Effect of Berberine on Glycation, Aldose Reductase Activity, and Oxidative Stress in the Lenses of Streptozotocin-Induced Diabetic Rats In Vivo—A Preliminary Study
Abstract
:1. Introduction
2. Results
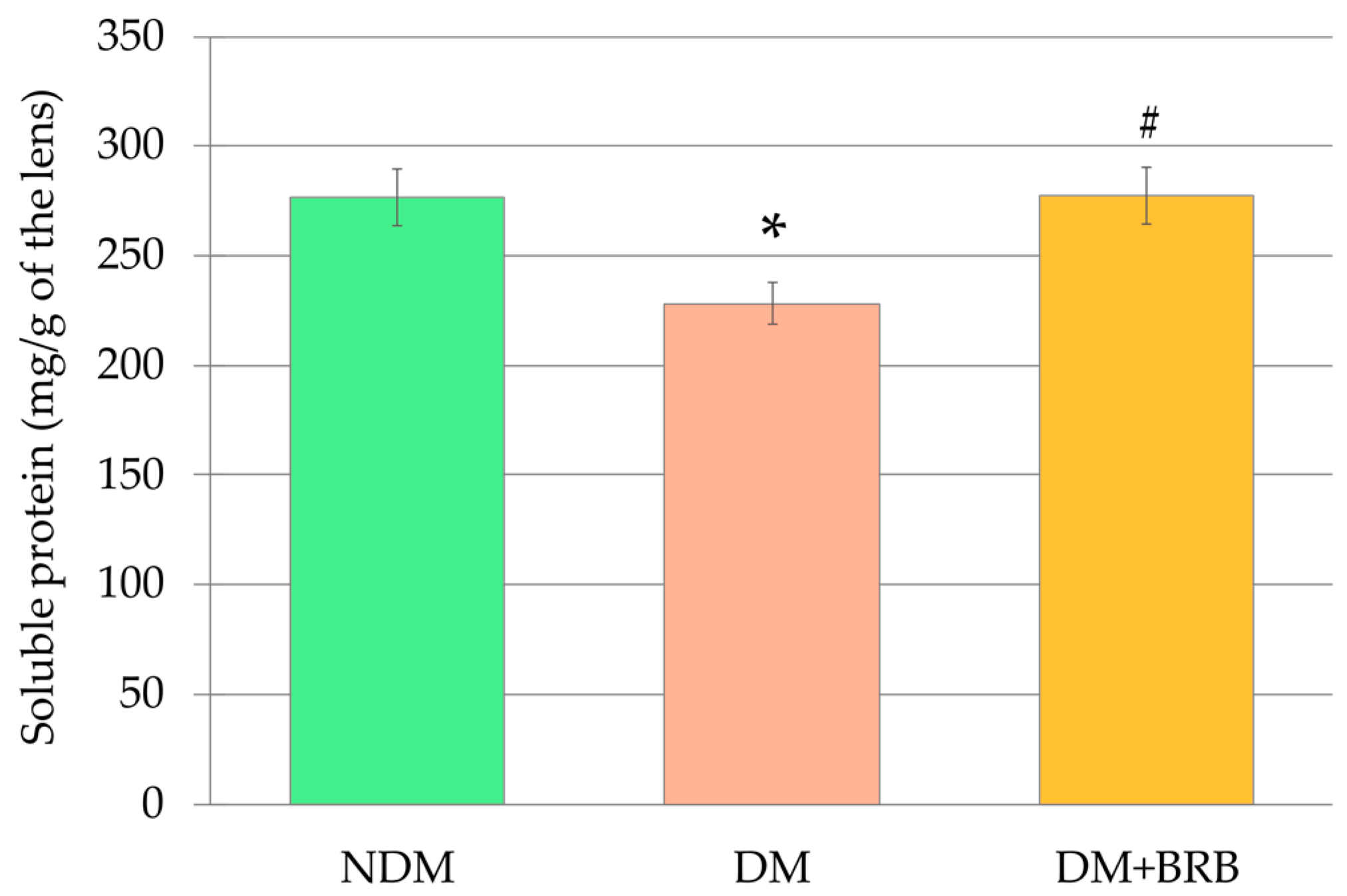
2.1. Effect of Berberine Administration on Lens Mass and Soluble Protein Level in the Lenses
2.2. Effect of Berberine Administration on the Advanced Glycation End-Products Level in the Lenses
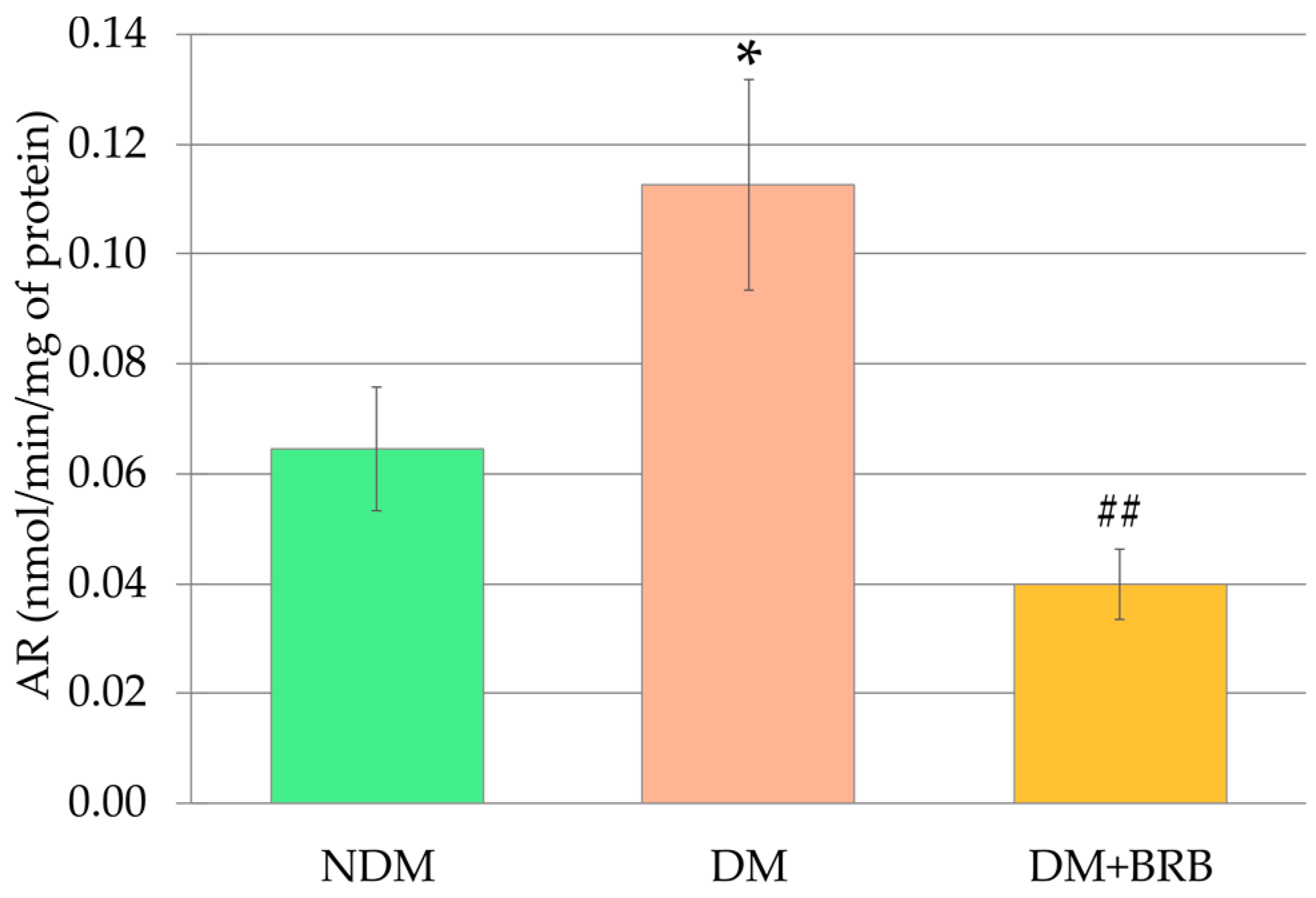
2.3. Effect of Berberine Administration on the Aldose Reductase Activity in the Lenses
2.4. Effect of Berberine Administration on the Activites of the Antioxidative Enzymes in the Lenses
2.5. Effect of Berberine Administration on the Non-Enzymatic Antioxidant Levels in the Lenses
2.6. Effect of Berberine Administration on Oxidative Damage Marker Levels in the Lenses
2.7. Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
- NDM: non-diabetic, control rats;
- DM: diabetic control rats;
- DM + BRB: diabetic rats treated orally (per os—p.o.) with berberine, at a dose of 50 mg/kg.
