Evaluation of the Impact of Cisplatin on Variances in the Expression Pattern of Leptin-Related Genes in Endometrial Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Cisplatin Cytotoxicity Assay
2.2. Leptin Cytotoxicity Assay
2.3. Morphology of Endometrial Cancer Cells Exposed to Cisplatin or/and Leptin
2.4. Evaluation of Caspase-3, -8 and -9 in the Ishikawa Cell Line Treated with Cisplatin
2.5. Evaluation of Caspase-3, -8 and -9 in the Ishikawa Cell Line Treated with Leptin
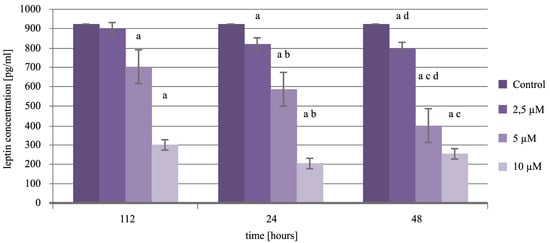
2.6. Expression Pattern of Leptin in Endometrial Cancer Cells Exposed to Cisplatin
2.7. Expression Pattern of Leptin-Receptors in the Endometrial Cancer Cell Line Treated with Cisplatin
2.8. Expression Pattern of JAK2 and STAT3 in Endometrial Cancer Cell Line Treated with Cisplatin
2.9. RNAi Analysis
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cytotoxicity Test
4.3. Induction of Apoptosis Activated via Analysis of Caspase-3, -8 and -9 Activity
4.4. RNA Isolation
4.5. Expression of Leptin and Leptin-Related Genes in the Ishikawa Cell Line Treated with Cisplatin
4.6. Level of Leptin and JAK 2 and STAT3 Obtained by ELISA Assay
4.7. RNA Interference
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| ELISA | Enzyme-linked immunosorbent assay |
| HGSOC | High grade serious epithelial ovarian carcinoma |
| LEPR | Leptin receptor |
| LEPROT | Leptin receptor overlapping transcript |
| LEPROTL1 | Leptin receptor overlapping transcript-like 1 |
| mRNA | Messenger RNA |
| RTqPCR | Real-time quantitative reverse transcription reaction |
| siRNA | Small interfering RNA |
References
- Münzberg, H.; Morrison, C.D. Structure, production, and signaling of leptin. Metabolism 2015, 64, 13–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.K.; AhAima, R.S. Physiology of leptin: Energy homeostasis, neuroendocrine function, and metabolism. Metabolism 2015, 64, 24–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, T.C.; Lee, T.C.; Hsu, S.L.; Yang, C.S. The molecular mechanism of leptin secretion and expression induced by aristolochic acid in kidney fibroblast. PLoS ONE 2011, 6, e16654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipsey, C.C.; Harbuzariu, A.; Daley-Brown, D.; Gonzalez-Perez, R.R. Oncogenic role of leptin and Notch interleukin-1 leptin crosstalk outcome in cancer. World J. Methodol 2016, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Abarzua-Catalan, L.; Trigo, C.; Delpiano, A.; Sanhueza, C.; García, K.; Ibañez, C.; Hormazábal, K.; Diaz, D.; Brañes, J.; et al. Leptin stimulates migration and invasion and maintains cancer stem-like properties in ovarian cancer cells: An explanation for poor outcomes in obese women. Oncotarget 2015, 6, 21100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milosevic, V.S.; Vukmirovic, F.C.; Krstic, M.S.; Zindovic, M.M.; Lj Stojanovic, D.; Jancic, S.A. Involvement of leptin receptors expression in proliferation and neoangiogenesis in colorectal carcinoma. J. Buon. 2015, 20, 100–108. [Google Scholar] [PubMed]
