Nutraceuticals in Neurological Disorders
Abstract
:1. Introduction
2. Methodology
3. Nutraceuticals and Its Categories
3.1. Food-Based Nutraceuticals or Traditional Nutraceuticals
3.1.1. Nutrients
3.1.2. Herbals or Extracts and Concentrates of Botanical Products
3.1.3. Probiotic Microorganisms
3.1.4. Nutraceutical Enzymes
3.2. Non-Traditional Nutraceuticals
3.2.1. Fortified Nutraceuticals
3.2.2. Recombinant Nutraceuticals
3.3. Based on the Mechanism of Action
3.4. Based on the Chemical Nature of the Products
4. Nutraceuticals in Ameliorating Neurodegeneration
4.1. Quercetin and Kaempferol
4.2. Withanine
4.3. Asiatic Acid
4.4. Bhilavanol A and Bhilavanol B
5. Nutraceuticals in Alzheimer’s Disease (AD)
5.1. Flavonoids
5.2. Carotenoids
5.3. Crocin
5.4. Cyanidin
5.5. Luteolin
6. Nutraceuticals in Parkinson’s Disease
6.1. Targeting the Dysfunction of Mitochondria and Oxidative Stress
6.2. Endoplasmic Reticulum (ER) Stress Pathway and Protein Misfolding and Aggregation
7. Nutraceuticals in Depression
8. Nutraceuticals in Psychotic Disorders
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Williams, R.J.; Mohanakumar, K.P.; Beart, P.M. Neuro-nutraceuticals: Further insights into their promise for brain health. Neurochem. Int. 2016, 95, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, R.J.; Mohanakumar, K.P.; Beart, P.M. Neuro-nutraceuticals: The path to brain health via nourishment is not so distant. Neurochem. Int. 2015, 89, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Bungau, S.G.; Popa, V.C. Between Religion and Science Some Aspects Concerning Illness and Healing in Antiquity. Transylv. Rev. 2015, 24, 3–18. [Google Scholar]
- Ramalingum, N.; Mahomoodally, M.F. The therapeutic potential of medicinal foods. Adv. Pharmacol. Sci. 2014, 2014, 354264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, V.; Boekel. Nutraceuticals: Possible future ingredients and food safety aspects. In Ensuring Global Food Safety; Academic Press: Cambridge, MA, USA, 2010; pp. 333–338. [Google Scholar]
- Orlando, J.M. Behavioral Nutraceuticals and Diets. Vet. Clin. Small Anim. Pract. 2018, 48, 473–495. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.; Prakash, D. Nutraceuticals for geriatrics. J. Tradit. Complement. Med. 2015, 5, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Daim, M.M.; El-Tawil, O.S.; Bungau, S.G.; Atanasov, A.G. Applications of Antioxidants in Metabolic Disorders and Degenerative Diseases: Mechanistic Approach. Oxidative Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Brown, L.A.; Riby, L.M.; Reay, J.L. Supplementing cognitive aging: A selective review of the effects of ginkgo biloba and a number of everyday nutritional substances. Exp. Aging Res. 2009, 36, 105–122. [Google Scholar] [CrossRef]
- van der Burg, K.P.; Cribb, L.; Firth, J.; Karmacoska, D.; Sarris, J. Nutrient and genetic biomarkers of nutraceutical treatment response in mood and psychotic disorders: A systematic review. Nutr. Neurosci. 2019, 1–17. [Google Scholar] [CrossRef]
- Georgiou, N.A.; Garssen, J.; Witkamp, R.F. Pharma-nutrition interface: The gap is narrowing. Eur. J. Pharmacol. 2011, 651, 1–8. [Google Scholar] [CrossRef]
