Class I KNOX Is Related to Determinacy during the Leaf Development of the Fern Mickelia scandens (Dryopteridaceae)
Abstract
:1. Introduction
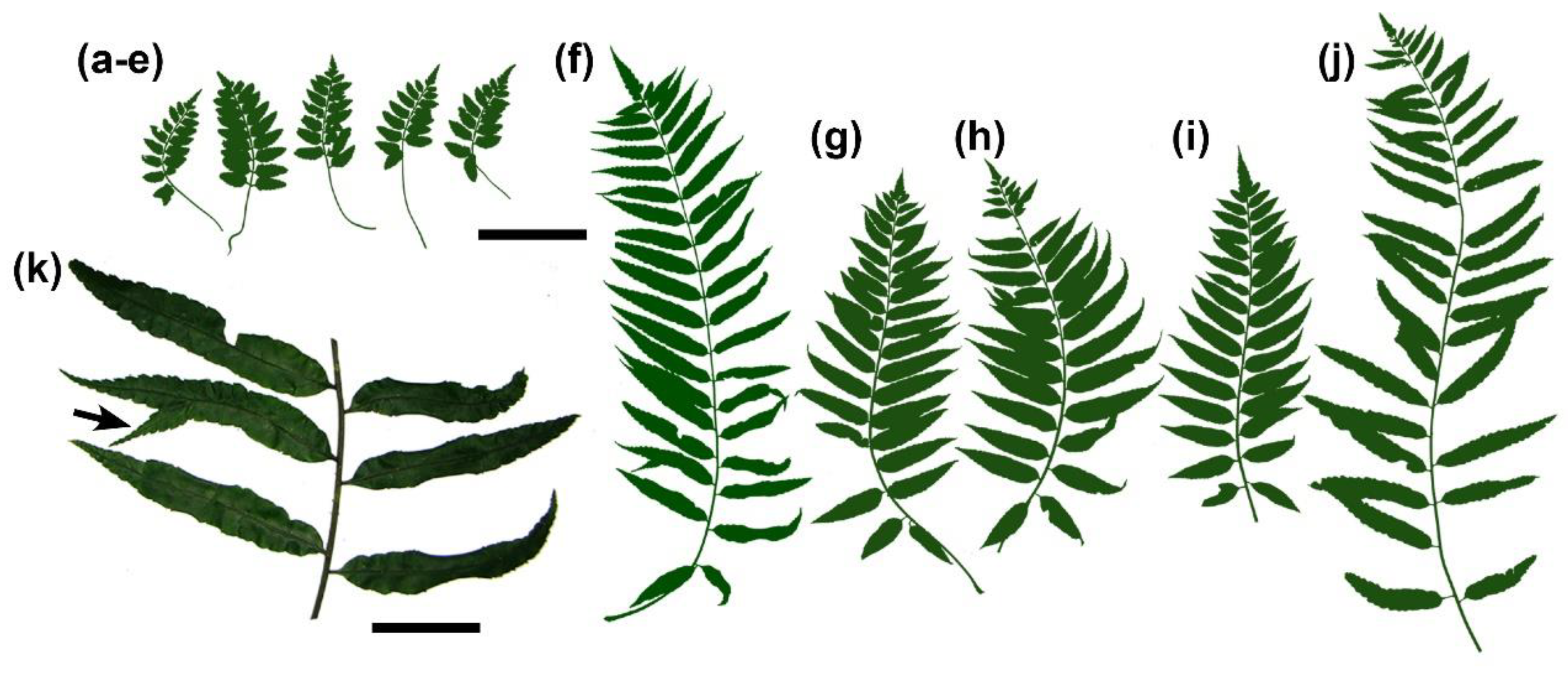
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. RNA extraction and cDNA Synthesis
4.3. Genes Isolation and Phylogenetic Analyses
4.4. Anatomy and In Situ Hybridization (ISH) Experiments
4.5. Quantitative Real-Time PCR
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Class I KNOX | Class I KNOTTED-LIKE HOMEOBOX |
| ISH | In-situ hybridization |
| LAM | Leaf apical meristem |
| MM | Marginal meristem |
| qRT-PCR | Real-time quantitative reverse transcription polymerase chain reaction |
| SAM | Shoot apical meristem |
References
- Kaplan, D.R. Fundamental concepts of leaf morphology and morphogenesis: A contribution to the interpratation of developmental mutants. Int. J. Plant Sci. 2001, 162, 465–474. [Google Scholar] [CrossRef]
- Dengler, N.G.; Tsukaya, H. Leaf Morphogenesis in Dicotyledons: Current Issues. Int. J. Plant Sci. 2001, 162, 459–464. [Google Scholar] [CrossRef] [Green Version]
- Frangedakis, E.; Saint-Marcoux, D.; Moody, L.A.; Rabbinowitsch, E.; Langdale, J.A. Nonreciprocal complementation of KNOX gene function in land plants. New Phytol. 2017, 216, 591–604. [Google Scholar] [CrossRef]
- Bürglin, T.R. Analysis of TALE superclass homeobox genes (MEIS, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic Acids Res. 1997, 25, 4173–4180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamant, O.; Pautot, V. Plant development: A TALE story. C. R. Biol. 2010, 333, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.G.; Greene, B.; Veit, B.; Hake, S. A dominant mutation in the maize homeobox gene, Knotted-1, causes its ectopic expression in leaf cells with altered fates. Development 1992, 116, 21–30. [Google Scholar] [PubMed]
- Bharathan, G.; Goliber, T.E.; Moore, C.; Kessler, S.; Pham, T.; Sinha, N.R. Homologies in leaf form inferred from KNOX1 gene expression during development. Science 2002, 296, 1858–1860. [Google Scholar] [CrossRef]
- Barton, M.K.; Poethig, R.S. Formation of the shoot apical meristem in Arabidopsis thaliana: An analysis of development in the wild type and in the shoot meristemless mutant. Development 1993, 119, 823–831. [Google Scholar]
- Endrizzi, K.; Moussian, B.; Haecker, A.; Levin, J.Z.; Laux, T. The SHOOT MERISTEMLESS gene is required for maintenance of undifferentiated cells in Arabidopsis shoot and acts at a different regulatory level than the meristem genes WUSCHEL and ZWILLE. Plant J. 1996, 10, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Kerstetter, R.A.; Laudencia-Chingcuanco, D.; Smith, L.G.; Hake, S. Loss-of-function mutations in the maize homeobox gene, knotted1, are defective in shoot meristem maintenance. Development 1997, 124, 3045–3054. [Google Scholar] [PubMed]
