The Impact of Photoperiod on the Leptin Sensitivity and Course of Inflammation in the Anterior Pituitary
Abstract
:1. Introduction
2. Results
2.1. Gene Expression of Interleukin-1β and Its Receptors
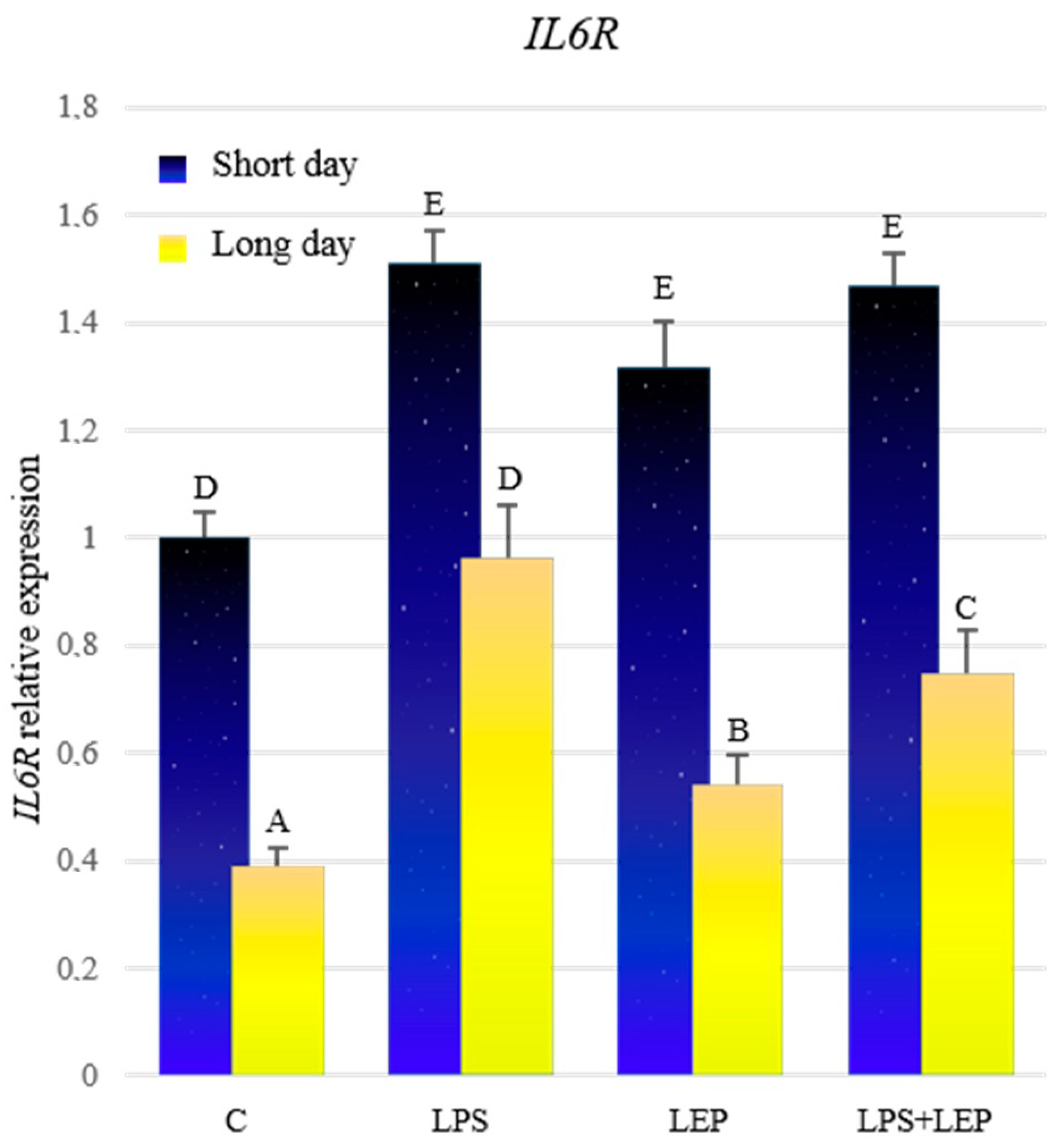
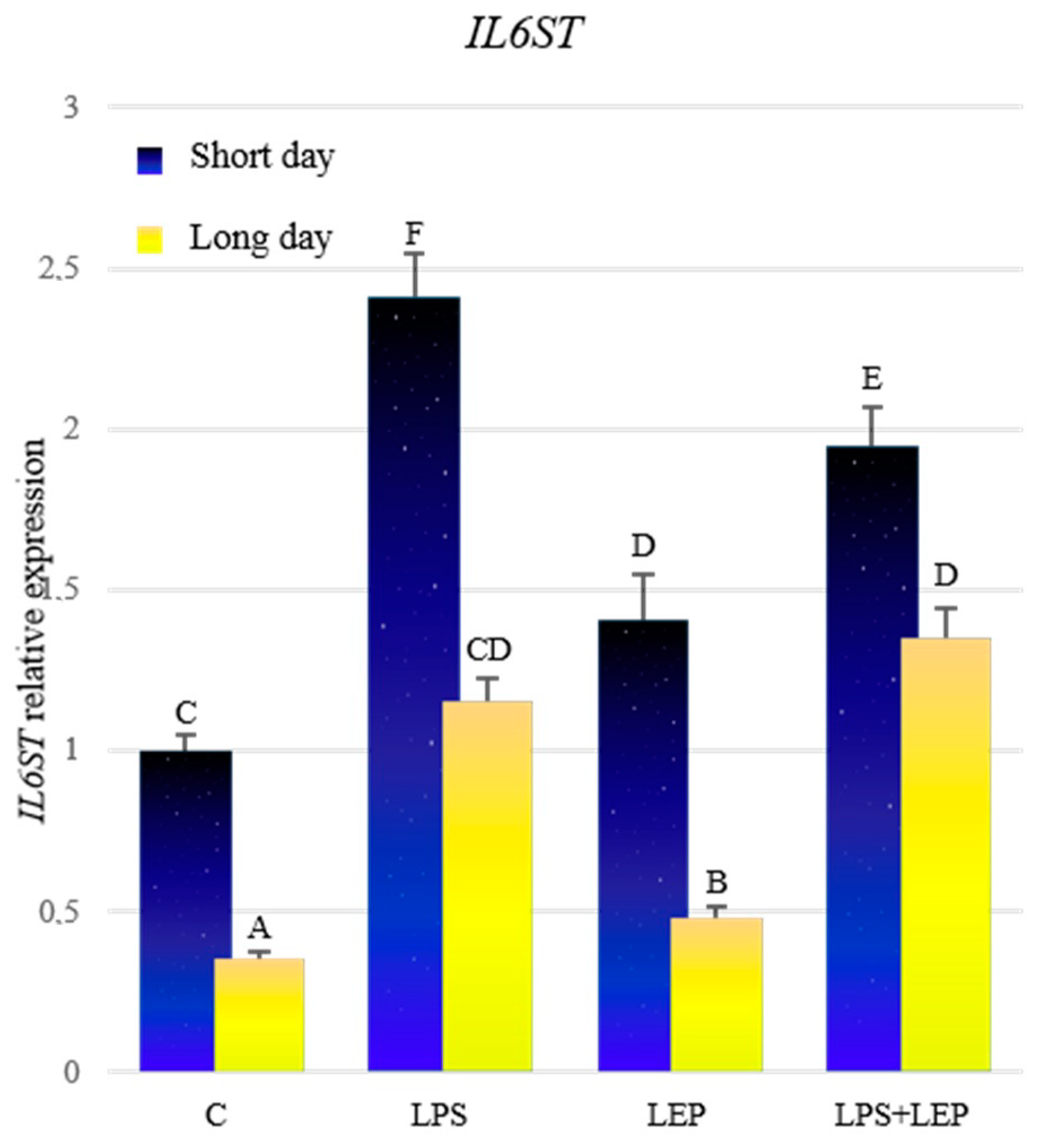
2.2. Gene Expression of Interleukin-6, Its Receptor and Signal Transducer
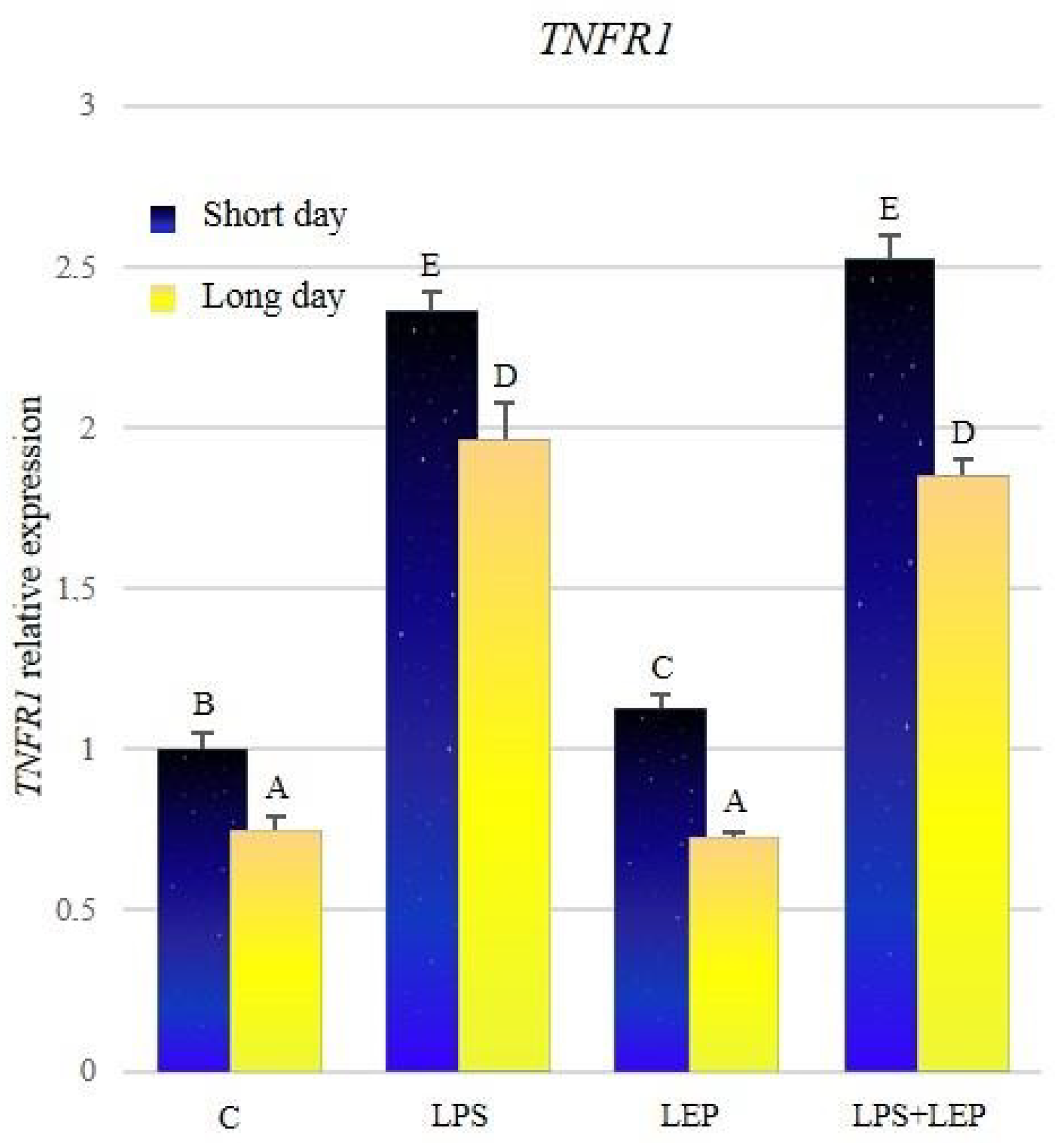
2.3. Gene Expression of Tumor Necrosis Factor-α and Its Receptors
3. Discussion
3.1. Leptin Effect on Cytokines Induction
3.2. The Influence of Leptin on Proinflammatory Cytokines during Inflammation
4. Material and Methods
4.1. Animals
- control—treated with saline in the volume equal to the experimental groups (0.9% w/v NaCl) (Baxter, Deerfield, IL, USA)
- LPS—intravenously injected with lipopolysaccharide (LPS) from Escherichia coli (400 ng/kg body weight); (Sigma-Aldrich, St. Louis, MO, USA)
- LEP—intravenously injected with ovine recombinant leptin (20 µg/kg body weight at a dose derived from experiments conducted on growing beef heifers [110]); [Protein Laboratories Rehovot (PLR) Ltd., Rehovot, Israel]
- LPS+LEP—intravenously injected with both LPS and leptin in the doses as mentioned above; the leptin injection was done 30 min after LPS injection.
4.2. Real-Time RT-PCR
4.3. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Al Maskari, M.Y.; Alnaqdy, A.A. Correlation between Serum Leptin Levels, Body Mass Index and Obesity in Omanis. Sultan Qaboos Univ. Med. J. 2006, 6, 27–31. [Google Scholar] [PubMed]
- Popovic, V.; Damjanovic, S.; Dieguez, C.; Casanueva, F.F. Leptin and the pituitary. Pituitary 2001, 4, 7–14. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Jin, L.; Tsumanuma, I.; Vidal, S.; Kovacs, K.; Horvath, E.; Scheithauer, B.W.; Couce, M.E.; Burguera, B. Leptin and Leptin Receptor in Anterior Pituitary Function. Pituitary 2001, 4, 33–47. [Google Scholar] [CrossRef]
- Yu, W.H.; Kimura, M.; Walczewska, A.; Karanth, S.; McCann, S.M. Role of leptin in hypothalamic-pituitary function. Proc. Natl. Acad. Sci. USA 1997, 94, 1023–1028. [Google Scholar] [CrossRef] [Green Version]
- Swerdloff, R.S.; Batt, R.A.; Bray, G.A. Reproductive hormonal function in the genetically obese (ob/ob) mouse. Endocrinology 1976, 98, 1359–1364. [Google Scholar] [CrossRef]
- Chehab, F.F.; Lim, M.E.; Lu, R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat. Genet. 1996, 12, 318–320. [Google Scholar] [CrossRef]
- Cioffi, J.A.; Shafer, A.W.; Zupancic, T.J.; Smith-Gbur, J.; Mikhail, A.; Platika, D.; Snodgrass, H.R. Novel B219/OB receptor isoforms: Possible role of leptin in hematopoiesis and reproduction. Nat. Med. 1996, 2, 585–589. [Google Scholar] [CrossRef]
- Barash, I.A.; Cheung, C.C.; Weigle, D.S.; Ren, H.; Kabigting, E.B.; Kuijper, J.L.; Clifton, D.K.; Steiner, R.A. Leptin is a metabolic signal to the reproductive system. Endocrinology 1996, 137, 3144–3147. [Google Scholar] [CrossRef] [Green Version]
- Chehab, F.F. Early Onset of Reproductive Function in Normal Female Mice Treated with Leptin. Science 1997, 275, 88–90. [Google Scholar] [CrossRef]
- Clément, K.; Vaisse, C.; Lahlou, N.; Cabrol, S.; Pelloux, V.; Cassuto, D.; Gourmelen, M.; Dina, C.; Chambaz, J.; Lacorte, J.-M.; et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 1998, 392, 398–401. [Google Scholar] [CrossRef]
- Childs, G.V.; Akhter, N.; Haney, A.; Syed, M.; Odle, A.; Cozart, M.; Brodrick, Z.; Gaddy, D.; Suva, L.J.; Akel, N.; et al. The Somatotrope as a Metabolic Sensor: Deletion of Leptin Receptors Causes Obesity. Endocrinology 2011, 152, 69–81. [Google Scholar] [CrossRef]
- Jin, L.; Burguera, B.G.; Couce, M.E.; Scheithauer, B.W.; Lamsan, J.; Eberhardt, N.L.; Kulig, E.; Lloyd, R.V. Leptin and Leptin Receptor Expression in Normal and Neoplastic Human Pituitary: Evidence of a Regulatory Role for Leptin on Pituitary Cell Proliferation 1. J. Clin. Endocrinol. Metab. 1999, 84, 2903–2911. [Google Scholar] [PubMed]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum Immunoreactive-Leptin Concentrations in Normal-Weight and Obese Humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Caro, J.F.; Kolaczynski, J.W.; Nyce, M.R.; Ohannesian, J.P.; Opentanova, I.; Goldman, W.H.; Lynn, R.B.; Zhang, P.-L.; Sinha, M.K.; Considine, R.V. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: A possible mechanism for leptin resistance. The Lancet 1996, 348, 159–161. [Google Scholar] [CrossRef]
- Szczesna, M.; Zieba, D.A. Phenomenon of leptin resistance in seasonal animals: The failure of leptin action in the brain. Domest. Anim. Endocrinol. 2015, 52, 60–70. [Google Scholar] [CrossRef]
