Circulating Levels of Brain-Enriched MicroRNAs Correlate with Neuron Specific Enolase after Cardiac Arrest—A Substudy of the Target Temperature Management Trial †
Abstract
:1. Introduction
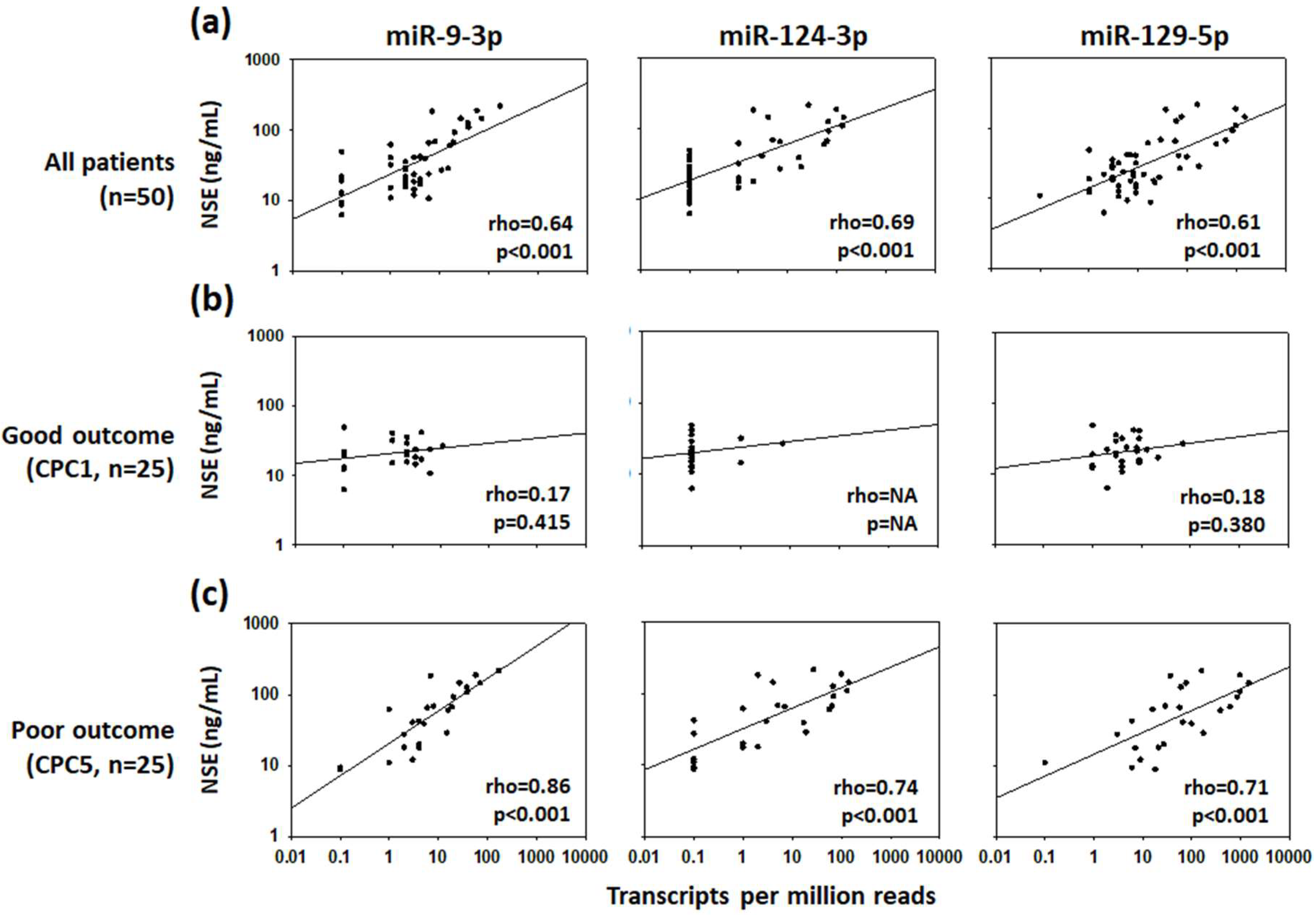
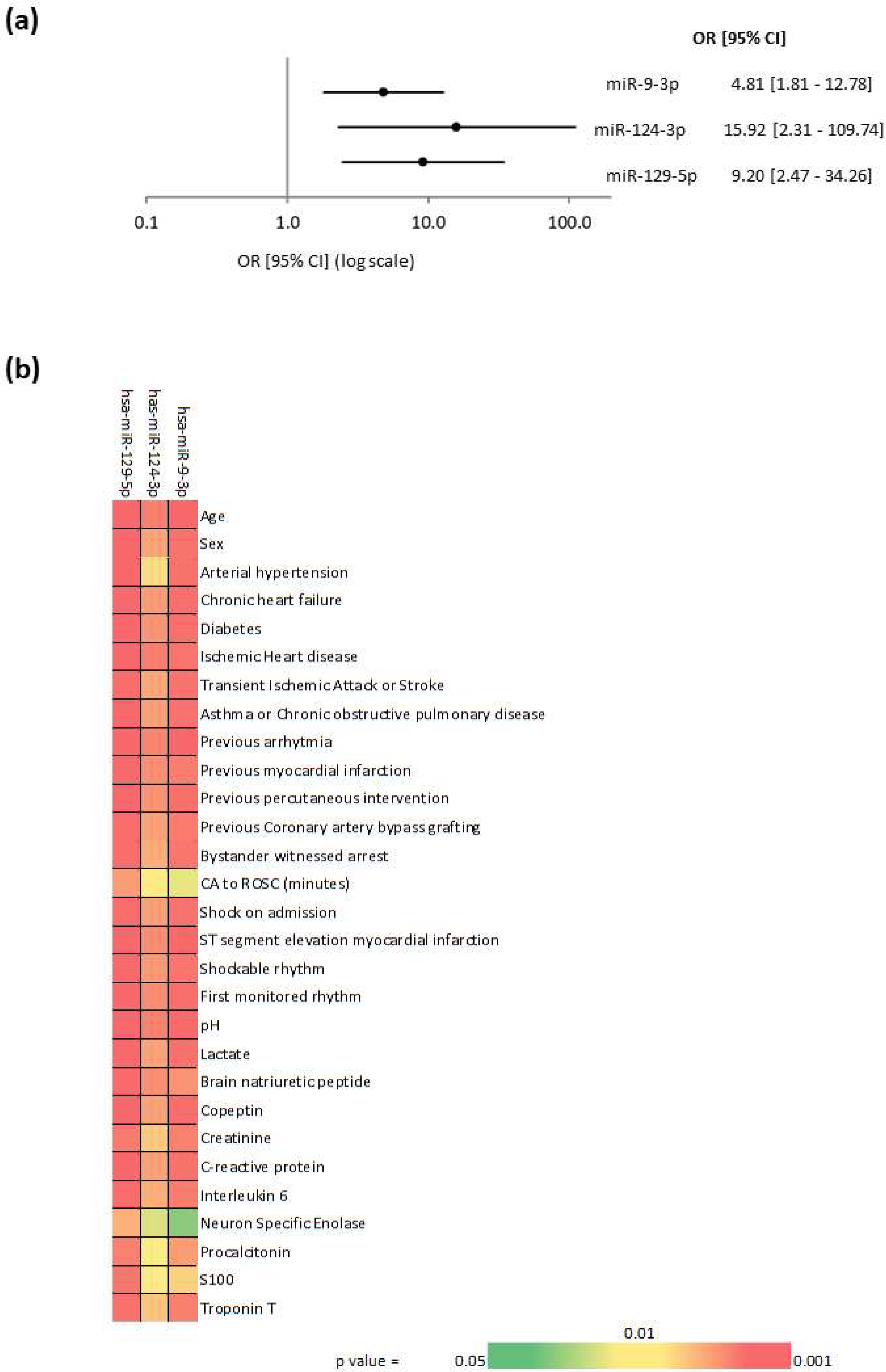
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Small RNA Sequencing
4.3. Neuron-Specific Enolase
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CA | Cardiac arrest |
| CI | Confidence intervals |
| CPC | Cerebral Performance Category |
| miRNAs | MicroRNAs |
| NSE | Neuron-specific enolase |
| OR | Odds ratio |
| ROSC | Return of spontaneous circulation |
| TTM | Targeted Temperature Management trial |
References
- Kim, J.H.; Kim, M.J.; You, J.S.; Lee, H.S.; Park, Y.S.; Park, I.; Chung, S.P. Multimodal approach for neurologic prognostication of out-of-hospital cardiac arrest patients undergoing targeted temperature management. Resuscitation 2019, 134, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandroni, C.; Cariou, A.; Cavallaro, F.; Cronberg, T.; Friberg, H.; Hoedemaekers, C.; Horn, J.; Nolan, J.P.; Rossetti, A.O.; Soar, J. Prognostication in comatose survivors of cardiac arrest: An advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Intensive Care Med. 2014, 40, 1816–1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stammet, P.; Collignon, O.; Hassager, C.; Wise, M.P.; Hovdenes, J.; Åneman, A.; Horn, J.; Devaux, Y.; Erlinge, D.; Kjaergaard, J.; et al. Neuron-specific enolase as a predictor of death or poor neurological outcome after out-of-hospital cardiac arrest and targeted temperature management at 33 °C and 36 °C. J. Am. Coll. Cardiol. 2015, 65, 2104–2114. [Google Scholar] [CrossRef] [PubMed]
- Samaniego, E.A.; Mlynash, M.; Caulfield, A.F.; Eyngorn, I.; Wijman, C.A. Sedation confounds outcome prediction in cardiac arrest survivors treated with hypothermia. Neurocrit. Care 2011, 15, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Isgro, M.A.; Bottoni, P.; Scatena, R. Neuron-specific enolase as a biomarker: Biochemical and clinical aspects. Adv. Exp. Med. Biol. 2015, 867, 125–143. [Google Scholar]