- Kidd, I.J. Biopiracy and the ethics of medical heritage: The case of India’s traditional knowledge digital library. J. Med. Humanit. 2012, 33, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Kuhnau, J. Flavonoids. A class of semi-essential food components: Their role in human nutrition. World Rev. Nutr. Diet. 2013, 24, 117–191. [Google Scholar]
- Chanda, S.; Tiwari, R.K.; Kumar, A.; Singh, K. Nutraceuticals inspiring the current therapy for lifestyle diseases. Adv. Pharmacol. Pharm. Sci. 2019, 2019, 6908716. [Google Scholar] [CrossRef] [PubMed]
- Menon, I.; Spudich, A. The Ashtavaidya physicians of Kerala: A tradition in transition. J. Ayurveda Integr. Med. 2010, 1, 245–250. [Google Scholar] [CrossRef] [Green Version]
- González-Sarrías, A.; Larrosa, M.; García-Conesa, M.T.; Tomás-Barberán, F.A.; Espín, J.C. Nutraceuticals for older people: Facts, fictions and gaps in knowledge. Maturitas 2013, 75, 313–334. [Google Scholar] [CrossRef]
- Yapijakis, C. Hippocrates of Kos, the father of clinical medicine, and Asclepiades of Bithynia, the father of molecular medicine. Vivo 2009, 23, 507–514. [Google Scholar]
- Andlauer, W.; Fürst, P. Nutraceuticals: A piece of history, present status and outlook. Food Res. Int. 2002, 35, 171–176. [Google Scholar] [CrossRef]
- Chauhan, N.B.; Mehla, J. Ameliorative Effects of Nutraceuticals in Neurological Disorders. In Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease; Elsevier: Amsterdam, The Netherlands, 2015; pp. 245–260. [Google Scholar]
- Peterson, C.T.; Denniston, K.; Chopra, D. Therapeutic uses of triphala in ayurvedic medicine. J. Altern. Complement. Med. 2017, 23, 607–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogue, J.; Collins, O.; Troy, A.J. Market analysis and concept development of functional foods. In Developing New Functional Food and Nutraceutical Products; Debasis Bagchi, S.N., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 29–45. [Google Scholar] [CrossRef]
- Champagne, C.P.; Gomes da Cruz, A.; Daga, M. Strategies to improve the functionality of probiotics in supplements and foods. Curr. Opin. Food Sci. 2018, 22, 160–166. [Google Scholar] [CrossRef]
- Casey, C.; Slawson, D.C.; Neal, L.R. Vitamin D supplementation in infants, children, and adolescents. Am. Fam. Physician 2010, 81, 745–748. [Google Scholar] [PubMed]
- Nicastro, H.L.; Ross, S.A.; Milner, J.A. Garlic and onions: Their cancer prevention properties. Cancer Prev. Res. 2015, 8, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhaskarachary, K. Traditional Foods, Functional Foods and Nutraceuticals. Proc. Indian Natl. Sci. Acad. 2016, 82, 1565–1577. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Bhat, H. Milk and Dairy Products as Functional Foods: A Review. Int. J. Dairy Sci. 2011, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Dohrmann, D.D.; Putnik, P.; Bursać Kovačević, D.; Simal-Gandara, J.; Lorenzo, J.M.; Barba, F.J. Japanese, Mediterranean and Argentinean Diets and Their Potential Roles in Neurodegenerative Diseases. Food Res. Int. 2019, 120, 464–477. [Google Scholar] [CrossRef]
- Barba, F.J.; Putnik, P.; Kovacevic, D.B. Agri-Food Industry Strategies for Healthy Diets and Sustainability: New Challenges in Nutrition and Public Health; Press, A., Ed.; MPS Limited Chennai India: Tamil Nadu, India, 2020. [Google Scholar]