- Zieba, D.A.; Klocek, B.; Williams, G.L.; Romanowicz, K.; Boliglowa, L.; Wozniak, M. In vitro evidence that leptin suppresses melatonin secretion during long days and stimulates its secretion during short days in seasonal breeding ewes. Domest. Anim. Endocrinol. 2007, 33, 358–365. [Google Scholar] [CrossRef]
- Szczesna, M.; Zieba, D.A.; Klocek-Gorka, B.; Misztal, T.; Stepien, E. Seasonal effects of central leptin infusion and prolactin treatment on pituitary SOCS-3 gene expression in ewes. J. Endocrinol. 2011, 208, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Williams, L.M.; Helliwell, R.J.A. Melatonin and seasonality in the sheep. Anim. Reprod. Sci. 1993, 33, 159–182. [Google Scholar] [CrossRef]
- Misztal, T.; Romanowicz, K.; Barcikowski, B. Seasonal changes of melatonin secretionin relation to the reproductive cycle in sheep. J. Anim. Feed Sci. 1996, 5, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Król, K.; Tomaszewska-Zaremba, D.; Herman, A. Photoperiod-dependent effect of inflammation on nocturnal gene expression of proinflammatory cytokines and their receptors in pars tuberalis of ewe. J. Anim. Feed Sci. 2016, 25, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Buonfiglio, D.; Parthimos, R.; Dantas, R.; Cerqueira Silva, R.; Gomes, G.; Andrade-Silva, J.; Ramos-Lobo, A.; Amaral, F.G.; Matos, R.; Sinésio, J.; et al. Melatonin Absence Leads to Long-Term Leptin Resistance and Overweight in Rats. Front. Endocrinol. 2018, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.J.; Drazen, D.L. Melatonin Mediates Seasonal Changes in Immune Function. Ann. N. Y. Acad. Sci. 2006, 917, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, X.; Ma, S.; Xu, Y.; Xu, Y.; Guo, X.; Li, D. Association of Melatonin Production with Seasonal Changes, Low Temperature, and Immuno-Responses in Hamsters. Molecules 2018, 23, 703. [Google Scholar] [CrossRef] [Green Version]
- Marie, M.; Findlay, P.; Thomas, L.; Adam, C. Daily patterns of plasma leptin in sheep: Effects of photoperiod and food intake. J. Endocrinol. 2001, 170, 277–286. [Google Scholar] [CrossRef] [Green Version]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef]
- Chilliard, Y.; Delavaud, C.; Bonnet, M. Leptin expression in ruminants: Nutritional and physiological regulations in relation with energy metabolism. Domest. Anim. Endocrinol. 2005, 29, 3–22. [Google Scholar] [CrossRef]
- Zieba, D.A.; Szczesna, M.; Klocek-Gorka, B.; Molik, E.; Misztal, T.; Williams, G.L.; Romanowicz, K.; Stepien, E.; Keisler, D.H.; Murawski, M. Seasonal effects of central leptin infusion on secretion of melatonin and prolactin and on SOCS-3 gene expression in ewes. J. Endocrinol. 2008, 198, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Hardeland, R. Melatonin and inflammation-Story of a double-edged blade. J. Pineal Res. 2018, 65, e12525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Li, N.; Bo, L.; Xu, Z. Melatonin and Hypothalamic-Pituitary-Gonadal Axis. Curr. Med. Chem. 2013, 20, 2017–2031. [Google Scholar] [CrossRef]
- Bojková, B.; Marková, M.; Ahlersová, E.; Ahlers, I.; Adámeková, E.; Kubatka, P.; Kassayová, M. Metabolic Effects of Prolonged Melatonin Administration and Short-Term Fasting in Laboratory Rats. Acta Vet. Brno 2006, 75, 21–32. [Google Scholar] [CrossRef]
- Marková, M.; Adámeková, E.; Kubatka, P.; Bojková, B.; Ahlersová, E.; Ahlers, I. Effect of Prolonged Melatonin Application on Metabolic Parameters and Organ Weights in Young Male and Female Sprague-Dawley rats. Acta Vet. Brno 2003, 72, 163–173. [Google Scholar] [CrossRef] [Green Version]
- Karamitri, A.; Jockers, R. Melatonin in type 2 diabetes mellitus and obesity. Nat. Rev. Endocrinol. 2019, 15, 105–125. [Google Scholar] [CrossRef]
- Opatrilova, R.; Caprnda, M.; Kubatka, P.; Valentova, V.; Uramova, S.; Nosal, V.; Gaspar, L.; Zachar, L.; Mozos, I.; Petrovic, D.; et al. Adipokines in neurovascular diseases. Biomed. Pharmacother. 2018, 98, 424–432. [Google Scholar] [CrossRef]
- Kubatka, P.; Zubor, P.; Busselberg, D.; Kwon, T.K.; Adamek, M.; Petrovic, D.; Opatrilova, R.; Gazdikova, K.; Caprnda, M.; Rodrigo, L.; et al. Melatonin and breast cancer: Evidences from preclinical and human studies. Crit. Rev. Oncol. Hematol. 2018, 122, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Gunata, M.; Parlakpinar, H.; Acet, H.A. Melatonin: A review of its potential functions and effects on neurological diseases. Rev. Neurol. 2020, 176, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Kirsz, K.; Szczesna, M.; Molik, E.; Misztal, T.; Wojtowicz, A.K.; Zieba, D.A. Seasonal changes in the interactions among leptin, ghrelin, and orexin in sheep1. J. Anim. Sci. 2012, 90, 2524–2531. [Google Scholar] [CrossRef]