- Champagne, C.E.M.; Goliber, T.E.; Wojciechowski, M.F.; Mei, R.W.; Townsley, B.T.; Wang, K.; Paz, M.M.; Geeta, R.; Sinha, N.R. Compound Leaf Development and Evolution in the Legumes. Plant Cell 2007, 19, 3369–3378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hay, A.; Tsiantis, M. The genetic basis for differences in leaf form between Arabidopsis thaliana and its wild relative Cardamine hirsuta. Nat. Genet. 2006, 38, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Venglat, S.P.; Dumonceaux, T.; Rozwadowski, K.; Parnell, L.; Babic, V.; Keller, W.; Martienssen, R.; Selvaraj, G.; Datla, R. The homeobox gene BREVIPEDICELLUS is a key regulator of inflorescence architecture in Arabidopsis. Proc. Natl. Acad. Sci. USA 2002, 99, 4730–4735. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.; Sinha, N. Role of KNOX genes in shoot development of Welwitschia mirabilis. Int. J. Plant Sci. 2003, 164, 333–343. [Google Scholar] [CrossRef]
- Champagne, C.; Sinha, N. Compound leaves: Equal to the sum of their parts? Development 2004, 131, 4401–4412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hay, A.; Tsiantis, M. KNOX genes: Versatile regulators of plant development and diversity. Development 2010, 137, 3153–3165. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, D.W. The Cytohistological and Cytohistochemical Zonation of the Shoot Apex of Botrychium multifidum. Am. J. Bot. 1976, 63, 852–856. [Google Scholar] [CrossRef]
- Stevenson, D.W. Observations on Shoot Apices of Eusporangiate Ferns. Kew Bull. 1978, 33, 279. [Google Scholar] [CrossRef]
- White, R.A.; Turner, M.D. Anatomy and development of the fern sporophyte. Bot. Rev. 1995, 61, 281–305. [Google Scholar] [CrossRef]
- Ambrose, B.A.; Vasco, A. Bringing the multicellular fern meristem into focus. New Phytol. 2016, 210, 790–793. [Google Scholar] [CrossRef]
- Bierhorst, D.W. On the stem apex, leaf initiation and early leaf ontogeny in filicalean ferns. Am. J. Bot. 1977, 64, 125–152. [Google Scholar] [CrossRef]
- Wardlaw, C.W. Reflections on the unity of the embryonitc tissues in ferns. Phytomorphology 1958, 8, 323–327. [Google Scholar]
- Bower, F.O. The comparative examination of the meristems of Ferns, as a Phylogenetic Study. Ann. Bot. 1889, 3, 305–392. [Google Scholar] [CrossRef]
- Imaichi, R. Surface-viewed shoot apex of Angiopteris lygodiifolia Ros. (Marattiaceae). Bot. Mag. Tokyo 1986, 99, 309–317. [Google Scholar] [CrossRef]
- Sano, R.; Juárez, C.M.; Hass, B.; Sakakibara, K.; Ito, M.; Banks, J.A.; Hasebe, M. KNOX homeobox genes potentially have similar function in both diploid unicellular and multicellular meristems, but not in haploid meristems. Evol. Dev. 2005, 7, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.J.; Coriey, S.B.; Moylan, E.C.; Alexander, D.L.; Scotland, R.W.; Langdale, J.A. Independent recruitment of a conserved developmental mechanism during leaf evolution. Nature 2005, 434, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Vasco, A.; Moran, R.C.; Ambrose, B.A. The evolution, morphology, and development of fern leaves. Front. Plant Sci. 2013, 4, 345. [Google Scholar] [CrossRef] [Green Version]
- Arber, A. The Natural Philosophy of Plant Form; Cambridge University Press: Cambridge, UK, 1950. [Google Scholar]
- Rutishauser, R.; Isler, B. Developmental genetics and morphological evolution of flowering plants, especially bladderworts (Utricularia): Fuzzy Arberian Morphology complements Classical Morphology. Ann. Bot. 2001, 88, 1173–1202. [Google Scholar] [CrossRef] [Green Version]
- Rutishauser, R.; Grob, V.; Pfeifer, E. Plants Are Used to Having Identity Crises; Cambridge University Press: Cambridge, UK, 2008; pp. 194–213. [Google Scholar] [CrossRef]