- Putnik, P.; Gabrić, D.; Roohinejad, S.; Barba, F.J.; Granato, D.; Lorenzo, J.M.; Bursać Kovačević, D. Bioavailability and food production of organosulfur compounds from edible Allium species. In Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Francisco, J., Barba, J.M.A.S., Giancarlo Cravotto, J., Lorenzo, M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 293–308. [Google Scholar] [CrossRef]
- Putnik, P.; Gabrić, D.; Roohinejad, S.; Barba, F.J.; Granato, D.; Mallikarjunan, K.; Lorenzo, J.M.; Bursać Kovačević, D. An Overview of Organosulfur Compounds From Allium Spp.: From Processing and Preservation to Evaluation of Their Bioavailability, Antimicrobial, and Anti-Inflammatory Properties. Food Chem. 2019, 276, 680–691. [Google Scholar] [CrossRef]
- Poojary, M.M.; Putnik, P.; Bursać Kovačević, D.; Barba, F.J.; Lorenzo, J.M.; Dias, D.A.; Shpigelman, A. Stability and extraction of bioactive sulfur compounds from Allium genus processed by traditional and innovative technologies. J. Food Compost. Anal. 2017, 61, 28–39. [Google Scholar] [CrossRef]
- Montesano, D.; Rocchetti, G.; Putnik, P.; Lucini, L. Bioactive profile of pumpkin: An overview on terpenoids and their health-promoting properties. Curr. Opin. Food Sci. 2018, 22, 81–87. [Google Scholar] [CrossRef]
- Pillitteri, J.L.; Shiffman, S.; Rohay, J.M.; Harkins, A.M.; Burton, S.L.; Wadden, T.A. Use of Dietary Supplements for Weight Loss in the United States: Results of a National Survey. Obesity 2008, 16, 790–796. [Google Scholar] [CrossRef]
- Rao, T.S.; Asha, M.R.; Ramesh, B.N.; Rao, K.S. Understanding Nutrition, Depression and Mental Illnesses. Indian J. Psychiatry 2008, 50, 77–82. [Google Scholar] [CrossRef]
- Gosálbez, L.; Ramón, D. Probiotics in Transition: Novel Strategies. Trends Biotechnol. 2015, 33, 195–196. [Google Scholar] [CrossRef]
- Zucko, J.; Starcevic, A.; Diminic, J.; Oros, D.; Mortazavian, A.M.; Putnik, P. Probiotic—Friend or foe? Curr. Opin. Food Sci. 2020, 32, 45–49. [Google Scholar] [CrossRef]
- Tapal, A.; Kaul Tiku, P. Nutritional and Nutraceutical Improvement by Enzymatic Modification of Food Proteins. In Enzymes in Food Biotechnology; Kuddus, M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 471–481. [Google Scholar] [CrossRef]
- Singh, J.; Sinha, S. Classification, regulatory acts and applications of nutraceuticals for health. Int. J. Pharma Bio Sci. 2012, 2, 177–187. [Google Scholar]
- Sapkale, A.P.; Thorat, M.S.; Vir, P.R.; Singh, M.C. Nutraceuticals—Global status and applications: A Review. Int. J. Chem. Pharm. 2012, 1, 1166–1181. [Google Scholar]
- Ottaway, P.B. Food Fortification and Supplementation: Technological, Safety and Regulatory Aspects; Woodhead Publishing: Cambridge, UK, 2008. [Google Scholar]
- Street, A. Food as Pharma: Marketing Nutraceuticals to India's Rural Poor. Crit. Public Health 2015, 25, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Dietary Supplement Health and Education Act of 1994. Available online: https://ods.od.nih.gov/About/DSHEA_Wording.aspx (accessed on 11 June 2020).
