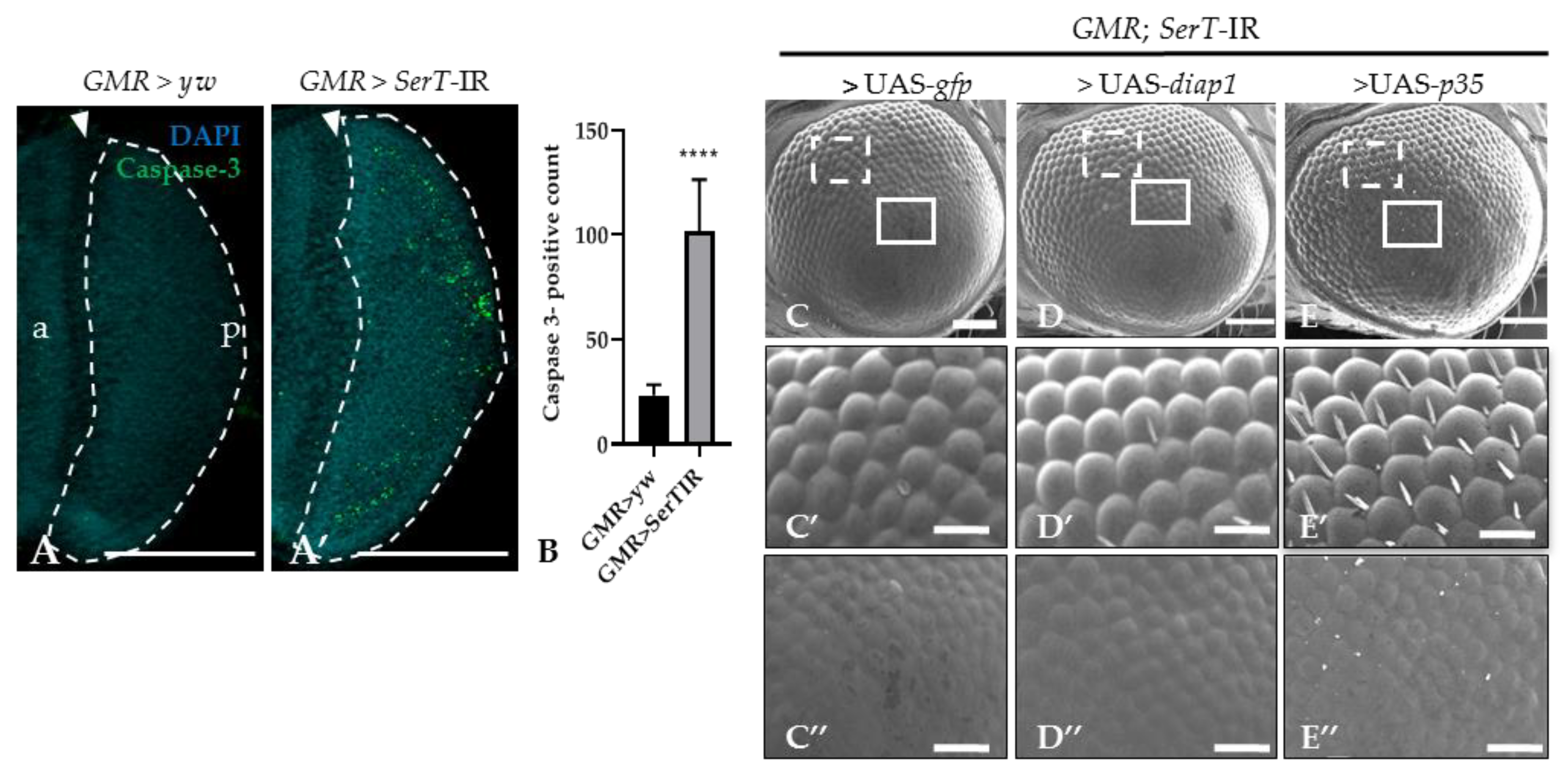
Previous reports suggested that the rough eye phenotype may be caused by excessive cell death [27,28]. Thus, we stained the eye disc of third-instar larvae with anti-caspase-3 antibody. The number of the caspase-3-positive signal was significantly higher (4.4-fold) in knockdown flies (SerT-kd) than in wild-type flies, suggesting an increase in caspase-dependent cell death (Figure 2A,A′). This result was further confirmed by introducing either the p35 or diap1 gene, both of which encoded apoptosis inhibitors, into knockdown flies. The flies carrying UAS-p35 or UAS-diap1 in the background of SerT knockdown showed less a severe phenotype (Figure 2D–D″,E–E″). The ommatidia structures slightly recovered, and the bristles were partially regenerated. Although flies carrying UAS-gfp and SerT-IR, which were generated as control, showed no significant rescue compared with the knockdown flies (Figure 2C–C″).
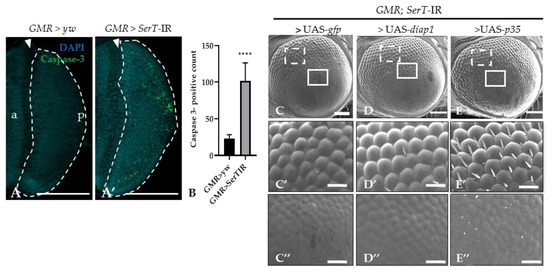
Figure 2.
