Effect of SOX2 Repression on Corneal Endothelial Cells
Abstract
:1. Introduction
2. Results
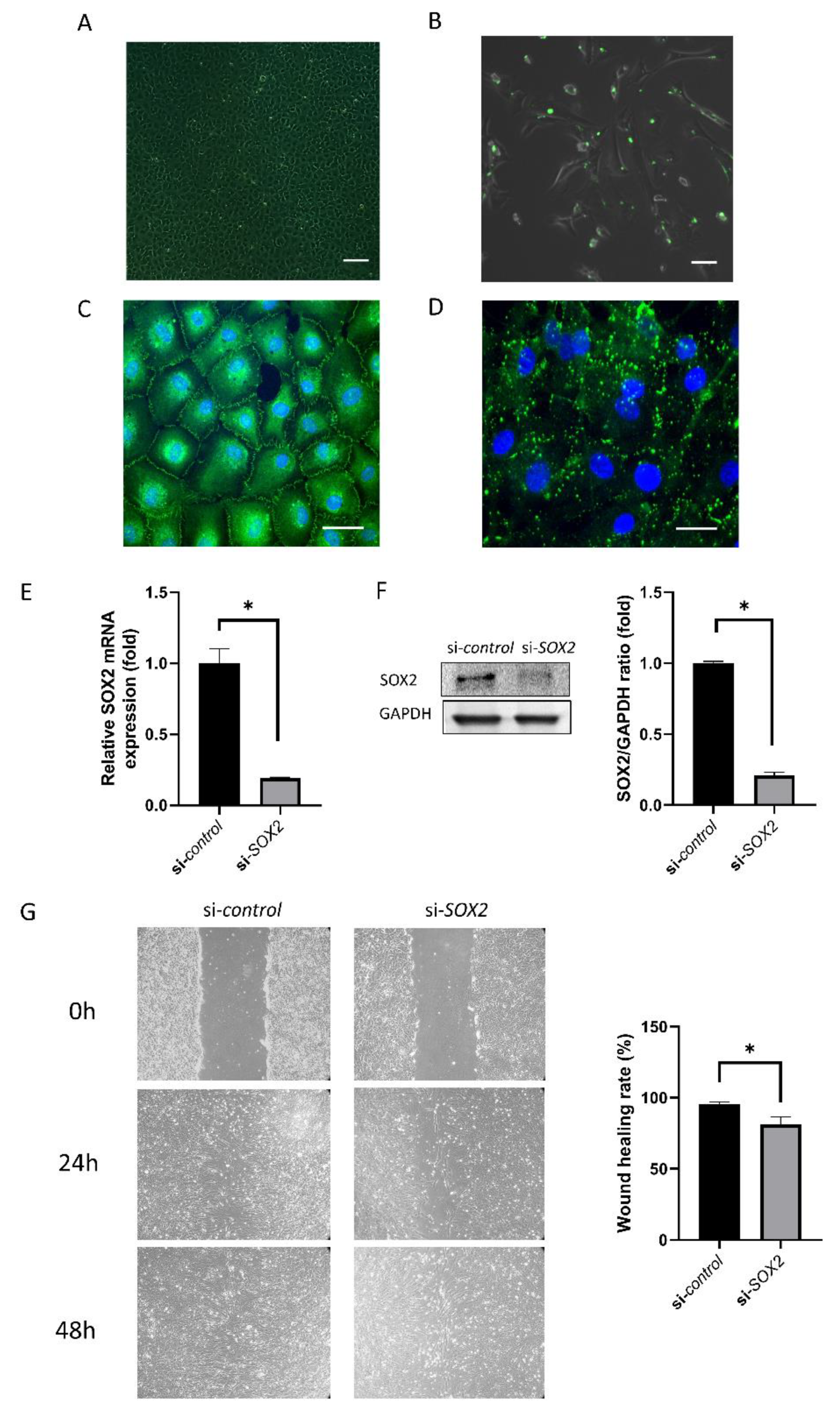
2.1. Cell Culture and Transfection
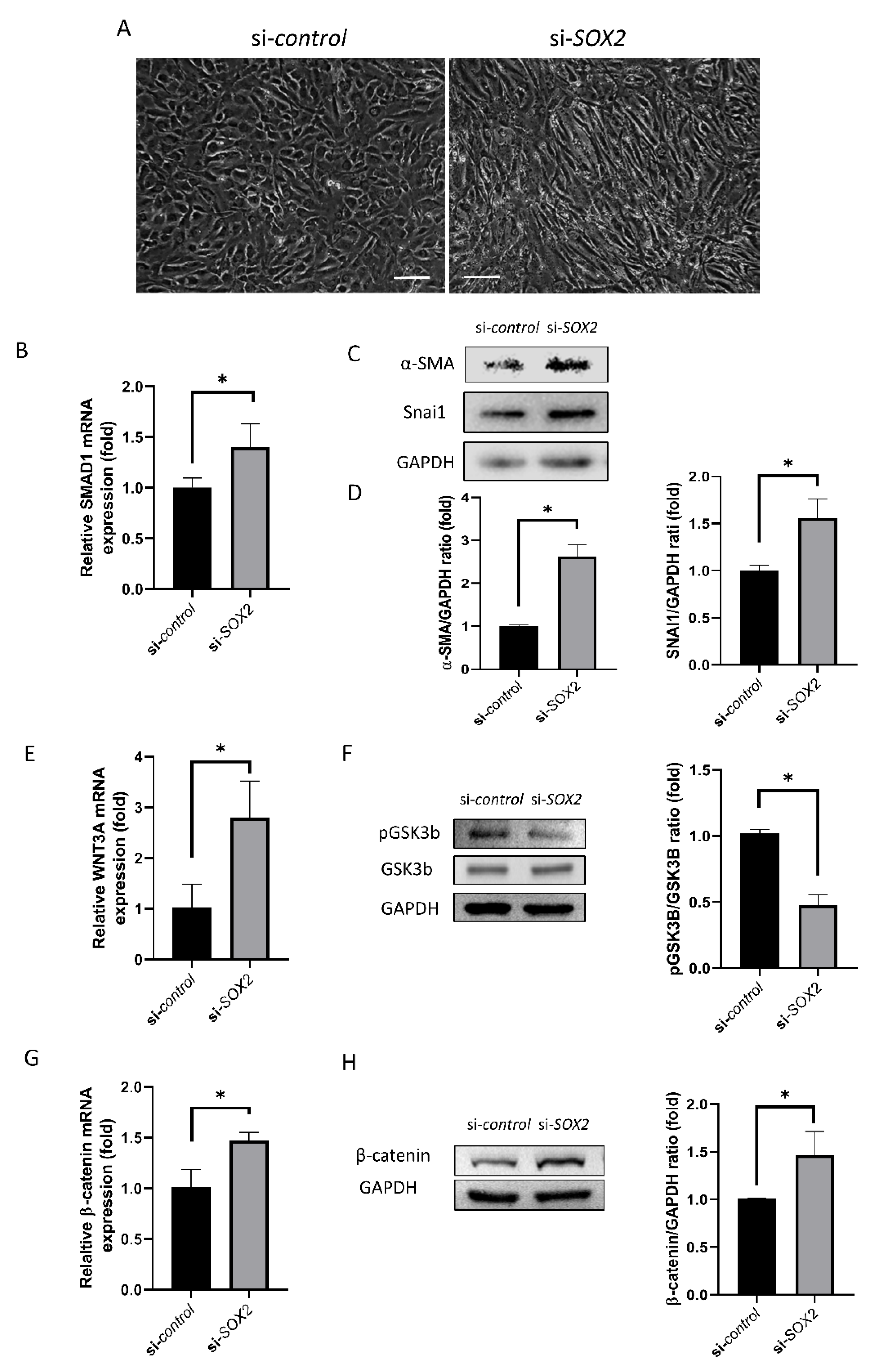
2.2. EndoMT and WNT Signaling
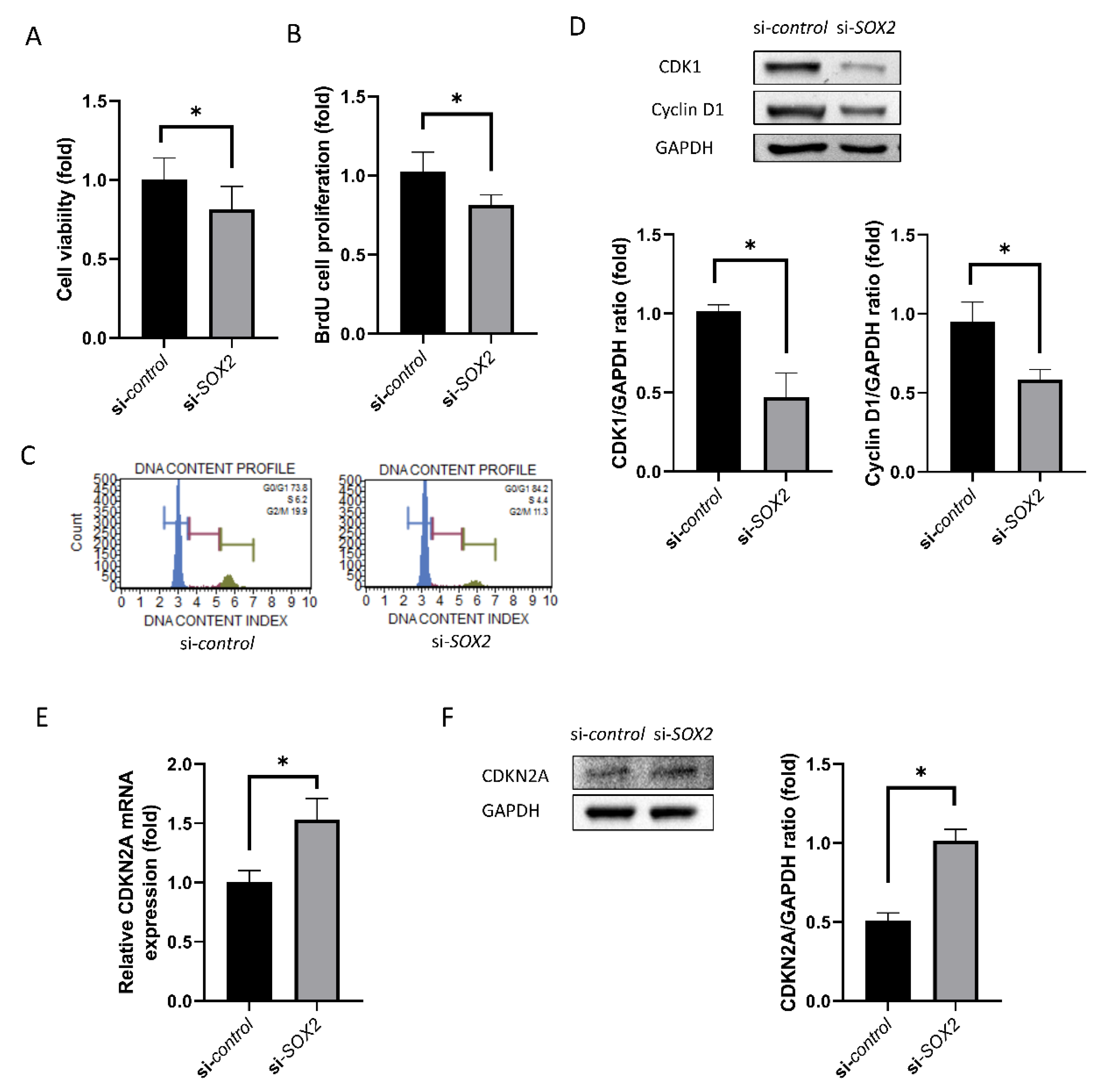
2.3. Cell Viability and Proliferation
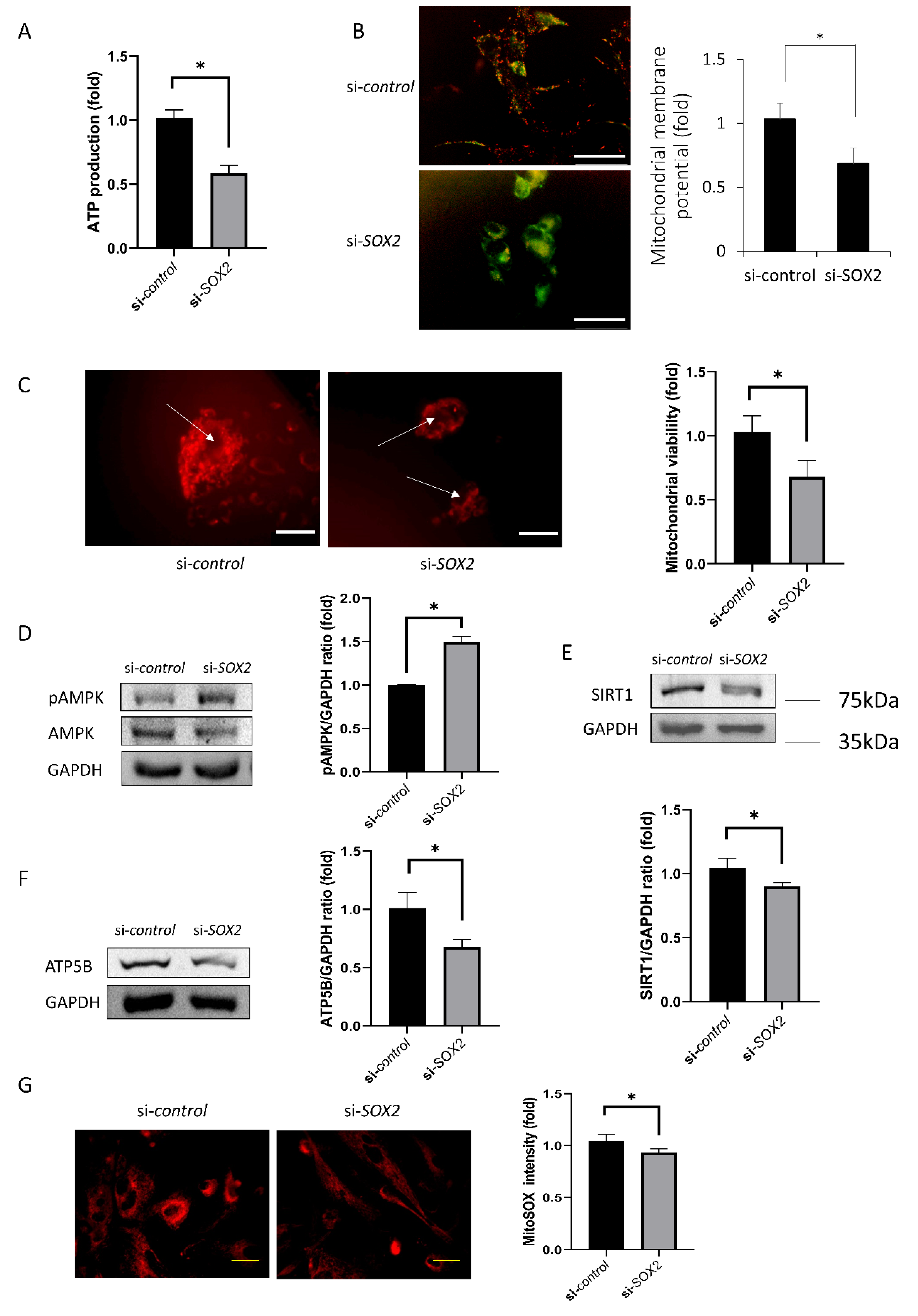
2.4. Mitochondrial Functions
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Transfection
4.2. Immunofluorescence Staining for Zonula Occludens-1 (ZO-1) and Connexin 43 (Cx43)
4.3. Cell Viability and Proliferation
4.4. Wound Healing Assay
4.5. ATP Production and Mitochondrial Oxidative Stress Measurement
4.6. Western Blotting
4.7. Real-Time Reverse Transcription PCR
4.8. Cell Cycle Analysis
4.9. Statistics
Author Contributions
Funding
Conflicts of Interest
References
- Yokoo, S.; Yamagami, S.; Yanagi, Y.; Uchida, S.; Mimura, T.; Usui, T.; Amano, S. Human Corneal Endothelial Cell Precursors Isolated by Sphere-Forming Assay. Investig. Opthalmol. Vis. Sci. 2005, 46, 1626–1631. [Google Scholar] [CrossRef] [Green Version]
- Bonanno, J.A. Molecular mechanisms underlying the corneal endothelial pump. Exp. Eye Res. 2011, 95, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsubara, M.; Tanishima, T. Wound-healing of the corneal endothelium in the monkey: A morphometric study. Jpn. J. Ophthalmol. 1982, 26, 264–273. [Google Scholar] [PubMed]
- Fujikawa, L.S.; Wickham, M.G.; Binder, P.S. Wound healing in cultured corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 1980, 19, 793–801. [Google Scholar]