- Gutiérrez-Del-Río, I.; Fernández, J.; Lombó, F. Plant Nutraceuticals as Antimicrobial Agents in Food Preservation: Terpenoids, Polyphenols and Thiols. Int. J. Antimicrob. Agents 2018, 52, 309–315. [Google Scholar] [CrossRef]
- Giavasis, I. Bioactive Fungal Polysaccharides as Potential Functional Ingredients in Food and Nutraceuticals. Curr. Opin. Biotechnol. 2014, 26, 162–173. [Google Scholar] [CrossRef]
- Al-Okbi, S.Y. Nutraceuticals of Anti-Inflammatory Activity as Complementary Therapy for Rheumatoid Arthritis. Toxicol. Ind. Health 2014, 30, 738–749. [Google Scholar] [CrossRef]
- Cornelli, U. Antioxidant Use in Nutraceuticals. Clin. Dermatol. 2009, 27, 175–194. [Google Scholar] [CrossRef]
- Chintale Ashwini, G.; Kadam Vaishali, S.; Sakhare Ram, S.; Birajdar Ganesh, O.; Nalwad Digambar, N. Role of nutraceuticals in various diseases: A comprehensive review. Int. J. Res. Pharm. Chem. 2013, 3, 290–299. [Google Scholar]
- Colín-González, A.L.; Ali, S.F.; Túnez, I.; Santamaría, A. On the antioxidant, neuroprotective and anti-inflammatory properties of S-allyl cysteine: An update. Neurochem. Int. 2015, 89, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Ferri, C.; Desideri, G. Brain protection and cognitive function: Cocoa flavonoids as nutraceuticals. Curr. Pharm. Des. 2016, 22, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Johnston, G.A. Flavonoid nutraceuticals and ionotropic receptors for the inhibitory neurotransmitter GABA. Neurochem. Int. 2015, 89, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, S.N.; Tollefsbol, T.O. The Role of Nutraceuticals in Chemoprevention and Chemotherapy and Their Clinical Outcomes. J. Oncol. 2012, 2012, 192464. [Google Scholar] [CrossRef] [Green Version]
- Asadi-Shekaari, M.; Kalantaripour, T.P.; Nejad, F.A.; Namazian, E.; Eslami, A. The anticonvulsant and neuroprotective effects of walnuts on the neurons of rat brain cortex. Avicenna J. Med Biotechnol. 2012, 4, 155. [Google Scholar]
- Kelsey, N.A.; Wilkins, H.M.; Linseman, D.A. Nutraceutical antioxidants as novel neuroprotective agents. Molecules 2010, 15, 7792–7814. [Google Scholar] [CrossRef] [Green Version]
- Barber, S.C.; Mead, R.J.; Shaw, P.J. Oxidative stress in ALS: A mechanism of neurodegeneration and a therapeutic target. Biochim. Biophys. Acta Mol. Basis Dis. 2006, 1762, 1051–1067. [Google Scholar] [CrossRef] [Green Version]
- Gonsette, R. Neurodegeneration in multiple sclerosis: The role of oxidative stress and excitotoxicity. J. Neurol. Sci. 2008, 274, 48–53. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Ghabaee, M.; Jabedari, B.; Al-E-Eshagh, N.; Ghaffarpour, M.; Asadi, F. Serum and cerebrospinal fluid antioxidant activity and lipid peroxidation in Guillain–Barre syndrome and multiple sclerosis patients. Int. J. Neurosci. 2010, 120, 301–304. [Google Scholar] [CrossRef]