SerT knockdown induces caspase-dependent cell death. Region posterior to the morphogenetic furrow (MF) in the eye discs of third-instar larvae carrying GMR > yw as a control (A) and GMR > SerT-IR as SerT-kd (A′) were stained with anti-caspase-3 antibody followed by secondary antibodies labeled with Alexa488. Quantification of caspase-3 positive signal count in the posterior region surrounded by dotted lines (B). Scanning electron micrograph of adult compound eyes of flies carrying GMR-Gal4/Y, SerT-IR/UAS-gfp, + (C), GMR-Gal4/Y, SerT-IR/UAS-diap1, + (D), and GMR-Gal4/Y, SerT-IR/UAS-p35, + (E). Larger images of the boxed regions with dotted white line and solid white line in (C–E) are shown in (C′–E′,C″–E″), respectively. Scale bar indicates 100 (A,A’,C–E) and 30 μm (C′–E′,C″–E″). The triangles point to the MF. a, anterior; p, posterior; ****, p < 0.0001.
2.3. SerT Knockdown Increases the Number of Cells in S-Phase in the Eye Imaginal Discs
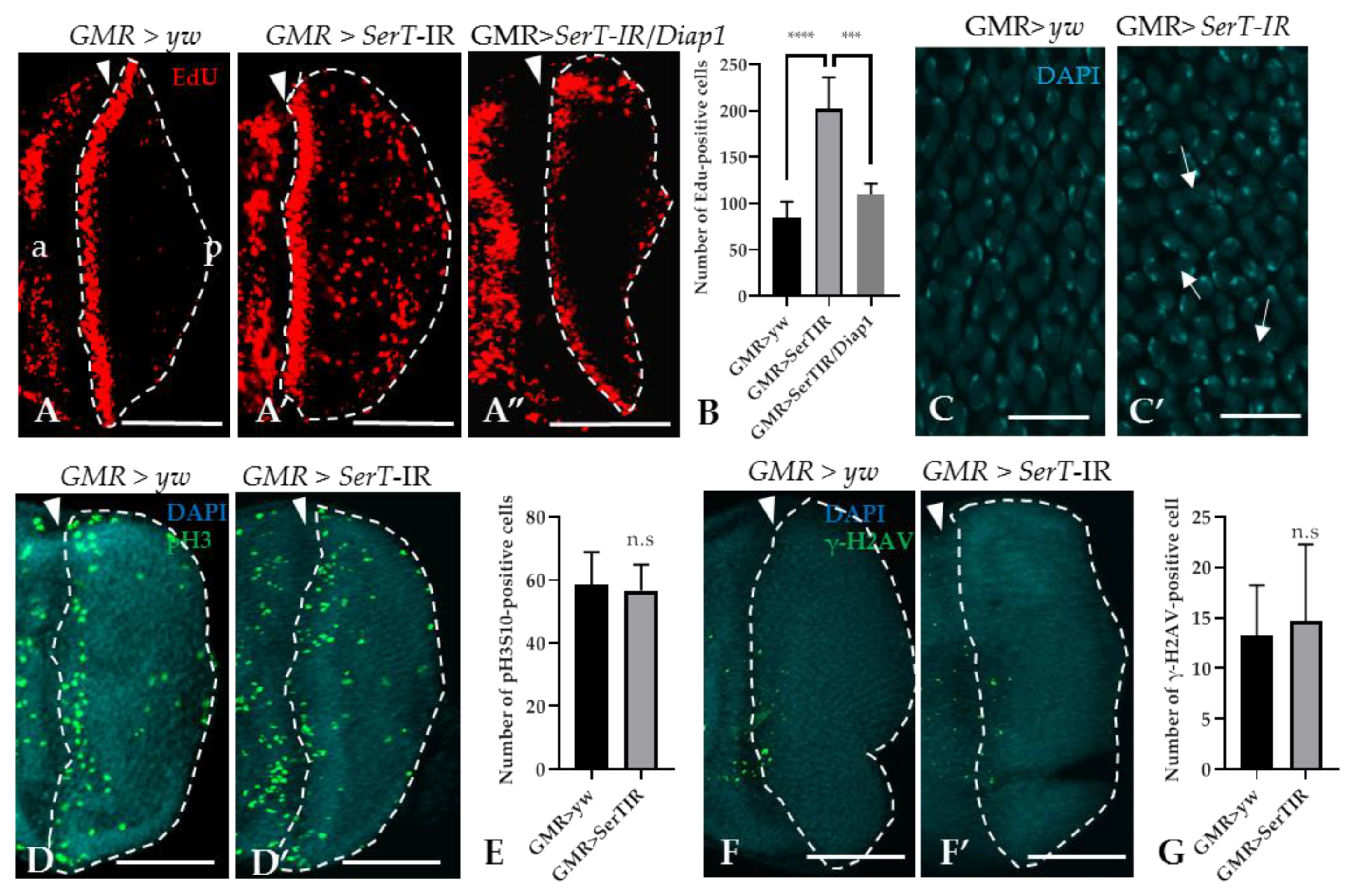
Given the increased level of cell death, we hypothesized that SerT-kd might cause cell cycle defects in the eye imaginal discs. To prove this hypothesis, we utilized the 5-ethynyl-2′deoxyuridine (EdU) incorporation assay, which can detect proliferating S-phase cells. The result showed that there was a significant increase (2.4-fold) in the number of EdU-positive cells in the posterior area of SerT-kd flies than in that of control flies (the region surrounded by dotted lines in Figure 3A,A′). Moreover, the inhibition of apoptosis by the expression of DIAP1 significantly decreased the number of S-phase cells (Figure 3A”), suggesting a link between two phenomena. Moreover, we also detected the abnormal organization of cone cell nuclei by DAPI staining, which might be a result of excessive proliferation (Figure 3C,C′). On the other hand, we found that there was no change in the number of cells in the M-phase, as determined by anti-phosphorylated histone 3 antibody, between SerT-kd and control flies (Figure 3D,D′,E). This suggests that only the number of cells in S-phase was affected, whereas the process of cell proliferation was not. In addition, we eliminated the possibility of DNA-damage causing excessive proliferation or cell death by staining the posterior eye disc with anti-phosphorylated-gamma-histone 2A, a known DNA-damage marker [29]. Low-signal intensity was observed in both the control and SerT-kd (Figure 3F,F′,G), suggesting that DNA-damage did not cause the phenotype.