- Tamori, Y.; Deng, W.-M. Compensatory cellular hypertrophy: The other strategy for tissue homeostasis. Trends Cell Boil. 2013, 24, 230–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.J.; Sato, T.; Matsubara, M.; Tanishima, T. [Wound healing of the corneal endothelium in the bullous keratopathy after keratoplasty]. Nippon Ganka Gakkai Zasshi 1983, 87, 701–707. [Google Scholar]
- Kizu, A.; Medici, D.; Kalluri, R. Endothelial–Mesenchymal Transition as a Novel Mechanism for Generating Myofibroblasts during Diabetic Nephropathy. Am. J. Pathol. 2009, 175, 1371–1373. [Google Scholar] [CrossRef] [Green Version]
- McCarey, B.E.; Edelhauser, H.F.; Lynn, M.J. Review of corneal endothelial specular microscopy for FDA clinical trials of refractive procedures, surgical devices, and new intraocular drugs and solutions. Cornea 2008, 27, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Sahu, P.K.; Das, G.K.; Agrawal, S.; Kumar, S. Comparative Evaluation of Corneal Endothelium in Patients with Diabetes Undergoing Phacoemulsification. Middle East Afr. J. Ophthalmol. 2017, 24, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Fong, H.; Hohenstein, K.A.; Donovan, P.J. Regulation of Self-Renewal and Pluripotency by Sox2 in Human Embryonic Stem Cells. Stem Cells 2008, 26, 1931–1938. [Google Scholar] [CrossRef]