- Lin, T.C.; Huang, K.W.; Liu, C.W.; Chang, Y.C.; Lin, W.M.; Yang, T.Y.; Hsiao, M. Leptin signaling axis specifically associates with clinical prognosis and is multifunctional in regulating cancer progression. Oncotarget 2018, 9, 17210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultana, R.; Kataki, A.C.; Borthakur, B.B.; Basumatary, T.K.; Bose, S. Imbalance in leptin-adiponectin levels and leptin receptor expression as chief contributors to triple-negative breast cancer progression in Northeast India. Gene 2017, 621, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Liu, Y.; Gong, C.; Ji, T.; Zhou, X.; Zhang, T.; Wan, D.; Xu, S.; Jin, P.; Yang, X.; et al. Targeting leptin as a therapeutic strategy against ovarian cancer peritoneal metastasis. Anti-cancer Agents Med. Chem. 2017, 17, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. The Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Cymbaluk, A.; Chudecka-Głaz, A.; Rzepka-Górska, I. Leptin levels in serum depending on Body Mass Index in patients with endometrial hyperplasia and cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 136, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Bain, G.H.; Collie-Duguid, E.; Murray, G.I.; Gilbert, F.J.; Denison, A.; McKiddie, F.; Ahearn, T.; Fleming, I.; Leeds, J.; Phull, P.; et al. Tumour expression of leptin is associated with chemotherapy resistance and therapy-independent prognosis in gastro-oesophageal adenocarcinomas. Br. J. Cancer 2014, 110, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.J.; Yang, Z.X.; Dong, Y.J.; Zhang, G.Y.; Sun, M.F.; An, X.K.; Zhang, S.L. Downregulation of leptin inhibits growth and induces apoptosis of lung cancer cells via the Notch and JAK/STAT3 signaling pathways. Biol. Open 2016, 5, 794–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, N.K.; Sharma, D.; Ding, X.; Lin, S.; Marra, F.; Merlin, D.; Anania, F.A. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in the leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007, 67, 2497–2507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manohar, S.; Leung, N. Cisplatin nephrotoxicity: A review of the literature. J. Nephrol. 2018, 31, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, P.; Tischkowitz, M. Genetics of gynaecological cancers. Best Pract Res. Clin. Obstet. Gynaecol. 2017, 42, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Dannenberg, A.J. The obese adipose tissue microenvironment in cancer development and progression. Nat. Rev. Endocrinol. 2019, 15, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Jochem, C.; Schlecht, I.; Leitzmann, M. Epidemiologic Relationship Between Obesity and Ovarian Cancer. In Focus on Gynecologic Malignancies; Springer: Cham, Switzerland, 2018; pp. 21–30. [Google Scholar]
- Garmendia, M.L.; Ruiz, P.; Uauy, R. Obesity, and cancer in Chile: Estimation of population attributable fractions. Rev. Med. Chil 2013, 141, 987–994. [Google Scholar]
- Shaw, E.; Farris, M.; McNeil, J.; Friedenreich, C. Obesity, and endometrial cancer. Recent Results Cancer Res. 2016, 208, 107–136. [Google Scholar] [PubMed]