- Lenaz, G. The Mitochondrial Production of Reactive Oxygen Species: Mechanisms and Implications in Human Pathology. IUBMB life 2001, 52, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Ott, M.; Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria, oxidative stress and cell death. Apoptosis 2007, 12, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Abdul Manap, A.S.; Vijayabalan, S.; Madhavan, P.; Chia, Y.Y.; Arya, A.; Wong, E.H.; Rizwan, F.; Bindal, U.; Koshy, S. Bacopa monnieri, a Neuroprotective Lead in Alzheimer Disease: A Review on Its Properties, Mechanisms of Action, and Preclinical and Clinical Studies. Drug Target Insights 2019, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Su, B.; Wang, X.; Smith, M.A.; Perry, G. Causes of Oxidative Stress in Alzheimer Disease. Cell Mol. Life Sci. 2007, 64, 2202–2210. [Google Scholar] [CrossRef]
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health Benefits of Polyphenols and Carotenoids in Age-Related Eye Diseases. Oxidative Med. Cell. Longev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Preethi Pallavi, M.C.; Sampath Kumar, H.M. Chapter 8—Nutraceuticals in Prophylaxis and Therapy of Neurodegenerative Diseases. In Discovery and Development of Neuroprotective Agents from Natural Products; Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 359–376. [Google Scholar] [CrossRef]
- Purza, L.; Abdel-Daim, M.; Belba, A.; Iovan, C.; Bumbu, A.; Lazar, L.; Bungau, S.; Tit, D.M. Monitoring the Effects of Various Combination of Specific Drug Therapies at Different Stages of Alzheimer's Dementia. Farmacia 2019, 67, 477–481. [Google Scholar] [CrossRef]
- Sivasankarapillai, V.S.; Madhu Kumar Nair, R.; Rahdar, A.; Bungau, S.; Zaha, D.C.; Aleya, L.; Tit, D.M. Overview of the anticancer activity of Withaferin A, an active constituent of the Indian Ginseng Withania somnifera. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef]
- Frisardi, V.; Panza, F.; Solfrizzi, V.; Seripa, D.; Pilotto, A. Plasma lipid disturbances and cognitive decline. J. Am. Geriatr. Soc. 2010, 58, 2429–2430. [Google Scholar] [CrossRef]
- Nunomura, A.; Perry, G.; Aliev, G.; Hirai, K.; Takeda, A.; Balraj, E.K.; Jones, P.K.; Ghanbari, H.; Wataya, T.; Shimohama, S.; et al. Oxidative Damage is the Earliest Event in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2001, 60, 759–767. [Google Scholar] [CrossRef] [Green Version]
- Nunomura, A.; Perry, G.; Pappolla, M.A.; Wade, R.; Hirai, K.; Chiba, S.; Smith, M.A. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer's disease. J. Neurosci. 1999, 19, 1959–1964. [Google Scholar] [CrossRef]
- Glevitzky, I.; Dumitrel, G.A.; Glevitzky, M.; Pasca, B.; Otrisal, P.; Bungau, S.; Cioca, G.; Pantis, C.; Popa, M. Statistical Analysis of the Relationship Between Antioxidant Activity and the Structure of Flavonoid Compounds. Rev. Chim. 2019, 70, 3103–3107. [Google Scholar] [CrossRef]
- Mecocci, P.; Tinarelli, C.; Schulz, R.J.; Polidori, M.C. Nutraceuticals in cognitive impairment and Alzheimer's disease. Front. Pharmacol. 2014, 5, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellani, R.J.; Harris, P.L.; Sayre, L.M.; Fujii, J.; Taniguchi, N.; Vitek, M.P.; Founds, H.; Atwood, C.S.; Perry, G.; Smith, M.A. Active glycation in neurofibrillary pathology of Alzheimer disease: Nε-(carboxymethyl) lysine and hexitol-lysine. Free Radic. Biol. Med. 2001, 31, 175–180. [Google Scholar] [CrossRef]
- Boccardi, V.; Tinarelli, C.; Mecocci, P. Nutraceuticals and Cognitive Dysfunction. Neuroprotective Eff. Phytochem. Neurol. Disord. 2017, 561–579. [Google Scholar] [CrossRef]