- Yao, J.; Guihard, P.J.; Blázquez-Medela, A.; Guo, Y.; Moon, J.H.; Jumabay, M.; Boström, K.I.; Yao, Y. Serine Protease Activation Essential for Endothelial-Mesenchymal Transition in Vascular Calcification. Circ. Res. 2015, 117, 758–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGowan, S.L.; Edelhauser, H.F.; Pfister, R.R.; Whikehart, D.R. Stem cell markers in the human posterior limbus and corneal endothelium of unwounded and wounded corneas. Mol. Vis. 2007, 13, 1984–2000. [Google Scholar] [PubMed]
- Chang, Y.K.; Hwang, J.S.; Chung, T.-Y.; Shin, Y.J. SOX2 Activation Using CRISPR/dCas9 Promotes Wound Healing in Corneal Endothelial Cells. STEM CELLS 2018, 36, 1851–1862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.O.; Jeon, H.S.; Hyon, J.Y.; Oh, Y.-J.; Wee, W.R.; Chung, T.-Y.; Shin, Y.J.; Kim, J.W. Recovery of Corneal Endothelial Cells from Periphery after Injury. PLoS ONE 2015, 10, e0138076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Ji, W.; Yu, Y.; Li, Z.; Niu, X.; Xia, W.; Lu, S. FGFR1-ERK1/2-SOX2 axis promotes cell proliferation, epithelial–mesenchymal transition, and metastasis in FGFR1-amplified lung cancer. Oncogene 2018, 37, 5340–5354. [Google Scholar] [CrossRef]
- He, Z.; Forest, F.; Gain, P.; Rageade, D.; Bernard, A.; Acquart, S.; Peoc’H, M.; Defoe, D.M.; Thuret, G. 3D map of the human corneal endothelial cell. Sci. Rep. 2016, 6, 29047. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Campolmi, N.; Gain, P.; Thi, B.M.H.; Dumollard, J.-M.; Duband, S.; Peoc’H, M.; Piselli, S.; Garraud, O.; Thuret, G. Revisited Microanatomy of the Corneal Endothelial Periphery: New Evidence for Continuous Centripetal Migration of Endothelial Cells in Humans. STEM CELLS 2012, 30, 2523–2534. [Google Scholar] [CrossRef]