- Luo, J.; Chlebowski, R.T.; Hendryx, M.; Rohan, T.; Wactawski-Wende, J.; Thomson, C.A.; Felix, A.S.; Chen, C.; Barrington, W.; Coday, M.; et al. Intentional weight loss and endometrial cancer risk. J. Clin. Oncol. 2017, 35, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Li, C.G.; Wang, Y.F.; Xu, L.H.; He, X.H.; Zeng, Q.Z.; Zeng, C.; Mai, F.; Hu, B.; Ouyang, D.Y. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis 2019, 24, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.T.; Lu, C.C.; Yang, J.S.; Chiang, J.H.; Li, T.C.; Ip, S.W.; Chung, J.G. Berberine induced apoptosis via promoting the expression of caspase-8,-9 and-3, apoptosis-inducing factor and endonuclease G in SCC-4 human tongue squamous carcinoma cancer cells. Anticancer Res. 2009, 29, 4063–4070. [Google Scholar] [PubMed]
- Song, H.; Sondak, V.K.; Barber, D.L.; Reid, T.J.; Lin, J. Modulation of Janus kinase 2 by cisplatin in cancer cells. Int. J. Oncol. 2004, 24, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Che, Q.; Xiao, X.; Liu, M.; Lu, Y.; Dong, X.; Liu, S. IL-6 promotes endometrial cancer cells invasion and migration through signal transducers and activators of transcription 3 signaling pathway. Pathol. Res. Pract. 2019, 215, 152392. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kwon, H.M.; Lim, H.J.; Hong, B.K.; Lee, J.Y.; Park, B.E.; Kim, H.S. Potential role of leptin in angiogenesis: Leptin induces endothelial cell proliferation and the expression of matrix metalloproteinases in vivo and in vitro. Exp. Mol. Med. 2001, 33, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Uchikova, E.; Uchikov, P.; Parahuleva, P. Obesity and endometrial carcinogenesis. Akush Ginekol (Sofiia) 2015, 54, 34–37. [Google Scholar] [PubMed]
- Daley-Brown, D.; Oprea-Ilies, G.M.; Lee, R.; Pattillo, R.; Gonzalez-Perez, R. Molecular cues on obesity signals, tumor markers, and endometrial cancer. Horm Mol. Biol. Clin. Investig. 2015, 21, 89–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, C.; Liu, Y.; Xiao, W.; Yin, J.; Wang, D.H.; Sheng, H. The role of ERK1/2 in leptin promoting the proliferation of human endometrial cancer cell line Ishikawa. Chin. J. Canc 2007, 26, 1211–1214. [Google Scholar]
- Liu, Y.; Lv, L.; Xiao, W.; Gong, C.; Yin, J.; Wang, D.; Sheng, H. Leptin activates STAT3 and ERK1/2 pathways and induces endometrial cancer cell proliferation. J. Huazhong U. Sci.-Med. 2011, 31, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Barbolosi, D.; Ciccolini, J.; Lacarelle, B.; Barlési, F.; André, N. Computational oncology—mathematical modelling of drug regimens for precision medicine. Nat. Rev. Clin. Oncol 2016, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Hebebrand, J.; Muller, T.D.; Holtkamp, K.; Herpertz-Dahlmann, B. The role of leptin in anorexia nervosa: Clinical implications. Mol. Psychiatry 2007, 12, 23–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, P. Anorexia-cachexia—The condition and its causes. Hospital Pharmacist 2007, 14, 249–253. [Google Scholar]
- Woo, S.M.; Choi, Y.K.; Kim, A.J.; Yun, Y.J.; Shin, Y.C.; Cho, S.G.; Ko, S.G. Sip-jeon-dea-bo-tang, a traditional herbal medicine, ameliorates cisplatin-induced anorexia via the activation of JAK1/STAT3-mediated leptin and IL-6 production in the fat tissue of mice. Mol. Med. Rep. 2016, 13, 2967–2972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, S.S.F.; Tsai, K.B.; Chung, Y.F.; Chan, T.F.; Yeh, Y.T.; Tsai, L.Y.; Su, J.H. Aberrant expression and the possible involvement of the leptin receptor in endometrial cancer. Gynecol. Oncol 2004, 92, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Matte, I.; Garde-Granger, P.; Bessette, P.; Piché, A. Serum CA125 and ascites leptin level ratio predicts baseline clinical resistance to first-line platinum-based treatment and poor prognosis in patients with high grade serous ovarian cancer. Am. J. Cancer Res. 2019, 9, 160. [Google Scholar] [PubMed]
