Role of Oxidative Stress in the Genesis of Ventricular Arrhythmias
Abstract
:1. Introduction
2. Oxidative Stress as a Factor for Increasing the Risk of Arrhythmias
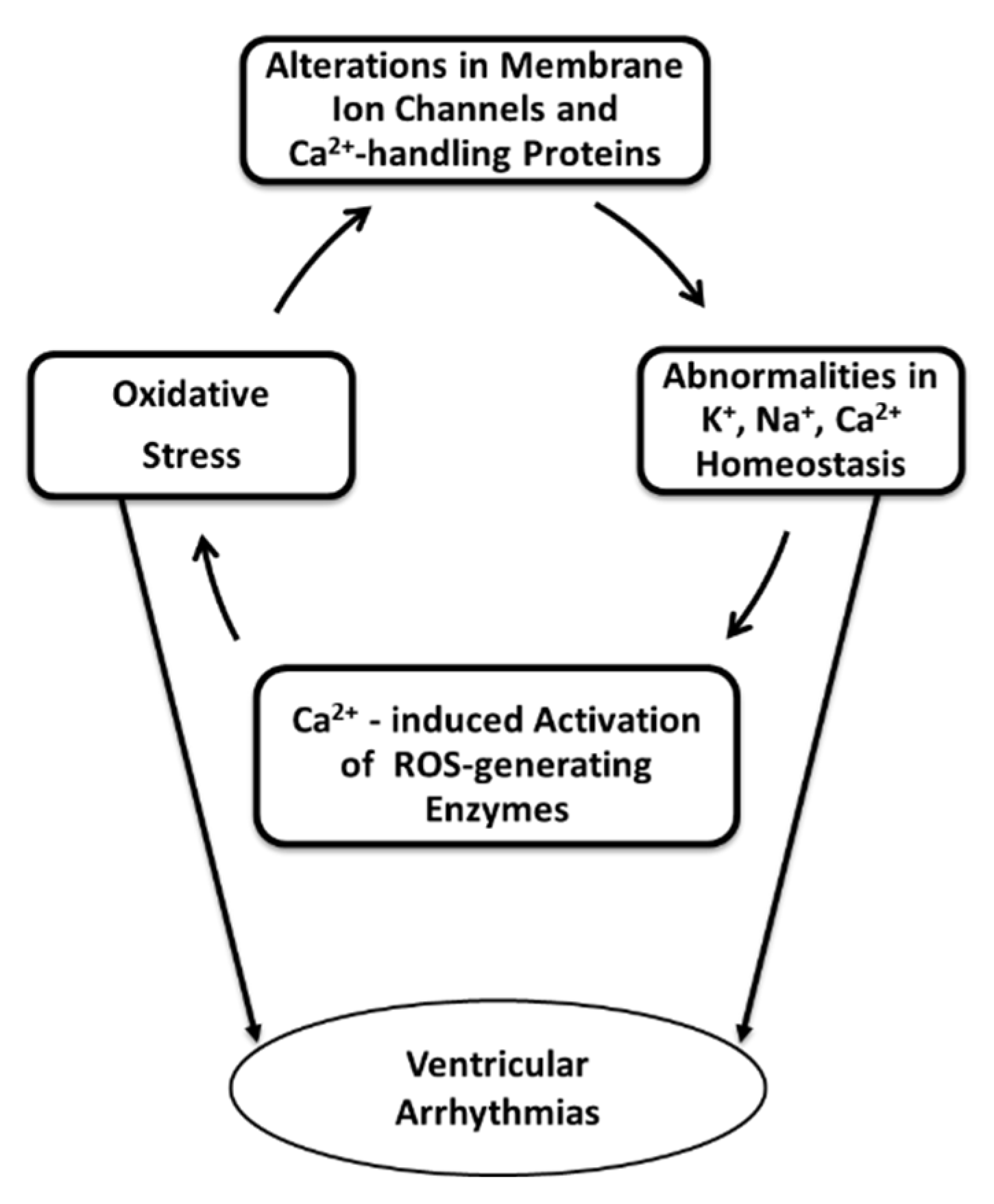
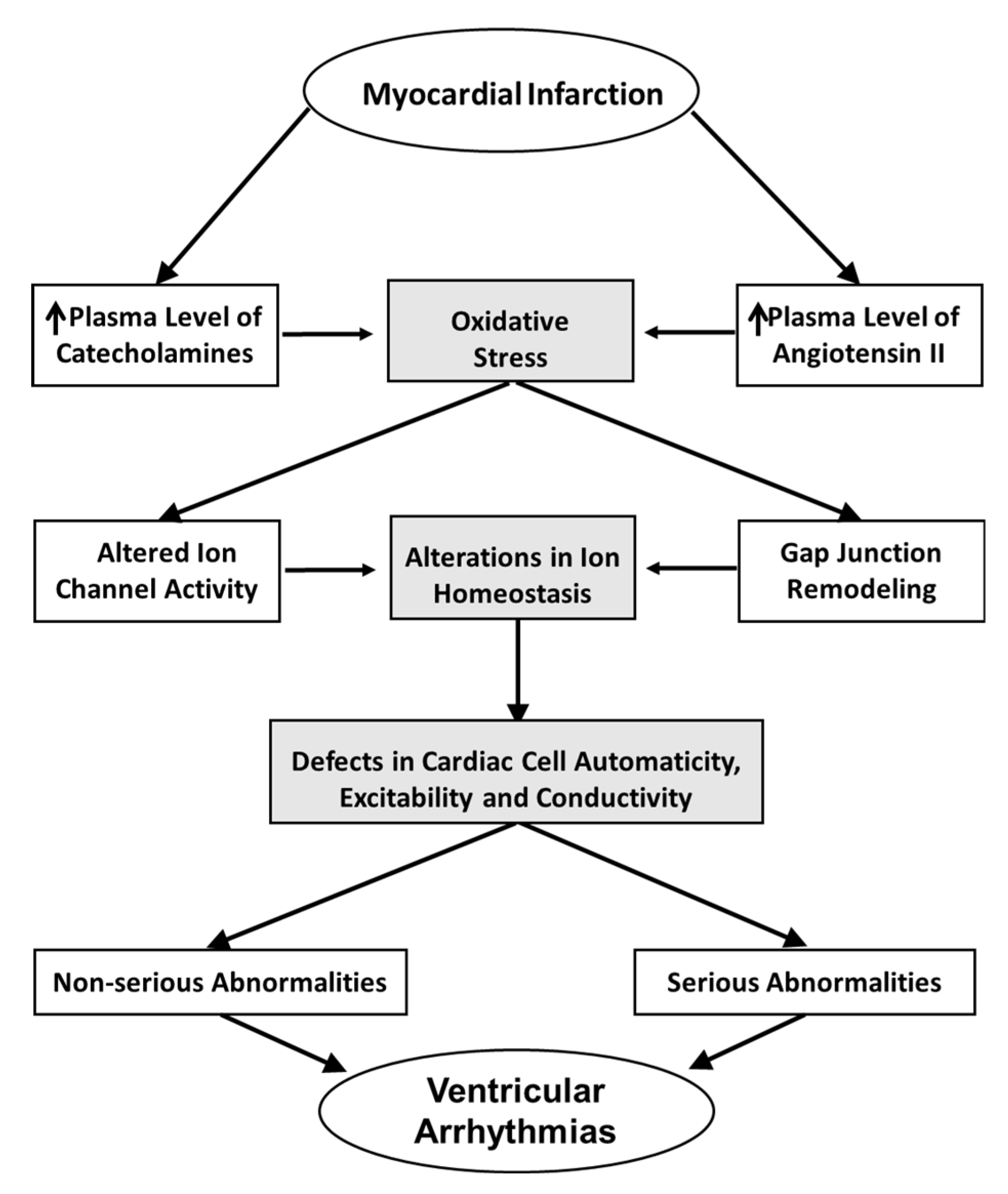
3. Potential Mechanisms of Oxidative Stress Induced Ventricular Arrhythmias
4. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Silvia, P.G.; Blomström-Lundqvist, C.; Mazzanti†, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Hear. J. 2015, 36, 2793–2867. [Google Scholar] [CrossRef] [Green Version]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castellà, M.; Diener, H.-C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Hear. J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [Green Version]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Circulation 2018, 138, e272–e391. [Google Scholar]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019, 140, e125–e151. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Elmoselhi, A.B.; Hata, T.; Makino, N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovasc. Res. 2000, 47, 446–456. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, J.H.; Lim, D.S.; Shim, W.J.; Ro, Y.M.; Park, G.H.; Becker, K.G.; Cho-Chung, Y.S.; Kim, M.-K. Gene expression profiling of oxidative stress on atrial fibrillation in humans. Exp. Mol. Med. 2003, 35, 336–349. [Google Scholar] [CrossRef]
- Zakkar, M.; Ascione, R.; James, A.; Angelini, G.; Suleiman, M.-S. Inflammation, oxidative stress and postoperative atrial fibrillation in cardiac surgery. Pharmacol. Ther. 2015, 154, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.-I.; Koo, S.-H.; Cha, T.-J.; Heo, J.-H.; Kim, H.-S.; Jo, G.-B.; Lee, J.W. Simvastatin attenuates the oxidative stress, endothelial thrombogenicity and the inducibility of atrial fibrillation in a rat model of ischemic heart failure. Int. J. Mol. Sci. 2014, 15, 14803–14818. [Google Scholar] [CrossRef] [Green Version]
- Sridhar, A.; Nishijima, Y.; Terentyev, D.; Khan, M.; Terentyeva, R.; Hamlin, R.L.; Nakayama, T.; Gyorke, S.; Cardounel, A.J.; Carnes, C.A. Chronic heart failure and the substrate for atrial fibrillation. Cardiovasc. Res. 2009, 84, 227–236. [Google Scholar] [CrossRef]
- Liu, T.; Takimoto, E.; Dimaano, V.L.; DeMazumder, D.; Kettlewell, S.; Smith, G.; Sidor, A.; Abraham, T.P.; O’Rourke, B. Inhibiting mitochondrial Na+/Ca2+ exchange prevents sudden death in a guinea pig model of heart failure. Circ. Res. 2014, 115, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Matejíková, J.; Kucharská, J.; Pancza, D.; Ravingerová, T. The effect of antioxidant treatment and NOS inhibition on the incidence of ischemia-induced arrhythmias in the diabetic rat heart. Physiol. Res. 2008, 57 (Suppl. 2), S55–S60. [Google Scholar]
- Nemcekova, M.; Carnicka, S.; Ferko, M.; Murarikova, M.; Ledvenyiova, V.; Ravingerova, T. Treatment of rats with hypolipidemic compound pirinixic acid protects their hearts against ischemic injury: Are mitochondrial K(ATP) channels and reactive oxygen species involved? Physiol. Res. 2013, 62, 577–584. [Google Scholar] [CrossRef]
- Kirshenbaum, L.A.; Singal, P.K. Increase in endogenous antioxidant enzymes protects hearts against reperfusion injury. Am. J. Physiol. Circ. Physiol. 1993, 265, H484–H493. [Google Scholar] [CrossRef]
- Sethi, R.; Adameova, A.; Dhalla, K.S.; Khan, M.; Elimban, V.; Dhalla, N.S. Modification of epinephrine-induced arrhythmias by N-acetyl-L-cysteine and vitamin E. J. Cardiovasc. Pharmacol. Ther. 2009, 14, 134–142. [Google Scholar] [CrossRef]
- Sethi, R.; Rehsia, N.S.; Jindal, K.; Dhalla, K.S.; Elimban, V.; Dhalla, N.S. Antiarrhythmic effects of some antioxidant vitamins in rats injected with epinephrine. Cardiovasc. Toxicol. 2009, 9, 177–184. [Google Scholar] [CrossRef]
- Barta, J.; Sanganalmath, S.K.; Kumamoto, H.; Takeda, N.; Édes, I.; Dhalla, N.S. Antiplatelet agents sarpogrelate and cilostazol affect experimentally-induced ventricular arrhythmias and mortality. Cardiovasc. Toxicol. 2008, 8, 127–135. [Google Scholar] [CrossRef]
- Cai, B.; Shan, L.; Gong, D.; Pan, Z.; Ai, J.; Xu, C.; Lu, Y.; Yang, B. Homocysteine modulates sodium channel currents in human atrial myocytes. Toxicology 2009, 256, 201–206. [Google Scholar] [CrossRef]
- Naji, F.; Suran, D.; Kanic, V.; Vokac, D.; Sabovic, M. High homocysteine levels predict the recurrence of atrial fibrillation after successful electrical cardioversion. Int. Hear. J. 2010, 51, 30–33. [Google Scholar] [CrossRef] [Green Version]
