The Strategic Alliance between Clinical and Molecular Science in the War against SARS-CoV-2, with the Rapid-Diagnostics Test as an Indispensable Weapon for Front Line Doctors
Abstract
:1. Introduction
2. Serological Assay for the Diagnosis of SARS-CoV-2 Infection: Background
3. Methods
3.1. The Immunochromatographic Rapid Test—GICA
3.2. Vivadiag™
Analysis of Preliminary Data on the Reliability of Colloidal Gold Rapid Test for Protocol Definition
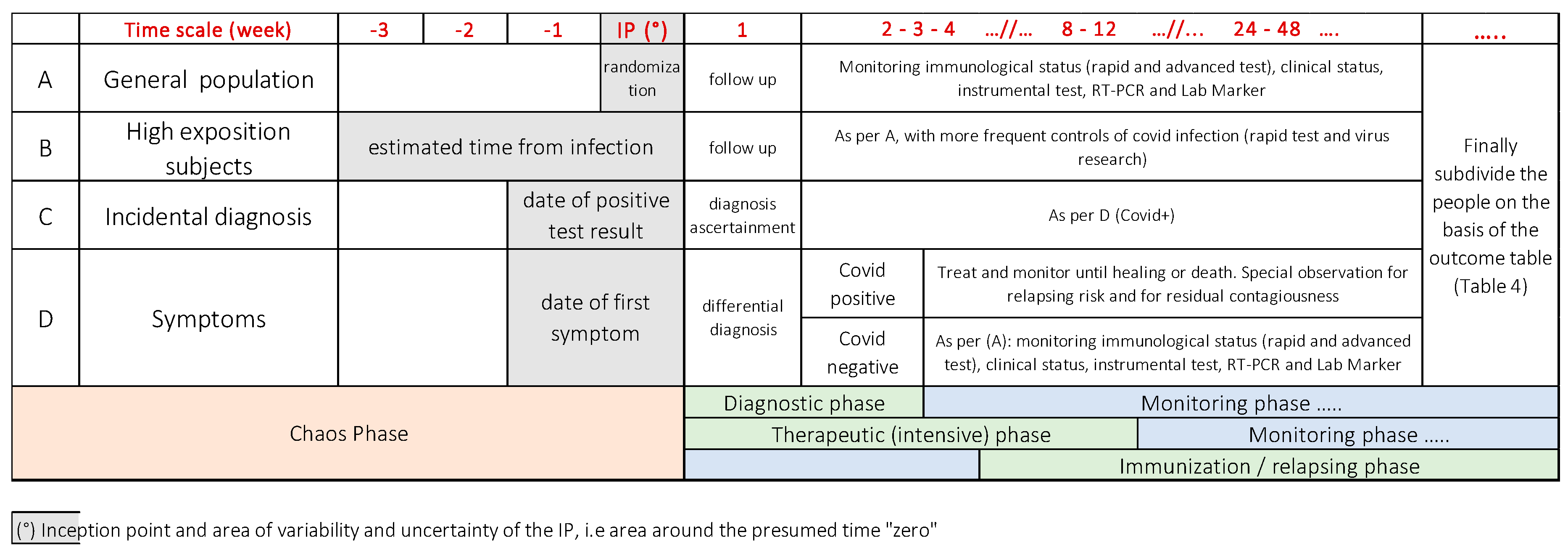
4. The Methodological Approach for the Design of a Research Protocol to Investigate COVID-19
- People with possible exposure to the virus (based on epidemiological context and/or on medical history). These people belong mainly to the general population or a specific group;
- High-risk groups (healthcare workers, ridders) that may have an increased risk of exposition to a significant viral load;
- People who incidentally underwent the test for several reasons, not related to clinical reasons;
- Patients who developed mild to severe symptoms of Covid-19.
Crafting a Research Protocol for a Diagnostic Rapid Test
- To establish the accordance of the test with the clinical features of the disease and to assess how the test results change over time in the different clusters of patients, as summarized in Table 5.
- To establish the reliability of the serologic rapid test to detect a SARS-CoV-2 infection and, therefore, its sensitivity and specificity when the integration of anamnestic, clinical, laboratory, molecular, imaging criteria are considered as the gold standard.
- If the test is positive, even in the presence of a negative PCR, the patient should be considered positive and treated according to his or her clinical picture. When IgM alone is positive, the serological test shows a recent infection, even if the patient has not developed any symptoms yet. On the other hand, if the test is positive for IgG alone and the patient is asymptomatic, the result can be used for epidemiological purposes. Some recent investigations [27], however, show an inconsistent response of IgM and IgG in different patients, with a seroconversion of both the immunoglobulin classes that reach 100% after a window period.