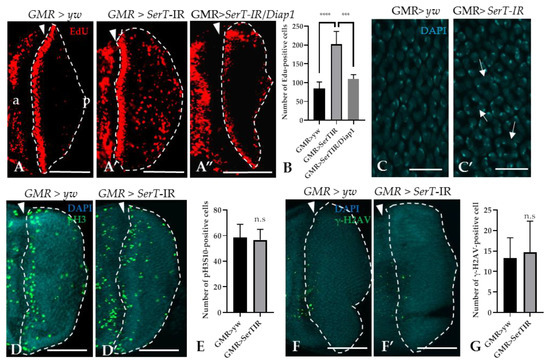
Figure 3.
Knockdown of SerT induces a high accumulation of cells in the S-phase, but not in the M-phase. The region posterior to the MF stained with EdU in third instar larvae carrying GMR > yw (A), GMR > SerT-IR (A′), and GMR > SerT-IR/diap1 (A”). Quantification of EdU-positive cells in the posterior region surrounded by dotted lines (B). DAPI staining of the nuclei of cone cells of third-instar larvae carrying GMR > yw (C) and GMR > SerT-IR (C′). Arrows point to the abnormal formation of the nuclei. Posterior region of third-instar larvae carrying GMR > yw and GMR > SerT-IR, respectively, were stained with anti-phosphorylated-histone 3 (Ser10) antibody conjugated with Alexa488 (D,D′) and with anti-phosphorylated-gamma-Histone 2A antibody (F,F′). Quantification of pH3S10-positive cells and γ-H2AV-positive cells in the region surrounded by dotted lines, respectively (E,G). Scale bars indicate 100 μm (A–A”,D,D′,F,F′) and 5 μm (C,C′). The triangles indicate the MF. a, anterior; p, posterior. ****, p < 0.0001; ***, p < 0.001; n.s., not significant.
2.4. SerT Knockdown Induces a Rough Eye Phenotype via the PI3K/Akt Pathway
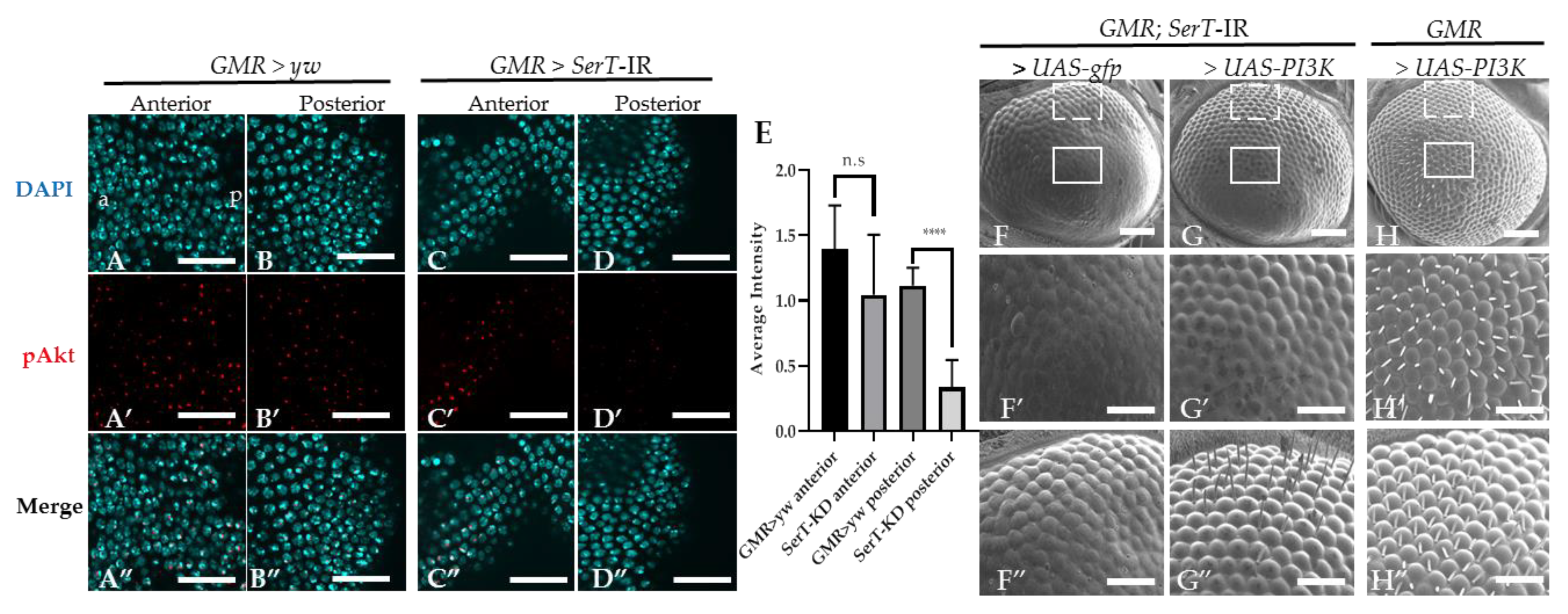
We suspected that the eye phenotype of the SerT knockdown fly might be influenced by the PI3k/Akt pathway. This pathway is well known to be involved in the regulation of the cell cycle, and there is also evidence that suggests its participation in the neurogenesis of the compound eye [22]. First, we visualized the Akt activation level using specific Drosophila anti-phosphorylated Akt at Ser505 (the equivalent of human Ser473). Figure 4A–D” shows a significant decrease in the level of phosphorylated Akt in the posterior region (the region affected by SerT-kd, Figure 4D–D”) of the eye disc of the SerT-kd fly compared with that in the control (Figure 4A–B”) and in the anterior region (the region unaffected by SerT-kd, Figure 4C–C”) of the same eye disc.