- Banks, W.A. Role of the blood-brain barrier in the evolution of feeding and cognition: The BBB and feeding. Ann. N.Y. Acad. Sci. 2012, 1264, 13–19. [Google Scholar] [CrossRef]
- Gruzdeva, O.; Borodkina, D.; Uchasova, E.; Dyleva, Y.; Barbarash, O. Leptin resistance: Underlying mechanisms and diagnosis. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Rumińska, M.; Witkowska-Sędek, E.; Majcher, A.; Pyrżak, B. Thyroid Function in Obese Children and Adolescents and Its Association with Anthropometric and Metabolic Parameters. In Prospect in Pediatric Diseases Medicine; Pokorski, M., Ed.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2016; Volume 912, pp. 33–41. ISBN 978-3-319-35103-2. [Google Scholar]
- Kosior-Korzecka, U.; Wojcik, M.; Longo, V.; Puzio, I.; Nowakiewicz, A.; Patkowski, K.; Gregula-Kania, M. Changes in growth hormone secretion and leptin receptor mRNA expression under the influence of leptin and adrenocorticotropin in pituitary cells of early weaned ewe lambs. J. Physiol. Pharmacol. 2019, 70. [Google Scholar] [CrossRef]
- Aschbacher, K.; Rodriguez-Fernandez, M.; van Wietmarschen, H.; Tomiyama, A.J.; Jain, S.; Epel, E.; Doyle, F.J.; van der Greef, J. The hypothalamic–pituitary–adrenal–leptin axis and metabolic health: A systems approach to resilience, robustness and control. Interface Focus 2014, 4, 20140020. [Google Scholar] [CrossRef] [Green Version]
- Tsatsanis, C.; Dermitzaki, E.; Avgoustinaki, P.; Malliaraki, N.; Mytaras, V.; Margioris, A. The impact of adipose tissue-derived factors on the hypothalamic-pituitary-gonadal (HPG) axis. HORMONES 2015, 14, 549–562. [Google Scholar] [CrossRef] [Green Version]
- Accorsi, P.A.; Munno, A.; Gamberoni, M.; Viggiani, R.; De Ambrogi, M.; Tamanini, C.; Seren, E. Role of Leptin on Growth Hormone and Prolactin Secretion by Bovine Pituitary Explants. J. Dairy Sci. 2007, 90, 1683–1691. [Google Scholar] [CrossRef] [Green Version]
- Entrican, G.; Wattegedera, S.R.; Griffiths, D.J. Exploiting ovine immunology to improve the relevance of biomedical models. Mol. Immunol. 2015, 66, 68–77. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Igaz, P. Cytokines in diseases of the endocrine system. Cell Biol. Int. 2000, 24, 663–668. [Google Scholar] [CrossRef]
- Lord, G.M. Leptin as a Proinflammatory Cytokine. In Contributions to Nephrology; Wolf, G., Ed.; Karger: Basel, Switzerland, 2006; pp. 151–164. ISBN 978-3-8055-8164-6. [Google Scholar]
- Otero, M.; Lago, R.; Lago, F.; Casanueva, F.F.; Dieguez, C.; Gómez-Reino, J.J.; Gualillo, O. Leptin, from fat to inflammation: Old questions and new insights. FEBS Lett. 2005, 579, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.S.; Qasim, A.; Reilly, M.P. Leptin Resistance. J. Am. Coll. Cardiol. 2008, 52, 1201–1210. [Google Scholar] [CrossRef] [Green Version]
- La Cava, A.; Alviggi, C.; Matarese, G. Unraveling the multiple roles of leptin in inflammation and autoimmunity. J. Mol. Med. 2004, 82, 4–11. [Google Scholar] [CrossRef]
- La Cava, A. Proinflammatory Activities of Leptin in Non-Autoimmune Conditions. Inflamm. Allergy-Drug Targets 2012, 11, 298–302. [Google Scholar] [CrossRef]
- Cava, A.L.; Matarese, G. The weight of leptin in immunity. Nat. Rev. Immunol. 2004, 4, 371–379. [Google Scholar] [CrossRef]
- Kiguchi, N.; Maeda, T.; Kobayashi, Y.; Fukazawa, Y.; Kishioka, S. Leptin enhances CC-chemokine ligand expression in cultured murine macrophage. Biochem. Biophys. Res. Commun. 2009, 384, 311–315. [Google Scholar] [CrossRef]
- Jitprasertwong, P.; Jaedicke, K.M.; Nile, C.J.; Preshaw, P.M.; Taylor, J.J. Leptin enhances the secretion of interleukin (IL)-18, but not IL-1β, from human monocytes via activation of caspase-1. Cytokine 2014, 65, 222–230. [Google Scholar] [CrossRef]
- Hosoi, T.; Okuma, Y.; Nomura, Y. Leptin regulates interleukin-1β expression in the brain via the STAT3-independent mechanisms. Brain Res. 2002, 949, 139–146. [Google Scholar] [CrossRef]
- Faggioni, R.; Fantuzzi, G.; Fuller, J.; Dinarello, C.A.; Feingold, K.R.; Grunfeld, C. IL-1β mediates leptin induction during inflammation. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1998, 274, R204–R208. [Google Scholar] [CrossRef]
- Finck, B.N.; Johnson, R.W. Tumor necrosis factor (TNF)-α induces leptin production through the p55 TNF receptor. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2000, 278, R537–R543. [Google Scholar] [CrossRef]