- If the test is negative in a symptomatic patient with a high exposure risk, the serological test should be routinely repeated, considering the window period between the time 0 of the infection and the production of circulating antibodies.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Practice, B.B. Coronavirus Disease 2019: Situation Report 76; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200405-sitrep-76-covid-19.pdf?sfvrsn=6ecf0977_4. [CrossRef] [Green Version]
- World Health Organization. WHO Director-General’s Opening Remarks at the Mission Briefing on COVID-19. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-mission-briefing-on-covid-19 (accessed on 16 April 2020).
- Lan, L.; Xu, D.; Ye, G.; Xia, C.; Wang, S.; Li, Y.; Xu, H. Positive RT-PCR Test Results in Patients Recovered From COVID-19. Jama 2020, 323, 1502–1503. [Google Scholar] [CrossRef] [Green Version]
- Lippi, G.; Simundic, A.-M.; Plebani, M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19). Clin. Chem. Lab. Med. 2020, 58, 1070–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winichakoon, P.; Chaiwarith, R.; Liwsrisakun, C.; Salee, P.; Goonna, A.; Limsukon, A.; Kaewpoowat, Q. Negative Nasopharyngeal and Oropharyngeal Swabs Do Not Rule Out COVID-19. J. Clin. Microbiol. 2020, 58, e00297-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, F.; Yan, L.; Wang, N.; Yang, S.; Wang, L.; Tang, Y.; Gao, G.; Wang, S.; Ma, C.; Xie, R.; et al. Quantitative Detection and Viral Load Analysis of SARS-CoV-2 in Infected Patients. Clin. Infect. Dis. 2020, ciaa345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caraguel, C.G.B.; Stryhn, H.; Gagné, N.; Dohoo, I.R.; Hammell, K.L. Selection of a cutoff value for real-time polymerase chain reaction results to fit a diagnostic purpose: Analytical and epidemiologic approaches. J. Vet. Diagn. Investig. 2011, 23, 2–15. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Laboratory Testing for 2019 Novel Coronavirus (2019-Ncov) in Suspected Human Cases; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Sheridan, C. Fast, portable tests come online to curb coronavirus pandemic. Nat. Biotechnol. 2020, 8, 515–518. [Google Scholar] [CrossRef]
- Li, Z.; Yi, Y.; Luo, X.; Xiong, N.; Liu, Y.; Li, S.; Sun, R.; Wang, Y.; Hu, B.; Chen, W.; et al. Development and Clinical Application of A Rapid IgM-IgG Combined Antibody Test for SARS-CoV-2 Infection Diagnosis. J. Med. Virol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Abbott. Abbot Launches Molecular Point-of-Care Test to Detect Novel Coronavirus in as Little as Five Minutes. Available online: https://abbott.mediaroom.com/2020-03-27-Abbott-Launches-Molecular-Point-of-Care-Test-to-Detect-Novel-Coronavirus-in-as-Little-as-Five-Minutes (accessed on 16 April 2020).
- WHO Population-Based Age-Stratified Seroepidemiological Investigation Protocol for Covid-19 Virus Infection, 2nd ed.; World Health Organization: Geneva, Switzerland, 2020; pp. 1–19.
