ESR Method in Monitoring of Nanoparticle Endocytosis in Cancer Cells
Abstract
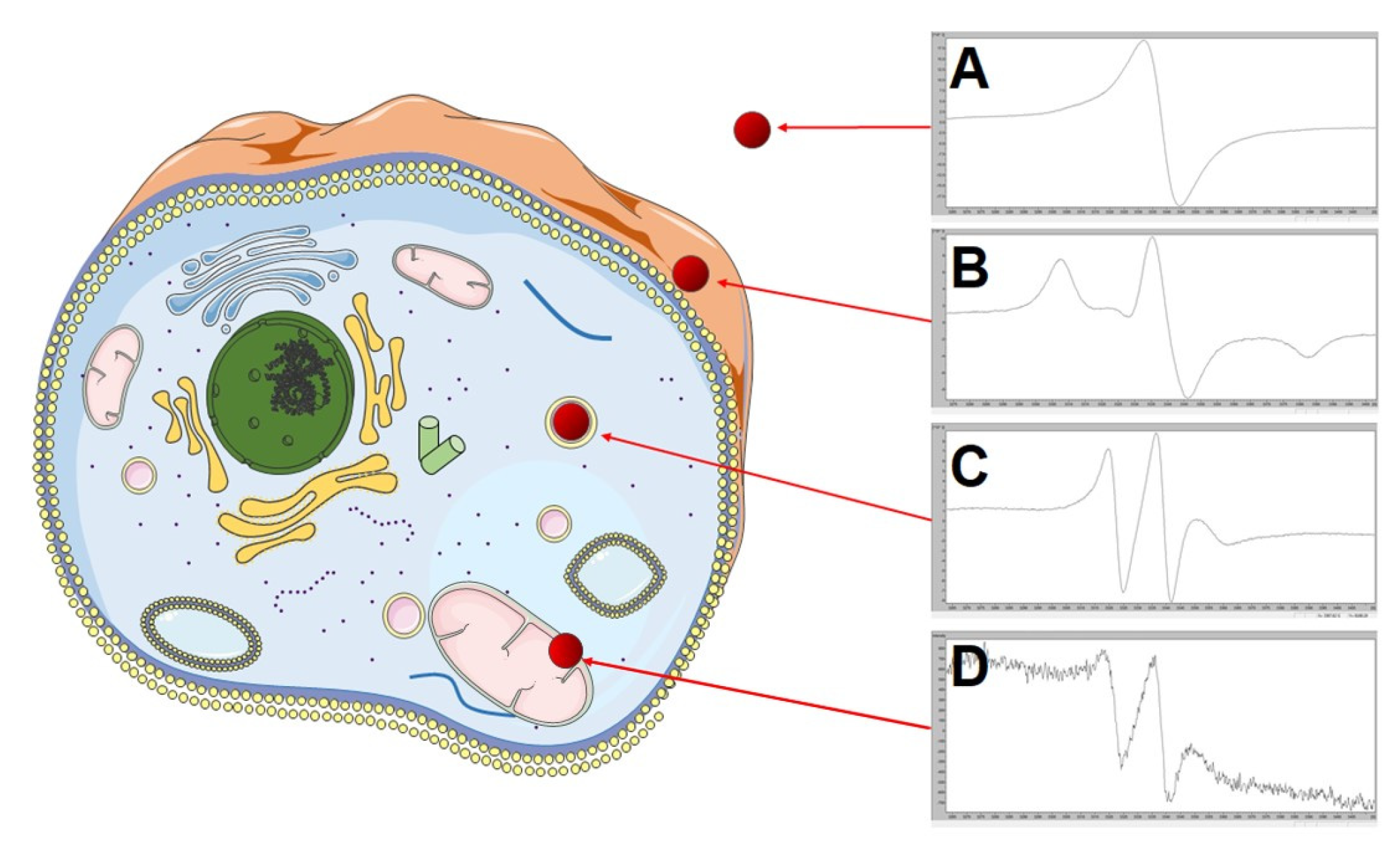
:1. Introduction
2. Results and Discussion
2.1. Effect of Temperature
2.2. Effect of Incubation Time
2.3. Modeling of Endocytosis Process
3. Materials and Methods
3.1. Materials
3.2. Synthesis Procedures
3.2.1. Synthesis of Fe3O4@SiO2@SiNHDOX@Dextran-TEMPOL
3.2.2. Synthesis of Fe3O4@SiO2@FITC-Dextran-TEMPOL
3.3. Procedures with Human Microvascular Endothelial, Breast Cancer, and Yeast Cells
3.4. ESR Measurements
3.5. Simulations in EasySpin
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ESR | Electron spin resonance |
| TEMPOL | 4-hydroxy-TEMPO spin label |
| HMEC | Human microvascular endothelial cells |
| TEOS | Tetraethyl orthosilate |
| DOX | Doxorubicin hydrochloride |
| FITC | Fluorescein isothiocyanate |
References
- Brzeziński, M.; Wedepohl, S.; Kost, B.; Calderón, M. Nanoparticles from supramolecular polylactides overcome drug resistance of cancer cells. Eur. Polym. J. 2018, 109, 117–123. [Google Scholar] [CrossRef]
- Peukert, D.; Kempson, I.; Douglass, M.; Bezak, E. Metallic nanoparticle radiosensitisation of ion radiotherapy: A review. Phys. Med. 2018, 47, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Vong, L.B.; Bui, T.Q.; Tomita, T.; Sakamoto, H.; Hiramatsu, Y.; Nagasaki, Y. Novel angiogenesis therapeutics by redox injectable hydrogel—Regulation of local nitric oxide generation for effective cardiovascular therapy. Biomaterials 2018, 167, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Shashni, B.; Nagasaki, Y. Nitroxide radical-containing nanoparticles attenuate tumorigenic potential of triple negative breast cancer. Biomaterials 2018, 178, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, Y. Design and application of redox polymers for nanomedicine. Polym. J. 2018, 50, 821–836. [Google Scholar] [CrossRef]
- Ma, Y.; Mou, Q.; Zhu, X.; Yan, D. Small molecule nanodrugs for cancer therapy. Mater. Today Chem. 2017, 4, 26–39. [Google Scholar] [CrossRef]
- Williams, H.M. The application of magnetic nanoparticles in the treatment and monitoring of cancer and infectious diseases. Biosci. Horiz. 2017, 10, hzx009. [Google Scholar] [CrossRef] [Green Version]
- Bakhtiary, Z.; Saei, A.A.; Hajipour, M.J.; Raoufi, M.; Vermesh, O.; Mahmoudi, M. Targeted superparamagnetic iron oxide nanoparticles for early detection of cancer: Possibilities and challenges. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 287–307. [Google Scholar] [CrossRef]