- Hebant-Mauri, R.; Gay, H. Morphogenesis and its relation to architecture in the dimorphic clonal fern Lomagramma guianensis (Aublet) Ching (Dryopteridaceae). Bot. J. Linn. Soc. 1993, 112, 257–276. [Google Scholar] [CrossRef]
- Gay, H. The architecture of a dimorphic clonal fern, Lomagramma guianensis (Aublet) Ching (Dryopteridaceae). Bot. J. Linn. Soc. 1993, 111, 343–358. [Google Scholar] [CrossRef]
- Moran, R.C.; Labiak, P.H.; Sundue, M. Synopsis of Mickelia, a newly recognized genus of bolbitidoid ferns (Dryopteridaceae). Brittonia 2010, 62, 337–356. [Google Scholar] [CrossRef]
- Zotz, G.; Wilhelm, K.; Becker, A. Heteroblasty—A Review. Bot. Rev. 2011, 77, 109–151. [Google Scholar] [CrossRef]
- Moran, R.C.; Labiak, P.H.; Sundue, M. Phylogeny and Character Evolution of the Bolbitidoid Ferns (Dryopteridaceae). Int. J. Plant Sci. 2010, 171, 547–559. [Google Scholar] [CrossRef]
- Vasco, A.; Lóriga, J.; Rouhan, G.; Ambrose, B.A.; Moran, R.C. Divided leaves in the genus Elaphoglossum (Dryopteridaceae): A phylogeny of Elaphoglossum section Squamipedia. Syst. Bot. 2015, 40, 46–55. [Google Scholar] [CrossRef]
- Smith, S.A.; Beaulieu, J.M.; Donoghue, M.J. An uncorrelated relaxed-clock analysis suggests an earlier origin for flowering plants. Proc. Natl. Acad. Sci. USA 2010, 107, 5897–5902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esau, K. Plant Anatomy, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 1953. [Google Scholar]
- Fahn, A. Plant Anatomy, 3rd ed.; Pergamon Press: Cambridge, UK, 1982. [Google Scholar]
- Evert, R.F. Esau’s Plant Anatomy—Meristems, Cells, and Tissues of the Plant Body—Their Structure, Function, and Development, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 9780471738435. [Google Scholar]
- Ogura, Y. Comparative Anatomy of Vegetative Organs of the Pteridophytes; Borntraeger: Berlin, Germany, 1972; ISBN 9783443140069. [Google Scholar]
- McAlpin, B.; White, R. Shoot organization in the Filicales: The promeristem. Am. J. Bot. 1974, 61, 562–579. [Google Scholar] [CrossRef]
- Beck, C.B. An Introduction to Plant Structure and Development, 2nd ed.; Cambridge University Press: Cambridge, UK, 2010; ISBN 9780521518055. [Google Scholar]
- Barnsley, M.F. Fractals Everywhere; Morgan Kaufmann: Burlington, MA, USA, 2000; ISBN 9780120790692. [Google Scholar]
- Sanders, H.L.; Darrah, P.R.; Langdale, J.A. Sector analysis and predictive modelling reveal iterative shoot-like development in fern fronds. Development 2011, 138, 2925–2934. [Google Scholar] [CrossRef] [Green Version]
- Plackett, A.R.G.; Di Stilio, V.S.; Langdale, J.A. Ferns: The missing link in shoot evolution and development. Front. Plant Sci. 2015, 6, 972. [Google Scholar] [CrossRef] [Green Version]
- Harrison, C.J.; Morris, J.L. The origin and early evolution of vascular plant shoots and leaves. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160496. [Google Scholar] [CrossRef]
- Matasci, N.; Hung, L.; Yan, Z.; Carpenter, E.J.; Wickett, N.J.; Mirarab, S.; Nguyen, N.; Warnow, T.; Ayyampalayam, S.; Barker, M.; et al. Data access for the 1,000 Plants (1KP) project. Gigascience 2014, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Banks, J.A. Fern genomes finally here. Nat. Plants 2018, 4, 404–405. [Google Scholar] [CrossRef] [PubMed]