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef] [Green Version]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. Jama 2020, 323, 1843–1844. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.Y.; Chen, Y.X.; Fang, J.Y. 2019 Novel coronavirus infection and gastrointestinal tract. J. Dig. Dis. 2020, 21, 125–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ai, T.; Yang, Z.; Hou, H.; Zhan, C.; Chen, C.; Lv, W.; Tao, Q.; Sun, Z.; Xia, L. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology 2020, 200642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Tholoth, M.; Bau, H.H.; Song, J. A Single and Two-Stage, Closed-Tube, Molecular Test for the 2019 Novel Coronavirus (COVID-19) at Home, Clinic, and Points of Entry. Chemrxiv 2020. preprint. [Google Scholar] [CrossRef]
- Porcheddu, R.; Serra, C.; Kelvin, D.; Kelvin, N.; Rubino, S. Similarity in Case Fatality Rates (CFR) of COVID-19/SARS-COV-2 in Italy and China. J. Infect. Dev. Ctries. 2020, 14, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, J.; Li, N.; Liu, Y.; Ye, R.; Qin, X.; Zheng, R. Serological detection of 2019-nCoV respond to the epidemic: A useful complement to nucleic acid testing. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Bai, S.L.; Wang, J.Y.; Zhou, Y.Q.; Yu, D.S.; Gao, X.M.; Li, L.L.; Yang, F. Analysis of the first cluster of cases in a family of novel coronavirus pneumonia in Gansu Province. Zhonghua Yu Fang Yi Xue Za Zhi 2020, 2020, E005. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Meyer, B.; Drosten, C.; Müller, M.A. Serological assays for emerging coronaviruses: Challenges and pitfalls. Virus Res. 2014, 194, 175–183. [Google Scholar] [CrossRef]
- Xiang, J.; Yan, M.; Li, H.; Liu, T.; Lin, C.; Huang, S.; Shen, C. Evaluation of Enzyme-Linked Immunoassay and Colloidal Gold- Immunochromatographic Assay Kit for Detection of Novel Coronavirus (SARS-Cov-2) Causing an Outbreak of Pneumonia (COVID-19). medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Liu, W.; Wang, S.; Zheng, S. A preliminary study on serological assay for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 238 admitted hospital patients. medRxiv 2020, 22, 206–211. [Google Scholar] [CrossRef] [Green Version]
- Cassaniti, I.; Novazzi, F.; Giardina, F.; Salivaro, F.; Sachs, M.; Perlini, S.; Bruno, R.; Mojoli, F.; Baldanti, F. Performance of VivaDiagTM COVID-19 IgM/IgG Rapid Test is inadequate for diagnosis of COVID-19 in acute patients referring to emergency room department. J. Med. Virol. 2020. [Google Scholar] [CrossRef] [Green Version]
- Long, Q.-X.; Liu, B.-Z.; Deng, H.-J.; Wu, G.-C.; Deng, K.; Chen, Y.-K.; Liao, P.; Qiu, J.-F.; Lin, Y.; Cai, X.-F.; et al. Not. Nat. Med. 2020. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, Q.; Wang, T.; Ke, Y.; Mo, F.; Jia, R.; Liu, W.; Liu, L.; Zheng, S.; Liu, Y.; et al. Evaluation of recombinant nucleocapsid and spike proteins for serological diagnosis of novel coronavirus disease 2019 (COVID-19). medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J.; et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Capello, F.; Cipolla, M.; Cosco, L.; Gnasso, A.; Mancini, R.; Nichelatti, M.; Savo, M.T.; Gaddi, A.V. The VivaDiag COVID-19 lgM/IgG Rapid Test for the Screening and Early Diagnosis of COVID-19 in patients with No Clinical Signs of the Disease. Int. J. Endocrinol. Metab. Disord. 2020, 6. [Google Scholar] [CrossRef]