- Padidar, S.; Farquharson, A.J.; Williams, L.M.; Kelaiditi, E.; Hoggard, N.; Arthur, J.R.; Drew, J.E. Leptin up-regulates pro-inflammatory cytokines in discrete cells within mouse colon. J. Cell. Physiol. 2011, 226, 2123–2130. [Google Scholar] [CrossRef]
- Herman, A.P.; Krawczyńska, A.; Bochenek, J.; Dobek, E.; Herman, A.; Tomaszewska-Zaremba, D. LPS-Induced Inflammation Potentiates the IL-1-Mediated Reduction of LH Secretion from the Anterior Pituitary Explants. Clin. Dev. Immunol. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Gaillard, R.C.; Turnill, D.; Sappino, P.; Muller, A.F. Tumor Necrosis Factor a Inhibits the Hormonal Response of the Pituitary Gland to Hypothalamic Releasing Factors*. Endocrinology 1990, 127, 101–106. [Google Scholar] [CrossRef]
- Ray, D.; Melmed, S. Pituitary Cytokine and Growth Factor Expression and Action. Endocr. Rev. 1997, 18, 206–228. [Google Scholar] [CrossRef]
- Renner, U.; Pagotto, U.; Arzt, E.; Stalla, G.K. Autocrine and paracrine roles of polypeptide growth factors, cytokines and vasogenic substances in normal and tumorous pituitary function and growth: A review. Eur. J. Endocrinol. 1996, 135, 515–532. [Google Scholar] [CrossRef]
- Renner, U.; Gloddek, J.; Pereda, M.P.; Arzt, E.; Stalla, G.K. Regulation and role of intrapituitary IL-6 production by folliculostellate cells. Domest. Anim. Endocrinol. 1998, 15, 353–362. [Google Scholar] [CrossRef]
- Wassen, F.W.; Moerings, E.P.; Van Toor, H.; De Vrey, E.A.; Hennemann, G.; Everts, M.E. Effects of interleukin-1 beta on thyrotropin secretion and thyroid hormone uptake in cultured rat anterior pituitary cells. Endocrinology 1996, 137, 1591–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, F.-Y.; Deng, J.-Y.; Shi, Y.-F. Stimulatory Effect of Interleukin-1β on Growth Hormone Gene Expression and Growth Hormone Release from Rat GH3 Cells. Neuroendocrinology 2005, 81, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, N.J. Cytokines in the Nervous System; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-1-4615-9695-0. [Google Scholar]
- Rachal Pugh, C.; Fleshner, M.; Watkins, L.R.; Maier, S.F.; Rudy, J.W. The immune system and memory consolidation: A role for the cytokine IL-1beta. Neurosci. Biobehav. Rev. 2001, 25, 29–41. [Google Scholar] [CrossRef]
- Dinarello, C.A. The interleukin-1 family: 10 years of discovery. FASEB J. 1994, 8, 1314–1325. [Google Scholar] [CrossRef] [Green Version]
- Peters, V.A.; Joesting, J.J.; Freund, G.G. IL-1 receptor 2 (IL-1R2) and its role in immune regulation. Brain. Behav. Immun. 2013, 32, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Krawczyńska, A.; Herman, A.P.; Antushevich, H.; Bochenek, J.; Wojtulewicz, K.; Zięba, D.A. The Influence of Photoperiod on the Action of Exogenous Leptin on Gene Expression of Proinflammatory Cytokines and Their Receptors in the Thoracic Perivascular Adipose Tissue (PVAT) in Ewes. Mediators Inflamm. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Opal, S.M. Endotoxins and Other Sepsis Triggers. In Contributions to Nephrology; Ronco, C., Piccinni, P., Rosner, M.H., Eds.; Karger: Basel, Switzerland, 2010; Volume 167, pp. 14–24. ISBN 978-3-8055-9484-4. [Google Scholar]
- Brade, H. (Ed.) Endotoxin in Health and Disease; Marcel Dekker: New York, NY, USA, 1999; ISBN 978-0-8247-1944-9. [Google Scholar]
- Brandenburg, K.; Wiese, A. Endotoxins: Relationships between structure, function, and activity. Curr. Top. Med. Chem. 2004, 4, 1127–1146. [Google Scholar] [CrossRef]
- Copeland, S.; Warren, H.S.; Lowry, S.F.; Calvano, S.E.; Remick, D. The Inflammation and the Host Response to Injury Investigators Acute Inflammatory Response to Endotoxin in Mice and Humans. Clin. Vaccine Immunol. 2005, 12, 60–67. [Google Scholar]
- Park, B.S.; Lee, J.-O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef] [Green Version]
- Raetz, C.R.H.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Ikejima, T.; Warner, S.J.; Orencole, S.F.; Lonnemann, G.; Cannon, J.G.; Libby, P. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J. Immunol. Baltim. Md 1950 1987, 139, 1902–1910. [Google Scholar]
- Dolasia, K.; Bisht, M.K.; Pradhan, G.; Udgata, A.; Mukhopadhyay, S. TLRs/NLRs: Shaping the landscape of host immunity. Int. Rev. Immunol. 2018, 37, 3–19. [Google Scholar] [CrossRef]
- Khameneh, H.J.; Ho, A.W.S.; Laudisi, F.; Derks, H.; Kandasamy, M.; Sivasankar, B.; Teng, G.G.; Mortellaro, A. C5a Regulates IL-1β Production and Leukocyte Recruitment in a Murine Model of Monosodium Urate Crystal-Induced Peritonitis. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Madej, M.P.; Töpfer, E.; Boraschi, D.; Italiani, P. Different Regulation of Interleukin-1 Production and Activity in Monocytes and Macrophages: Innate Memory as an Endogenous Mechanism of IL-1 Inhibition. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef]