- Shizukuda, Y.; Rosing, U.R. Iron overload and arrhythmias: Influence of confounding factors. J. Arrhythmia 2019, 35, 575–583. [Google Scholar] [CrossRef]
- Persad, S.; Panagia, V.; Dhalla, N.S. Role of H2O2 in changing beta-adrenoceptor and adenylyl cyclase in ischemia-reperfused hearts. Mol. Cell. Biochem. 1998, 186, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Manning, A.S.; Coltart, D.J.; Hearse, D.J. Ischemia and reperfusion-induced arrhythmias in the rat. Effects of xanthine oxidase inhibition with allopurinol. Circ. Res. 1984, 55, 545–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodward, B.; Zakaria, M. Effect of some free radical scavengers on reperfusion induced arrhythmias in the isolated rat heart. J. Mol. Cell. Cardiol. 1985, 17, 485–493. [Google Scholar] [CrossRef]
- Sovari, A.A.; Gu, L.; Mitchell, D.; Jeong, E.; Bonini, M.G.; Dudley, S.C.; Iravanian, S. Mitochondria-targeted antioxidant, mito-tempo, prevents angiotensin II mediated connexin 43 remodeling and sudden cardiac death. J. Investig. Med. 2011, 59, 693–694. [Google Scholar]
- Hearse, D.J. Reperfusion-induced injury: A possible role for oxidant stress and its manipulation. Cardiovasc. Drugs Ther. 1991, 5, 225–235. [Google Scholar] [CrossRef]
- Adameova, A.; Tappia, P.S.; Hatala, R.; Dhalla, N.S. Potential of sulphur-containing amino acids in the prevention of catecholamine-induced arrhythmias. Curr. Med. Chem. 2018, 25, 346–354. [Google Scholar] [CrossRef]
- Goldhaber, J.I.; Weiss, J.N. Oxygen free radicals and cardiac reperfusion abnormalities. Hypertension 1992, 20, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Grover, A.; Samson, S.E.; Fomin, V.P.; Werstiuk, E.S. Effects of peroxide and superoxide on coronary artery: ANG II response and sarcoplasmic reticulum Ca2+ pump. Am. J. Physiol. Physiol. 1995, 269, C546–C553. [Google Scholar] [CrossRef] [PubMed]
- Elmoselhi, A.B.; Butcher, A.; Samson, S.E.; Grover, A. Free radicals uncouple the sodium pump in pig coronary artery. Am. J. Physiol. Physiol. 1994, 266, C720–C728. [Google Scholar] [CrossRef]
- Grover, A.; Samson, S.E.; Fomin, V.P. Peroxide inactivates calcium pumps in pig coronary artery. Am. J. Physiol. Circ. Physiol. 1992, 263, H537–H543. [Google Scholar] [CrossRef]
- Sano, M.; Fukuda, K.; Sato, T.; Kawaguchi, H.; Suematsu, M.; Matsuda, S.; Koyasu, S.; Matsui, H.; Yamauchi-Takihara, K.; Harada, M.; et al. ERK and p38 MAPK, but not NF-kappaB, are critically involved in reactive oxygen species-mediated induction of IL-6 by angiotensin II in cardiac fibroblasts. Circ. Res. 2001, 89, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Engler, R.L.; Dahlgren, M.D.; Peterson, M.A.; Dobbs, A.; Schmid-Schönbein, G.W. Accumulation of polymorphonuclear leukocytes during 3-h experimental myocardial ischemia. Am. J. Physiol. Circ. Physiol. 1986, 251, H93–H100. [Google Scholar] [CrossRef] [PubMed]
- Corbalan, R.; Verrier, R.L.; Lown, B. Differing mechanisms for ventricular vulnerability during coronary artery occlusion and release. Am. Hear. J. 1976, 92, 223–230. [Google Scholar] [CrossRef]
- Balke, C.W.; Kaplinsky, E.; Michelson, E.L.; Naito, M.; Dreifus, L.S. Reperfusion ventricular tachyarrhythmias: Correlation with antecedent coronary artery occlusion tachyarrhythmias and duration of myocardial ischemia. Am. Hear. J. 1981, 101, 449–456. [Google Scholar] [CrossRef]
- Ravingerová, T.; Slezák, J.; Tribulová, N.; Džurba, A.; Uhrík, B.; Ziegelhöffer, A. Free oxygen radicals contribute to high incidence of reperfusion-induced arrhythmias in isolated rat heart. Life Sci. 1999, 65, 1927–1930. [Google Scholar] [CrossRef]
- Podzuweit, T. Cyclic AMP levels in ischaemic and non-ischaemic myocardium following coronary artery ligation: Relation to ventricular fibrillation. J. Mol. Cell. Cardiol. 1978, 10, 81–94. [Google Scholar] [CrossRef]
- Menken, U.; Wiegand, V.; Bucher, P.; Meesmann, W. Prophylaxis of ventricular fibrillation after acute experimental coronary occlusion by chronic beta- adrenoceptor blockade with atenolol. Cardiovasc. Res. 1979, 13, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Leprán, I.; Koltai, M.; Siegmund, W.; Szekeres, L. Coronary artery ligation, early arrhythmias, and determination of the ischemic area in conscious rats. J. Pharmacol. Methods 1983, 9, 219–230. [Google Scholar] [CrossRef]