- Grabarek, B.; Wcisło-Dziadecka, D.; Gola, J.; Kruszniewska-Rajs, C.; Brzezinska-Wcislo, L.; Zmarzly, N.; Mazurek, U. Changes in the expression profile of Jak/Stat signaling pathway genes and miRNAs regulating their expression under the adalimumab therapy. Curr. Pharm. Biotech. 2018, 19, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Dziobek, K.; Opławski, M.; Grabarek, B.O.; Zmarzły, N.; Tomala, B.; Halski, T.; Boroń, D. Changes in the Expression Profile of VEGF-A, VEGF-B, VEGFR-1, VEGFR-2 in Different Grades of Endometrial Cancer. Curr. Pharm. Biotech. 2019, 11, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Cao, F.L.; Li, N.; Gao, X.; Su, X.; Jiang, X. Leptin induces epithelial-to-mesenchymal transition via activation of the ERK signaling pathway in lung cancer cells. Oncol. Lett. 2018, 16, 4782–4788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabarek, B.O.; Wcisło-Dziadecka, D.; Sanakiewicz, A.; Kruszniewska-Rajs, C.; Gola, J. Evaluation of changes in the expression pattern of oxidative stress genes under the influence of adalimumab. Derm. Ther. 2019, 32, e13141. [Google Scholar] [CrossRef] [PubMed]






| The Oncentration of Cisplatin (µM) | Time (h) | Microarray Data | RTqPCR | ELISA Assay Leptin (pg/mL) | |
|---|---|---|---|---|---|
| FC | FC | Mean | Standard Deviation | ||
| Control (untreated cells) | - | - | 921.977 | 0.345 | |
| 12 | −4.96 a | −5.12 a | 899.553 | 0.518 | |
| 2.5 | 24 | −7.89 a | −7.99 a,b | 820.800 a | 0.381 |
| 48 | −9.01 a | −8.54 a,c,d | 800.380 a,d | 0.532 | |
| 12 | −11.69 a | −12.03 a | 704.453 a | 0.402 | |
| 5 | 24 | −13.55 a | −12.98 a,b | 585.997 a,b | 1.721 |
| 48 | −17.01 a | −18.44 a,c,d | 401.317 a,c,d | 0.938 | |
| 12 | −18.58 a | −18.99 a | 300.209 a | 0.180 | |
| 10 | 24 | −19.03 a | −19.30 a,b | 205.909 a,b | 0.992 |
| 48 | −21.48 a | −21.78 a,c,d | 253.673 a,c | 1.773 | |
| Exposure Time Cells with 5 µM Cisplatin (h) | mRNA | ID | Microarray Data Fold Change | RTqPCR Data Fold Change |
|---|---|---|---|---|
| 12 | LEPROT | 202377_at 202378_s_at | −5.82 * −5.36 * | −6.18 * |
| LEPROTL1 | 202594_at 202595_s_at | −6.12 * −6.58 * | −6.77 * | |
| LEPR | 209894_at 209959_at 211167_s_at 211354_s_at 211355_x_at 211356_x_at | −11.02 * −12.22 * −13.36 * −11.58 * −13.44 * −14.02 * | −12.09 * | |
| 24 | LEPROT | 202377_at 202378_s_at | −5.96 * −6.88 * | −7.04 * |
| LEPROTL1 | 202594_at 202595_s_at | −7.55 * −8.02 * | −7.69 * | |
| LEPR | 209894_at 209959_at 211167_s_at 211354_s_at 211355_x_at 211356_x_at | −14.33 * −14.02 * −11.02 * −12.08 * −14.01 * −14.77 * | −13.03 * | |
| 48 | LEPROT | 202377_at 202378_s_at | −8.11 −8.60 | −9.58 |
| LEPROTL1 | 202594_at 202595_s_at | −9.02 −9.03 | −9.14 | |
| LEPR | 209894_at 209959_at 211167_s_at 211354_s_at 211355_x_at 211356_x_at | −15.12 −15.73 −12.11 −13.08 −13.99 −13.02 | −16.25 |
| Group | Time (h) | mRNA | ID | Microarray Data Fold Change | RTqPCR Data Fold Change | ELISA (pg/mL) |