- Krawczyńska, A.; Antushevich, H.; Bochenek, J.; Wojtulewicz, K.; Pawlina, B.; Herman, A.; Zięba, D. Photoperiodic conditions as a factor modulating leptin influence on pro-inflammatory cytokines and their receptors gene expression in ewe’s aorta. J. Anim. Feed Sci. 2019, 28, 128–137. [Google Scholar] [CrossRef]
- Herman, A.; Misztal, T.; Herman, A. Dorota Tomaszewska - Zaremba Expression of Interleukin (IL)-1β and IL-1 Receptors Genes in the Hypothalamus of Anoestrous Ewes after Lipopolysaccharide Treatment: Interleukin -1β in the Hypothalamus after LPS Treatment. Reprod. Domest. Anim. 2010, 45, e426–e433. [Google Scholar] [CrossRef]
- Chesnokova, V.; Kariagina, A.; Melmed, S. Opposing effects of pituitary leukemia inhibitory factor and SOCS-3 on the ACTH axis response to inflammation. Am. J. Physiol.-Endocrinol. Metab. 2002, 282, E1110–E1118. [Google Scholar] [CrossRef] [Green Version]
- Mihara, M.; Hashizume, M.; Yoshida, H.; Suzuki, M.; Shiina, M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin. Sci. 2012, 122, 143–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wójcik, M.; Krawczyńska, A.; Antushevich, H.; Herman, A. Post-Receptor Inhibitors of the GHR-JAK2-STAT Pathway in the Growth Hormone Signal Transduction. Int. J. Mol. Sci. 2018, 19, 1843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamimura, D.; Ishihara, K.; Hirano, T. IL-6 signal transduction and its physiological roles: The signal orchestration model. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2004; Volume 149, pp. 1–38. ISBN 978-3-540-20213-4. [Google Scholar]
- Nyati, K.K.; Masuda, K.; Zaman, M.M.-U.; Dubey, P.K.; Millrine, D.; Chalise, J.P.; Higa, M.; Li, S.; Standley, D.M.; Saito, K.; et al. TLR4-induced NF-κB and MAPK signaling regulate the IL-6 mRNA stabilizing protein Arid5a. Nucleic Acids Res. 2017, 45, 2687–2703. [Google Scholar] [CrossRef]
- Sedger, L.M.; McDermott, M.F. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants – past, present and future. Cytokine Growth Factor Rev. 2014, 25, 453–472. [Google Scholar] [CrossRef] [Green Version]
- Shalaby, M.R.; Waage, A.; Aarden, L.; Espevik, T. Endotoxin, tumor necrosis factor-α and interleukin 1 induce interleukin 6 production in vivo. Clin. Immunol. Immunopathol. 1989, 53, 488–498. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Lin, J.X.; Yip, Y.K.; Vilcek, J. Enhancement of cAMP levels and of protein kinase activity by tumor necrosis factor and interleukin 1 in human fibroblasts: Role in the induction of interleukin 6. Proc. Natl. Acad. Sci. USA 1988, 85, 6802–6805. [Google Scholar] [CrossRef] [Green Version]
- Tomaszewska-Zaremba, D.; Haziak, K.; Tomczyk, M.; Herman, A.P. Inflammation and LPS-Binding Protein Enable the Stimulatory Effect of Endotoxin on Prolactin Secretion in the Ovine Anterior Pituitary: Ex Vivo Study. Mediators Inflamm. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Herman, A.; Skipor, J.; Krawczyńska, A.; Bochenek, J.; Wojtulewicz, K.; Pawlina, B.; Antushevich, H.; Herman, A.; Tomaszewska-Zaremba, D. Effect of Central Injection of Neostigmine on the Bacterial Endotoxin Induced Suppression of GnRH/LH Secretion in Ewes during the Follicular Phase of the Estrous Cycle. Int. J. Mol. Sci. 2019, 20, 4598. [Google Scholar] [CrossRef] [Green Version]
- Herman, A.P.; Bochenek, J.; Król, K.; Krawczyńska, A.; Antushevich, H.; Pawlina, B.; Herman, A.; Romanowicz, K.; Tomaszewska-Zaremba, D. Central Interleukin-1 β Suppresses the Nocturnal Secretion of Melatonin. Mediators Inflamm. 2016, 2016, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Herman, A.P.; Tomaszewska-Zaremba, D.; Kowalewska, M.; Szczepkowska, A.; Oleszkiewicz, M.; Krawczyńska, A.; Wójcik, M.; Antushevich, H.; Skipor, J. Neostigmine Attenuates Proinflammatory Cytokine Expression in Preoptic Area but Not Choroid Plexus during Lipopolysaccharide-Induced Systemic Inflammation. Mediators Inflamm. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Herman, A.P.; Krawczyńska, A.; Bochenek, J.; Antushevich, H.; Herman, A.; Tomaszewska-Zaremba, D. Peripheral Injection of SB203580 Inhibits the Inflammatory-Dependent Synthesis of Proinflammatory Cytokines in the Hypothalamus. BioMed Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Tartaglia, L.A.; Rothe, M.; Hu, Y.-F.; Goeddel, D.V. Tumor necrosis factor’s cytotoxic activity is signaled by the p55 TNF receptor. Cell 1993, 73, 213–216. [Google Scholar] [CrossRef]