- Clements-Jewery, H.; Andrag, E.; Hearse, D.J.; Curtis, M. Complex adrenergic and inflammatory mechanisms contribute to phase 2 ventricular arrhythmias in anaesthetized rats. Br. J. Pharmacol. 2009, 156, 444–453. [Google Scholar] [CrossRef] [Green Version]
- Åblad, B.; Bjurö, T.; Björkman, J.-A.; Edström, T. Prevention of ventricular fibrillation requires central β-adrenoceptor blockade in rabbits. Scand. Cardiovasc. J. 2007, 41, 221–229. [Google Scholar] [CrossRef]
- Adameova, A.D.; Bhullar, S.K.; Elimban, V.; Dhalla, N.S. Activation of beta1-adrenoceptors may not be involved in arrhythmogenesis in ischemic heart disease. Rev. Cardiovasc. Med. 2018, 19, 97–101. [Google Scholar] [PubMed]
- Chernecki, W.; Das, P.K.; Dhalla, N.S.; Sharma, G.P. Cardiovascular effects of acebutolol following coronary-artery occlusion and reperfusion in anesthetized dog. Brit. J. Pharmacol. 1978, 64, 265–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adameova, A.; Elimban, V.; Ganguly, P.K.; Dhalla, N.S. beta-1 adrenoceptors and AT1 receptors may not be involved in catecholamine-induced lethal arrhythmias. Can. J. Physiol. Pharmacol. 2019, 97, 570–576. [Google Scholar] [CrossRef]
- Romaschin, A.; Rebeyka, I.; Wilson, G.; Mickle, D. Conjugated dienes in ischemic and reperfused myocardium: An in vivo chemical signature of oxygen free radical mediated injury. J. Mol. Cell. Cardiol. 1987, 19, 289–302. [Google Scholar] [CrossRef]
- Adameova, A.; Abdellatif, Y.; Dhalla, N.S. Role of the excessive amounts of circulating catecholamines and glucocorticoids in stress-induced heart disease. Can. J. Physiol. Pharmacol. 2009, 87, 493–514. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Adameova, A.; Kaur, M. Role of catecholamine oxidation in sudden cardiac death. Fundam. Clin. Pharmacol. 2010, 24, 539–546. [Google Scholar] [CrossRef]
- Persad, S.; Takeda, S.; Panagia, V.; Dhalla, N.S. Beta-adrenoceptor-linked signal transduction in ischemic-reperfused heart and scavenging of oxyradicals. J. Mol. Cell Cardiol. 1997, 29, 545–558. [Google Scholar] [CrossRef]
- Mialet-Perez, J.; Santin, Y.; Parini, A. Monoamine oxidase-A, serotonin and norepinephrine: Synergistic players in cardiac physiology and pathology. J. Neural Transm. 2018, 125, 1627–1634. [Google Scholar] [CrossRef]
- Walker, M.K.; Vergely, C.; Lecour, S.; Abadie, C.; Maupoil, V.; Rochette, L. Vitamin E analogues reduce the incidence of ventricular fibrillations and scavenge free radicals. Fundam. Clin. Pharmacol. 1998, 12, 164–172. [Google Scholar] [CrossRef]
- Nagai, S.; Miyazaki, Y.; Ogawa, K.; Satake, T.; Sugiyama, S.; Ozawa, T. The effect of coenzyme Q10 on reperfusion injury in canine myocardium. J. Mol. Cell. Cardiol. 1985, 17, 873–884. [Google Scholar] [CrossRef]
- Nishinaka, Y.; Sugiyama, S.; Yokota, M.; Saito, H.; Ozawa, T. The effects of a high dose of ascorbate on ischemia-reperfusion-induced mitochondrial dysfunction in canine hearts. Hear. Vessel. 1992, 7, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Sebbag, L.; Forrat, R.; Canet, E.; Renaud, S.; Delaye, J.; De Lorgeril, M. Effects of dietary supplementation with alpha-tocopherol on myocardial infarct size and ventricular arrhythmias in a dog model of ischemia-reperfusion. J. Am. Coll. Cardiol. 1994, 24, 1580–1585. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yin, C.; Yang, Y.; Fan, Z.; Shang, J.; Tan, W. Inhibition of rapid delayed rectifier potassium current (I Kr) by ischemia/reperfusion and its recovery by vitamin E in ventricular myocytes. J. Electrocardiol. 2017, 50, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Sochman, J.; Kolc, J.; Vrána, M.; Fabián, J. Cardioprotective effects of N-acetylcysteine: The reduction in the extent of infarction and occurrence of reperfusion arrhythmias in the dog. Int. J. Cardiol. 1990, 28, 191–196. [Google Scholar] [CrossRef]
- Forrat, R.; De Lorgeril, M.; Hadour, G.; Sebbag, L.; Delaye, J.; Ferrera, R. Effect of chronic oral supplementation with α-tocopherol on myocardial stunning in the dog. J. Cardiovasc. Pharmacol. 1997, 29, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Demirag, K.; Askar, F.Z.; Uyar, M.; Cevik, A.; Ozmen, D.; Mutaf, I.; Bayindir, O. The protective effects of high dose ascorbic acid and diltiazem on myocardial ischaemia-reperfusion injury. Middle East J. Anaesthesiol. 2001, 16, 67–79. [Google Scholar]