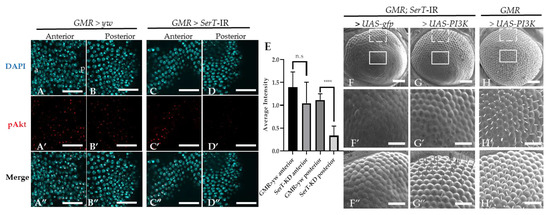
Figure 4.
Knockdown of SerT reduces the levels of phosphorylated Akt protein. The anterior and posterior region to the MF in the eye discs of third-instar larvae carrying GMR > yw as a control (A–A”,B–B”, respectively) and GMR > SerT-IR as SerT-kd (C–C”,D–D”, respectively) were stained with DAPI (blue) and anti-phosphorylated-Akt antibody followed by secondary antibodies labeled with Alexa594 (red). Merged images are also shown (A”,B”,C”,D”). The images are representative of 5 eye imaginal discs. Quantification of the phosphorylated Akt average intensity (E). Scanning electron micrograph of the adult compound eyes of flies carrying GMR-Gal4/Y, SerT-IR/UAS-gfp, + (F), GMR-Gal4/Y, SerT-IR/UAS-PI3K, + (G), and GMR-Gal4/Y, UAS-PI3K, + (H). Larger images of regions surrounded by box and dotted lines are shown (F′–H′,F”–H”, respectively). Scale bar indicates 15 μm (A–D”), 100 μm (F–H) and 30 μm (F′–H”). a, anterior; p, posterior; ****, p < 0.0001.
Since Akt activation is regulated via PI3K, we tried to rescue the phenotype by introducing PI3K in SerT-kd flies. The result showed the significant restoration of ommatidia shape and bristle formation (Figure 4G–G”), compared with those in the control flies carrying gfp (Figure 4F–F”). A representative image of the compound eyes of flies with PI3K overexpression, but without SerT-RNAi, is also shown (Figure 4H–H”). Taken together, the results indicated that the levels of Akt phosphorylation were reduced by SerT knockdown. The levels of phosphorylated Akt are negatively regulated by the feedback of Akt itself, and it is often difficult to interpret changes in Akt phosphorylation. This observation alone is not proof of reduced PI3K/Akt activity, but it is suggestive.
3. Discussion
SerT plays important roles in various processes in the peripheral system [30]. In this study, we visualized the expression of SerT in Drosophila imaginal disc by specific antibody (Figure 1B,B′), which led us to elucidate the function of SerT in eye development. The effect of the knockdown of the SerT gene using a GMR-GAL4 driver resulted in a noticeable rough eye phenotype, represented by the abnormal organization of ommatidia and the complete loss of bristles (Figure 1D,D′,E,E′). Previous studies indicated that the rough eye phenotype was possibly caused by the disruption of the cell cycle in the development of the compound eyes, which may lead to the premature termination of cells via a caspase-dependent pathway [27,31]. Our results indeed showed an increase in caspase-dependent cell death in knockdown flies, which was effectively rescued by the caspase inhibitor DIAP1 or p35 (Figure 2D–D”,E–E”). Figure 3A′ also showed that, under SerT-kd, the number of cells in the S-phase accumulated in the posterior region was significantly higher in knockdown flies than in the control, though the first mitotic wave (indicated by MF) and the second wave had passed.
Although a large number of cells were in S-phase, pH3S10 staining showed no change in the number of cells undergoing mitosis between SerT-kd and the controls. A previous study showed that rux mutation caused defects in the cell cycle regulation that led to the premature entry into S phase (represented by substantial increases in 5-bromo-2’-deoxyuridine staining), resulting in a similar but more severe rough eye phenotype to that observed in the present study and in the absence of changes in mitosis [32]. Moreover, our recent study of the regulation of the developing wing suggested that the knockdown of dLipin by an sd-GAL4 driver induces the accumulation of cells in S-phase while also decreasing the number of mitotic cells [33]. These studies suggest that increases in S- and M-phase cells can be regulated differently due to cell cycle defects; however, the underlying mechanism needs to be investigated further. The result shown in Figure 3F,F′, describing a lack of DNA damage, excludes the involvement of the DNA-repair mechanism in the phenotype. An alternative hypothesis might be that the excess numbers of S-phase cells might be induced by compensatory mechanisms related to cell death. Although this is suggestive, key evidence is missing, especially the cause of differential regulation of the mitotic phase. The abnormal organization of cone cell nuclei (Figure 3C′) suggested that excessive S-phase proliferation may cause the elevated recruitment of DNA in this cell type, strengthening our claim in the link between S-phase proliferation and rough eye phenotype. However, whether extra cone cells are formed or not is unclear and requires further investigation.