- Mlynash, M.; Buckwalter, M.S.; Okada, A.; Caulfield, A.F.; Venkatasubramanian, C.; Eyngorn, I.; Verbeek, M.M.; Wijman, C.A. Serum neuron-specific enolase levels from the same patients differ between laboratories: Assessment of a prospective post-cardiac arrest cohort. Neurocrit. Care 2013, 19, 161–166. [Google Scholar] [CrossRef]
- Rundgren, M.; Cronberg, T.; Friberg, H.; Isaksson, A. Serum neuron specific enolase-impact of storage and measuring method. BMC Res. Notes 2014, 7, 726. [Google Scholar] [CrossRef] [Green Version]
- Marchi, N.; Rasmussen, P.; Kapural, M.; Fazio, V.; Kight, K.; Mayberg, M.R.; Kanner, A.; Ayumar, B.; Albensi, B.; Cavaglia, M.; et al. Peripheral markers of brain damage and blood-brain barrier dysfunction. Restor. Neurol. Neurosci. 2003, 21, 109–121. [Google Scholar]
- Devaux, Y.; Stammet, P.; Cardiolinc, N. What’s new in prognostication after cardiac arrest: microRNAs? Intensive Care Med. 2018, 44, 897–899. [Google Scholar] [CrossRef]
- Izzotti, A.; Longobardi, M.; La Maestra, S.; Micale, R.T.; Pulliero, A.; Camoirano, A.; Geretto, M.; D’Agostini, F.; Balansky, R.; Miller, M.S.; et al. Release of microRNAs into body fluids from ten organs of mice exposed to cigarette smoke. Theranostics 2018, 8, 2147–2160. [Google Scholar] [CrossRef]
- de Gonzalo-Calvo, D.; Iglesias-Gutierrez, E.; Llorente-Cortes, V. Epigenetic biomarkers and cardiovascular disease: Circulating microRNAs. Rev. Esp. Cardiol. (Engl. Ed.) 2017, 70, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K. Extracellular microRNAs as biomarkers in human disease. Chonnam Med. J. 2015, 51, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devaux, Y.; Dankiewicz, J.; Salgado-Somoza, A.; Stammet, P.; Collignon, O.; Gilje, P.; Gidlof, O.; Zhang, L.; Vausort, M.; Hassager, C.; et al. Association of circulating microrna-124-3p levels with outcomes after out-of-hospital cardiac arrest: A substudy of a randomized clinical trial. JAMA Cardiol. 2016, 1, 305–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilje, P.; Gidlof, O.; Rundgren, M.; Cronberg, T.; Al-Mashat, M.; Olde, B.; Friberg, H.; Erlinge, D. The brain-enriched microRNA miR-124 in plasma predicts neurological outcome after cardiac arrest. Crit. Care 2014, 18, R40. [Google Scholar] [CrossRef] [Green Version]