4.2. Soluble Protein Level in the Lenses
4.3. Advanced Oxidation End-Products in the Lenses
4.4. Activity of Enzymes in the Lenses
4.5. Vitamin C and Non-Protein Sulfhydryl Groups in the Lenses
4.6. Oxidative Damage Markers in the Lenses
4.7. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGEs | Advanced glycation end-products |
| AOPP | Advanced oxidation protein products |
| AR | Aldose reductase |
| CAT | Catalase |
| DM | Diabetic control rats |
| DM + BRB | Diabetic rats treated with berberine at a dose of 50 mg/kg p.o. |
| GPx | Glutathione peroxidase |
| GSH | Reduced glutathione |
| NDM | Non-diabetic control rats |
| NPSH | Non-protein sulfhydryl groups |
| PCA | Principal component analysis |
| PC 1, PC 2 | Principal component 1, principal component 2 |
| SOD | Superoxide dismutase |
| TAC | Total antioxidant capacity |
| TBARS | Thiobarbituric acid reactive substances |
Appendix A

References
- Kumar, A.; Ekavali; Chopra, K.; Mukherjee, M.; Pottabathini, R.; Dhull, D.K. Current knowledge and pharmacological profile of berberine: An update. Eur. J. Pharmacol. 2015, 761, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.P.; Mahajan, S. Berberine and its derivatives: A patent review (2009–2012). Expert Opin. Ther. Pat. 2013, 23, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Feng, X.; Chai, L.; Cao, S.; Qiu, F. The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab. Rev. 2017, 49, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Khadka, D.B.; Cho, W.-J. Pharmacological effects of berberine and its derivatives: A patent update. Expert Opin. Ther. Pat. 2016, 26, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Vuddanda, P.R.; Chakraborty, S.; Singh, S. Berberine: A potential phytochemical with multispectrum therapeutic activities. Expert Opin. Investig. Drugs 2010, 19, 1297–1307. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Tartagni, E. Antidiabetic properties of berberine: From cellular pharmacology to clinical effects. Hosp. Pract. 2012, 40, 56–63. [Google Scholar] [CrossRef]
- Tabeshpour, J.; Imenshahidi, M.; Hosseinzadeh, H. A review of the effects of berberis vulgaris and its major component, berberine, in metabolic syndrome. Iran. J. Basic Med. Sci. 2017, 20, 557–568. [Google Scholar]
- Ahmed, T.; Gilani, A.U.H.; Abdollahi, M.; Daglia, M.; Nabavi, S.F.; Nabavi, S.M. Berberine and neurodegeneration: A review of literature. Pharmacol. Reports 2015, 67, 970–979. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, C.; Yang, W. Role of berberine in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2016, 12, 2509–2520. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Sheng, J.; Li, G.; Zhao, L.; Wang, Y.; Yang, W.; Yao, X.; Sun, L.; Zhang, Z.; Cui, R. Effects of berberine and its derivatives on cancer: A systems pharmacology review. Front. Pharmacol. 2020, 10, 1461. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Zhang, K.; Jin, Y.; Li, B.; Gao, S.; Zhu, J.; Cui, R. Pharmacological effects of berberine on mood disorders. J. Cell. Mol. Med. 2019, 23, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Zhao, L.-H.; Zhou, Q.; Zhao, T.-Y.; Wang, H.; Gu, C.-J.; Tong, X.-L. Application of berberine on treating type 2 diabetes mellitus. Int. J. Endocrinol. 2015, 2015, 905749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Sureda, A.; Jafari, S.; Memariani, Z.; Tewari, D.; Annunziata, G.; Barrea, L.; Hassan, S.T.S.; Smejkal, K.; Malaník, M.; et al. Berberine in cardiovascular and metabolic diseases: From mechanisms to therapeutics. Theranostics 2019, 9, 1923–1951. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xiao, Y.; Yin, J.; Hou, W.; Yu, X.; Shen, L.; Liu, F.; Wei, L.; Jia, W. Berberine promotes glucose consumption independently of AMP-activated protein kinase activation. PLoS ONE 2014, 9, e103702. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Pan, Y.; Xu, L.; Tang, D.; Dorfman, R.G.; Zhou, Q.; Yin, Y.; Li, Y.; Zhou, L.; Zhao, S.; et al. Berberine promotes glucose uptake and inhibits gluconeogenesis by inhibiting deacetylase SIRT3. Endocrine 2018, 62, 576–587. [Google Scholar] [CrossRef]