- Kjenseth, A.; Fykerud, T.A.; Sirnes, S.; Bruun, J.; Yohannes, Z.; Kolberg, M.; Omori, Y.; Rivedal, E.; Leithe, E. The Gap Junction Channel Protein Connexin 43 is Covalently Modified and Regulated by SUMOylation*. J. Biol. Chem. 2012, 287, 15851–15861. [Google Scholar] [CrossRef] [Green Version]
- Nakano, Y.; Oyamada, M.; Dai, P.; Nakagami, T.; Kinoshita, S.; Takamatsu, T. Connexin43 Knockdown Accelerates Wound Healing but Inhibits Mesenchymal Transition after Corneal Endothelial Injury In Vivo. Investig. Opthalmol. Vis. Sci. 2008, 49, 93–104. [Google Scholar] [CrossRef]
- Petroll, W.M.; Barry-Lane, P.A.; Cavanagh, H.; Jester, J.V. ZO-1 Reorganization and Myofibroblast Transformation of Corneal Endothelial Cells after Freeze Injury in the Cat. Exp. Eye Res. 1997, 64, 257–267. [Google Scholar] [CrossRef]
- Daly, A.C.; Randall, R.A.; Hill, C.S. Transforming Growth Factor β-Induced Smad1/5 Phosphorylation in Epithelial Cells Is Mediated by Novel Receptor Complexes and Is Essential for Anchorage-Independent Growth. Mol. Cell. Biol. 2008, 28, 6889–6902. [Google Scholar] [CrossRef] [Green Version]
- Fuentealba, L.C.; Eivers, E.; Ikeda, A.; Hurtado, C.; Kuroda, H.; Pera, E.M.; De Robertis, E.M. Integrating Patterning Signals: Wnt/GSK3 Regulates the Duration of the BMP/Smad1 Signal. Cell 2007, 131, 980–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yook, J.I.; Li, X.-Y.; Ota, I.; Hu, C.; Kim, H.S.; Kim, N.H.; Cha, S.Y.; Ryu, J.K.; Choi, Y.J.; Kim, J.; et al. A Wnt–Axin2–GSK3β cascade regulates Snail1 activity in breast cancer cells. Nature 2006, 8, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xiao, L.; Sun, L.; Liu, F. Wnt/beta-catenin signaling: A promising new target for fibrosis diseases. Physiol. Res. 2012, 61, 337–346. [Google Scholar] [CrossRef]