- Coombes, J.S.; Powers, S.K.; Demirel, H.; Hamilton, K.; Jessup, J.; Vincent, H.K.; Shanely, A.R. Vitamin E deficiency fails to affect myocardial performance during in vivo ischemia-reperfusion. Int. J. Vitam. Nutr. Res. 2000, 70, 293–300. [Google Scholar] [CrossRef]
- Nikas, D.N.; Chatziathanasiou, G.; Kotsia, A.; Papamichael, N.; Thomas, C.; Papafaklis, M.I.; Naka, K.K.; Kazakos, N.; Milionis, H.J.; Vakalis, K.; et al. Effect of intravenous administration of antioxidants alone and in combination on myocardial reperfusion injury in an experimental pig model. Curr. Ther. Res. 2008, 69, 423–439. [Google Scholar] [CrossRef] [Green Version]
- Sethi, R.; Takeda, N.; Nagano, M.; Dhalla, N.S. Beneficial effects of vitamin E treatment in acute myocardial infarction. J. Cardiovasc. Pharmacol. Ther. 2000, 5, 51–58. [Google Scholar] [CrossRef]
- Opitz, C.F.; Mitchell, G.F.; A Pfeffer, M.; Pfeffer, J.M. Arrhythmias and death after coronary artery occlusion in the rat. Continuous telemetric ECG monitoring in conscious, untethered rats. Circulation 1995, 92, 253–261. [Google Scholar] [CrossRef]
- Pahor, M.; Gambassi, G.; Carbonin, P. Antiarrhythmic effects of ACE inhibitors: A matter of faith or reality? Cardiovasc. Res. 1994, 28, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Linz, W.; Schölkens, B.A.; Kaiser, J.; Just, M.; Bei-Yin, Q.; Albus, U.; Petry, P. Cardiac arrhythmias are ameliorated by local inhibition of angiotensin formation and bradykinin degradation with the converting-enzyme inhibitor ramipril. Cardiovasc. Drugs Ther. 1989, 3, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Olmez, E.; Birincioglu, M.; Aksoy, T.; Acet, A. Effects of captopril on ischaemia-reperfusion-induced arrhythmias in an in vivo rat model. Pharmacol. Res. 1995, 32, 37–41. [Google Scholar] [CrossRef]
- Van Gilst, W.H.; De Graeff, P.A.; Kingma, J.; Wesseling, H.; De Langen, C.D. Captopril reduces purine loss and reperfusion arrhythmias in the rat heart after coronary artery occlusion. Eur. J. Pharmacol. 1984, 100, 113–117. [Google Scholar] [CrossRef]
- Bussmann, W.-D.; Micke, G.; Hildenbrand, R.; Klepzig, H. Captopril in acute myocardial infarction: Beneficial effects on infarct size and arrhythmias. Clin. Cardiol. 1995, 18, 465–470. [Google Scholar] [CrossRef]
- Ren, B.; Lukas, A.; Shao, Q.; Guo, M.; Takeda, N.; Aitken, R.M.; Dhalla, N.S. Electrocardiographic changes and mortality due to myocardial infarction in rats with or without imidapril treatment. J. Cardiovasc. Pharmacol. Ther. 1998, 3, 11–22. [Google Scholar] [CrossRef]
- De Boer, R.A.; Van Geel, P.P.; Pinto, Y.M.; Suurmeijer, A.J.; Crijns, H.J.G.M.; Van Gilst, W.H.; Van Veldhuisen, D.J. Efficacy of angiotensin II type 1 receptor blockade on reperfusion-induced arrhythmias and mortality early after myocardial infarction is increased in transgenic rats with cardiac angiotensin II type 1 overexpression. J. Cardiovasc. Pharmacol. 2002, 39, 610–619. [Google Scholar] [CrossRef] [Green Version]
- De Langen, C.D.J.; De Graeff, P.A.; Van Gilst, W.H.; Bel, K.J.; Kingma, J.H.; Wesseling, H. Effects of angiotensin II and captopril on inducible sustained ventricular tachycardia two weeks after myocardial infarction in the pig. J. Cardiovasc. Pharmacol. 1989, 13, 186–191. [Google Scholar] [CrossRef]
- Lee, Y.M.; Peng, Y.Y.; A Ding, Y.; Yen, M.H. Losartan attenuates myocardial ischemia-induced ventricular arrythmias and reperfusion injury in spontaneously hypertensive rats. Am. J. Hypertens. 1997, 10, 852–858. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, K.; Kumagai, K.; Annoura, M.; Yamanouchi, Y.; Handa, K.; Nakashima, Y.; Hiroki, T.; Arakawa, K. Effects of an angiotensin II antagonist on reperfusion arrhythmias in dogs. Pacing Clin. Electrophysiol. 1997, 20, 938–945. [Google Scholar] [CrossRef]
- Pourdjabbar, A.; Parker, T.G.; Nguyen, Q.T.; Desjardins, J.-F.; Lapointe, N.; Tsoporis, J.N.; Rouleau, J.-L. Effects of pre-, peri-, and postmyocardial infarction treatment with losartan in rats: Effect of dose on survival, ventricular arrhythmias, function, and remodeling. Am. J. Physiol. Circ. Physiol. 2005, 288, H1997–H2005. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Komuro, I.; Hayashi, D.; Sugaya, T.; Murakami, K.; Yazaki, Y. Angiotensin II type 1a receptor is involved in the occurrence of reperfusion arrhythmias. Circulation 1998, 97, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Cat, A.N.D.; Montezano, A.C.; Burger, D.; Touyz, R. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxidants Redox Signal. 2013, 19, 1110–1120. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Tian, J.; Sun, Y.; Xu, T.-R.; Chi, R.-F.; Zhang, X.-L.; Hu, X.-L.; Zhang, Y.-A.; Qin, F.-Z.; Zhang, W.-F. Activation of NADPH oxidase mediates increased endoplasmic reticulum stress and left ventricular remodeling after myocardial infarction in rabbits. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2015, 1852, 805–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brasil, D.; Temsah, R.M.; Kumar, K.; Kumamoto, H.; Takeda, N.; Dhalla, N.S. Blockade of 5-HT(2A) receptors by sarpogrelate protects the heart against myocardial infarction in rats. J. Cardiovasc. Pharmacol. Ther. 2002, 7, 53–59. [Google Scholar] [CrossRef]
- Campbell, D.L.; Stamler, J.S.; Strauss, H.C. Redox modulation of L-type calcium channels in ferret ventricular myocytes. Dual mechanism regulation by nitric oxide and S-nitrosothiols. J. Gen. Physiol. 1996, 108, 277–293. [Google Scholar] [CrossRef]
- Fearon, I.M.; Palmer, A.C.V.; Balmforth, A.J.; Ball, S.G.; Varadi, G.; Peers, C. Modulation of recombinant human cardiac L-type Ca2+ channel α1C subunits by redox agents and hypoxia. J. Physiol. 1999, 514, 629–637. [Google Scholar] [CrossRef]
- Ho, H.-T.; Stevens, S.C.W.; Terentyeva, R.; Carnes, C.A.; Terentyev, D.; Györke, S. Arrhythmogenic adverse effects of cardiac glycosides are mediated by redox modification of ryanodine receptors. J. Physiol. 2011, 589, 4697–4708. [Google Scholar] [CrossRef]
- Terentyev, D.; Györke, I.; Belevych, A.E.; Terentyeva, R.; Sridhar, A.; Nishijima, Y.; De Blanco, E.C.; Khanna, S.; Sen, C.K.; Cardounel, A.J.; et al. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ. Res. 2008, 103, 1466–1472. [Google Scholar] [CrossRef] [Green Version]
- Belevych, A.E.; Terentyev, D.; Viatchenko-Karpinski, S.; Terentyeva, R.; Sridhar, A.; Nishijima, Y.; Wilson, L.D.; Cardounel, A.J.; Laurita, K.R.; Carnes, C.A.; et al. Redox modification of ryanodine receptors underlies calcium alternans in a canine model of sudden cardiac death. Cardiovasc. Res. 2009, 84, 387–395. [Google Scholar] [CrossRef]
- Cooper, L.L.; Li, W.; Lu, Y.; Centracchio, J.; Terentyeva, R.; Koren, G.; Terentyev, D. Redox modification of ryanodine receptors by mitochondria-derived reactive oxygen species contributes to aberrant Ca2+ handling in ageing rabbit hearts. J. Physiol. 2013, 591, 5895–5911. [Google Scholar] [CrossRef] [PubMed]
- Leenhardt, A.; Denjoy, I.; Guicheney, P. Catecholaminergic polymorphic ventricular tachycardia. Circ. Arrhythmia Electrophysiol. 2012, 5, 1044–1052. [Google Scholar] [CrossRef] [Green Version]
- Priori, S.G.; Napolitano, C.; Tiso, N.; Memmi, M.; Vignati, G.; Bloise, R.; Sorrentino, V.; Danieli, G.A. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation 2001, 103, 196–200. [Google Scholar] [CrossRef] [Green Version]
- Laitinen, P.; Brown, K.; Piippo, K. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. ACC Curr. J. Rev. 2001, 10, 69. [Google Scholar] [CrossRef] [Green Version]
- Scherer, N.M.; Deamer, D.W. Oxidation of thiols in the Ca2+-ATPase of sarcoplasmic reticulum microsomes. Biochim. Biophys. Acta (BBA) Biomembr. 1986, 862, 309–317. [Google Scholar] [CrossRef]
- Xu, K.Y.; Zweier, J.L.; Becker, L.C. Hydroxyl radical inhibits sarcoplasmic reticulum Ca2+-ATPpase function by direct attack on the ATP binding Site. Circ. Res. 1997, 80, 76–81. [Google Scholar] [CrossRef]
- Turrens, J.F.; Thornton, J.; Barnard, M.L.; Snyder, S.; Liu, G.; Downey, J.M. Protection from reperfusion injury by preconditioning hearts does not involve increased antioxidant defenses. Am. J. Physiol. Circ. Physiol. 1992, 262, H585–H589. [Google Scholar] [CrossRef]
- Temsah, R.M.; Netticadan, T.; Chapman, D.; Takeda, S.; Mochizuki, S.; Dhalla, N.S. Alterations in sarcoplasmic reticulum function and gene expression in ischemic-reperfused rat heart. Am. J. Physiol. Content 1999, 277, H584–H594. [Google Scholar] [CrossRef]
- Hinata, M.; Matsuoka, I.; Iwamoto, T.; Watanabe, Y.; Kimura, J. Mechanism of Na+/Ca2+ exchanger activation by hydrogen peroxide in guinea-pig ventricular myocytes. J. Pharmacol. Sci. 2007, 103, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Pogwizd, S.M.; Bers, D.M. Na/Ca exchange in heart failure: Contractile dysfunction and arrhythmogenesis. Ann. N. Y. Acad. Sci. 2002, 976, 454–465. [Google Scholar] [CrossRef]