- Yin, J.; Gao, Z.; Liu, D.; Liu, Z.; Ye, J. Berberine improves glucose metabolism through induction of glycolysis. Am. J. Physiol. - Endocrinol. Metab. 2008, 294, E148–E156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Hao, G.; Zhang, Q.; Hua, W.; Wang, M.; Zhou, W.; Zong, S.; Huang, M.; Wen, X. Berberine induces GLP-1 secretion through activation of bitter taste receptor pathways. Biochem. Pharmacol. 2015, 97, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Cok, A.; Plaisier, C.; Salie, M.J.; Oram, D.S.; Chenge, J.; Louters, L.L. Berberine acutely activates the glucose transport activity of GLUT1. Biochimie 2011, 93, 1187–1192. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhao, Y.; Zhang, M.; Pang, X.; Xu, J.; Kang, C.; Li, M.; Zhang, C.; Zhang, Z.; Zhang, Y.; et al. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS ONE 2012, 7, e42529. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, X.; Zou, D.; Liu, W.; Yang, J.; Zhu, N.; Huo, L.; Wang, M.; Hong, J.; Wu, P.; et al. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J. Clin. Endocrinol. Metab. 2008, 93, 2559–2565. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Rubio, K.G.; González-Ortiz, M.; Martínez-Abundis, E.; Robles-Cervantes, J.A.; Espinel-Bermúdez, M.C. Effect of berberine administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab. Syndr. Relat. Disord. 2013, 11, 366–369. [Google Scholar] [CrossRef]
- Ni, W.J.; Ding, H.H.; Tang, L.Q. Berberine as a promising anti-diabetic nephropathy drug: An analysis of its effects and mechanisms. Eur. J. Pharmacol. 2015, 760, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zhang, S.; Chen, Z.; Zhang, R.; Tian, L.; Cheng, L.; Shang, F.; Sun, J. Berberine could ameliorate cardiac dysfunction via interfering myocardial lipidomic profiles in the rat model of diabetic cardiomyopathy. Front. Physiol. 2018, 9, 1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.O.; Kim, H.J. Berberine ameliorates cold and mechanical allodynia in a rat model of diabetic neuropathy. J. Med. Food 2013, 16, 511–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Zhou, S. Effect of berberine on PPAR α/δ/γ expression in type 2 diabetic rat retinae. Acta Pharm. Sin. Yao Xue Xue Bao 2007, 42, 1243–1249. [Google Scholar]
- Payne, A.J.; Kaja, S.; Naumchuk, Y.; Kunjukunju, N.; Koulen, P. Antioxidant drug therapy approaches for neuroprotection in chronic diseases of the retina. Int. J. Mol. Sci. 2014, 15, 1865–1886. [Google Scholar] [CrossRef] [Green Version]
- Rhone, M.; Basu, A. Phytochemicals and age-related eye diseases. Nutr. Rev. 2008, 66, 465–472. [Google Scholar] [CrossRef]
- Jang, M.H.; Kim, H.Y.; Kang, K.S.; Yokozawa, T.; Park, J.H. Hydroxyl radical scavenging activities of isoquinoline alkaloids isolated from Coptis chinensis. Arch. Pharm. Res. 2009, 32, 341–345. [Google Scholar] [CrossRef]
- Chandirasegaran, G.; Elanchezhiyan, C.; Ghosh, K. Modulatory effects of berberine chloride on lipid profile, oxidant status and insulin signaling molecules in streptozotocin induced diabetic rats. Indian J. Clin. Biochem. 2019, 34, 254–262. [Google Scholar] [CrossRef]
- Hasanein, P.; Ghafari-Vahed, M.; Khodadadi, I. Effects of isoquinoline alkaloid berberine on lipid peroxidation, antioxidant defense system, and liver damage induced by lead acetate in rats. Redox Rep. Commun. 2017, 22, 42–50. [Google Scholar] [CrossRef]
- Li, Z.; Geng, Y.-N.; Jiang, J.-D.; Kong, W.-J. Antioxidant and anti-inflammatory activities of Berberine in the treatment of diabetes mellitus. Evidence-based Complement. Altern. Med. 2014, 2014, 289264. [Google Scholar] [CrossRef] [PubMed]
- Imanshahidi, M.; Hosseinzadeh, H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phyther. Res. 2008, 22, 999–1012. [Google Scholar] [CrossRef]