- Devaux, Y.; Salgado-Somoza, A.; Dankiewicz, J.; Boileau, A.; Stammet, P.; Schritz, A.; Zhang, L.; Vausort, M.; Gilje, P.; Erlinge, D.; et al. Incremental value of circulating mir-122-5p to predict outcome after out of hospital cardiac arrest. Theranostics 2017, 7, 2555–2564. [Google Scholar] [CrossRef]
- Park, J.S.; You, Y.; Min, J.H.; Yoo, I.; Jeong, W.; Cho, Y.; Ryu, S.; Lee, J.; Kim, S.W.; Cho, S.U.; et al. Study on the timing of severe blood-brain barrier disruption using cerebrospinal fluid-serum albumin quotient in post cardiac arrest patients treated with targeted temperature management. Resuscitation 2019, 135, 118–123. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, L.; Pearce, W.J. MicroRNAs in brain development and cerebrovascular pathophysiology. Am. J. Physiol. Cell Physiol. 2019, 317, C3–C19. [Google Scholar] [CrossRef]
- Sim, S.E.; Lim, C.S.; Kim, J.I.; Seo, D.; Chun, H.; Yu, N.K.; Lee, J.; Kang, S.J.; Ko, H.G.; Choi, J.H.; et al. The brain-enriched microrna mir-9-3p regulates synaptic plasticity and memory. J. Neurosci. 2016, 36, 8641–8652. [Google Scholar] [CrossRef]
- Zeng, A.; Yin, J.; Li, Y.; Li, R.; Wang, Z.; Zhou, X.; Jin, X.; Shen, F.; Yan, W.; You, Y. MiR-129-5p targets Wnt5a to block PKC/ERK/NF-kappaB and JNK pathways in glioblastoma. Cell Death Dis. 2018, 9, 394. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Zhang, X.; Chen, P.; Ruan, X.; Liu, W.; Li, Y.; Sun, C.; Hou, L.; Yin, B.; Qiang, B.; et al. MicroRNA-129 modulates neuronal migration by targeting Fmr1 in the developing mouse cortex. Cell Death Dis. 2019, 10, 287. [Google Scholar] [CrossRef]
- Rajman, M.; Metge, F.; Fiore, R.; Khudayberdiev, S.; Aksoy-Aksel, A.; Bicker, S.; Ruedell Reschke, C.; Raoof, R.; Brennan, G.P.; Delanty, N.; et al. A microRNA-129-5p/Rbfox crosstalk coordinates homeostatic downscaling of excitatory synapses. EMBO J. 2017, 36, 1770–1787. [Google Scholar] [CrossRef] [PubMed]
- Stammet, P.; Goretti, E.; Vausort, M.; Zhang, L.; Wagner, D.R.; Devaux, Y. Circulating microRNAs after cardiac arrest. Crit. Care Med. 2012, 40, 3209–3214. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.; Wetterslev, J.; Al-Subaie, N.; Andersson, B.; Bro-Jeppesen, J.; Bishop, G.; Brunetti, I.; Cranshaw, J.; Cronberg, T.; Edqvist, K.; et al. Target temperature management after out-of-hospital cardiac arrest—A randomized, parallel-group, assessor-blinded clinical trial--rationale and design. Am. Heart J. 2012, 163, 541–548. [Google Scholar] [CrossRef]