- Li, F.W.; Brouwer, P.; Carretero-Paulet, L.; Cheng, S.; De Vries, J.; Delaux, P.M.; Eily, A.; Koppers, N.; Kuo, L.Y.; Li, Z.; et al. Fern genomes elucidate land plant evolution and cyanobacterial symbioses. Nat. Plants 2018, 4, 460–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandstädter, J.; Rossbach, C.; Theres, K. The pattern of histone H4 expression in the tomato shoot apex changes during development. Planta 1994, 192, 69–74. [Google Scholar] [CrossRef]
- Groot, E.P.; Sinha, N.; Gleissberg, S. Expression patterns of STM-like KNOX and Histone H4 genes in shoot development of the dissected-leaved basal eudicot plants Chelidonium majus and Eschscholzia californica (Papaveraceae). Plant Mol. Biol. 2005, 58, 317–331. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef] [Green Version]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Lanfear, R.; Calcott, B.; Ho, S.Y.W.; Guindon, S. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef] [Green Version]
- Johansen, D. Plant Microtechnique; McGraw-Hill Book Company Inc.: New York, NY, USA, 1940. [Google Scholar]
- Ambrose, B.A.; Lerner, D.R.; Ciceri, P.; Padilla, C.M.; Yanofsky, M.F.; Schmidt, R.J. Molecular and genetic analyses of the silky1 gene reveal conservation in floral organ specification between eudicots and monocots. Mol. Cell 2000, 5, 569–579. [Google Scholar] [CrossRef]
- Vasco, A.; Smalls, T.L.; Graham, S.W.; Cooper, E.D.; Wong, G.K.S.; Stevenson, D.W.; Moran, R.C.; Ambrose, B.A. Challenging the paradigms of leaf evolution: Class III HD-Zips in ferns and lycophytes. New Phytol. 2016, 212, 745–758. [Google Scholar] [CrossRef]
- Cantero, A.; Barthakur, S.; Bushart, T.J.; Chou, S.; Morgan, R.O.; Fernandez, M.P.; Clark, G.B.; Roux, S.J. Expression profiling of the Arabidopsis annexin gene family during germination, de-etiolation and abiotic stress. Plant Physiol. Biochem. 2006, 44, 13–24. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, R.; Melo-de-Pinna, G.F.A.; Vasco, A.; Prado, J.; Ambrose, B.A. Class I KNOX Is Related to Determinacy during the Leaf Development of the Fern Mickelia scandens (Dryopteridaceae). Int. J. Mol. Sci. 2020, 21, 4295. https://doi.org/10.3390/ijms21124295
Cruz R, Melo-de-Pinna GFA, Vasco A, Prado J, Ambrose BA. Class I KNOX Is Related to Determinacy during the Leaf Development of the Fern Mickelia scandens (Dryopteridaceae). International Journal of Molecular Sciences. 2020; 21(12):4295. https://doi.org/10.3390/ijms21124295
Chicago/Turabian StyleCruz, Rafael, Gladys F. A. Melo-de-Pinna, Alejandra Vasco, Jefferson Prado, and Barbara A. Ambrose. 2020. "Class I KNOX Is Related to Determinacy during the Leaf Development of the Fern Mickelia scandens (Dryopteridaceae)" International Journal of Molecular Sciences 21, no. 12: 4295. https://doi.org/10.3390/ijms21124295
APA StyleCruz, R., Melo-de-Pinna, G. F. A., Vasco, A., Prado, J., & Ambrose, B. A. (2020). Class I KNOX Is Related to Determinacy during the Leaf Development of the Fern Mickelia scandens (Dryopteridaceae). International Journal of Molecular Sciences, 21(12), 4295. https://doi.org/10.3390/ijms21124295




