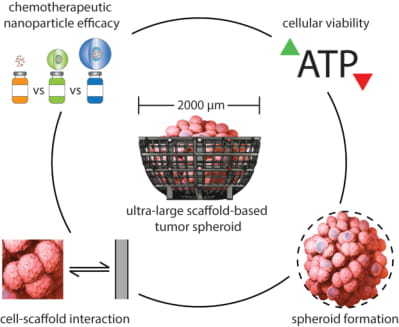
Assessing Advantages and Drawbacks of Rapidly Generated Ultra-Large 3D Breast Cancer Spheroids: Studies with Chemotherapeutics and Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
2.1. Preliminary Cell Line Evaluation
2.2. Chemotherapeutic Effects on Small Spheroid Cell Proliferation
- When examining change in area over the four-day period, the spheroid size has an inverse relationship with increasing drug concentration for all three drugs. However, a notable observation is that all cancer cell spheroids would increase in size during this period of time with or without the influence (verified by the control) of the drugs. The drugs resulted in retardation to the cellular expansion in proportion to the drug strength; it was theorized that the cell expansion could be attributed by either cellular growth or due to cellular disintegration.
- The viability results of the experiment demonstrated that all three drugs had an inhibitive effect against the cancer spheroids. However, the effects of the drugs did not become significantly apparent until they reached 5 µM in concentration (see Figure 4C).
2.3. Chemotherapeutic Effects on Ultra-Large Spheroid Cell Proliferation
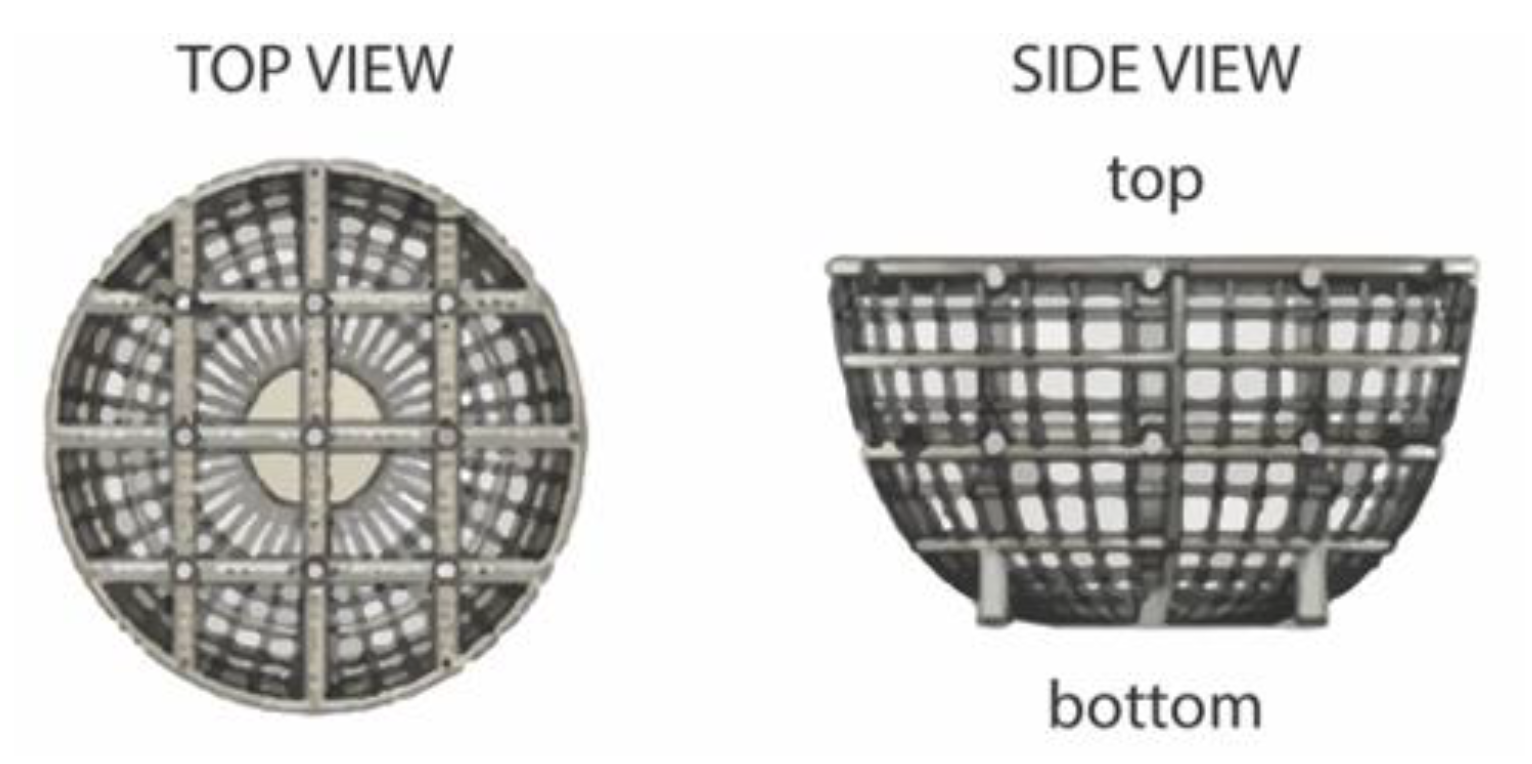
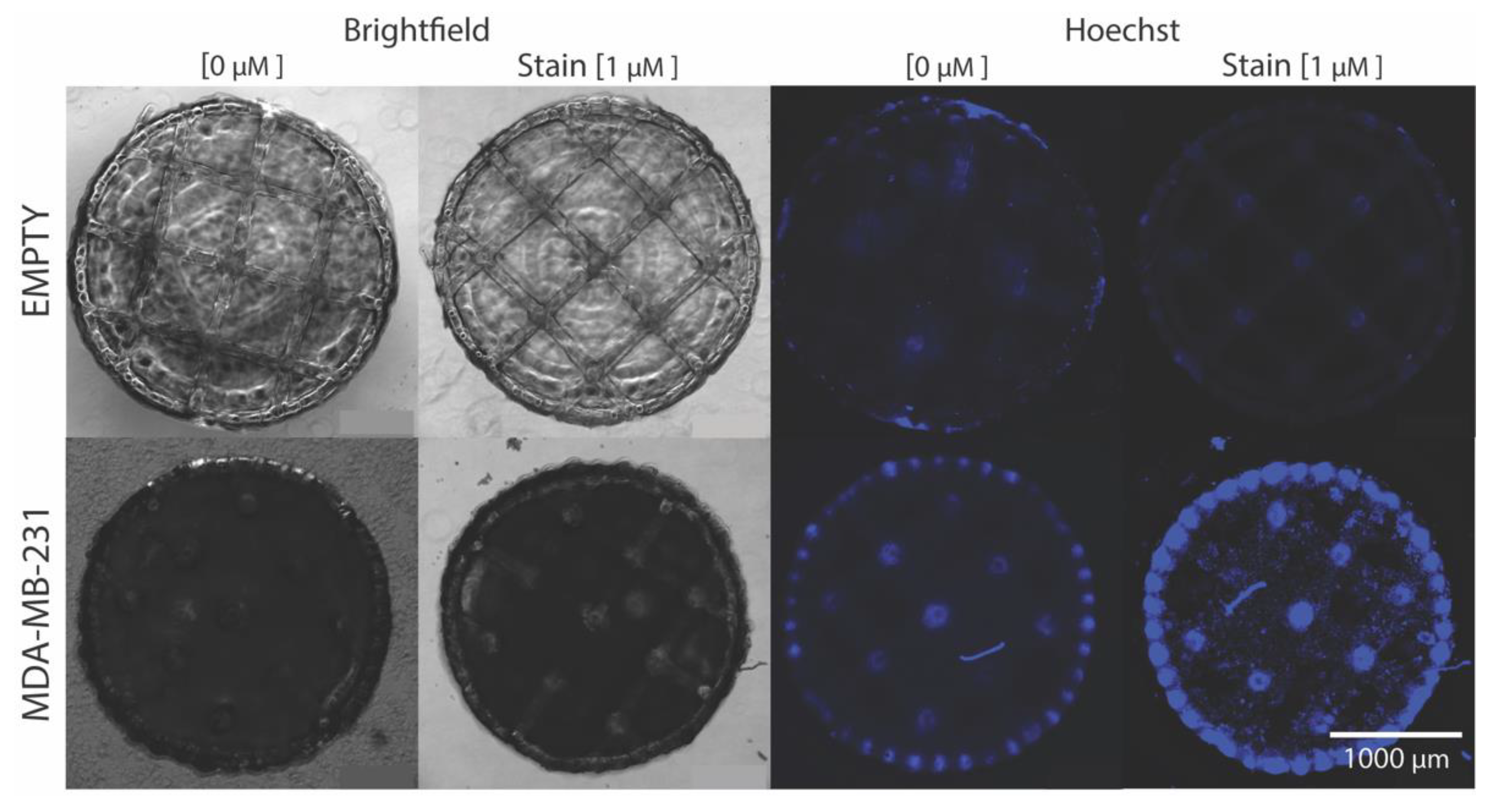