- Sawmiller, D.; Li, S.; Shahaduzzaman, M.; Smith, A.J.; Obregon, D.; Giunta, B.; Borlongan, C.V.; Sanberg, P.R.; Tan, J. Luteolin Reduces Alzheimer's Disease Pathologies Induced by Traumatic Brain Injury. Int. J. Mol. Sci. 2014, 15, 895–904. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, H.; Cheng, H.; Che, Z. Ameliorating Effect of Luteolin on Memory Impairment in an Alzheimer's Disease Model. Mol. Med. Rep. 2016, 13, 4215–4220. [Google Scholar] [CrossRef] [Green Version]
- Mythri, R.; Kumar, A.; Mms, B. Nutraceuticals and Other Natural Products in Parkinson’s Disease Therapy: Focus on Clinical Applications. In Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease; Academic Press: Cambridge, MA, USA, 2015; pp. 421–431. [Google Scholar] [CrossRef]
- Mandel, S.; Grünblatt, E.; Riederer, P.; Gerlach, M.; Levites, Y.; Youdim, M.B. Neuroprotective strategies in Parkinson's disease : An update on progress. CNS Drugs 2003, 17, 729–762. [Google Scholar] [CrossRef]
- Tatton, W.; Chalmers-Redman, R.; Brown, D.; Tatton, N. Apoptosis in Parkinson's disease: Signals for neuronal degradation. Ann. Neurol. 2003, 53 (Suppl. 3), S61–S70. [Google Scholar] [CrossRef]
- Jenner, P.; Olanow, C.W. Oxidative stress and the pathogenesis of Parkinson's disease. Neurology 1996, 47, 161S–170S. [Google Scholar] [CrossRef]
- Henchcliffe, C.; Beal, M.F. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat. Clin. Pract. Neurol. 2008, 4, 600–609. [Google Scholar] [CrossRef]
- Navarro, A.; Boveris, A. Brain mitochondrial dysfunction and oxidative damage in Parkinson's disease. J. Bioenerg. Biomembr. 2009, 41, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Lama, A.; Pirozzi, C.; Avagliano, C.; Annunziata, C.; Mollica, M.P.; Calignano, A.; Meli, R.; Mattace Raso, G. Nutraceuticals: An integrative approach to starve Parkinson’s disease. Brain Behav. Immun. Health 2020, 2, 100037. [Google Scholar] [CrossRef]
- Ceskova, E.; Silhan, P. Novel treatment options in depression and psychosis. Neuropsychiatr. Dis. Treat. 2018, 14, 741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fodor, K.; Tit, D.M.; Pasca, B.; Bustea, C.; Uivarosan, D.; Endres, L.; Iovan, C.; Abdel-Daim, M.M.; Bungau, S. Long-Term Resveratrol Supplementation as a Secondary Prophylaxis for Stroke. Oxidative Med. Cell. Longev. 2018. [Google Scholar] [CrossRef]
- Ceskova, E. Novel Treatment Options in Depression and Psychosis. Clin. Ther. 2017, 39, e103. [Google Scholar] [CrossRef] [Green Version]
- Hiemke, C.; Baumann, P.; Bergemann, N.; Conca, A.; Dietmaier, O.; Egberts, K.; Fric, M.; Gerlach, M.; Greiner, C.; Gründer, G. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: Update 2011. Pharmacopsychiatry 2011, 21, 195–235. [Google Scholar] [CrossRef] [Green Version]
- Butterweck, V.; Schmidt, M. St. John's Wort: Role of Active Compounds for Its Mechanism of Action and Efficacy. Wien. Med. Wochenschr. 2007, 157, 356–361. [Google Scholar] [CrossRef]
- Cloutier, M.; Aigbogun, M.S.; Guerin, A.; Nitulescu, R.; Ramanakumar, A.V.; Kamat, S.A.; DeLucia, M.; Duffy, R.; Legacy, S.N.; Henderson, C. The economic burden of schizophrenia in the United States in 2013. J. Clin. Psychiatry 2016, 77, 764–771. [Google Scholar] [CrossRef]