- Wu, Y.; Ginther, C.; Kim, J.; Mosher, N.; Chung, S.; Slamon, D.; Vadgama, J.V. Expression of Wnt3 activates Wnt/β-catenin pathway and promotes EMT-like phenotype in trastuzumab-resistant HER2-overexpressing breast cancer cells. Mol. Cancer Res. 2012, 10, 1597–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastías-Candia, S.; Martínez, M.; Zolezzi, J.M.; Tapia-Rojas, C. Wnt Signaling Upregulates Teneurin-3 Expression via Canonical and Non-canonical Wnt Pathway Crosstalk. Front. Mol. Neurosci. 2019, 13, 505. [Google Scholar] [CrossRef]
- Dorsky, R.I.; Moon, R.; Raible, D.W. Control of neural crest cell fate by the Wnt signalling pathway. Nature 1998, 396, 370–373. [Google Scholar] [CrossRef]
- Okumura, N.; Nakamura, T.; Kay, E.P.; Nakahara, M.; Kinoshita, S.; Koizumi, N. R-spondin1 Regulates Cell Proliferation of Corneal Endothelial Cells via the Wnt3a/ -Catenin Pathway. Investig. Opthalmol. Vis. Sci. 2014, 55, 6861–6869. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Hao, H.; Reynolds, K.; McMahon, M.; Zhou, C.J. Wnt Signaling in Neural Crest Ontogenesis and Oncogenesis. Cells 2019, 8, 1173. [Google Scholar] [CrossRef] [Green Version]