2.4. Cellular Hoechst 33,342 Fluorescence Interaction with Ultra-Large Basket Scaffold
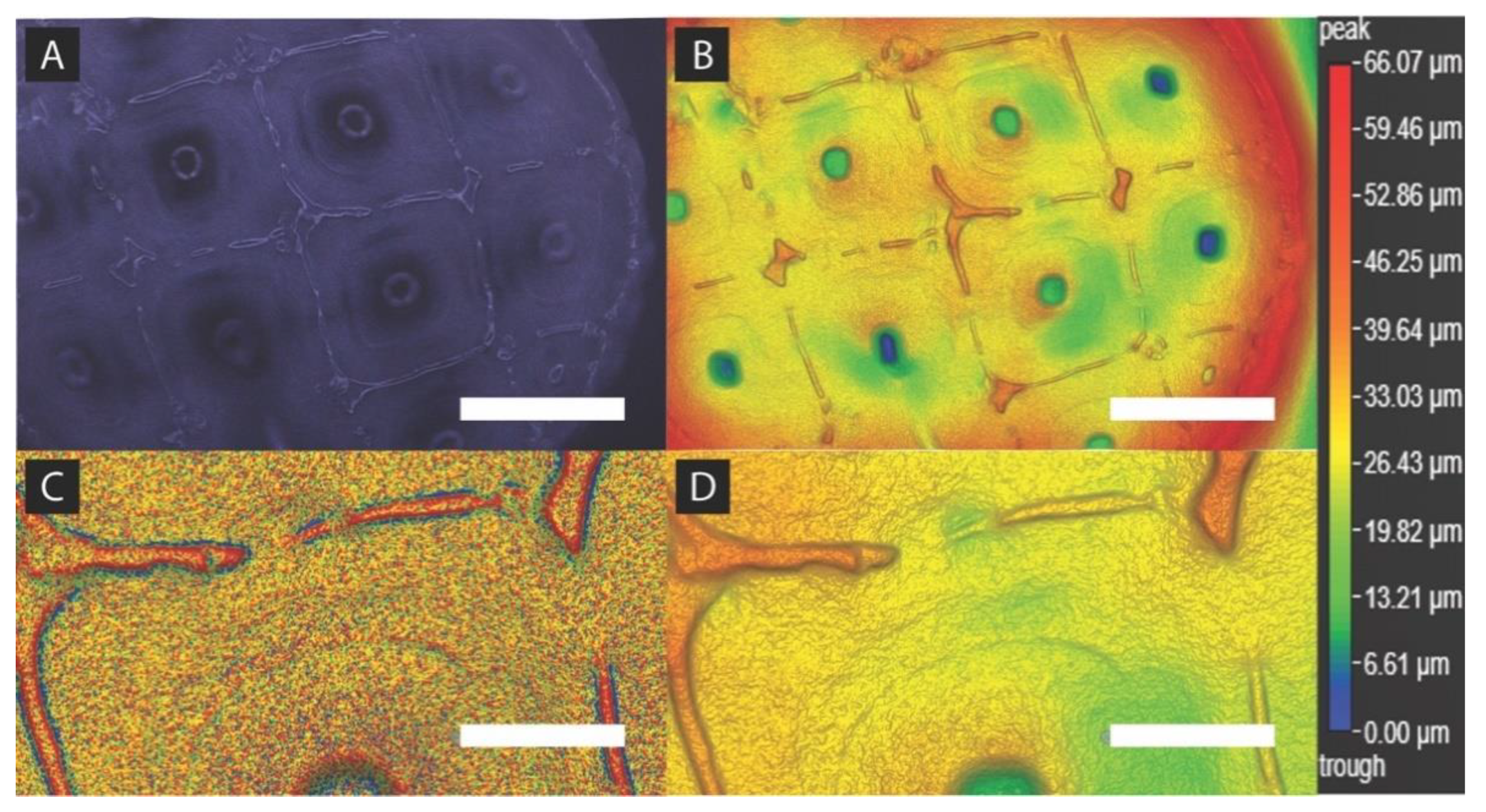
2.5. Spheroid Formation Analysis
3. Materials and Methods
3.1. Culture Preparation and Cell Spheroid Placement
3.2. Drug Preparation and Administration
3.3. Spheroid Imaging and Drug Treatment
3.4. ATP Viability Assay
3.5. Size Measurement
3.6. Image Binary Processing
3.7. Statistical Analysis
3.8. Hoechst Staining
3.9. Dark-Field 3D Imaging
3.10. Overview
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on November 2018 submission data (1999–2016): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Available online: http://www.cdc.gov/cancer/dataviz (accessed on 24 April 2020).