- McCoy, M.K.; Tansey, M.G. TNF signaling inhibition in the CNS: Implications for normal brain function and neurodegenerative disease. J. Neuroinflammation 2008, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Gehr, G.; Gentz, R.; Brockhaus, M.; Loetscher, H.; Lesslauer, W. Both tumor necrosis factor receptor types mediate proliferative signals in human mononuclear cell activation. J. Immunol. 1992, 149, 911. [Google Scholar]
- Wajant, H.; Siegmund, D. TNFR1 and TNFR2 in the Control of the Life and Death Balance of Macrophages. Front. Cell Dev. Biol. 2019, 7. [Google Scholar] [CrossRef]
- Bradley, J. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Borges, B.C.; Garcia-Galiano, D.; Rorato, R.; Elias, L.L.K.; Elias, C.F. PI3K p110β subunit in leptin receptor expressing cells is required for the acute hypophagia induced by endotoxemia. Mol. Metab. 2016, 5, 379–391. [Google Scholar] [CrossRef]
- Gan, L.; Guo, K.; Cremona, M.L.; McGraw, T.E.; Leibel, R.L.; Zhang, Y. TNF-α Up-Regulates Protein Level and Cell Surface Expression of the Leptin Receptor by Stimulating Its Export via a PKC-Dependent Mechanism. Endocrinology 2012, 153, 5821–5833. [Google Scholar] [CrossRef] [Green Version]
- Han, C.; Wu, W.; Ale, A.; Kim, M.S.; Cai, D. Central Leptin and Tumor Necrosis Factor-α (TNFα) in Diurnal Control of Blood Pressure and Hypertension. J. Biol. Chem. 2016, 291, 15131–15142. [Google Scholar] [CrossRef] [Green Version]
- Wauman, J.; Zabeau, L.; Tavernier, J. The Leptin Receptor Complex: Heavier Than Expected? Front. Endocrinol. 2017, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-M.; Choi, H.-J.; Oh, C.-H.; Oh, J.-W.; Han, J.-S. Leptin Increases TNF-α Expression and Production through Phospholipase D1 in Raw 264.7 Cells. PLoS ONE 2014, 9, e102373. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Sakaida, I.; Uchida, K.; Terai, S.; Okita, K. Leptin enhances TNF-α production via p38 and JNK MAPK in LPS-stimulated Kupffer cells. Life Sci. 2005, 77, 1502–1515. [Google Scholar] [CrossRef]
- Song, I.-H.; Lee; Kim, H.-S.; Dong, -E.; Kim, H.-Y.; Kim, S.-K.; Seul, J.-H. Effect of Leptin on the Expression ofLipopolysaccharide-Induced ChemokineKC mRNA in the Mouse Peritoneal Macrophages. J. Microbiol. Biotechnol. 2004, 14, 722–729. [Google Scholar]
- Maciel, M.N.; Zieba, D.A.; Amstalden, M.; Keisler, D.H.; Neves, J.P.; Williams, G.L. Chronic administration of recombinant ovine leptin in growing beef heifers: Effects on secretion of LH, metabolic hormones, and timing of puberty1. J. Anim. Sci. 2004, 82, 2930–2936. [Google Scholar] [CrossRef]
- Bliss, C.I. The toxicity of poisons applied jointly. Ann. Appl. Biol. 1939, 26, 585–615. [Google Scholar] [CrossRef]
- Roell, K.R.; Reif, D.M.; Motsinger-Reif, A.A. An Introduction to Terminology and Methodology of Chemical Synergy—Perspectives from Across Disciplines. Front. Pharmacol. 2017, 8, 158. [Google Scholar] [CrossRef]
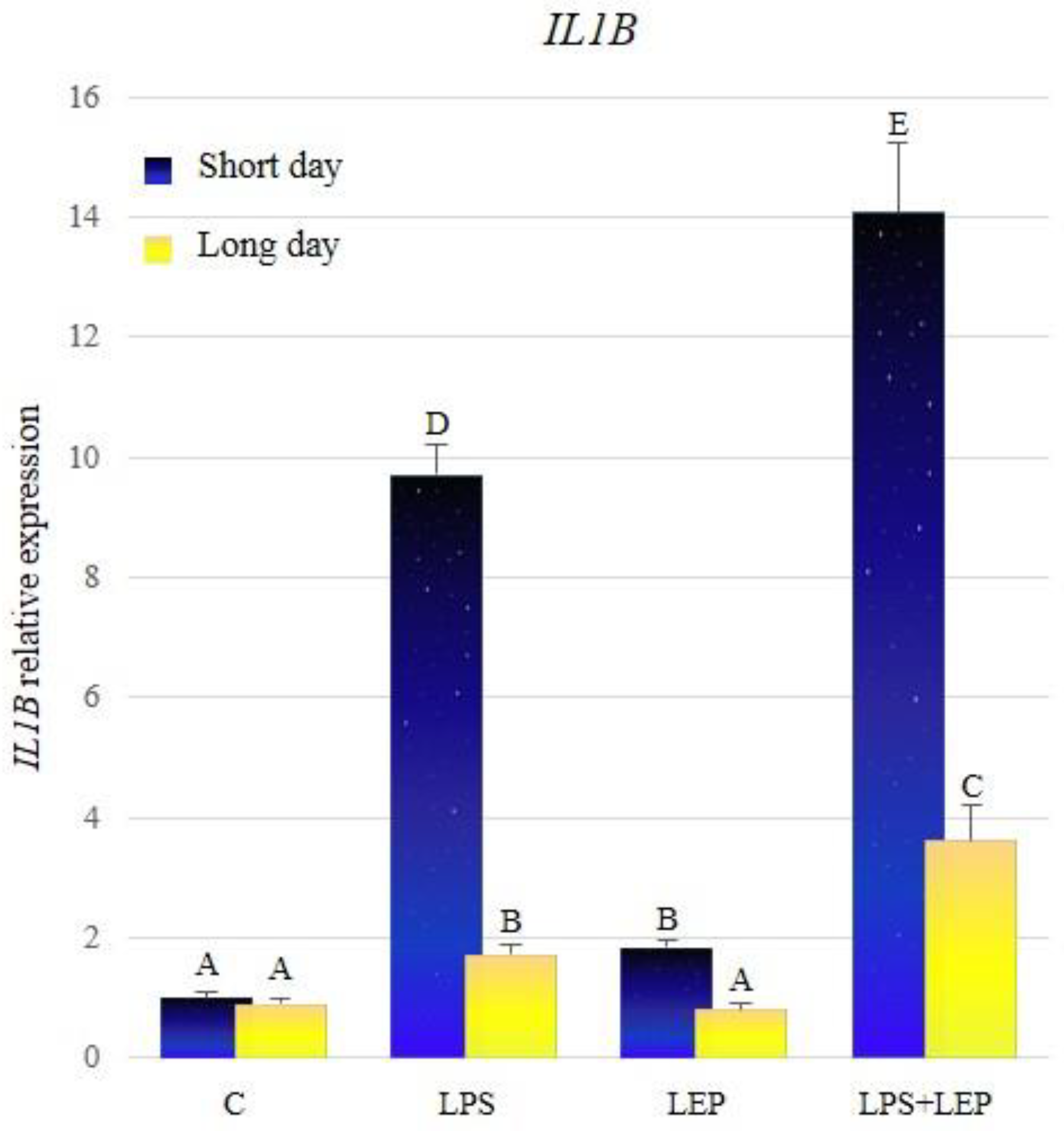
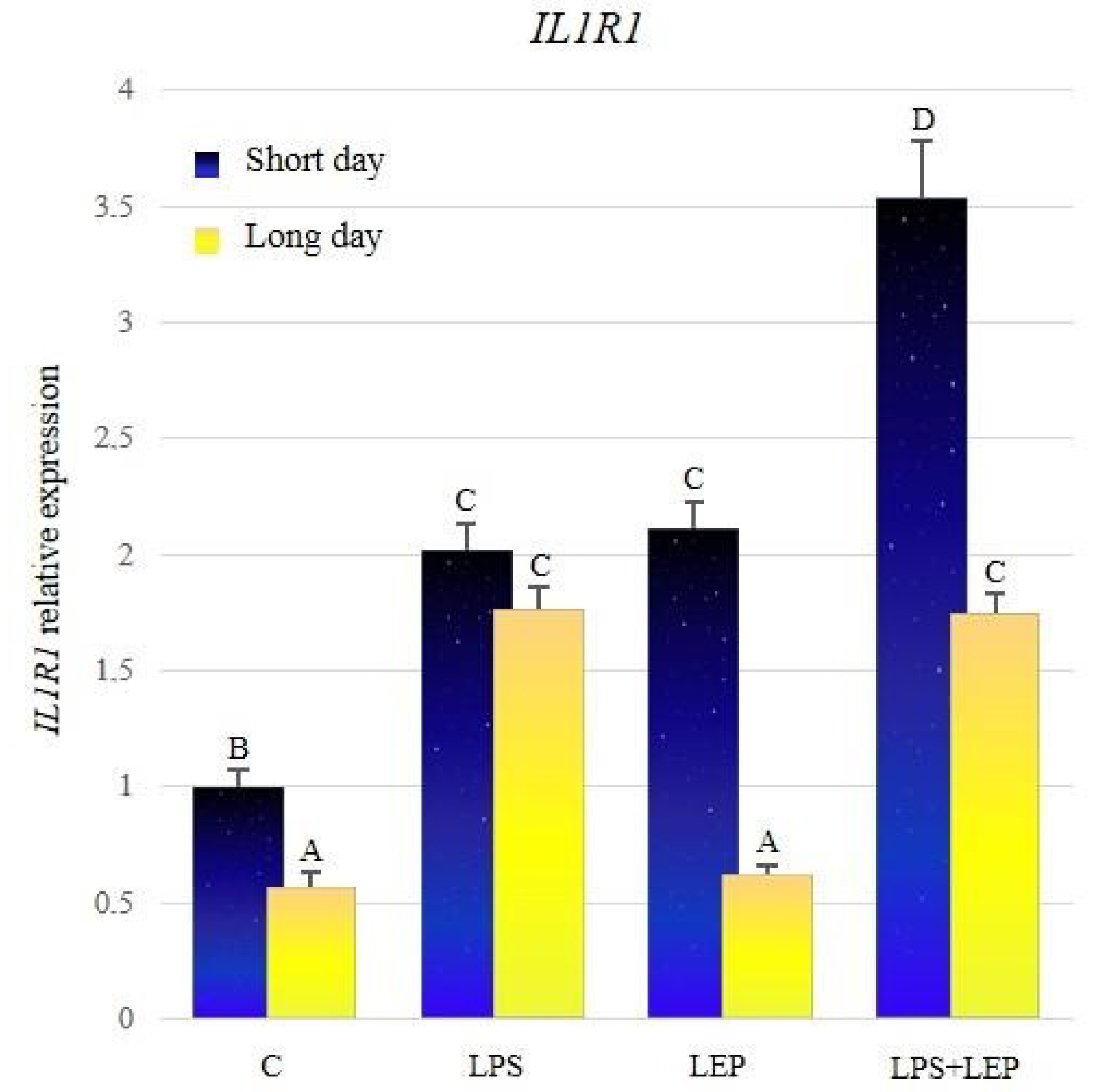
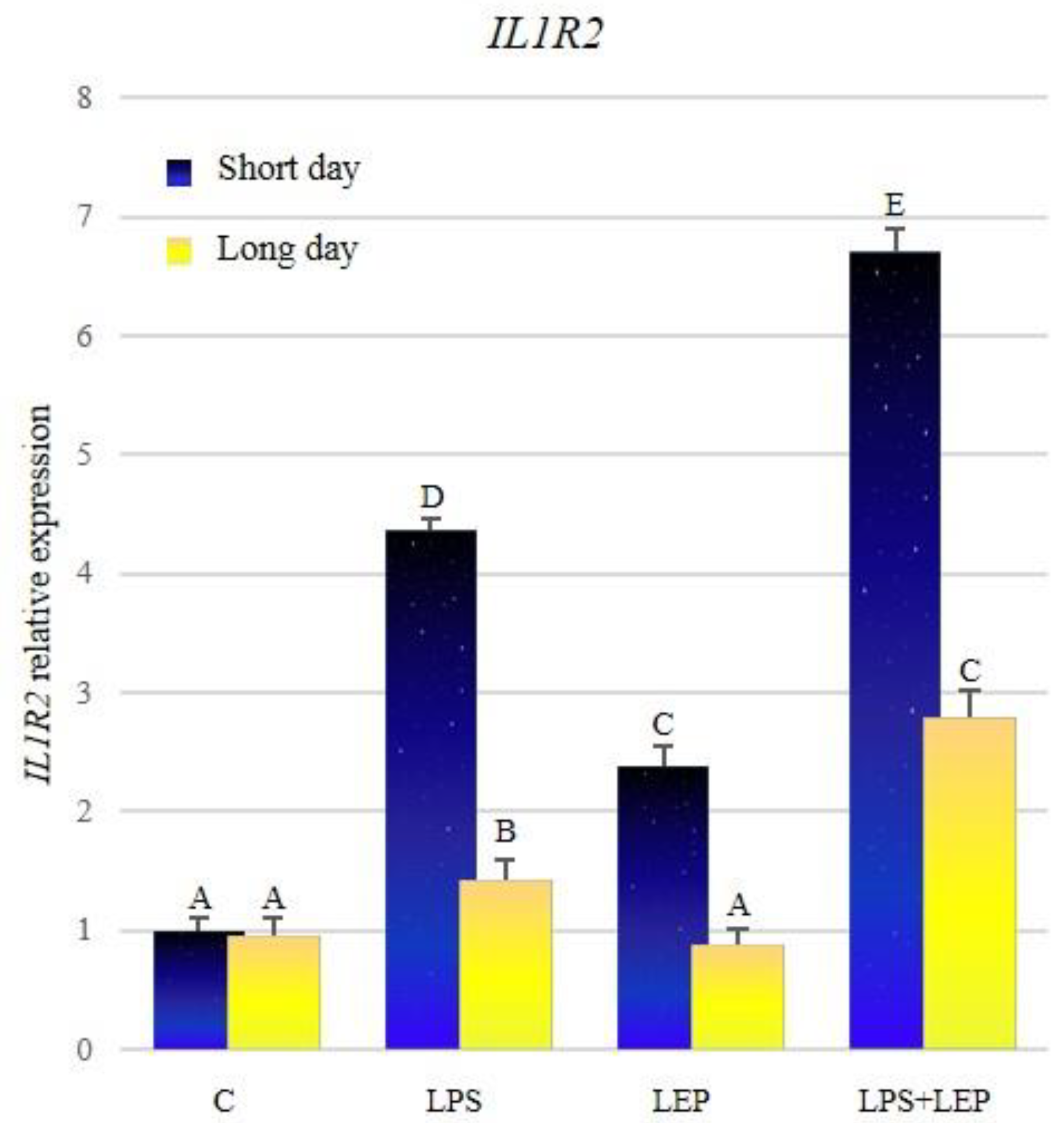
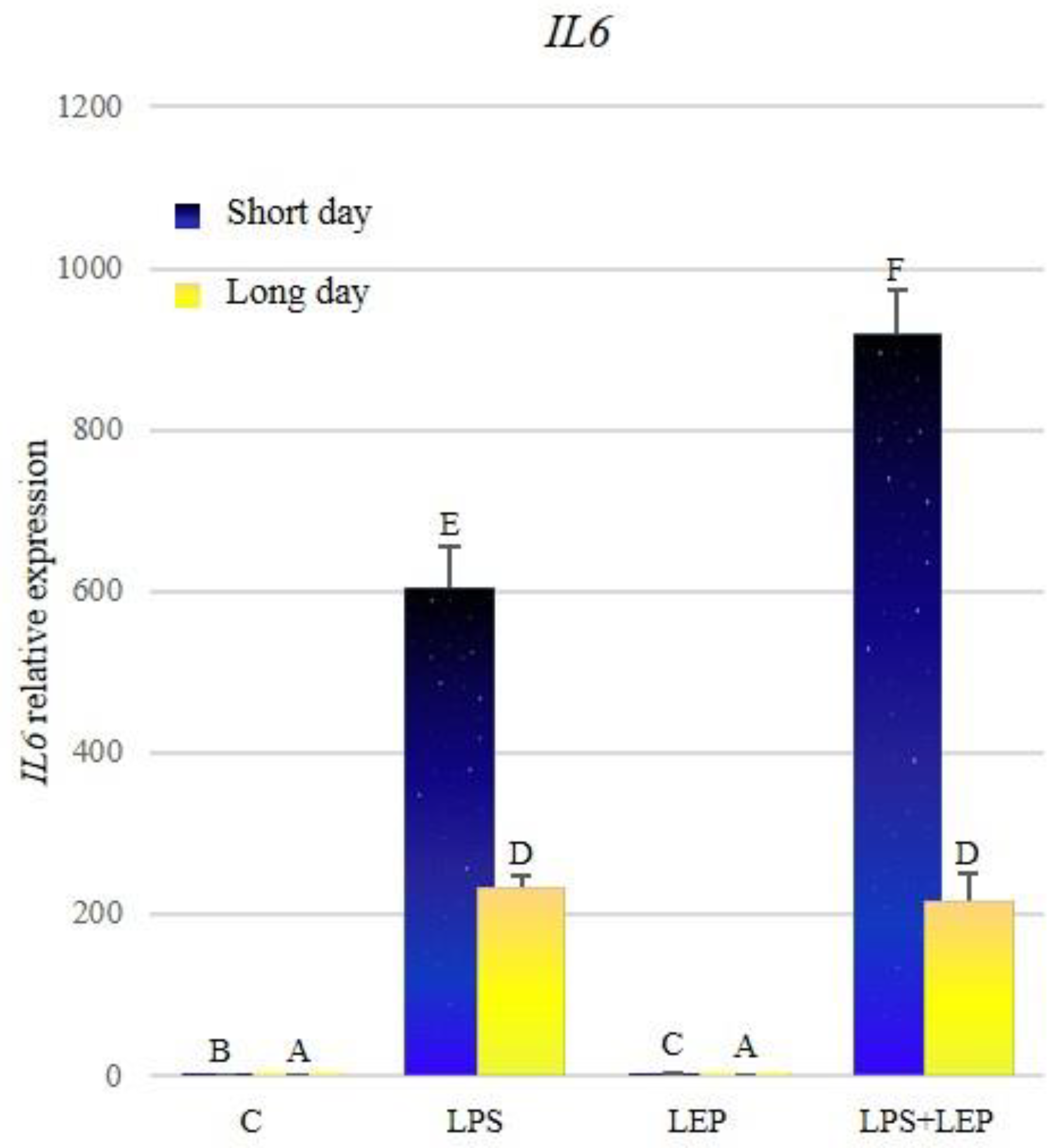


| Groups | Short Day | Long Day | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | LEP | LPS | LPS+LEP | C | LEP | LPS | LPS+LEP | ||
| Short day | C | 0.000164 * | 0.000000 * | 0.000000 * | 0.503301 | 0.133028 | 0.000635 * | 0.000000 * | |
| LEP | 0.000164 * | 0.000000 * | 0.000000 * | 0.000020 * | 0.000001 * | 0.653650 | 0.000125 * | ||
| LPS | 0.000000 * | 0.000000 * | 0.019915 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | ||
| LPS+LEP | 0.000000 * | 0.000000 * | 0.019915 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | ||
| Long day | C | 0.503301 | 0.000020 * | 0.000000 * | 0.000000 * | 0.395964 | 0.000083 * | 0.000000 * | |
| LEP | 0.133028 | 0.000001 * | 0.000000 * | 0.000000 * | 0.395964 | 0.000005 * | 0.000000 * | ||
| LPS | 0.000635 * | 0.653650 | 0.000000 * | 0.000000 * | 0.000083 * | 0.000005 * | 0.000031 * | ||
| LPS+LEP | 0.000000 * | 0.000125 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000031 * | ||
| Groups | Short Day | Long Day | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | LEP | LPS | LPS+LEP | C | LEP | LPS | LPS+LEP | ||
| Short day | C | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000013 * | 0.000000 * | 0.000001 * | |
| LEP | 0.000000 * | 0.716176 | 0.000003 * | 0.000000 * | 0.000000 * | 0.071536 | 0.059186 | ||
| LPS | 0.000000 * | 0.716176 | 0.000001 * | 0.000000 * | 0.000000 * | 0.145376 | 0.122910 | ||