- Bergsland, M.; Ramsköld, D.; Zaouter, C.; Klum, S.; Sandberg, R.; Muhr, J. Sequentially acting Sox transcription factors in neural lineage development. Genes Dev. 2011, 25, 2453–2464. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Shi, J.; Zhang, K.; Xue, J.; Li, J.; Yang, J.; Chen, J.; Wei, J.; Ren, H.; Liu, X. Sox2 inhibits Wnt-β-catenin signaling and metastatic potency of cisplatin-resistant lung adenocarcinoma cells. Mol. Med. Rep. 2017, 15, 1693–1701. [Google Scholar] [CrossRef] [Green Version]
- Daugherty, R.L.; Gottardi, C.J. Phospho-regulation of Beta-catenin adhesion and signaling functions. Physiology 2007, 22, 303–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hydbring, P.; Malumbres, M.; Sicinski, P. Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases. Nat. Rev. Mol. Cell Biol. 2016, 17, 280–292. [Google Scholar] [CrossRef]
- Diril, M.K.; Ratnacaram, C.K.; Padmakumar, V.C.; Du, T.; Wasser, M.; Coppola, V.; Tessarollo, L.; Kaldis, P. Cyclin-dependent kinase 1 (Cdk1) is essential for cell division and suppression of DNA re-replication but not for liver regeneration. Proc. Natl. Acad. Sci. USA 2012, 109, 3826–3831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhejaily, A.; Day, A.G.; Feilotter, H.E.; Baetz, T.; Lebrun, D.P. Inactivation of the CDKN2A Tumor-Suppressor Gene by Deletion or Methylation Is Common at Diagnosis in Follicular Lymphoma and Associated with Poor Clinical Outcome. Clin. Cancer Res. 2014, 20, 1676–1686. [Google Scholar] [CrossRef] [Green Version]
- Arciuch, V.G.A.; Elguero, M.E.; Poderoso, J.J.; Carreras, M.C. Mitochondrial Regulation of Cell Cycle and Proliferation. Antioxid. Redox Signal. 2012, 16, 1150–1180. [Google Scholar] [CrossRef] [Green Version]