- Mahajan, V.M.; Sharma, A.; Rattan, A. Antimycotic activity of berberine sulphate: An alkaloid from an indian medicinal herb. Sabouraudia 1982, 20, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Chignell, C.F.; Sik, R.H.; Watson, M.A.; Wielgus, A.R. Photochemistry and photocytotoxicity of alkaloids from goldenseal (Hydrastis canadensis L.) 3. Effect on human lens and retinal pigment epithelial cells. Photochem. Photobiol. 2007, 83, 938–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, D.; Song, J.; Wang, C.; Li, Y.; Dunaief, J.L. Berberine protects against light-induced photoreceptor degeneration in the mouse retina. Exp. Eye Res. 2016, 145, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Gaur, U.; Chong, C.M.; Lin, S.; Fang, J.; Zeng, Z.; Wang, H.; Zheng, W. Berberine protects human retinal pigment epithelial cells from hydrogen peroxide-induced oxidative damage through activation of AMPK. Int. J. Mol. Sci. 2018, 19, 1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Ji, Y.; Yan, X.; Su, G.; Chen, L.; Xiao, J. Berberine attenuates apoptosis in rat retinal Müller cells stimulated with high glucose via enhancing autophagy and the AMPK/mTOR signaling. Biomed. Pharmacother. 2018, 108, 1201–1207. [Google Scholar] [CrossRef]
- Fu, D.; Yu, J.Y.; Connell, A.R.; Yang, S.; Hookham, M.B.; McLeese, R.; Lyons, T.J. Beneficial effects of berberine on oxidized LDL-induced cytotoxicity to human retinal Müller cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3369–3379. [Google Scholar] [CrossRef] [Green Version]
- Gehlot, V.; Dave, K.; Goyal, S.; Chounhary, N. Berberine from roots of Berberis aristata prevents cataract formation in isolated goat eye lens: An in-vitro study. Int. J. Pharm. Biol. Arch. 2012, 3, 1265–1270. [Google Scholar]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef]
- King, A.J.F. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, M.; Ong, L.; Smith, M.T.; Ross, F.B.; Schmid, K.; Hoey, A.J.; Burstow, D.; Brown, L. The streptozotocin-diabetic rat as a model of the chronic complications of human diabetes. Hear. Lung Circ. 2003, 12, 44–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrilatha, B.; Muralidhara. Early oxidative stress in testis and epididymal sperm in streptozotocin-induced diabetic mice: Its progression and genotoxic consequences. Reprod. Toxicol. 2007, 23, 578–587. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas Ninth Edition 2019, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; ISBN 978-2-930229-87-4. [Google Scholar]
- Fowler, M.J. Microvascular and macrovascular complications of diabetes. Clin. Diabetes 2011, 29, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Vieira-Potter, V.J.; Karamichos, D.; Lee, D.J. Ocular complications of diabetes and therapeutic approaches. Biomed Res. Int. 2016, 2016, 3801570. [Google Scholar] [CrossRef] [Green Version]
- Kiziltoprak, H.; Tekin, K.; Inanc, M.; Goker, Y.S. Cataract in diabetes mellitus. World J. Diabetes 2019, 10, 140–153. [Google Scholar] [CrossRef]
- Lim, J.C.; Umapathy, A.; Grey, A.C.; Vaghe, E.; Donaldson, P.J. Novel roles for the lens in preserving overall ocular health. Exp. Eye Res. 2017, 156, 117–123. [Google Scholar] [CrossRef]
- Kaur, A.; Gupta, V.; Christopher, A.F.; Malik, M.A.; Bansal, P. Nutraceuticals in prevention of cataract—An evidence based approach. Saudi J. Ophthalmol. 2017, 31, 30–37. [Google Scholar] [CrossRef]
- Sella, R.; Afshari, N.A. Nutritional effect on age-related cataract formation and progression. Curr. Opin. Ophthalmol. 2019, 30, 63–69. [Google Scholar] [CrossRef]
- Liu, W.; Hei, Z.; Nie, H.; Tang, F.; Huang, H.; Li, X.; Deng, Y.; Chen, S.; Guo, F.; Huang, W.; et al. Berberine ameliorates renal injury in streptozotocin-induced diabetic rats by suppression of both oxidative stress and aldose reductase. Chin. Med. J. (Engl.) 2008, 121, 706–712. [Google Scholar] [CrossRef]
- Qiu, Y.; Tang, L.; Wei, W. Berberine exerts renoprotective effects by regulating the AGEs-RAGE signaling pathway in mesangial cells during diabetic nephropathy. Mol. Cell. Endocrinol. 2017, 443, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-W.; Seol, I.-C.; Son, C.-G. Interpretation of animal dose and human equivalent dose for drug development. J. Korean Orient. Med. 2010, 31, 1–7. [Google Scholar]