- Zgajnar, J. Clinical Presentation, Diagnosis and Staging of Breast Cancer. Breast Cancer Manag. Surg. 2017, 159–176. [Google Scholar] [CrossRef]
- Shafei, A.; Al Haleem, E.N.A.; Sobhy, A.; Wagdy, O.; Reda, A.; Aboelenin, O.; Marzouk, A.; El Habak, K.; Mostafa, R.; Ali, M.A.; et al. A review on the efficacy and toxicity of different doxorubicin nanoparticles for targeted therapy in metastatic breast cancer. Biomed. Pharmacother. 2017, 95, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-S.; Zhao, Z.; Yang, Z.-N.; Xu, F.; Lu, H.-J.; Zhu, Z.-Y.; Shi, W.; Jiang, J.; Yao, P.-P.; Zhu, H.-P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Wörmann, B. Breast cancer: Basics, screening, diagnostics and treatment. Med. Monatsschr. Pharm. 2017, 40, 55–64. [Google Scholar] [PubMed]
- La Mare, J.-A.; Contu, L.; Hunter, M.C.; Moyo, B.; Sterrenberg, J.; Dhanani, K.C.H.; Mutsvunguma, L.Z.; Edkins, A.L.; De La Mare, J.-A. Breast cancer: Current developments in molecular approaches to diagnosis and treatment. Recent Patents Anti-Cancer Drug Discov. 2014, 9, 153–175. [Google Scholar] [CrossRef] [PubMed]
- PDQ Adult Treatment Editorial Board. Breast Cancer Treatment (Adult) (PDQ®): Health Professional Version. In PDQ Cancer Information Summaries; National Cancer Institute: Bethesda, MD, USA, 2020. [Google Scholar]
- Gill, D.; Bruce, D.; Tan, P. Controlling the cost of breast cancer. Eur. J. Cancer Care 2011, 20, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Doxorubicin (Adriamycin) | Cancer Drugs | Cancer Research UK. Available online: https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/cancer-drugs/drugs/doxorubicin (accessed on 24 April 2020).
- Rivankar, S. An overview of doxorubicin formulations in cancer therapy. J. Cancer Res. Ther. 2014, 10, 853. [Google Scholar] [CrossRef] [PubMed]
- Adriamycin PFS Side Effects Center Available. Available online: https://www.rxlist.com/adriamycin-pfs-side-effects-drug-center.htm (accessed on 12 May 2020).
- Wibroe, P.P.; Ahmadvand, D.; Oghabian, M.A.; Yaghmur, A.; Moghimi, S.M. An integrated assessment of morphology, size, and complement activation of the PEGylated liposomal doxorubicin products Doxil®, Caelyx®, DOXOrubicin, and SinaDoxosome. J. Control. Release 2016, 221, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Parot, J.; Caputo, F.; Mehn, D.; Hackley, V.; Calzolai, L. Physical characterization of liposomal drug formulations using multi-detector asymmetrical-flow field flow fractionation. J. Control. Release 2020, 320, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Product Is Cytotoxic And Handle With Care! Product Is For Research Only, Not For Human Use. Available online: https://tribiosciences.com/wp-content/uploads/2019/05/CofA_F30204B-D_10171101_Doxoves.pdf (accessed on 13 May 2020).
- Dunkern, T.R.; Mueller-Klieser, W. Quantification of apoptosis induction by doxorubicin in three types of human mammary carcinoma spheroids. Anticancer. Res. 2000, 19, 3141–3146. [Google Scholar]
- Mellor, H.R.; Callaghan, R. Accumulation and distribution of doxorubicin in tumour spheroids: The influence of acidity and expression of P-glycoprotein. Cancer Chemother. Pharmacol. 2011, 68, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Potiron, V.; Clément-Colmou, K.; Jouglar, E.; Pietri, M.; Chiavassa, S.; Delpon, G.; Paris, F.; Supiot, S. Tumor vasculature remodeling by radiation therapy increases doxorubicin distribution and efficacy. Cancer Lett. 2019, 457, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, S.; Wang, X.; Wang, M.; Tian, Q.; Yang, J.; Wang, J.; Wang, B.; Liu, P.-J.; Yang, J. Tumor vasculature remolding by thalidomide increases delivery and efficacy of cisplatin. J. Exp. Clin. Cancer Res. 2019, 38, 427. [Google Scholar] [CrossRef] [PubMed]
- Prellis Bio Announces that it has Generated 300 Human Antibodies that have Neutralization Potential Against SARS-CoV-2. Available online: https://www.prellisbio.com (accessed on 13 May 2020).