Then, our results suggested that the excessive caspase-dependent cell death in the posterior region of the eye disc was induced by the decreased level of the PI3K/Akt pathway activation. Clinical and experimental data highlighted the insulin-induced PI3K/Akt pathway, a universal pathway in yeast, insects, mice, and mammals, as a common pathway in the stabilization of cell growth under the control of nutrition [34]. The PI3K/Akt pathway is widely known as a crucial inhibitor of apoptotic effectors in the growth-signaling pathway. The activation of this pathway can reduce apoptosis in various types of cells, including neuronal, ischemia-inducing myocardial, and tumor cells [35,36,37,38]. In the rat hippocampus, the PI3K/Akt signaling pathway plays a pivotal role in neuronal apoptosis after inducing subarachnoid hemorrhage [39]. Furthermore, the inhibition of the PI3K/Akt pathway promotes the activation of caspase-3, subsequently increasing apoptotic cell death in diabetic rats [40]. Moreover, the activation of PI3K/Akt is effectively controlled by the insulin network [41,42]. The inhibition of SerT via a genetic or pharmacological mechanism results in insulin resistance prior to adiposity [43,44,45]. Previous research revealed that in SerT-deficient mice, JNK activity is exalted, and insulin-induced Akt activation is declined, whereas the elevation of AKT signaling by PTEN deficiency rescues the glucose tolerance phenotype. This study also claimed that SerT-deficiency downregulates insulin action and is responsible for impaired PI3K/Akt signaling in the peripheral system [43]. Consistent with these previous findings, our study also showed a link between SerT and PI3K/Akt signaling.
SerT functions are tightly connected to serotonin. SerT knockdown will diminish the clearance of excessive serotonin, which may, in turn, elevates the serotonin accumulation in cells. In mammals, serotonin induces the synthesis and release of insulin, as well as enhances the sensitivity of its target tissue [46]. In Drosophila, serotonergic neurons express a GTPase NS3, which controls growth via insulin signaling. Furthermore, Nässel et al. provided a more detailed mechanism that one of the serotonin receptors, 5-HT1A, inhibits adenylate cyclase and protein kinase A, thus inactivating cAMP response element-binding protein, which in turn stimulates insulin signaling [47]. Multiple studies showed that increasing serotonin levels activates the PI3K/Akt pathway in cancer cells and neurodegenerative Parkinson’s disease cellular model [48,49]. This discrepancy can be explained by the different serotonin regulatory processes in serotonergic network interactions. The serotonergic system is complex and regulated by multiple factors, and the regulatory feedback caused by excessive serotonin levels have been previously recorded [50,51]. In order to clarify the detailed interaction within the serotonergic system, the assay of serotonin levels in SerT knockdown flies is further needed. Further studies to reveal the specific cell types affected by SerT during eye disc development are warranted.
In conclusion, the cell fate of Drosophila imaginal eye disc is strictly regulated by a series of events, and numerous molecules have been found to control these events. In this study, we showed that the cell death induced by the suppressed activation of the PI3K/Akt pathway resulted in a rough eye phenotype in SerT-kd flies. Thus, we concluded that SerT played a role in normal eye development by controlling caspase-dependent cell death through the PI3K/Akt pathway. The detailed mechanism of the link between SerT and this cascade requires further investigations.
4. Materials and Methods
4.1. Fly Stocks
Fly stocks were maintained at 25 °C on standard food containing 0.65% agar, 10% glucose, 4% dry yeast, and 5% cornmeal. Transgenic flies with UAS-dSerT-IR686–1079 (SerT-IR1) and UAS-dSerT-IR1740–1760 (SerT-IR2) were obtained from the Vienna Drosophila Resource Center (#100584; Vienna, Austria) and Bloomington Drosophila Stock Center (#62985; Bloomington, IN, USA), respectively. These flies carried an IR of the SerT gene (targeting regions from nucleotide 686 to 1079 and from 1740 to 1760, respectively) downstream of the UAS sequence, on the second chromosome. All other flies used in this study were obtained from Bloomington Drosophila Stock Center: UAS-gfp (#1522), UAS-p35 (#5072), UAS-diap1 (#6657), UAS-PI3K (#8287). yw flies were used as the wild-type strain.
Scanning Electron Microscopy
All flies were anesthetized by CO2 and mounted on a holder. A VE-7800 scanning electron microscope (Keyence, Osaka, Japan) was used to observe the compound eyes of adult flies. At least five adult male flies were observed in each experiment.
4.2. Immunostaining
Cells in the S-phase were detected using Click-iT EdU (5-ethynyl-2′-deoxyuridine) labeling Alexa Fluor 594 Imaging Kit (Invitrogen, Carlsbad, CA, USA) [33]. Third instar larvae were dissected in phosphate buffer saline (PBS), and the eye discs were fixed in 4% paraformaldehyde for 20 min at 25 °C. After washing with PBS containing 0.3% Triton X-100 (PBST), the samples were blocked with 0.1% PBST and 10% normal goat serum for 30 min at 25 °C, and incubated with diluted primary antibodies in 0.1% PBST and 10% normal goat serum for 16 h at 4 °C [52,53]. The following antibodies were used as primary antibodies: rabbit anti-Drosophila SerT antibody (1:200; S1001-25H, USBio, Salem, MA, USA), rabbit anti-cleaved caspase-3 antibody (1:500; Sigma-Aldrich, St. Louis, MO, USA), anti-Phospho-Histone H3 (Ser10) (D2C8) XP Rabbit mAb conjugated with Alexa488 (1:400; Cell Signaling Technology, Danvers, MA, USA), anti-phosphorylated-histone 2A gamma variant antibody (1:400; DSHB, Iowa, IA, USA), and anti-phosphorylated-Drosophila Akt (Ser505) antibody (1:200; Cell Signaling Technology, Danvers, MA, USA). After washing with 0.3% PBST, samples were incubated with secondary antibodies labeled with either Alexa488 or Alexa 594 (Goat anti-rabbit IgG 1:1000 and Goat anti-mouse IgG 1:800; Abcam, Cambridge, UK) for 2 h at 25 °C. After further washing with 0.1% PBST, samples were mounted in a Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA) and observed by using a Fluoview Fv10i-0 confocal laser scanning microscope (Olympus, Tokyo, Japan). The signals were analyzed by the MetaMorph software (Molecular Devices, Sunnyvale, CA, USA).