|---|---|---|---|---|---|---|
| Control | - | - | 489 | |||
| Cells+ 5 µM cisplatin | 12 | JAK2 | 205841_at | −1.74 a | −1.79 a | 208 a |
| 24 | −1.71 a | −1.41 a | 216 a | |||
| 48 | −1.81 a | −1.77 a | 298 a | |||
| Cells+ 10 ng/mL of leptin | 12 | +3.02 a | +3.09 a | 1111 a | ||
| 24 | +3.14 a | +3.11 a | 1196 a | |||
| 48 | +2.98 a | +3.04 a | 1120 a | |||
| Cells+ 20 ng/mL of leptin | 12 | +2.74 a | +2.29 a | 987 a | ||
| 24 | +3.58 a | +3.77 a | 1417 a | |||
| 48 | +3.47 a,c | +3.68 a | 1402 a | |||
| Cells+ 40 ng/mL of leptin | 12 | +4.02 a | +4.14 a | 1854 a | ||
| 24 | +4.14 a | +4.21 a | 1902 a | |||
| 48 | +4.84 a,c | +4.77 a | 1869 a | |||
| Cells+ 5 µM cisplatin | 12 | STAT3 | 208991_at | −2.03 a | −2.36 a | 499 |
| 24 | −1.98 a | −2.01 a | 454 | |||
| 48 | −1.47 a | −1.51 a | 450 | |||
| Cells+ 10 ng/mL of leptin | 12 | +2.07 a | +2.14 a | 850 a | ||
| 24 | +2.11 a | +2.19 a | 896 a | |||
| 48 | +2.36 a | +2.41 a | 884 a | |||
| Cells+ 20 ng/mL of leptin | 12 | +3.04 a | +3.09 a | 914 a | ||
| 24 | +3.89 a | +3.74 a | 948 a | |||
| 48 | +4.01 a,c | +4.15 a | 1100 a | |||
| Cells+ 40 ng/mL of leptin | 12 | +4.87 a,b | +4.81 a | 1126 a | ||
| 24 | +4.22 a | +4.23 a | 1161 a | |||
| 48 | +4.74 a | +4.69 a | 1198 a |
| mRNA | Probe Set ID on the Microarray Plate | Sequence |
| LEP | 207092_at | Forward GAAGACCACATCCACACACG Reverse AGCTCAGCCAGACCCATCTA |
| LEPROT | 202377_at 202378_s_at | Forward GCTTGGAGAGGCAGATAACG Reverse AATGTCCTGGGTCCAGAGTG |
| LEPROTL1 | 202594_at 202595_s_at | Forward TGCAATGTGGGAAGAAATGA Reverse AAGGAGGAAGCAGAGGAAGG |
| LEPR | 209894_at 209959_at 211167_s_at 211354_s_at 211355_x_at 211356_x_at | Forward ACAGTCCCTTTGTGGGTCAG Reverse TATCCGAGCTCCAGCGTACT |
| JAK2 | 205841_at | Forward AGTAAAAGTCCACCAGCGGA Reverse AGGAGGGGCGTTGATTTACA |
| STAT3 | 208991_at | Forward AAAGCAGCAAAGAAGGAGGC Reverse CTGGCCGACAATACTTTCCG |
| ACTB | - | Forward TCACCCACACTGTGCCCATCTACGA Reverse CAGCGGAACCGCTCATTGCCAATGG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbruś, D.; Kiełbasiński, R.; Grabarek, B.O.; Boroń, D. Evaluation of the Impact of Cisplatin on Variances in the Expression Pattern of Leptin-Related Genes in Endometrial Cancer Cells. Int. J. Mol. Sci. 2020, 21, 4135. https://doi.org/10.3390/ijms21114135
Dąbruś D, Kiełbasiński R, Grabarek BO, Boroń D. Evaluation of the Impact of Cisplatin on Variances in the Expression Pattern of Leptin-Related Genes in Endometrial Cancer Cells. International Journal of Molecular Sciences. 2020; 21(11):4135. https://doi.org/10.3390/ijms21114135
Chicago/Turabian StyleDąbruś, Dariusz, Robert Kiełbasiński, Beniamin Oskar Grabarek, and Dariusz Boroń. 2020. "Evaluation of the Impact of Cisplatin on Variances in the Expression Pattern of Leptin-Related Genes in Endometrial Cancer Cells" International Journal of Molecular Sciences 21, no. 11: 4135. https://doi.org/10.3390/ijms21114135
APA StyleDąbruś, D., Kiełbasiński, R., Grabarek, B. O., & Boroń, D. (2020). Evaluation of the Impact of Cisplatin on Variances in the Expression Pattern of Leptin-Related Genes in Endometrial Cancer Cells. International Journal of Molecular Sciences, 21(11), 4135. https://doi.org/10.3390/ijms21114135