| LPS+LEP | 0.000000 * | 0.000003 * | 0.000001 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | ||
| Long day | C | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.271236 | 0.000000 * | 0.000000 * | |
| LEP | 0.000013 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.271236 | 0.000000 * | 0.000000 * | ||
| LPS | 0.000000 * | 0.071536 | 0.145376 | 0.000000 * | 0.000000 * | 0.000000 * | 0.927990 | ||
| LPS+LEP | 0.000001 * | 0.059186 | 0.122910 | 0.000000 * | 0.000000 * | 0.000000 * | 0.927990 | ||
| Groups | Short Day | Long Day | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | LEP | LPS | LPS+LEP | C | LEP | LPS | LPS+LEP | ||
| Short day | C | 0.000001 * | 0.000000 * | 0.000000 * | 0.590985 | 0.309193 | 0.030987 * | 0.000000 * | |
| LEP | 0.000001 * | 0.000296 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.001146 * | 0.325885 | ||
| LPS | 0.000000 * | 0.000296 * | 0.008222 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.005016 * | ||
| LPS+LEP | 0.000000 * | 0.000000 * | 0.008222 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000001 * | ||
| Long day | C | 0.590985 | 0.000000 * | 0.000000 * | 0.000000 * | 0.628042 | 0.008290 * | 0.000000 * | |
| LEP | 0.309193 | 0.000000 * | 0.000000 * | 0.000000 * | 0.628042 | 0.002241 * | 0.000000 * | ||
| LPS | 0.030987 * | 0.001146 * | 0.000000 * | 0.000000 * | 0.008290 * | 0.002241 * | 0.000058 * | ||
| LPS+LEP | 0.000000 * | 0.325885 | 0.005016 * | 0.000001 * | 0.000000 * | 0.000000 * | 0.000058 * | ||
| Groups | Short Day | Long Day | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | LEP | LPS | LPS+LEP | C | LEP | LPS | LPS+LEP | ||
| Short day | C | 0.000073 * | 0.000000 * | 0.000000 * | 0.000916 * | 0.000203 * | 0.000000 * | 0.000000 * | |
| LEP | 0.000073 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | ||
| LPS | 0.000000 * | 0.000000 * | 0.021568 * | 0.000000 * | 0.000000 * | 0.000004 * | 0.000000 * | ||
| LPS+LEP | 0.000000 * | 0.000000 * | 0.021568 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | ||
| Long day | C | 0.000916 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.613919 | 0.000000 * | 0.000000 * | |
| LEP | 0.000203 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.613919 | 0.000000 * | 0.000000 * | ||
| LPS | 0.000000 * | 0.000000 * | 0.000004 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.460984 | ||
| LPS+LEP | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.460984 | ||
| Groups | Short Day | Long Day | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | LEP | LPS | LPS+LEP | C | LEP | LPS | LPS+LEP | ||
| Short day | C | 0.021227 * | 0.000839 * | 0.001664 * | 0.000000 * | 0.000002 * | 0.620013 | 0.009028 * | |
| LEP | 0.021227 * | 0.232119 | 0.335857 | 0.000000 * | 0.000000 * | 0.006067 * | 0.000008 * | ||
| LPS | 0.000839 * | 0.232119 | 0.812154 | 0.000000 * | 0.000000 * | 0.000190 * | 0.000000 * | ||
| LPS+LEP | 0.001664 * | 0.335857 | 0.812154 | 0.000000 * | 0.000000 * | 0.000390 * | 0.000000 * | ||
| Long day | C | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.007271 * | 0.000000 * | 0.000001 * | |
| LEP | 0.000002 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.007271 * | 0.000009 * | 0.007181 * | ||
| LPS | 0.620013 | 0.006067 * | 0.000190 * | 0.000390 * | 0.000000 * | 0.000009 * | 0.030371 * | ||
| LPS+LEP | 0.009028 * | 0.000008 * | 0.000000 * | 0.000000 * | 0.000001 * | 0.007181 * | 0.030371 * | ||
| Groups | Short Day | Long Day | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | LEP | LPS | LPS+LEP | C | LEP | LPS | LPS+LEP | ||
| Short day | C | 0.001158 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.137910 | 0.003346 * | |
| LEP | 0.001158 * | 0.000001 * | 0.000954 * | 0.000000 * | 0.000000 * | 0.053906 | 0.706263 | ||
| LPS | 0.000000 * | 0.000001 * | 0.029708 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | ||
| LPS+LEP | 0.000000 * | 0.000954 * | 0.029708 * | 0.000000 * | 0.000000 * | 0.000002 * | 0.000312 * | ||
| Long day | C | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.002213 * | 0.000000 * | 0.000000 * | |
| LEP | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.002213 * | 0.000000 * | 0.000000 * | ||
| LPS | 0.137910 | 0.053906 | 0.000000 * | 0.000002 * | 0.000000 * | 0.000000 * | 0.116029 | ||
| LPS+LEP | 0.003346 * | 0.706263 | 0.000000 * | 0.000312 * | 0.000000 * | 0.000000 * | 0.116029 | ||
| Groups | Short Day | Long Day | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | LEP | LPS | LPS+LEP | C | LEP | LPS | LPS+LEP | ||
| Short day | C | 0.000431 * | 0.004784 * | 0.000000 * | 0.000203 * | 0.006401 * | 0.354653 | 0.042266 * | |