- Paradiso, A.V.; Summa, S.D.; Loconsole, D.; Procacci, V.; Sallustio, A.; Centrone, F.; Silvestris, N.; Cafagna, V.; Palma, G.D.; Tufaro, A.; et al. Clinical meanings of rapid serological assay in patients tested for SARS-Co2 RT-PCR. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. Br. Med. J. 2003, 321, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conti, P.; Ronconi, G.; Caraffa, A.L.; Gallenga, C.E.; Ross, R.; Frydas, I.; Kritas, S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents 2020, 34, 1. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wang, B.; Mao, J. Cytokine Storm in COVID-19 and Treatment. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
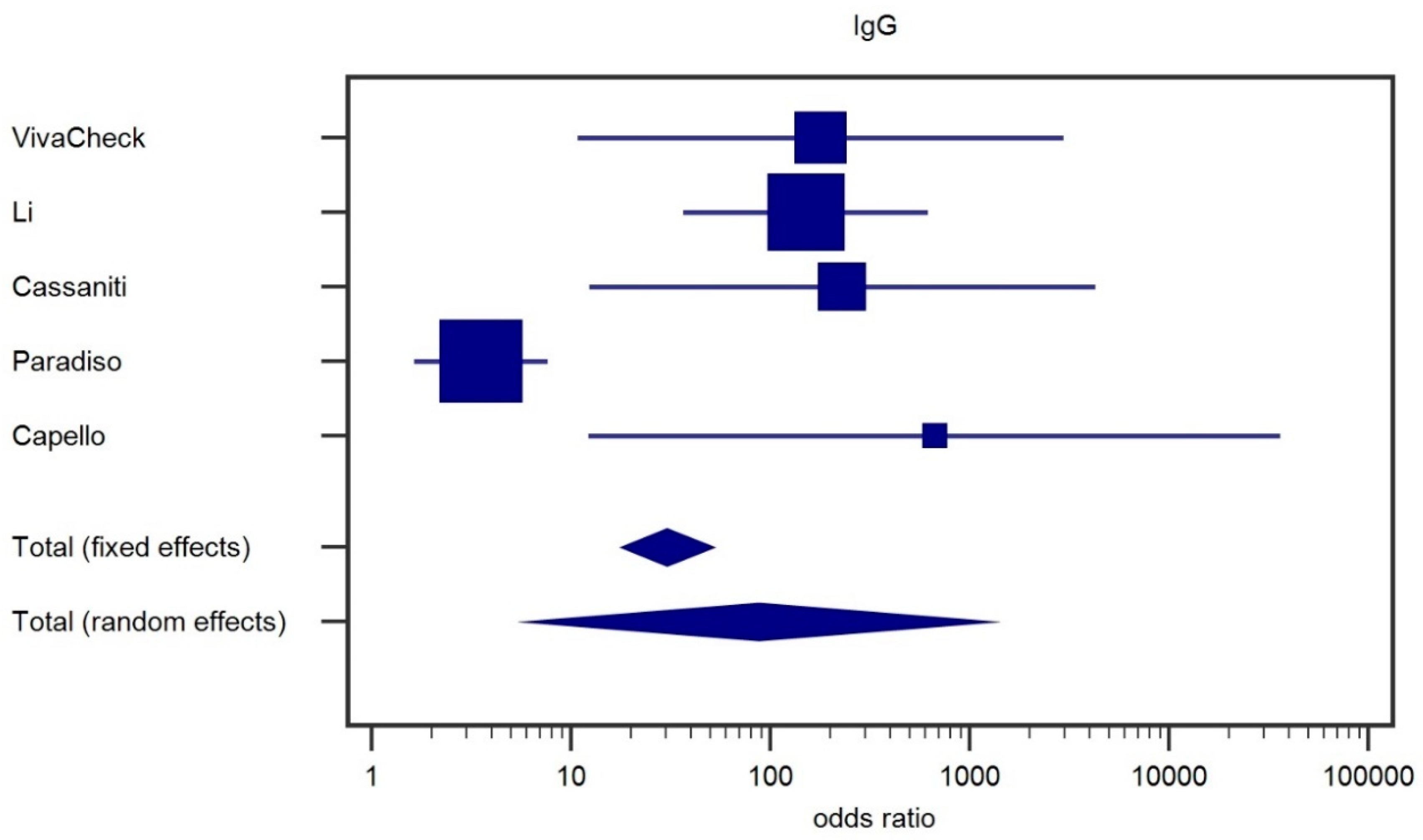

| VivaCheck | Li | Cassaniti-a (*) | Cassaniti-B | Paradiso | Capello | |
|---|---|---|---|---|---|---|
| Test (n) | 350 | 397 | 60 | 50 | 191 | 26 |
| Sensitivity | 81.2 (^) | 88.6 | 83.3 | 18.4 | 30.0 | 100.0 |
| Specificity | 100.0 | 90.6 | 100.0 | 9.7 | 89.0 | 100.0 |
| Type of Variable | Possible Independent Variable |
|---|---|
| Demographic and environmental feature | Age |
| Sex | |
| Ethnicity | |
| Occupation | |
| Geographical location and Climatic factors, Pollution | |
| Socio-economic status | |
| Physiological Features | Diet |
| Level of physical activity | |
| Immunization Status | |
| Genetic Subtype | |
| Comorbidity | Health patients |
| Concomitant acute condition | |
| Chronic conditions and frailties | |
| Smoke and other risk factors | |
| Viral Physiology | Viral strain |
| Tropism | |
| Elusion mechanisms of virus | |
| Physio-pathology of the disease | Virulence |
| Immune response of the host | |
| Clinical assessment and investigations | Symptoms (*) |
| Clinical signs (*) | |
| Laboratory findings (*) | |
| Instrumental investigation (*) | |
| Analytes Considered (*) | |
| Drugs |
| Clinical and Laboratory |
|---|
| Symptoms |
| Fever (temperature ≥ 37.3 °C); cough, sputum, shortness of breath, myalgia, fatigue, diarrhea, nausea and vomiting, conjunctivitis, anosmia, dysgeusia… |
| Comorbidities |
| Hypertension, heart failure, coronary heart disease, diabetes, kidney failure, cancer, chronic obstructive lung disease, immunodeficiency, stroke, cerebrovascular accident, gastrointestinal disease, transplant… |
| Laboratory findings |
| White blood cell count, lymphocyte count, hemoglobin, platelet count, albumin, creatinine, ALT/AST, lactate dehydrogenase, high-sensitivity cardiac troponin I, prothrombin time, D-dimer, IL-6, IL-1, IL-8, IL 38; IL-39, TNFα, CCl-2, CCL-3, CCL-5, IP-10, MCP-1, procalcitonin, C-reactive protein, pH, lactate, vitamin D |
| Score |
| Curb-65; quick-SOFA, SOFA, APACHE II |
| Imagine findings |
| Consolidation, ground-glass opacity, bilateral pulmonary infiltration |
| Histo- and cytopathological findings |
| Sign of inflammation, alveolar damage with exudate, lymphocyte, multinucleated giant cells… |
| Outcomes | Clinicians/Epidemiologists | Research/Protocols (Examples) | Notes |
|---|---|---|---|
| Death | Clinical observation and autopsy | Nested case-control, pathophysiological approach | Monitoring of all parameters, including in-deep laboratory investigations, PCR, microscopy, to define the initial cause of death, the final one and contributing cause |