- Bers, D.M.; Pogwizd, S.M.; Schlotthauer, K. Upregulated Na/Ca exchange is involved in both contractile dysfunction and arrhythmogenesis in heart failure. Basic Res. Cardiol. 2002, 97 (Suppl. 1), I36–I42. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.R.; A Joiner, M.-L.; Guan, X.; Kutschke, W.; Yang, J.; Oddis, C.V.; Bartlett, R.K.; Lowe, J.S.; O’Donnell, S.E.; Aykin-Burns, N.; et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 2008, 133, 462–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, M.E.; Braun, A.P.; Wu, Y.; Lu, T.; Wu, Y.; Schulman, H.; Sung, R.J. KN-93, an inhibitor of multifunctional Ca++/calmodulin-dependent protein kinase, decreases early afterdepolarizations in rabbit heart. J. Pharmacol. Exp. Ther. 1998, 287, 996–1006. [Google Scholar] [PubMed]
- Wu, Y.; Roden, D.M.; Anderson, M.E. Calmodulin kinase inhibition prevents development of the arrhythmogenic transient inward current. Circ. Res. 1999, 84, 906–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajtik, T.; Carnicka, S.; Szobi, A.; Giricz, Z.; Hassova, V.; Svec, P.; Ferdinandy, P.; Ravingerova, T.; Adameova, A. Oxidative activation of CaMKIIdelta in acute myocardial ischemia/reperfusion injury: A role of angiotensin AT1 receptor-NOX2 signaling axis. Eur. J. Pharmacol. 2016, 771, 114–122. [Google Scholar] [CrossRef] [PubMed]
- A Kuehl, F.; Humes, J.L.; A Ham, E.; Egan, R.W.; Dougherty, H.W. Inflammation: The role of peroxidase-derived products. Adv. Prostaglandin Thromboxane Res. 1980, 6, 77–86. [Google Scholar]
- Bérubé, J.; Caouette, D.; Daleau, P. Hydrogen peroxide modifies the kinetics of HERG channel expressed in a mammalian cell line. J. Pharmacol. Exp. Ther. 2001, 297, 96–102. [Google Scholar]
- Gómez, R.; Caballero, R.; Barana, A.; Amorós, I.; Calvo, E.; Lopez, J.A.; Klein, H.; Vaquero, L.M.; Osuna, L.; Atienza, F.; et al. Nitric oxide increases cardiac IK1 by nitrosylation of cysteine 76 of Kir2.1 channels. Circ. Res. 2009, 105, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Shang, L.L.; Sanyal, S.; Pfahnl, A.E.; Jiao, Z.; Allen, J.; Liu, H.; Dudley, S.C.J. NF-kappaB-dependent transcriptional regulation of the cardiac scn5a sodium channel by angiotensin II. Am. J. Physiol. Physiol. 2007, 294, C372–C379. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, R.; Komuro, I.; Yamazaki, T.; Zou, Y.; Kudoh, S.; Tanaka, M.; Shiojima, I.; Hiroi, Y.; Yazaki, Y. Oxidative stress activates extracellular signal-regulated kinases through Src and Ras in cultured cardiac myocytes of neonatal rats. J. Clin. Investig. 1997, 100, 1813–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kieken, F.; Mutsaers, N.; Dolmatova, E.; Virgil, K.; Wit, A.L.; Kellezi, A.; Hirst-Jensen, B.J.; Duffy, H.S.; Sorgen, P.L. Structural and molecular mechanisms of gap junction remodeling in epicardial border zone myocytes following myocardial infarction. Circ. Res. 2009, 104, 1103–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sovari, A.A.; Iravanian, S.; Dolmatova, E.; Jiao, Z.; Liu, H.; Zandieh, S.; Kumar, V.; Wang, K.; Bernstein, K.; Bonini, M.G.; et al. Inhibition of c-Src tyrosine kinase prevents angiotensin II–mediated connexin-43 remodeling and sudden cardiac death. J. Am. Coll. Cardiol. 2011, 58, 2332–2339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorov, D.B.; Filburn, C.R.; Klotz, L.-O.; Zweier, J.L.; Sollott, S.J. Reactive Oxygen Species (Ros-Induced) Ros Release. J. Exp. Med. 2000, 192, 1001–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [Green Version]
- Biary, N.; Xie, C.; Kauffman, J.; Akar, F.G. Biophysical properties and functional consequences of reactive oxygen species (ROS)-induced ROS release in intact myocardium. J. Physiol. 2011, 589, 5167–5179. [Google Scholar] [CrossRef]
- Le Grand, B.; Hatem, S.N.; Le Heuzey, J.-Y.; Deroubaix, E.; Benitah, J.-P.; Coraboeuf, E. Pro-arrhythmic effect of nicorandil in isolated rabbit atria and its suppression by tolbutamide and quinidine. Eur. J. Pharmacol. 1992, 229, 91–96. [Google Scholar] [CrossRef]
- Aidonidis, I.; Poyatzi, A.; Stamatiou, G.; Lymberi, M.; Stamatoyannis, N.; Molyvdas, P.-A. Dose-related shortening of ventricular tachycardia cycle length after administration of the KATP channel opener bimakalim in a 4-day-old chronic infarct anesthetized pig model. J. Cardiovasc. Pharmacol. Ther. 2009, 14, 222–230. [Google Scholar] [CrossRef]