| LEP | 0.000431 * | 0.399832 | 0.030589 * | 0.000000 * | 0.000000 * | 0.000024 * | 0.089413 | ||
| LPS | 0.004784 * | 0.399832 | 0.003607 * | 0.000000 * | 0.000001 * | 0.000334 * | 0.378914 | ||
| LPS+LEP | 0.000000 * | 0.030589 * | 0.003607 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000280 * | ||
| Long day | C | 0.000203 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.232630 | 0.003060 * | 0.000000 * | |
| LEP | 0.006401 * | 0.000000 * | 0.000001 * | 0.000000 * | 0.232630 | 0.059313 | 0.000013 * | ||
| LPS | 0.354653 | 0.000024 * | 0.000334 * | 0.000000 * | 0.003060 * | 0.059313 | 0.004221 * | ||
| LPS+LEP | 0.042266 * | 0.089413 | 0.378914 | 0.000280 * | 0.000000 * | 0.000013 * | 0.004221 * | ||
| Groups | Short Day | Long Day | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | LEP | LPS | LPS+LEP | C | LEP | LPS | LPS+LEP | ||
| Short day | C | 0.033938 * | 0.000000 * | 0.000000 * | 0.000009 * | 0.000003 * | 0.000000 * | 0.000000 * | |
| LEP | 0.033938 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000* | 0.000000 * | ||
| LPS | 0.000000 * | 0.000000 * | 0.230573 | 0.000000 * | 0.000000 * | 0.001927 * | 0.000108 * | ||
| LPS+LEP | 0.000000 * | 0.000000 * | 0.230573 | 0.000000 * | 0.000000 * | 0.000051* | 0.000002 * | ||
| Long day | C | 0.000009 * | 0.000000 * | 0.000000 * | 0.000000* | 0.723256 | 0.000000 * | 0.000000 * | |
| LEP | 0.000003 * | 0.000000* | 0.000000 * | 0.000000 * | 0.723256 | 0.000000 * | 0.000000 * | ||
| LPS | 0.000000 * | 0.000000 * | 0.001927 * | 0.000051 * | 0.000000 * | 0.000000 * | 0.335630 | ||
| LPS+LEP | 0.000000 * | 0.000000 * | 0.000108 * | 0.000002 * | 0.000000 * | 0.000000 * | 0.335630 | ||
| Groups | Short Day | Long Day | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | LEP | LPS | LPS+LEP | C | LEP | LPS | LPS+LEP | ||
| Short day | C | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000254 * | 0.000000 * | |
| LEP | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.053487 | 0.002733 * | ||
| LPS | 0.000000 * | 0.000000 * | 0.015297 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | ||
| LPS+LEP | 0.000000 * | 0.000000 * | 0.015297 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | ||
| Long day | C | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.819236 | 0.000000 * | 0.000000 * | |
| LEP | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.819236 | 0.000000 * | 0.000000 * | ||
| LPS | 0.000254 * | 0.053487 | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000007 * | ||
| LPS+LEP | 0.000000 * | 0.002733 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000000 * | 0.000007 * | ||
| Gene Symbol | Primer | Gene Bank, Accession Number | References | |
|---|---|---|---|---|
| Forward | Reverse | |||
| ACTB | GCCAACCGTGAGAAGATGAC | TCCATCACGATGCCAGTG | NM_001009784.2 | [83] |
| HDAC1 | CTGGGGACCTACGGGATATT | GACATGACCGGCTTGAAAAT | XM_004005023.3 | [97] |
| GAPDH | TGACCCCTTCATTGACCTTC | GATCTCGCTCCTGGAAGATG | NM_001190390.1 | [84] |
| IL1B | CAGCCGTGCAGTCAGTAAAA | GAAGCTCATGCAGAACACCA | NM_001009465.2 | [84] |
| IL1R1 | GGGAAGGGTCCACCTGTAAC | ACAATGCTTTCCCCAACGTA | NM_001206735.1 | [97] |
| IL1R2 | CGCCAGGCATACTCAGAAA | GAGAACGTGGCAGCTTCTTT | NM_001046210.2 | [83] |
| IL6 | GTTCAATCAGGCGATTTGCT | CCTGCGATCTTTTCCTTCAG | NM_001009392.1 | [97] |
| IL6R | TCAGCGACTCCGGAAACTAT | CCGAGGACTCCACTCACAAT | NM_001110785.3 | [97] |
| IL6ST | GGCTTGCCTCCTGAAAAACC | ACTTCTCTGTTGCCCACTCAG | XM_012096909.2 | [20] |
| TNF | CAAATAACAAGCCGGTAGCC | AGATGAGGTAAAGCCCGTCA | NM_001024860.1 | [97] |
| TNFR1 (TNFRSF1A) | AGGTGCCGGGATGAAATGTT | CAGAGGCTGCAGTTCAGACA | NM_001166185.1 | [97] |
| TNFR2 (TNFRSF1B) | ACCTTCTTCCTCCTCCCAAA | AGAAGCAGACCCAATGCTGT | NM_001040490.2 | [97] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik, M.; Herman, A.P.; Zieba, D.A.; Krawczyńska, A. The Impact of Photoperiod on the Leptin Sensitivity and Course of Inflammation in the Anterior Pituitary. Int. J. Mol. Sci. 2020, 21, 4153. https://doi.org/10.3390/ijms21114153
Wójcik M, Herman AP, Zieba DA, Krawczyńska A. The Impact of Photoperiod on the Leptin Sensitivity and Course of Inflammation in the Anterior Pituitary. International Journal of Molecular Sciences. 2020; 21(11):4153. https://doi.org/10.3390/ijms21114153
Chicago/Turabian StyleWójcik, Maciej, Andrzej Przemysław Herman, Dorota Anna Zieba, and Agata Krawczyńska. 2020. "The Impact of Photoperiod on the Leptin Sensitivity and Course of Inflammation in the Anterior Pituitary" International Journal of Molecular Sciences 21, no. 11: 4153. https://doi.org/10.3390/ijms21114153