- Organoids are Biocompatible Structures that Hold Your Precious Cells in Place with an Oxygen Permeable 3D Scaffold, Guaranteeing Your 3D Tissue Culture will Work Every Time. Available online: https://www.prellisbio.com/organoids (accessed on 24 April 2020).
- Ziperstein, M.J.; Guzman, A.; Kaufman, L.J. Breast Cancer Cell Line Aggregate Morphology Does Not Predict Invasive Capacity. PLoS ONE 2015, 10, e0139523. [Google Scholar] [CrossRef] [PubMed]
- Ivascu, A.; Kubbies, M. Rapid Generation of Single-Tumor Spheroids for High-Throughput Cell Function and Toxicity Analysis. J. Biomol. Screen. 2006, 11, 922–932. [Google Scholar] [CrossRef] [PubMed]
- CellTiter-Glo® Luminescent Cell Viability Assay Technical Bulletin. Available online: https://www.promega.com/-/media/files/resources/protocols/technical-bulletins/0/celltiter-glo-luminescent-cell-viability-assay-protocol.pdf?la=en (accessed on 18 May 2020).





| Concentration [µM] | Area Difference | Cell Viability |
|---|---|---|
| p-Value | p-Value | |
| 0.05 | 0.143 | 0.005 |
| 0.10 | 0.311 | 0.694 |
| 0.50 | 0 | 0.006 |
| 1.00 | 0 | 0.004 |
| 5.00 | 0 | 0 |
| 10.0 | 0 | 0 |
| Cell Type | Data Type | Control | Molecular DOX | Dox-NPⓇ | DoxovesTM | ||||
|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 0 | Day 3 | Day 0 | Day 3 | Day 0 | Day 3 | ||
| MCF-7 | scaffold coverage (% area) | 34.7 | 54.9 | 51.4 | 27.3 | 67.2 | 37.5 | 49.4 | 41.5 |
| # black pixels | 425,922 | 674,276 | 631,632 | 334,961 | 825,351 | 460,759 | 606,451 | 509,364 | |
| MDA-MB-231 | scaffold coverage (% area) | 57.6 | 54.4 | 52.2 | 34.6 | 47.2 | 46.8 | 46.2 | 46.5 |
| # black pixels | 707,343 | 668,893 | 640,883 | 424,733 | 580,588 | 575,383 | 568,289 | 571,259 | |
| Concentration [µM] | MCF-7 Cell Viability | MDA-MB-231 Cell Viability |
|---|---|---|
| p-Value | p-Value | |
| 0.05 | 0.280 | 0.324 |
| 0.50 | 0.019 | 0.012 |
| 5.00 | 0.020 | 0.023 |
| 50.0 | 0.033 | 0.012 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holub, A.R.; Huo, A.; Patel, K.; Thakore, V.; Chhibber, P.; Erogbogbo, F. Assessing Advantages and Drawbacks of Rapidly Generated Ultra-Large 3D Breast Cancer Spheroids: Studies with Chemotherapeutics and Nanoparticles. Int. J. Mol. Sci. 2020, 21, 4413. https://doi.org/10.3390/ijms21124413
Holub AR, Huo A, Patel K, Thakore V, Chhibber P, Erogbogbo F. Assessing Advantages and Drawbacks of Rapidly Generated Ultra-Large 3D Breast Cancer Spheroids: Studies with Chemotherapeutics and Nanoparticles. International Journal of Molecular Sciences. 2020; 21(12):4413. https://doi.org/10.3390/ijms21124413
Chicago/Turabian StyleHolub, Austin R., Anderson Huo, Kavil Patel, Vishal Thakore, Pranav Chhibber, and Folarin Erogbogbo. 2020. "Assessing Advantages and Drawbacks of Rapidly Generated Ultra-Large 3D Breast Cancer Spheroids: Studies with Chemotherapeutics and Nanoparticles" International Journal of Molecular Sciences 21, no. 12: 4413. https://doi.org/10.3390/ijms21124413
APA StyleHolub, A. R., Huo, A., Patel, K., Thakore, V., Chhibber, P., & Erogbogbo, F. (2020). Assessing Advantages and Drawbacks of Rapidly Generated Ultra-Large 3D Breast Cancer Spheroids: Studies with Chemotherapeutics and Nanoparticles. International Journal of Molecular Sciences, 21(12), 4413. https://doi.org/10.3390/ijms21124413