- Balejcikova, L.; Molcan, M.; Kovac, J.; Kubovcikova, M.; Saksl, K.; Mitroova, Z.; Timko, M.; Kopcansky, P. Hyperthermic effect in magnetoferritin aqueous colloidal solution. J. Mol. Liq. 2019, 283, 39–44. [Google Scholar] [CrossRef]
- Kaczmarek, K.; Hornowski, T.; Dobosz, B.; Józefczak, A. Influence of magnetic nanoparticles on the focused ultrasound hyperthermia. Materials 2018, 11, 1607. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.; Yi, T.; Park, J.S.; Kim, D.K. Electron spin resonance (ESR) and microwave absorption studies of superparamagnetic iron oxide nanoparticles (SPIONs) for hyperthermia applications. J. Korean Ceram. Soc. 2011, 48, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Li, B.; Qiao, Y. Fe3O4 nanoparticles in targeted drug/gene delivery systems. Materials 2018, 11, 324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, B.; Jalodia, K.; Kumar, P.; Gautam, H.K. Recent advances in nanoparticle-mediated drug delivery. J. Drug Deliv. Sci. Technol. 2017, 41, 260–268. [Google Scholar] [CrossRef]
- Hałupka-Bryl, M.; Bednarowicz, M.; Dobosz, B.; Krzyminiewski, R.; Zalewski, T.; Wereszczyńska, B.; Nagasaki, Y. Doxorubicin loaded PEG-b-poly(4-vinylbenzylphosphonate) coated magnetic iron oxide nanoparticles for targeted drug delivery. J. Magn. Magn. Mater. 2015, 384, 320–327. [Google Scholar] [CrossRef]
- Ghorbani, H.R.; Pazoki, H.; Rad, A.S. Synthesis of magnetite nanoparticles by biological technique. Biosci. Biotechnol. Res. Asia 2017, 14, 631–633. [Google Scholar] [CrossRef]
- Man, D.; Słota, R.; Broda, M.A.; Mele, G.; Li, J. Metalloporphyrin intercalation in liposome membranes: ESR study. J. Biol. Inorg. Chem. 2011, 16, 173–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Huang, H.; Zhang, L.; Bai, Y.; Chen, H. PCM and TAT co-modified liposome with improved myocardium delivery: In vitro and in vivo evaluations. Drug Deliv. 2017, 24, 339–345. [Google Scholar] [CrossRef] [Green Version]
- Yoshitomi, T.; Kuramochi, K.; Vong, L.B.; Nagasaki, Y. Development of nitroxide radicals–containing polymer for scavenging reactive oxygen species from cigarette smoke. Sci. Technol. Adv. Mater. 2014, 15, 035002. [Google Scholar] [CrossRef] [Green Version]
- Yoshitomi, T.; Nagasaki, Y. Design and preparation of a nanoprobe for imaging inflammation sites. Biointerphases 2012, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Bednarowicz, M.; Dobosz, B.; Krzyminiewski, R.; Hałupka-Bryl, M.; Deptuła, T.; Nagasaki, Y. ESR studies of redox-active PMNT-PEG-PMNT polymer. Mater. Chem. Phys. 2015, 161, 250–255. [Google Scholar] [CrossRef]