- O’Rourke, B.; Ramza, B.; Marban, E. Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science 1994, 265, 962–966. [Google Scholar] [CrossRef]
- Brown, D.A.; O’Rourke, B. Cardiac mitochondria and arrhythmias. Cardiovasc. Res. 2010, 88, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Akar, F.G.; Aon, M.A.; Tomaselli, G.F.; O’Rourke, B. The mitochondrial origin of postischemic arrhythmias. J. Clin. Investig. 2005, 115, 3527–3535. [Google Scholar] [CrossRef]
- Xiao, X.-H.; Holley, L.K. Reducing electrical defibrillation thresholds with glibenclamide in an isolated rabbit heart preparation. J. Cardiovasc. Pharmacol. 1997, 30, 576–582. [Google Scholar] [CrossRef] [PubMed]
- E Billman, G.; Englert, H.C.; A Schölkens, B. HMR 1883, a novel cardioselective inhibitor of the ATP-sensitive potassium channel. Part II: Effects on susceptibility to ventricular fibrillation induced by myocardial ischemia in conscious dogs. J. Pharmacol. Exp. Ther. 1998, 286, 1465–1473. [Google Scholar]
- Billman, G.E.; Houle, M.S.; Englert, H.C.; Gögelein, H. Effects of a Novel Cardioselective ATP-Sensitive Potassium Channel Antagonist, 1-[[5-[2-(5-Chloro-o-anisamido)ethyl]-β-methoxyethoxyphenyl]sulfonyl]-3-methylthiourea, Sodium Salt (HMR 1402), on Susceptibility to Ventricular Fibrillation Induced by Myocardial Ischemia: In Vitro and in Vivo Studies. J. Pharmacol. Exp. Ther. 2004, 309, 182–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, T.; Takizawa, T.; Saito, T.; Kobayashi, S.; Hara, Y.; Nakaya, H. Amiodarone inhibits sarcolemmal but not mitochondrial KATP channels in guinea pig ventricular cells. J. Pharmacol. Exp. Ther. 2003, 307, 955–960. [Google Scholar] [CrossRef] [Green Version]
- Cacciapuoti, F.; Spiezia, R.; Bianchi, U.; Lama, D.; D’Avino, M.; Varricchio, M. Effectiveness of glibenclamide on myocardial ischemic ventricular arrhythmias in non-insulin-dependent diabetes mellitus. Am. J. Cardiol. 1991, 67, 843–847. [Google Scholar] [CrossRef]
- Chicco, A.J.; Johnson, M.S.; Armstrong, C.J.; Lynch, J.M.; Gardner, R.T.; Fasen, G.S.; Gillenwater, C.P.; Moore, R. Sex-specific and exercise-acquired cardioprotection is abolished by sarcolemmal KATP channel blockade in the rat heart. Am. J. Physiol. Circ. Physiol. 2007, 292, H2432–H2437. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.A.; Chicco, A.J.; Jew, K.N.; Johnson, M.S.; Lynch, J.M.; Watson, P.A.; Moore, R. Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not the mitochondrial, isoform of the KATP channel in the rat. J. Physiol. 2005, 569, 913–924. [Google Scholar] [CrossRef]
- Leducq, N.; Bono, F.; Sulpice, T.; Vin, V.É.; Janiak, P.; Le Fur, G.; O’Connor, S.E.; Herbert, J.-M. Role of peripheral benzodiazepine receptors in mitochondrial, cellular, and cardiac damage induced by oxidative stress and ischemia-reperfusion. J. Pharmacol. Exp. Ther. 2003, 306, 828–837. [Google Scholar] [CrossRef] [Green Version]
- Morin, D.; Musman, J.; Pons, S.; Berdeaux, A.; Ghaleh, B. Mitochondrial translocator protein (TSPO): From physiology to cardioprotection. Biochem. Pharmacol. 2016, 105, 1–13. [Google Scholar] [CrossRef]
- Mestre, M.; Bouetard, G.; Uzan, A.; Gueremy, C.; Renault, C.; Dubroeucq, M.-C.; Le Fur, G. PK 11195, an antagonist of peripheral benzodiazepine receptors, reduces ventricular arrhythmias during myocardial ischemia and reperfusion in the dog. Eur. J. Pharmacol. 1985, 112, 257–260. [Google Scholar] [CrossRef]
- Vegh, A.; Parratt, J.R. The role of mitochondrial K(ATP) channels in antiarrhythmic effects of ischaemic preconditioning in dogs. Br. J. Pharmacol. 2002, 137, 1107–1115. [Google Scholar] [CrossRef] [Green Version]
- Matejíková, J.; Kucharská, J.; Pintérová, M.; Pancza, D.; Ravingerová, T. Protection against ischemia-induced ventricular arrhythmias and myocardial dysfunction conferred by preconditioning in the rat heart: Involvement of mitochondrial K(ATP) channels and reactive oxygen species. Physiol. Res. 2008, 58, 9–19. [Google Scholar] [PubMed]
- Liu, Q.; Yao, J.-Y.; Qian, C.; Chen, R.; Li, X.-Y.; Liu, S.-W.; Sun, B.-G.; Song, L.-S.; Hong, J. Effects of propofol on ischemia-induced ventricular arrhythmias and mitochondrial ATP-sensitive potassium channels. Acta Pharmacol. Sin. 2012, 33, 1495–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imani, A.; Faghihi, M.; Sadr, S.S.; Keshavarz, M.; Niaraki, S.S. Noradrenaline reduces ischemia-induced arrhythmia in anesthetized rats: Involvement of α1-adrenoceptors and mitochondrial KATP channels. J. Cardiovasc. Electrophysiol. 2008, 19, 309–315. [Google Scholar] [CrossRef]