- Balanzá-Martínez, V. Nutritional supplements in psychotic disorders. Actas Esp. Psiquiatr. 2017, 45 (Suppl. 1), 16–25. [Google Scholar]
- Davis, J.; Moylan, S.; Harvey, B.H.; Maes, M.; Berk, M. Neuroprogression in schizophrenia: Pathways underpinning clinical staging and therapeutic corollaries. Aust. N. Z. J. Psychiatry 2014, 48, 512–529. [Google Scholar] [CrossRef]
- Howes, O.D.; Kapur, S. The dopamine hypothesis of schizophrenia: Version III—The final common pathway. Schizophr. Bull. 2009, 35, 549–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savitz, A.J.; Xu, H.; Gopal, S.; Nuamah, I.; Ravenstijn, P.; Janik, A.; Schotte, A.; Hough, D.; Fleischhacker, W.W. Efficacy and Safety of Paliperidone Palmitate 3-Month Formulation for Patients with Schizophrenia: A Randomized, Multicenter, Double-Blind, Noninferiority Study. Int. J. Neuropsychopharmacol. 2016, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, H.E.; Roffman, J.L. Emerging treatments in schizophrenia: Highlights from recent supplementation and prevention trials. Harv. Rev. Psychiatry 2016, 24, e1–e7. [Google Scholar] [CrossRef]
- Sarris, J.; Logan, A.C.; Akbaraly, T.N.; Amminger, G.P.; Balanzá-Martínez, V.; Freeman, M.P.; Hibbeln, J.; Matsuoka, Y.; Mischoulon, D.; Mizoue, T.; et al. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry 2015, 2, 271–274. [Google Scholar] [CrossRef]
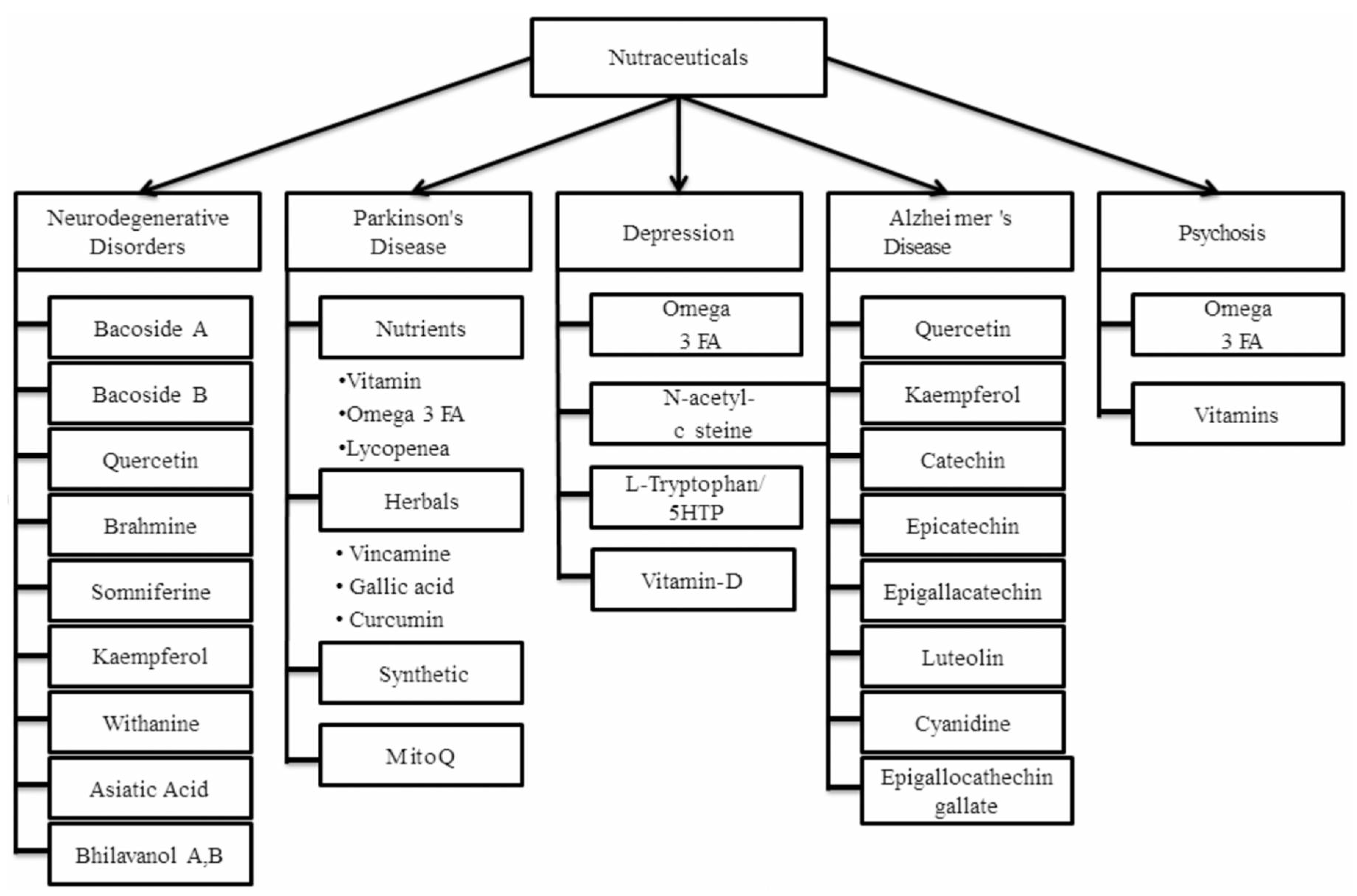

| Compound | Mechanism of Action |
|---|---|
| Omega-3 Fatty Acid Molecules | They act by inhibiting re-uptake of monoamines during neurological transmission and benefits neurotransmission by increasing the fluidity in membranes of cell. These molecules decrease inflammatory mediators and their synthesis, enhancing neurogenesis and prevents depressive episodes [85]. |
| N-acetyl Cysteine | It mainly comprises of anti-inflammatory and antioxidant activities which leads to replenishment of glutathione levels and enhances neurogenesis. It also protects the individual against mitochondrial toxicity and modulates glutamate pathway thereby preventing depression. |
| S-adenosyl Methionine | It mainly influences the production and biotransformation of neurotransmitters as it is an important methyl donor of methyl groups. It also decreases the secretion of prolactin and increases the conversion of phosphatidylcholine [86]. |
| L-Tryptophan/5-HTP | Tryptophan is required for conversion into serotonin in the presence of B6 and magnesium to actively form 5-HTP through intermediate processes. The augmentation of tryptophan with a range of antidepressants has been found to be effective in increasing effect. It is used in concert with a range of antidepressants, protein deficient, or in patients with dysregulated serotonergic pathways. |
| Vitamin D | Vitamin D is a ‘neurosteroid’ compound that acts as a ligand for receptors that are present in the hypothalamus, substantia nigra and prefrontal cortex region of the brain. It chiefly regulates the genetic expression leading to coding of protein tyrosine hydroxylase. |