- Wang, B.; Fielding, A.J.; Dryfe, R.A.W. Electron paramagnetic resonance investigation of the structure of graphene oxide: pH-dependence of the spectroscopic response. ACS Appl. Nano Mater. 2019, 2, 19–27. [Google Scholar] [CrossRef]
- Dobosz, B.; Krzyminiewski, R.; Schroeder, G.; Kurczewska, J. Electron paramagnetic resonance as an effective method for a characterization of functionalized iron oxide. J. Phys. Chem. Solids 2014, 75, 594–598. [Google Scholar] [CrossRef]
- Dobosz, B.; Krzyminiewski, R.; Schroeder, G.; Kurczewska, J. Diffusion of functionalized magnetite nanoparticles forced by a magnetic field studied by EPR method. Curr. Appl. Phys. 2016, 16, 562–567. [Google Scholar] [CrossRef]
- Dobosz, B.; Krzyminiewski, R.; Kurczewska, J.; Schroeder, G. The dynamics of functionalized magnetite nanoparticles in various solutions studied by ESR method. Mater. Chem. Phys. 2017, 198, 297–302. [Google Scholar] [CrossRef]
- Dobosz, B.; Krzyminiewski, R.; Kurczewska, J.; Schroeder, G. The influence of surface modification, coating agents and pH value of aqueous solutions on physical properties of magnetite nanoparticles investigated by ESR method. J. Magn. Magn. Mater. 2017, 429, 203–210. [Google Scholar] [CrossRef]
- Yurenyaa, A.Y.; Polikarpov, M.A.; Chukalova, A.A.; Moskaleva, E.Y.; Taldenkov, A.N.; Panchenko, V.Y. The magnetic introduction of magnetite nanoparticles into live cells for radiosensibility enhancement. J. Magn. Magn. Mater. 2017, 427, 111–113. [Google Scholar] [CrossRef]
- Vegerhof, A.; Barnoy, E.A.; Motiei, M.; Malka, D.; Danan, Y.; Zalevsky, Z.; Popovtzer, R. Targeted magnetic nanoparticles for mechanical lysis of tumor cells by low-amplitude alternating magnetic field. Materials 2016, 9, 943. [Google Scholar] [CrossRef] [Green Version]
- Feliciano, C.P.; Nagasaki, Y. Oral nanotherapeutics: Redox nanoparticles attenuate ultraviolet B radiation-induced skin inflammatory disorders in Kud: Hr- hairless mice. Biomaterials 2017, 142, 162–170. [Google Scholar] [CrossRef]
- Feliciano, C.P.; Tsuboi, K.; Suzuki, K.; Kimura, H.; Nagasaki, Y. Long-term bioavailability of redox nanoparticles effectively reduces organ dysfunctions and death in whole-body irradiated mice. Biomaterials 2017, 129, 68–82. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, S.; You, H.; Zhang, Y.; Yang, B.; Sun, X.; Yang, L.; Chen, Y.; Fu, S.; Wu, J. In vivo synergistic anti-tumor effect of paclitaxel nanoparticles combined with radiotherapy on human cervical carcinoma. Drug Deliv. 