- Nielsen, N.; Wetterslev, J.; Cronberg, T.; Erlinge, D.; Gasche, Y.; Hassager, C.; Horn, J.; Hovdenes, J.; Kjaergaard, J.; Kuiper, M.; et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N. Engl. J. Med. 2013, 369, 2197–2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennett, B.; Bond, M. Assessment of outcome after severe brain damage: A practical scale. Lancet 1975, 305, 480–484. [Google Scholar] [CrossRef]
- Kozomara, A.; Griffiths-Jones, S. MiRBase: Integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011, 39, D152–D157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristic | ALL (n = 50) | Good (n = 25) | Poor (n = 25) | p-Value |
|---|---|---|---|---|
| Age | 63 (59–74) | 62 (59–69) | 70 (60–80) | 0.039 |
| Sex | 41 (82%) | 21 (84%) | 21 (84%) | 1 |
| COMORBIDITIES | ||||
| Arterial hypertension | 21 (42%) | 10 (40%) | 11 (44%) | 1 |
| Chronic heart failure | 3 (6%) | 1 (4%) | 2 (8%) | 1 |
| Diabetes | 3 (6%) | 1 (4%) | 2 (8%) | 1 |
| Ischemic heart disease | 11 (22%) | 3 (12%) | 8 (32%) | 0.17 |
| Transient ischemic attack or stroke | 4 (8%) | 2 (8%) | 2 (8%) | 1 |
| Asthma or chronic obstructive pulmonary disease | 5 (10%) | 1 (4%) | 4 (16%) | 0.35 |
| Previous arrhythmia | 11 (22%) | 2 (8%) | 9 (36%) | 0.04 |
| Previous myocardial infarction | 8 (16%) | 2 (8%) | 6 (24%) | 0.25 |
| ARREST CONDITIONS | ||||
| Bystander witnessed arrest | 41 (82%) | 22 (88%) | 19 (76%) | 0.46 |
| Bystander cardiopulmonary resuscitation | 37 (74%) | 19 (76%) | 18 (72%) | 1 |
| Time from CA to ROSC (minutes) | 22 (19–30) | 20 (17–22) | 30 (22–37) | 0.001 |
| Shock on admission | 8 (16%) | 3 (12%) | 5 (20%) | 0.7 |
| ST segment elevation myocardial infarction | 29 (58%) | 16 (64%) | 13 (52%) | 0.57 |
| Shockable rhythm | 46 (92%) | 25 (100%) | 21 (84%) | 0.11 |
| First monitored rhythm | 44 (88%) | 24 (96%) | 20 (80%) | 0.19 |
| LABORATORY MEASUREMENTS | ||||
| pH | 7.25 (7.15–7.32) | 7.29 (7.18–7.33) | 7.21 (7.13–7.29) | 0.221 |
| Lactate (mmol/L) | 4.9 (2.9–8.7) | 4.8 (3.1–7.2) | 5 (2.9–9.5) | 0.992 |
| Brain natriuretic peptide (NT-proBNP, pg/mL) | 1856 (946–2932) | 1327 (407–1872) | 2324 (1494–4009) | 0.005 |
| Copeptin (pmol/L) | 48.74 (25.4–112.87) | 53.43 (27.43–119.73) | 31.63 (23.63–82.95) | 0.491 |