| Relapse of the disease | In-deep permanent clinical observation | Ecological study, clinical reports, case control | Study immune response and virus variability |
| Chronicity of the disease | Clinical observation | Cross-sectional survey, clinical reports, case control | Evaluate comorbidities, aging, chronic drug intake |
| Relapse of the disease in a healed patient | In-deep permanent clinical observation | Epidemiological surveys, case controls, nested case control | Study immune response and virus variability |
| Patient Healed, immunized | Follow-up | Epidemiological surveys, cohort study | Patients need to be studied in the long-time to avoid unexpected relapse |
| Non infected, healthy | Special follow-up, particularly in exposed/working subjects | Ecological study, epidemiological surveys | Prevent the infection with appropriate measures of disease control, waiting for the vaccine. At least two serological tests should be administered. |
| Not infected, healthy, elderly or high-risk subject | In-deep follow-up | Ecological study, longitudinal cohort study | Prevent the infection, studying the major comorbidities which can modify the prognosis. |
| Group Classification | Main Clusters |
|---|---|
| Exposure/risk classification |
|
| Selection Criteria |
|
| Diagnostic criterium |
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaddi, A.V.; Capello, F.; Aluigi, L.; Antignani, P.L.; Callegaro, A.; Casu, G.; Cipolla, E.; Cipolla, M.; Cosco, L.; Culzoni, F.; et al. The Strategic Alliance between Clinical and Molecular Science in the War against SARS-CoV-2, with the Rapid-Diagnostics Test as an Indispensable Weapon for Front Line Doctors. Int. J. Mol. Sci. 2020, 21, 4446. https://doi.org/10.3390/ijms21124446
Gaddi AV, Capello F, Aluigi L, Antignani PL, Callegaro A, Casu G, Cipolla E, Cipolla M, Cosco L, Culzoni F, et al. The Strategic Alliance between Clinical and Molecular Science in the War against SARS-CoV-2, with the Rapid-Diagnostics Test as an Indispensable Weapon for Front Line Doctors. International Journal of Molecular Sciences. 2020; 21(12):4446. https://doi.org/10.3390/ijms21124446
Chicago/Turabian StyleGaddi, Antonio Vittorino, Fabio Capello, Leonardo Aluigi, Pier Luigi Antignani, Annapaola Callegaro, Gavino Casu, Enrico Cipolla, Maurizio Cipolla, Lucio Cosco, Federico Culzoni, and et al. 2020. "The Strategic Alliance between Clinical and Molecular Science in the War against SARS-CoV-2, with the Rapid-Diagnostics Test as an Indispensable Weapon for Front Line Doctors" International Journal of Molecular Sciences 21, no. 12: 4446. https://doi.org/10.3390/ijms21124446
APA StyleGaddi, A. V., Capello, F., Aluigi, L., Antignani, P. L., Callegaro, A., Casu, G., Cipolla, E., Cipolla, M., Cosco, L., Culzoni, F., Dentali, F., Elexpuru-Zabaleta, M., Forbes-Hernandez, T. Y., Fragiacomo, C., Giampieri, F., Gnasso, A., Mancini, R., Modena, M. G., Nichelatti, M., ... Battino, M. (2020). The Strategic Alliance between Clinical and Molecular Science in the War against SARS-CoV-2, with the Rapid-Diagnostics Test as an Indispensable Weapon for Front Line Doctors. International Journal of Molecular Sciences, 21(12), 4446. https://doi.org/10.3390/ijms21124446