2017, 24, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Di Fiore, P.P.; von Zastrow, M. Endocytosis, signaling, and beyond. Cold Spring Harb. Perspect. Biol. 2014, 6, a016865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, M.; Karni, M.; Baranes, K.; Levy, I.; Alon, N.; Margel, S.; Shefi, O. Iron oxide nanoparticles for neuronal cell applications: Uptake study and magnetic manipulations. J. Nanobiotechnology 2016, 14, 37. [Google Scholar] [CrossRef] [Green Version]
- de Castro, C.E.; Ribeiro, C.A.S.; Alavarse, A.C.; Albuquerque, L.J.C.; da Silva, M.C.C.; Jäger, E.; Surman, F.; Schmidt, V.; Giacomelli, C.; Giacomelli, F.C. Nanoparticle−cell interactions: Surface chemistry effects on the cellular uptake of biocompatible block copolymer assemblies. Langmuir 2018, 34, 2180–2188. [Google Scholar] [CrossRef]
- Nakamura, H.; Watano, S. Direct permeation of nanoparticles across cell membrane: A review. Kona Powder Part. J. 2018, 35, 49–65. [Google Scholar] [CrossRef] [Green Version]
- Fürthauer, M.; Smythe, E. Systems dynamics in endocytosis. Traffic 2014, 15, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Sun, B.; Liu, Y.; Shen, Q.-D.; Jiang, S. Dual-color fluorescence imaging of magnetic nanoparticles in live cancer cells using conjugated polymer probes. Sci. Rep. 2016, 6, 22368. [Google Scholar] [CrossRef] [Green Version]
- Lewandowski, M.; Gwozdzinski, K. Nitroxides as antioxidants and anticancer drugs. Int. J. Mol. Sci. 2017, 18, 2490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzyminiewski, R.; Dobosz, B.; Schroeder, G.; Kurczewska, J. ESR as a monitoring method of the interactions between TEMPO-functionalized magnetic nanoparticles and yeast cells. Sci. Rep. 2019, 9, 18733. [Google Scholar] [CrossRef] [PubMed]
- Ades, E.W.; Candal, F.J.; Swerlick, R.A.; George, V.G.; Summers, S.; Bosse, D.C.; Lawley, T.J. HMEC-1: Establishment of an immortalized human microvascular endothelial cell line. J. Investig. Dermatol. 1992, 99, 683–690. [Google Scholar] [CrossRef] [Green Version]
- Cailleau, R.; Olive, M.; Cruciger, Q.V. Long-term human breast carcinoma cell lines of metastatic origin: Preliminary characterization. In Vitro 1978, 14, 911–915. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]












| Replicate | Control | 005 µM | 01 µM | 05 µM | 1 µM | 5 µM | 25 µM |
|---|---|---|---|---|---|---|---|
| 1st | 100.0000 | 90.916570 | 92.000170 | 72.982670 | 76.579710 | 67.436440 | 63.858640 |
| 2nd | 100.0000 | 90.628040 | 88.633960 | 74.951110 | 85.383160 | 73.950870 | 78.958500 |