- Headrick, J.P.; Willems, L.; Ashton, K.J.; Holmgren, K.; Peart, J.; Matherne, G.P. Ischaemic tolerance in aged mouse myocardium: The role of adenosine and effects of a1adenosine receptor overexpression. J. Physiol. 2003, 549, 823–833. [Google Scholar] [CrossRef]
- Fryer, R.M.; Hsu, A.K.; Nagase, H.; Gross, G.J. Opioid-induced cardioprotection against myocardial infarction and arrhythmias: Mitochondrial versus sarcolemmal ATP-sensitive potassium channels. J. Pharmacol. Exp. Ther. 2000, 294, 451–457. [Google Scholar]
- Fischbach, P.S.; Barrett, T.D.; Reed, N.J.; Lucchesi, B.R. SNC-80-induced preconditioning: Selective activation of the mitochondrial adenosine triphosphate-gated potassium channel. J. Cardiovasc. Pharmacol. 2003, 41, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Baharvand, B.; Esmailidehaj, M.; Rasoulian, B.; Namdari, M.; Shikhani, Y.; Kiani, A.A.; Baharvand-Ahmadi, B. Delayed anti-arrhythmic effect of nitroglycerin in anesthetized rats: Involvement of CGRP, PKC and mKATP channels. Int. J. Cardiol. 2009, 135, 187–192. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Incidence (%) | Duration (s) | ||
|---|---|---|---|---|
| Untreated | Atenolol-Treated | Untreated | Atenolol-Treated | |
| Ventricular premature beats | 100 | 100 | 1.98 ± 0.45 | 3.95 ± 1.44 |
| Bigemines | 73 | 77 | 5.23 ± 2.38 | 7.21 ± 2.11 |
| Trigemines | ND | 9 | ND | 0.19 ± 0.19 |
| Salvos | 63 | 65 | 0.54 ± 0.19 | 0.66 ± 0.25 |
| Ventricular tachycardia | 100 | 90 | 44.9 ± 17.70 | 32.3 ± 19.25 |
| Ventricular fibrillation | 70 | 75 | 274 ± 78.54 | 212 ± 95.24 |
| Parameters | Untreated | Atenolol-Treated | Losartan-Treated |
|---|---|---|---|
| Incidence (%) | 90 | 100 | 90 |
| Episodes (number) | 5 ± 1.9 | 14 ± 4.8 * | 8 ± 1.8 |
| Onset (s) | 21.5 ± 7.5 | 24.6 ± 8.8 | 16.3 ± 2.8 |
| Duration (s) | 1.2 ± 0.4 | 3.4 ± 1.2 | 2.0 ± 0.9 |
| QTc intervals (s) | 0.23 ± 0.007 | 0.22 ± 0.006 | 0.21 ± 0.008 |
| QRS interval (s) | 0.06 ± 0.002 | 0.06 ± 0.002 | 0.06 ± 0.001 |
| Parameters | Untreated | Vitamin E-Treated | N-Acetyl-l-Cysteine-Treated |
|---|---|---|---|
| Onset of arrhythmias (s) | 12 ± 1.2 | 18 ± 1.8 * | 20 ± 1.2 * |
| Duration of arrhythmias (s) | 145 ± 5.3 | 24 ± 1.6 * | 22 ± 0.8 * |
| Singles (number/5 min) | 22 ± 2.2 | 9 ± 0.7 * | 10 ± 1.1 * |
| Salvos (number/5 min) | 12 ± 1.0 | 3 ± 0.5 * | 5 ± 0.4 * |
| Ventricular tachycardia (number/5 min) | 45 ± 6.7 | 22 ± 3.6 * | 20 ± 3.2 * |
| MDA levels before epinephrine (µmol/L) | 1.53 ± 0.10 | 1.25 ± 0.08 * | 1.43 ± 0.03 |
| MDA levels after epinephrine (µmol/L) | 154 ± 8.21 | 67 ± 4.90 * | 21 ± 2.82 * |
| Parameters | Control | Untreated | Vitamin E—Treated |
|---|---|---|---|
| ST segment (mV) | 0.02 ± 0.003 | 0.11 ± 0.01 * | 0.04 ± 0.002 # |
| QTc interval (ms) | 340 ± 20 | 510 ± 26 * | 320 ± 18 # |
| Q wave appearance (%) | ND | 28 | 4.0 |
| PVC incidence (%) | ND | 18 | 4.0 |
| Conjugated dienes (µmol/mg tissue lipids) | 35.8 ± 2.7 | 62.2 ± 3.1 * | 42.5 ± 2.6 # |
| Malondialdehyde levels (µmol/mg tissue lipids) | 3.1 ± 0.2 | 4.8 ± 0.2 * | 2.6 ± 0.1 # |
| Parameters | Control | Untreated | Imidapril-Treated |
|---|---|---|---|
| ST segment (mV) | 0.03 ± 0.004 | 0.13 ± 0.02 * | 0.04 ± 0.003 # |
| QTc interval (ms) | 378 ± 14 | 548 ± 9 * | 423 ± 9 # |
| Q wave appearance (%) | ND | 25 | 25 |
| PVC incidence (%) | ND | 14 | 5 |
| QRS duration (ms) | 0.10 ± 0.01 | 0.09 ± 0.01 | 0.10 ± 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adameova, A.; Shah, A.K.; Dhalla, N.S. Role of Oxidative Stress in the Genesis of Ventricular Arrhythmias. Int. J. Mol. Sci. 2020, 21, 4200. https://doi.org/10.3390/ijms21124200
Adameova A, Shah AK, Dhalla NS. Role of Oxidative Stress in the Genesis of Ventricular Arrhythmias. International Journal of Molecular Sciences. 2020; 21(12):4200. https://doi.org/10.3390/ijms21124200
Chicago/Turabian StyleAdameova, Adriana, Anureet K. Shah, and Naranjan S. Dhalla. 2020. "Role of Oxidative Stress in the Genesis of Ventricular Arrhythmias" International Journal of Molecular Sciences 21, no. 12: 4200. https://doi.org/10.3390/ijms21124200
APA StyleAdameova, A., Shah, A. K., & Dhalla, N. S. (2020). Role of Oxidative Stress in the Genesis of Ventricular Arrhythmias. International Journal of Molecular Sciences, 21(12), 4200. https://doi.org/10.3390/ijms21124200