| Zinc | Zinc is the most predominant trace element found in the hippocampus, amygdala and neocortex regions of the brain. It mainly leads to amplification of neurogenesis in hippocampal regions by increasing BNDF. The activity of glutamate and NMDA receptors is also modified. |
| Disease | Mechanism of Action and Commonly Used Nutraceuticals |
|---|---|
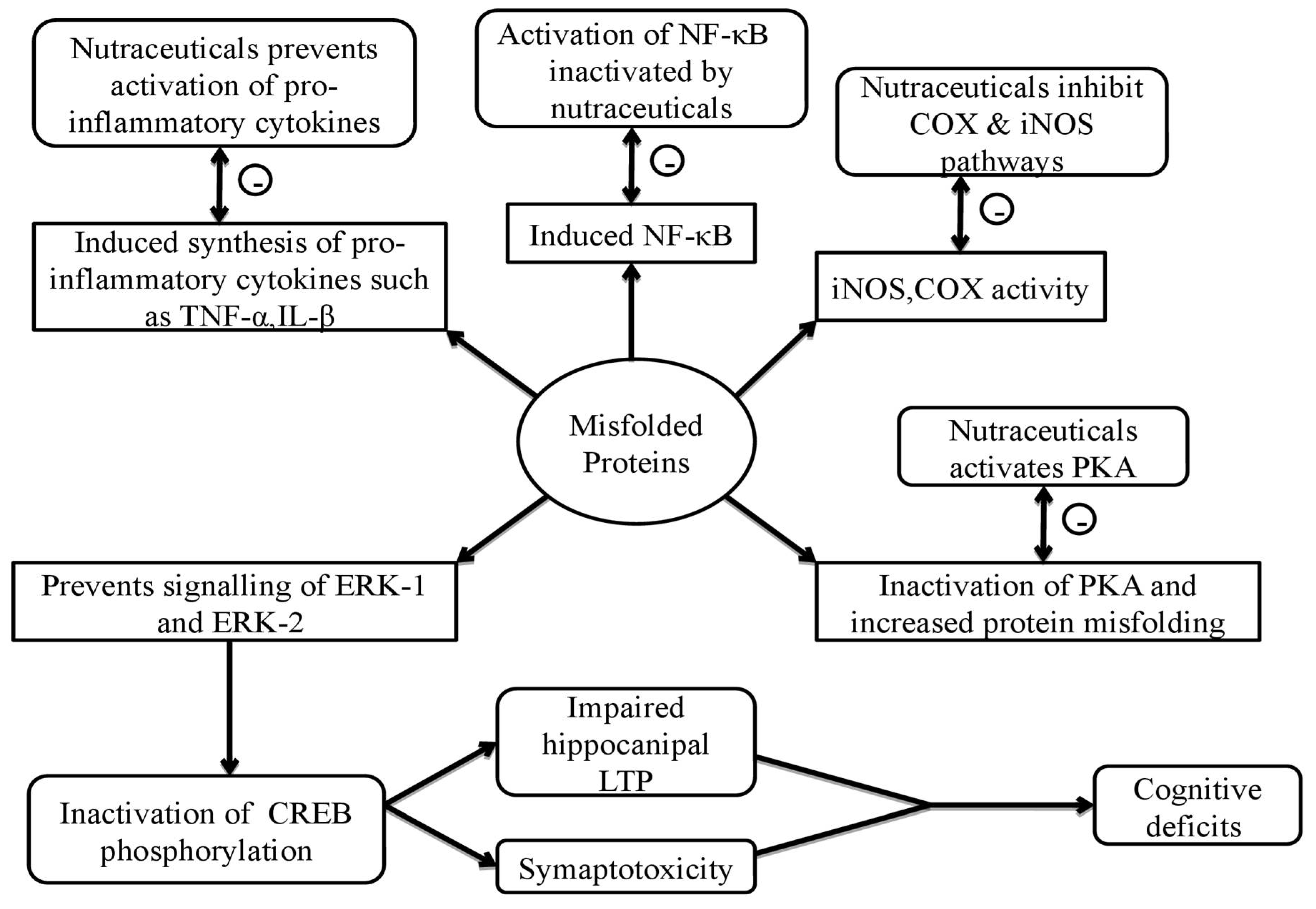
| Neurodegenerative Disorders | Neurodegenerative disorders are mainly developed by protein misfolding. Nutraceuticals mainly prevent misfolding of proteins by inhibiting the activation and synthesis of proinflammatory cytokines and associated pathways.Example: bacoside A, bacoside B, brahmine, quercetin, kaempferol, withanine, somniferine, asiatic acid, bhilavanol A and B. |
| Alzheimer’s Disease | AD is mainly associated with increase in oxidative stress and free radicals. The nutraceuticals typically antioxidant in nature are mostly employed in the management of this disease. Examples: flavonoids (fruits, vegetables, tea, wine, coffee); carotenoids (lutein, zeaxanthin, lycopene, β-cryptoxanthin including α and β carotenes); anthocyanidins (cyanidin); flavones (luteolin, apigenin). |
| Parkinson’s Disease | The uninhibited oxidative stress and free radicals in association with abnormally misfolded proteins, neuroinflammation, and dysfunctional mitochondria lead to compromised cellular metabolism and energy thereby impacting the functioning of the brain and leading to neurodegenerative disorders including PD.Examples: Vitamin A, Omega-3 fatty acids, lycopene, vincamine, gallic acid, curcumin, Mito Q. |
| Depression | Nutraceuticals that act by inhibiting re-uptake of monoamines, possess anti-inflammatory and antioxidant properties which are well suited for management of depression.Examples: Omega-3 fatty acids, folic acid, S-adenosyl methionine, zinc, N-acetyl cysteine, L-Tryptophan/5-HTP, Vitamin-D. |
| Psychosis | Nutraceuticals that can improve neurotransmission in dopaminergic serotoninergic neurons can be employed in management of psychosis. These mainly includes all types of vitamins and omega 3 fatty acids. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makkar, R.; Behl, T.; Bungau, S.; Zengin, G.; Mehta, V.; Kumar, A.; Uddin, M.S.; Ashraf, G.M.; Abdel-Daim, M.M.; Arora, S.; et al. Nutraceuticals in Neurological Disorders. Int. J. Mol. Sci. 2020, 21, 4424. https://doi.org/10.3390/ijms21124424
Makkar R, Behl T, Bungau S, Zengin G, Mehta V, Kumar A, Uddin MS, Ashraf GM, Abdel-Daim MM, Arora S, et al. Nutraceuticals in Neurological Disorders. International Journal of Molecular Sciences. 2020; 21(12):4424. https://doi.org/10.3390/ijms21124424
Chicago/Turabian StyleMakkar, Rashita, Tapan Behl, Simona Bungau, Gokhan Zengin, Vineet Mehta, Arun Kumar, Md. Sahab Uddin, Ghulam Md. Ashraf, Mohamed M. Abdel-Daim, Sandeep Arora, and et al. 2020. "Nutraceuticals in Neurological Disorders" International Journal of Molecular Sciences 21, no. 12: 4424. https://doi.org/10.3390/ijms21124424
APA StyleMakkar, R., Behl, T., Bungau, S., Zengin, G., Mehta, V., Kumar, A., Uddin, M. S., Ashraf, G. M., Abdel-Daim, M. M., Arora, S., & Oancea, R. (2020). Nutraceuticals in Neurological Disorders. International Journal of Molecular Sciences, 21(12), 4424. https://doi.org/10.3390/ijms21124424