- Shibata, S.; Natori, Y.; Nishihara, T.; Tomisaka, K.; Matsumoto, K.; Sansawa, H.; Nguen, C. Antioxidant and anti-cataract effect of Chlorella on rats with streptozotocin-induced diabetes. J. Nutr. Sci. Vitaminol. (Tokyo) 2003, 49, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, F.; Bathaie, S.Z.; Aldavood, S.J.; Ghahghaei, A. Glycine therapy inhibits the progression of cataract in streptozotocin-induced diabetic rats. Mol. Vis. 2012, 18, 439–448. [Google Scholar] [PubMed]
- Sengupta, P. The laboratory rat: Relating its age with human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar] [PubMed]
- Pollreisz, A.; Schmidt-Erfurth, U. Diabetic cataract—pathogenesis, epidemiology and treatment. J. Ophthalmol. 2010, 2010, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Falck, A.; Laatikainen, L. Diabetic cataract in children. Acta Ophthalmol. Scand. 1998, 76, 238–240. [Google Scholar] [CrossRef]
- Khorsand, M.; Akmali, M.; Sharzad, S.; Beheshtitabar, M. Melatonin reduces cataract formation and aldose reductase activity in lenses of streptozotocin-induced diabetic rat. Iran. J. Med. Sci. 2016, 41, 305–313. [Google Scholar] [PubMed]
- Kilari, E.K.; Putta, S. Delayed progression of diabetic cataractogenesis and retinopathy by Litchi chinensis in STZ-induced diabetic rats. Cutan. Ocul. Toxicol. 2017, 36, 52–59. [Google Scholar] [CrossRef]
- Pradeep, S.R.; Srinivasan, K. Ameliorative influence of dietary fenugreek (Trigonella foenum-graecum) seeds and onion (Allium cepa) on eye lens abnormalities via modulation of crystallin proteins and polyol pathway in experimental diabetes. Curr. Eye Res. 2018, 43, 1108–1118. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, Q.; Tan, S. Inhibitory effect of r-hirudin variant III on streptozotocin-induced diabetic cataracts in rats. Sci. World J. 2013, 2013, 630651. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S. Rat lens aldose reductase inhibitory activities of Coptis japonica root-derived isoquinoline alkaloids. J. Agric. Food Chem. 2002, 50, 7013–7016. [Google Scholar] [CrossRef]
- Paul, M.; Hemshekhar, M.; Kemparaju, K.; Girish, K.S. Free Radical Biology and Medicine Berberine mitigates high glucose-potentiated platelet aggregation and apoptosis by modulating aldose reductase and NADPH oxidase activity. Free Radic. Biol. Med. 2019, 130, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, P.; Tao, S.; Deng, Y.; Li, X.; Lan, T.; Zhang, X.; Guo, F.; Huang, W.; Chen, F.; et al. Berberine inhibits aldose reductase and oxidative stress in rat mesangial cells cultured under high glucose. Arch. Biochem. Biophys. 2008, 475, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, E.; Taylor, A. Too sweet: Problems of protein glycation in the eye. Exp. Eye Res. 2019, 178, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N. Advanced glycation endproducts—Role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. [Google Scholar] [CrossRef]
- Rowan, S.; Bejarano, E.; Taylor, A. Mechanistic targeting of advanced glycation end-products in age-related diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3631–3643. [Google Scholar] [CrossRef] [PubMed]
- Kuhla, B.; Haase, C.; Flach, K.; Lüth, H.J.; Arendt, T.; Münch, G. Effect of pseudophosphorylation and cross-linking by lipid peroxidation and advanced glycation end product precursors on tau aggregation and filament formation. J. Biol. Chem. 2007, 282, 6984–6991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, H.; Mitsuhashi, T.; Kuwajima, S.; Aoki, S.; Kuroda, Y.; Nagakawa, S. Immunochemical detection of advanced glycation end products in lens crystallins from streptozocin-induced diabetic rat. Diabetes 1993, 42, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Aspects Med. 2009, 30, 42–59. [Google Scholar] [CrossRef] [Green Version]