4.3. Statistical Analysis
Signals in the posterior region of the MF were counted from at least six eye imaginal discs. All experiments were repeated at least three times. Statistical analyses were performed using the Student’s t-test and one-way ANOVA. Error bars represent the standard error of the mean (SEM), and all the data are shown as means ± SEM. Differences with p-values of <0.05 were considered significant.
Author Contributions
Conceptualization, T.L.A.P., and K.K.; methodology, T.L.A.P., T.D.B., G.L., and R.S.; writing—original draft preparation, T.L.A.P.; writing—review and editing, T.D.B., K.K., T.Q.C.N., Y.D.H.N., T.T.M.; supervision, K.K. All authors have read and agreed to the published version of the manuscript
Funding
This work was partially supported by Grants-in-Aid from the JSPS Core-to-Core program, B. Asia–Africa Science Platforms.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| SerT | Serotonin transporter |
| MF | Morphogenetic furrow |
| PI3K | Phosphatidylinositol 3-kinase |
| TOR | Target of rapamycin |
References
- Rillich, J.; Stevenson, P.A. Serotonin Mediates Depression of Aggression After Acute and Chronic Social Defeat Stress in a Model Insect. Front. Behav. Neurosci. 2018, 12. [Google Scholar] [CrossRef]
- Lin, S.H.; Lee, L.T.; Yang, Y.K. Serotonin and mental disorders: A concise review on molecular neuroimaging evidence. Clin. Psychopharmacol. Neurosci. 2014, 12, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Nutt, D.J. Serotonin and brain function: A tale of two receptors. J. Psychopharmacol. 2017, 31, 1091–1120. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.L.; Azmitia, E.C. Structure and function of the brain serotonin system. Physiol. Rev. 1992, 72, 165–229. [Google Scholar] [CrossRef] [PubMed]
- Visser, A.K.; Ramakrishnan, N.K.; Willemsen, A.T.; Di Gialleonardo, V.; de Vries, E.F.; Kema, I.P.; Dierckx, R.A.; van Waarde, A. [(11)C]5-HTP and microPET are not suitable for pharmacodynamic studies in the rodent brain. J. Cereb. Blood Flow Metab. 2014, 34, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Terry, N.; Margolis, K.G. Serotonergic mechanisms regulating the GI tract: Experimental evidence and therapeutic relevance. Handb. Exp. Pharmacol. 2017, 239, 319–342. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.M.; Namkung, J.; Go, Y.; Shong, K.E.; Kim, K.; Kim, H.; Park, B.Y.; Lee, H.W.; Jeon, Y.H.; Song, J.; et al. Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nat. Commun. 2015, 6, 6794. [Google Scholar] [CrossRef] [PubMed]
- Brindley, R.L.; Bauer, M.B.; Blakely, R.D.; Currie, K.P. Serotonin and Serotonin Transporters in the Adrenal Medulla: A Potential Hub for Modulation of the Sympathetic Stress Response. ACS Chem. Neurosci. 2017. [Google Scholar] [CrossRef]
- Genet, N.; Billaud, M.; Rossignol, R.; Dubois, M.; Gillibert-Duplantier, J.; Isakson, B.E.; Marthan, R.; Savineau, J.P.; Guibert, C. Signaling Pathways Linked to Serotonin-Induced Superoxide Anion Production: A Physiological Role for Mitochondria in Pulmonary Arteries. Front. Physiol. 2017, 8, 76. [Google Scholar] [CrossRef]
- Solmi, M.; Gallicchio, D.; Collantoni, E.; Correll, C.U.; Clementi, M.; Pinato, C.; Forzan, M.; Cassina, M.; Fontana, F.; Giannunzio, V.; et al. Serotonin transporter gene polymorphism in eating disorders: Data from a new biobank and META-analysis of previous studies. World J. Biol. Psychiatry 2016, 17, 244–257. [Google Scholar] [CrossRef]
- Yamakawa, K.; Matsunaga, M.; Isowa, T.; Ohira, H. Serotonin transporter gene polymorphism modulates inflammatory cytokine responses during acute stress. Sci. Rep. 2015, 5, 13852. [Google Scholar] [CrossRef] [PubMed]
- Haase, J.; Grudzinska-Goebel, J.; Muller, H.K.; Munster-Wandowski, A.; Chow, E.; Wynne, K.; Farsi, Z.; Zander, J.F.; Ahnert-Hilger, G. Serotonin Transporter Associated Protein Complexes Are Enriched in Synaptic Vesicle Proteins and Proteins Involved in Energy Metabolism and Ion Homeostasis. ACS Chem. Neurosci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Thanh, M.T.; Pham, T.L.A.; Tran, B.D.; Nguyen, Y.D.H.; Kaeko, K. Drosophila model for studying the link between lipid metabolism and development. Front. Biosci. (Landmark Ed.) 2020, 25, 147–158. [Google Scholar] [PubMed]