- Aebert, H.; Regel, G. Paranasal sinusitis and sepsis in ICU patients with nasotracheal intubation. Intensiv. Care Med. 1988, 15, 27–30. [Google Scholar] [CrossRef]
- Taylor, J.; Gaze, R. The induction of an anomalous ipsilateral retinotectal projection in Xenopus laevis. Brain Struct. Funct. 1990, 181, 393–404. [Google Scholar] [CrossRef]
- Dimroth, P.; Kaim, G.; Matthey, U. Crucial role of the membrane potential for ATP synthesis by F(1)F(o) ATP synthases. J. Exp. Biol. 2000, 203, 51–59. [Google Scholar]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nature 2011, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Poulose, N.; Raju, R. Sirtuin regulation in aging and injury. Biochim. Biophys. Acta (BBA) Bioenerg 2015, 1852, 2442–2455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iijima, T.; Mishima, T.; Tohyama, M.; Akagawa, K.; Iwao, Y. Mitochondrial membrane potential and intracellular ATP content after transient experimental ischemia in the cultured hippocampal neuron. Neurochem. Int. 2003, 43, 263–269. [Google Scholar] [CrossRef]
- Vayssier-Taussat, M.; Kreps, E.S.; Adrie, C.; Dall’Ava, J.; Christiani, D.; Polla, B.S. Mitochondrial membrane potential: A novel biomarker of oxidative environmental stress. Environ. Health Perspect. 2002, 110, 301–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moleiro, A.F.; Conceição, G.; Leite-Moreira, A.; Rocha-Sousa, A. A Critical Analysis of the AvailableIn VitroandEx VivoMethods to Study Retinal Angiogenesis. J. Ophthalmol. 2017, 2017, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, Y.J.; Cho, D.Y.; Chung, T.Y.; Han, S.B.; Hyon, J.Y.; Wee, W.R. Rapamycin Reduces Reactive Oxygen Species in Cultured Human Corneal Endothelial Cells. Curr. Eye Res. 2011, 36, 1116–1122. [Google Scholar] [CrossRef]