- Pastore, A.; Federici, G.; Bertini, E.; Piemonte, F. Analysis of glutathione: Implication in redox and detoxification. Clin. Chim. Acta 2003, 333, 19–39. [Google Scholar] [CrossRef]
- Umapathy, A.; Li, B.; Donaldson, P.J.; Lim, J.C. Functional characterisation of glutathione export from the rat lens. Exp. Eye Res. 2018, 166, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Monnier, V.M.; Whitson, J. Lens glutathione homeostasis: Discrepancies and gaps in knowledge standing in the way of novel therapeutic approaches. Exp. Eye Res. 2017, 156, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Wojnar, W.; Zych, M.; Kaczmarczyk-Sedlak, I. Wpływ naryngeniny na odpowiedź antyoksydacyjną oraz status oksydacyjny w soczewkach szczurów z cukrzycą. Herbalism 2018, 1, 17–30. [Google Scholar]
- Hao, M.; Li, S.; Sun, C.; Lin, Y.; Liu, K.; Wang, L.; Li, C.; Zhou, Q.; Du, J.; Li, H. Amelioration effects of berberine on diabetic microendothelial injury model by the combination of high glucose and advanced glycation end products in vitro. Eur. J. Pharmacol. 2011, 654, 320–325. [Google Scholar] [CrossRef]
- Zhao, W.; Devamanoharan, P.S.; Henein, M.; Ali, A.H.; Varma, S.D. Diabetes-induced biochemical changes in rat lens: Attenuation of cataractogenesis by pyruvate. Diabetes, Obes. Metab. 2000, 2, 165–174. [Google Scholar] [CrossRef]
- Wojnar, W.; Kaczmarczyk-Sedlak, I.; Zych, M. Diosmin ameliorates the effects of oxidative stress in lenses of streptozotocin-induced type 1 diabetic rats. Pharmacol. Reports 2017, 69, 995–1000. [Google Scholar] [CrossRef]
- Wojnar, W.; Zych, M.; Kaczmarczyk-Sedlak, I. Antioxidative effect of flavonoid naringenin in the lenses of type 1 diabetic rats. Biomed. Pharmacother. 2018, 108, 974–984. [Google Scholar] [CrossRef]
- Piwowar, A. Zaawansowane produkty utleniania białek. Część I. Mechanizm powstawania, struktura i właściwości. Pol. Merkur. Lek. 2010, 28, 166–169. [Google Scholar]
- Yamamoto, Y.; Yamamoto, H. Controlling the receptor for advanced glycation end-products to conquer diabetic vascular complications. J. Diabetes Investig. 2012, 3, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Colombo, G.; Clerici, M.; Giustarini, D.; Portinaro, N.; Badalamenti, S.; Rossi, R.; Milzani, A.; Dalle-Donne, I. A central role for intermolecular dityrosine cross-linking of fibrinogen in high molecular weight advanced oxidation protein product (AOPP) formation. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayana, P.; Saraswat, M.; Petrash, J.M.; Reddy, G.B. Emblica officinalis and its enriched tannoids delay streptozotocin-induced diabetic cataract in rats. Mol. Vis. 2007, 13, 1291–1297. [Google Scholar] [PubMed]
- Wojnar, W.; Zych, M.; Borymski, S.; Kaczmarczyk-Sedlak, I. Chrysin reduces oxidative stress but does not affect polyol pathway in the lenses of type 1 diabetic rats. Antioxidants 2020, 9, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedlak, L.; Wojnar, W.; Zych, M.; Wyględowska-Promieńska, D.; Mrukwa-Kominek, E.; Kaczmarczyk-Sedlak, I. Effect of resveratrol, a dietary-derived polyphenol, on the oxidative stress and polyol pathway in the lens of rats with streptozotocin-induced diabetes. Nutrients 2018, 10, 1423. [Google Scholar] [CrossRef] [Green Version]
- Hashim, Z.; Zarina, S. Osmotic stress induced oxidative damage: Possible mechanism of cataract formation in diabetes. J. Diabetes Complications 2012, 26, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 175–190. [Google Scholar] [CrossRef]
- Olofsson, E.M.; Marklund, S.L.; Behndig, A. Enhanced diabetes-induced cataract in copper-zinc superoxide dismutase-null mice. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2913–2918. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Miao, Y.Q.; Fan, D.J.; Yang, S.S.; Lin, X.; Meng, L.K.; Tang, X. Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats. AAPS PharmSciTech 2011, 12, 705–711. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.Y.; Lin, L.C.; Tseng, T.Y.; Wang, S.C.; Tsai, T.H. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 853, 183–189. [Google Scholar] [CrossRef]