- Treisman, J.E. Retinal differentiation in Drosophila. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Escudero, L.M.; Freeman, M. Mechanism of G1 arrest in the Drosophila eye imaginal disc. BMC Dev. Biol. 2007, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Sukhanova, M.J.; Du, W. Control of cell cycle entry and exiting from the second mitotic wave in the Drosophila developing eye. BMC Dev. Biol. 2008, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Meserve, J.H.; Duronio, R.J. A population of G2-arrested cells are selected as sensory organ precursors for the interommatidial bristles of the Drosophila eye. Dev. Biol. 2017, 430, 374–384. [Google Scholar] [CrossRef]
- Baker, N.E.; Li, K.; Quiquand, M.; Ruggiero, R.; Wang, L.H. Eye development. Methods 2014, 68, 252–259. [Google Scholar] [CrossRef][Green Version]
- Zhang, H.; Stallock, J.P.; Ng, J.C.; Reinhard, C.; Neufeld, T.P. Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Genes Dev. 2000, 14, 2712–2724. [Google Scholar] [CrossRef]
- Wang, B.; Chen, N.; Wei, Y.; Li, J.; Sun, L.; Wu, J.; Huang, Q.; Liu, C.; Fan, C.; Song, H. Akt signaling-associated metabolic effects of dietary gold nanoparticles in Drosophila. Sci. Rep. 2012, 2, 1–7. [Google Scholar] [CrossRef]
- Palomero, T.; Sulis, M.L.; Cortina, M.; Real, P.J.; Barnes, K.; Ciofani, M.; Caparros, E.; Buteau, J.; Brown, K.; Perkins, S.L.; et al. Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. Nat. Med. 2007, 13, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Bateman, J.M. Mechanistic insights into the role of mTOR signaling in neuronal differentiation. Neurogenesis 2015, 2, e1058684. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hietakangas, V.; Cohen, S.M. Re-evaluating AKT regulation: Role of TOR complex 2 in tissue growth. Genes Dev. 2007, 21, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Slade, J.D.; Staveley, B.E. Compensatory growth in novel Drosophila Akt1 mutants Developmental Biology. BMC Res. Notes 2015, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Giang, T.; Rauchfuss, S.; Ogueta, M.; Scholz, H. The Serotonin Transporter Expression in Drosophila melanogaster. J. Neurogenet. 2011, 25, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, S.; Molina-Mateo, D.; Escobedo, P.; Zárate, R.V.; Fritz, E.; Fierro, A.; Perez, E.G.; Iturriaga-Vasquez, P.; Reyes-Parada, M.; Varas, R.; et al. Characterization of a Novel Drosophila SERT Mutant: Insights on the Contribution of the Serotonin Neural System to Behaviors. ACS Chem. Neurosci. 2017, 8, 2168–2179. [Google Scholar] [CrossRef]
- Tanaka, R.; Miyata, S.; Yamaguchi, M.; Yoshida, H. Role of the smallish gene during Drosophila eye development. Gene 2019, 684, 10–19. [Google Scholar] [CrossRef]
- Ly, L.L.; Suyari, O.; Yoshioka, Y.; Tue, N.T.; Yoshida, H.; Yamaguchi, M. DNF-YB plays dual roles in cell death and cell differentiation during Drosophila eye development. Gene 2013, 520, 106–118. [Google Scholar] [CrossRef]
- Lake, C.M.; Holsclaw, J.K.; Bellendir, S.P.; Sekelsky, J.; Hawley, R.S. The development of a monoclonal antibody recognizing the Drosophila melanogaster phosphorylated histone H2A variant (γ-H2AV). G3 (Bethesda) 2013, 3, 1539–1543. [Google Scholar] [CrossRef]
- Watanabe, H.; Rose, M.; Kanayama, Y.; Shirakawa, H.; Aso, H. Energy Homeostasis by the Peripheral Serotonergic System. In Serotonin—A Chemical Messenger Between All Types of Living Cells; InTech: Vienna, Austria, 2017. [Google Scholar]
- Rimkus, S.A.; Katzenberger, R.J.; Trinh, A.T.; Dodson, G.E.; Tibbetts, R.S.; Wassarman, D.A. Mutations in String/CDC25 inhibit cell cycle re-entry and neurodegeneration in a Drosophila model of Ataxia telangiectasia. Genes Dev. 2008, 22, 1205–1220. [Google Scholar] [CrossRef]
- Thomas, B.J.; Gunning, D.A.; Cho, J.; Zipursky, S.L. Cell cycle progression in the developing Drosophila eye: Roughex encodes a novel protein required for the establishment of G1. Cell 1994, 77, 1003–1014. [Google Scholar] [CrossRef]
- Duy Binh, T.; L. A. Pham, T.; Nishihara, T.; Thanh Men, T.; Kamei, K. The Function of Lipin in the Wing Development of Drosophila melanogaster. Int. J. Mol. Sci. 2019, 20, 3288. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Carreño, S.; Morante, J.; Dominguez, M. Systemic signalling and local effectors in developmental stability, body symmetry, and size. Cell Stress 2018, 2, 340–361. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Ren, J.Q.; Li, H.; Kong, Z.L.; Zhu, H.G. Downregulation of wild-type p53 protein by HER-2/neu mediated PI3K pathway activation in human breast cancer cells: Its effect on cell proliferation and implication for therapy. Cell Res. 2004, 14, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Noshita, N.; Lewén, A.; Sugawara, T.; Chan, P.H. Evidence of phosphorylation of Akt and neuronal survival after transient focal cerebral ischemia in mice. J. Cereb. Blood Flow Metab. 2001, 21, 1442–1450. [Google Scholar] [CrossRef]