| Creatinine (mg/dL) | 0.99 (0.82–1.47) | 0.9 (0.71–1.08) | 1.33 (0.89–1.55) | 0.007 |
| C-reactive protein (µg/mL) | 143 (102–201) | 142 (115–192) | 144 (100–205) | 0.961 |
| Interleukin 6 (pg/mL) | 166 (74–337) | 120 (73–295) | 196 (77–545) | 0.332 |
| Neuron specific enolase (ng/mL) | 27 (17–57) | 20 (15–29) | 60 (20–110) | 0.003 |
| Procalcitonin (µg/L) | 1.11 (0.4–3.61) | 0.64 (0.34–1.38) | 3 (0.57–5.27) | 0.015 |
| S100 (S100A1B and S100BB, µg/L) | 0.12 (0.07–0.21) | 0.09 (0.07–0.14) | 0.16 (0.1–0.26) | 0.035 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanizzi, F.M.; Nielsen, N.; Zhang, L.; Dankiewicz, J.; Stammet, P.; Gilje, P.; Erlinge, D.; Hassager, C.; Wise, M.P.; Kuiper, M.; et al. Circulating Levels of Brain-Enriched MicroRNAs Correlate with Neuron Specific Enolase after Cardiac Arrest—A Substudy of the Target Temperature Management Trial. Int. J. Mol. Sci. 2020, 21, 4353. https://doi.org/10.3390/ijms21124353
Stefanizzi FM, Nielsen N, Zhang L, Dankiewicz J, Stammet P, Gilje P, Erlinge D, Hassager C, Wise MP, Kuiper M, et al. Circulating Levels of Brain-Enriched MicroRNAs Correlate with Neuron Specific Enolase after Cardiac Arrest—A Substudy of the Target Temperature Management Trial. International Journal of Molecular Sciences. 2020; 21(12):4353. https://doi.org/10.3390/ijms21124353
Chicago/Turabian StyleStefanizzi, Francesca Maria, Niklas Nielsen, Lu Zhang, Josef Dankiewicz, Pascal Stammet, Patrik Gilje, David Erlinge, Christian Hassager, Matthew P. Wise, Michael Kuiper, and et al. 2020. "Circulating Levels of Brain-Enriched MicroRNAs Correlate with Neuron Specific Enolase after Cardiac Arrest—A Substudy of the Target Temperature Management Trial" International Journal of Molecular Sciences 21, no. 12: 4353. https://doi.org/10.3390/ijms21124353
APA StyleStefanizzi, F. M., Nielsen, N., Zhang, L., Dankiewicz, J., Stammet, P., Gilje, P., Erlinge, D., Hassager, C., Wise, M. P., Kuiper, M., Friberg, H., Devaux, Y., & Salgado-Somoza, A. (2020). Circulating Levels of Brain-Enriched MicroRNAs Correlate with Neuron Specific Enolase after Cardiac Arrest—A Substudy of the Target Temperature Management Trial. International Journal of Molecular Sciences, 21(12), 4353. https://doi.org/10.3390/ijms21124353