- Merlini, L.; Angelin, A.; Tiepolo, T.; Braghetta, P.; Sabatelli, P.; Zamparelli, A.; Ferlini, A.; Maraldi, N.M.; Bonaldo, P.; Bernardi, P. Cyclosporin A corrects mitochondrial dysfunction and muscle apoptosis in patients with collagen VI myopathies. Proc. Natl. Acad. Sci. USA 2008, 105, 5225–5229. [Google Scholar] [CrossRef] [Green Version]
- Kazumi, T.; Hirose, Y.; Ishihara, K.; Makimura, H.; Yoshino, G.; Utsumi, M.; Baba, S. Thyrotropin-Releasing Hormone and Insulin in Chemically Induced Pancreatic Islet Cell Tumors in Rats. Horm. Metab. Res. 1986, 18, 584–586. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.S.; Yi, H.C.; Shin, Y.J. Effect of SOX2 Repression on Corneal Endothelial Cells. Int. J. Mol. Sci. 2020, 21, 4397. https://doi.org/10.3390/ijms21124397
Hwang JS, Yi HC, Shin YJ. Effect of SOX2 Repression on Corneal Endothelial Cells. International Journal of Molecular Sciences. 2020; 21(12):4397. https://doi.org/10.3390/ijms21124397
Chicago/Turabian StyleHwang, Jin Sun, Ho Chul Yi, and Young Joo Shin. 2020. "Effect of SOX2 Repression on Corneal Endothelial Cells" International Journal of Molecular Sciences 21, no. 12: 4397. https://doi.org/10.3390/ijms21124397
APA StyleHwang, J. S., Yi, H. C., & Shin, Y. J. (2020). Effect of SOX2 Repression on Corneal Endothelial Cells. International Journal of Molecular Sciences, 21(12), 4397. https://doi.org/10.3390/ijms21124397