- Noshita, N.; Lewén, A.; Sugawara, T.; Chan, P.H. Akt phosphorylation and neuronal survival after traumatic brain injury in mice. Neurobiol. Dis. 2002, 9, 294–304. [Google Scholar] [CrossRef]
- Xu, X.; Cao, Z.; Cao, B.; Li, J.; Guo, L.; Que, L.; Ha, T.; Chen, Q.; Li, C.; Li, Y. Carbamylated erythropoietin protects the myocardium from acute ischemia/reperfusion injury through a PI3K/Akt-dependent mechanism. Surgery 2009, 146, 506–514. [Google Scholar] [CrossRef]
- Zhuang, Z.; Zhao, X.; Wu, Y.; Huang, R.; Zhu, L.; Zhang, Y.; Shi, J. The anti-apoptotic effect of PI3K-Akt signaling pathway after subarachnoid hemorrhage in rats. Ann. Clin. Lab. Sci. 2011, 41, 364–372. [Google Scholar]
- Meng, Y.; Wang, W.; Kang, J.; Wang, X.; Sun, L. Role of the PI3K/AKT signalling pathway in apoptotic cell death in the cerebral cortex of streptozotocin-induced diabetic rats. Exp. Ther. Med. 2017, 13, 2417–2422. [Google Scholar] [CrossRef]
- Kulkarni, M.M.; Kulkarni, M.M.; Sopko, R.; Sun, X.; Hu, Y.; Nand, A.; Villalta, C.; Moghimi, A.; Yang, X.; Mohr, S.E.; et al. An Integrative Analysis of the InR/PI3K/Akt Network Identifies the Dynamic Response to Insulin Signaling. Cell Rep. 2016, 16, 3062–3074. [Google Scholar] [CrossRef]
- Galagovsky, D.; Katz, M.J.; Acevedo, J.M.; Sorianello, E.; Glavic, A.; Wappner, P. The Drosophila insulin-degrading enzyme restricts growth by modulating the PI3K pathway in a cell-autonomous manner. Mol. Biol. Cell 2014, 25, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Margolis, K.J.; Gershon, M.D.; Schwartz, G.J.; Sze, J.Y. Reduced serotonin reuptake transporter (SERT) function causes insulin resistance and hepatic steatosis independent of food intake. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Zha, W.; Ho, H.T.B.; Hu, T.; Hebert, M.F.; Wang, J. Serotonin transporter deficiency drives estrogen-dependent obesity and glucose intolerance. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Giannaccini, G.; Betti, L.; Palego, L.; Marsili, A.; Santini, F.; Pelosini, C.; Fabbrini, L.; Schmid, L.; Giusti, L.; Maffei, M.; et al. The expression of platelet serotonin transporter (SERT) in human obesity. BMC Neurosci. 2013, 14, 128. [Google Scholar] [CrossRef]
- Lam, D.D.; Heisler, L.K. Serotonin and energy balance: Molecular mechanisms and implications for type 2 diabetes. Expert Rev. Mol. Med. 2007, 9, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Nässel, D.R.; Kubrak, O.I.; Liu, Y.; Luo, J.; Lushchak, O.V. Factors that regulate insulin producing cells and their output in drosophila. Front. Physiol. 2013, 4, 252. [Google Scholar] [CrossRef]
- Dizeyi, N.; Hedlund, P.; Bjartell, A.; Tinzl, M.; Austild-Taskén, K.; Abrahamsson, P.A. Serotonin activates MAP kinase and PI3K/Akt signaling pathways in prostate cancer cell lines. Urol. Oncol. Semin. Orig. Investig. 2011, 29, 436–445. [Google Scholar] [CrossRef]
- Nakano, N.; Matsuda, S.; Ichimura, M.; Minami, A.; Ogino, M.; Murai, T.; Kitagishi, Y. PI3K/AKT signaling mediated by G protein-coupled receptors is involved in neurodegenerative Parkinson’s disease (Review). Int. J. Mol. Med. 2017, 39, 253–260. [Google Scholar] [CrossRef]
- Bari, A.; Theobald, D.E.; Caprioli, D.; Mar, A.C.; Aidoo-Micah, A.; Dalley, J.W.; Robbins, T.W. Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology 2010, 35, 1290–1301. [Google Scholar] [CrossRef]
- Daubert, E.A.; Heffron, D.S.; Mandell, J.W.; Condron, B.G. Serotonergic dystrophy induced by excess serotonin. Mol. Cell. Neurosci. 2010, 44, 297–306. [Google Scholar] [CrossRef]
- Binh, T.D.; Pham, T.L.A.; Men, T.T.; Dang, T.T.P.; Kamei, K. LSD-2 dysfunction induces dFoxO-dependent cell death in the wing of Drosophila melanogaster. Biochem. Biophys. Res. Commun. 2019, 509, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Binh, T.D.; Pham, T.L.A.; Men, T.T.; Kamei, K. Dysfunction of LSD-1 induces JNK signaling pathway-dependent abnormal development of thorax and apoptosis cell death in Drosophila melanogaster. Biochem. Biophys. Res. Commun. 2019, 516, 451–456. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).