- Radomska-Leśniewska, D.M.; Osiecka-Iwan, A.; Hyc, A.; Góźdź, A.; Dąbrowska, A.M.; Skopiński, P. Therapeutic potential of curcumin in eye diseases. Cent. Eur. J. Immunol. 2019, 44, 181–189. [Google Scholar] [CrossRef]
- Suryanarayana, P.; Saraswat, M.; Mrudula, T.; Krishna, T.P.; Krishnaswamy, K.; Reddy, G.B. Curcumin and turmeric delay streptozotocin-induced diabetic cataract in rats. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2092–2099. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, Y.; Oka, M.; Bando, M.; Takehana, M. Hesperetin prevents selenite-induced cataract in rats. Mol. Vis. 2015, 21, 804–810. [Google Scholar] [PubMed]
- Nakazawa, Y.; Nagai, N.; Ishimori, N.; Oguchi, J.; Tamura, H. Administration of antioxidant compounds affects the lens chaperone activity and prevents the onset of cataracts. Biomed. Pharmacother. 2017, 95, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Madany, J. Serum malondialdehyde level and activity of total antioxidant status of dogs with age-related cataract. Pol. J. Vet. Sci. 2016, 19, 429–431. [Google Scholar] [CrossRef] [Green Version]
- Folwarczna, J.; Kisiel, E.; Kocik, S.; Londzin, P.; Trawczyński, M.; Janas, A. Effects of berberine and diosgenin on the skeletal system in rats with experimental type 1 diabetes. In Proceedings of the 20th International Congress of the Polish Pharmacological Society, Lublin, Poland, 5–7 June 2019; pp. 131–132. [Google Scholar]
- Hayman, S.; Kinoshita, J.H. Isolation and properties of lLens aldose reductase. J. Biol. Chem. 1965, 240, 877–882. [Google Scholar] [PubMed]
- Halder, N.; Joshi, S.; Gupta, S.K. Lens aldose reductase inhibiting potential of some indigenous plants. J. Ethnopharmacol. 2003, 86, 113–116. [Google Scholar] [CrossRef]
- Patel, D.K.; Kumar, R.; Kumar, M.; Sairam, K.; Hemalatha, S. Evaluation of in vitro aldose reductase inhibitory potential of different fraction of Hybanthus enneaspermus Linn F. Muell. Asian Pac. J. Trop. Biomed. 2012, 2, 134–139. [Google Scholar] [CrossRef] [Green Version]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Jagota, S.K.; Dani, H.M. A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal. Biochem. 1982, 127, 178–182. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]






| Principal Component | NDM | DM | DM + BRB |
|---|---|---|---|
| PC 1 | −1.21 ± 0.38 | 2.01 ± 0.36 *** | −0.80 ± 0.47 ### |
| PC 2 | 0.77 ± 0.46 | −0.63 ± 0.37 * | −0.14 ± 0.32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zych, M.; Wojnar, W.; Kielanowska, M.; Folwarczna, J.; Kaczmarczyk-Sedlak, I. Effect of Berberine on Glycation, Aldose Reductase Activity, and Oxidative Stress in the Lenses of Streptozotocin-Induced Diabetic Rats In Vivo—A Preliminary Study. Int. J. Mol. Sci. 2020, 21, 4278. https://doi.org/10.3390/ijms21124278
Zych M, Wojnar W, Kielanowska M, Folwarczna J, Kaczmarczyk-Sedlak I. Effect of Berberine on Glycation, Aldose Reductase Activity, and Oxidative Stress in the Lenses of Streptozotocin-Induced Diabetic Rats In Vivo—A Preliminary Study. International Journal of Molecular Sciences. 2020; 21(12):4278. https://doi.org/10.3390/ijms21124278
Chicago/Turabian StyleZych, Maria, Weronika Wojnar, Magdalena Kielanowska, Joanna Folwarczna, and Ilona Kaczmarczyk-Sedlak. 2020. "Effect of Berberine on Glycation, Aldose Reductase Activity, and Oxidative Stress in the Lenses of Streptozotocin-Induced Diabetic Rats In Vivo—A Preliminary Study" International Journal of Molecular Sciences 21, no. 12: 4278. https://doi.org/10.3390/ijms21124278
APA StyleZych, M., Wojnar, W., Kielanowska, M., Folwarczna, J., & Kaczmarczyk-Sedlak, I. (2020). Effect of Berberine on Glycation, Aldose Reductase Activity, and Oxidative Stress in the Lenses of Streptozotocin-Induced Diabetic Rats In Vivo—A Preliminary Study. International Journal of Molecular Sciences, 21(12), 4278. https://doi.org/10.3390/ijms21124278