| 3rd | 100.0000 | 95.475390 | 91.718050 | 77.919780 | 89.916330 | 80.580700 | 77.092660 |
| Replicate | Control | 0.05% | 0.1% | 0.5% | 1% | 5% | 10% | 25% | 50% |
|---|---|---|---|---|---|---|---|---|---|
| 1st | 100.0000 | 80.381940 | 96.283270 | 91.974530 | 90.006090 | 93.295360 | 86.575760 | 90.570340 | 53.766420 |
| 2nd | 100.0000 | 91.685990 | 84.241850 | 82.004130 | 81.734830 | 74.053450 | 86.043580 | 68.443100 | 79.279100 |
| 3rd | 100.0000 | 74.527930 | 84.171330 | 73.816220 | 93.276120 | 88.159480 | 81.318060 | 85.716580 | 76.740010 |
| Bonferroni’s Multiple Comparison Test | Mean Diff. | t | Significant? p < 0.05? | Summary | 95% CI of diff |
|---|---|---|---|---|---|
| Control vs. 05 µM | 24.72 | 6.071 | Yes | *** | 9.656 to 39.77 |
| Control vs. 1 µM | 16.04 | 3.940 | Yes | * | 0.9810 to 31.10 |
| Control vs. 5 µM | 26.01 | 6.389 | Yes | *** | 10.95 to 41.07 |
| Control vs. 25 µM | 26.70 | 6.558 | Yes | *** | 11.64 to 41.76 |
| Bonferroni’s Multiple Comparison Test | Mean Diff. | t | Significant? p < 0.05? | Summary | 95% CI of diff |
|---|---|---|---|---|---|
| Control vs. 50% | 30.07 | 4.227 | Yes | * | 3.222 to 56.92 |
| Replicate | Yeast Cells with Nanoparticles Growth | Yeast Cells without Nanoparticles Growth |
|---|---|---|
| 1 | 2.4 | 2.6 |
| 2 | 2.5 | 2.6 |
| 3 | 2.4 | 2.7 |
| 4 | 2.4 | 2.3 |
| 5 | 2.3 | 2.8 |
| Mean | 2.4 | 2.6 |
| SD | 0.07 | 0.19 |
| SE | 0.03 | 0.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzyminiewski, R.; Dobosz, B.; Krist, B.; Schroeder, G.; Kurczewska, J.; Bluyssen, H.A.R. ESR Method in Monitoring of Nanoparticle Endocytosis in Cancer Cells. Int. J. Mol. Sci. 2020, 21, 4388. https://doi.org/10.3390/ijms21124388
Krzyminiewski R, Dobosz B, Krist B, Schroeder G, Kurczewska J, Bluyssen HAR. ESR Method in Monitoring of Nanoparticle Endocytosis in Cancer Cells. International Journal of Molecular Sciences. 2020; 21(12):4388. https://doi.org/10.3390/ijms21124388
Chicago/Turabian StyleKrzyminiewski, Ryszard, Bernadeta Dobosz, Bart Krist, Grzegorz Schroeder, Joanna Kurczewska, and Hans A.R. Bluyssen. 2020. "ESR Method in Monitoring of Nanoparticle Endocytosis in Cancer Cells" International Journal of Molecular Sciences 21, no. 12: 4388. https://doi.org/10.3390/ijms21124388
APA StyleKrzyminiewski, R., Dobosz, B., Krist, B., Schroeder, G., Kurczewska, J., & Bluyssen, H. A. R. (2020). ESR Method in Monitoring of Nanoparticle Endocytosis in Cancer Cells. International Journal of Molecular Sciences, 21(12), 4388. https://doi.org/10.3390/ijms21124388






