Characterization of the Biogenic Volatile Organic Compounds (BVOCs) and Analysis of the PR1 Molecular Marker in Vitis vinifera L. Inoculated with the Nematode Xiphinema index
Abstract
:1. Introduction
2. Results
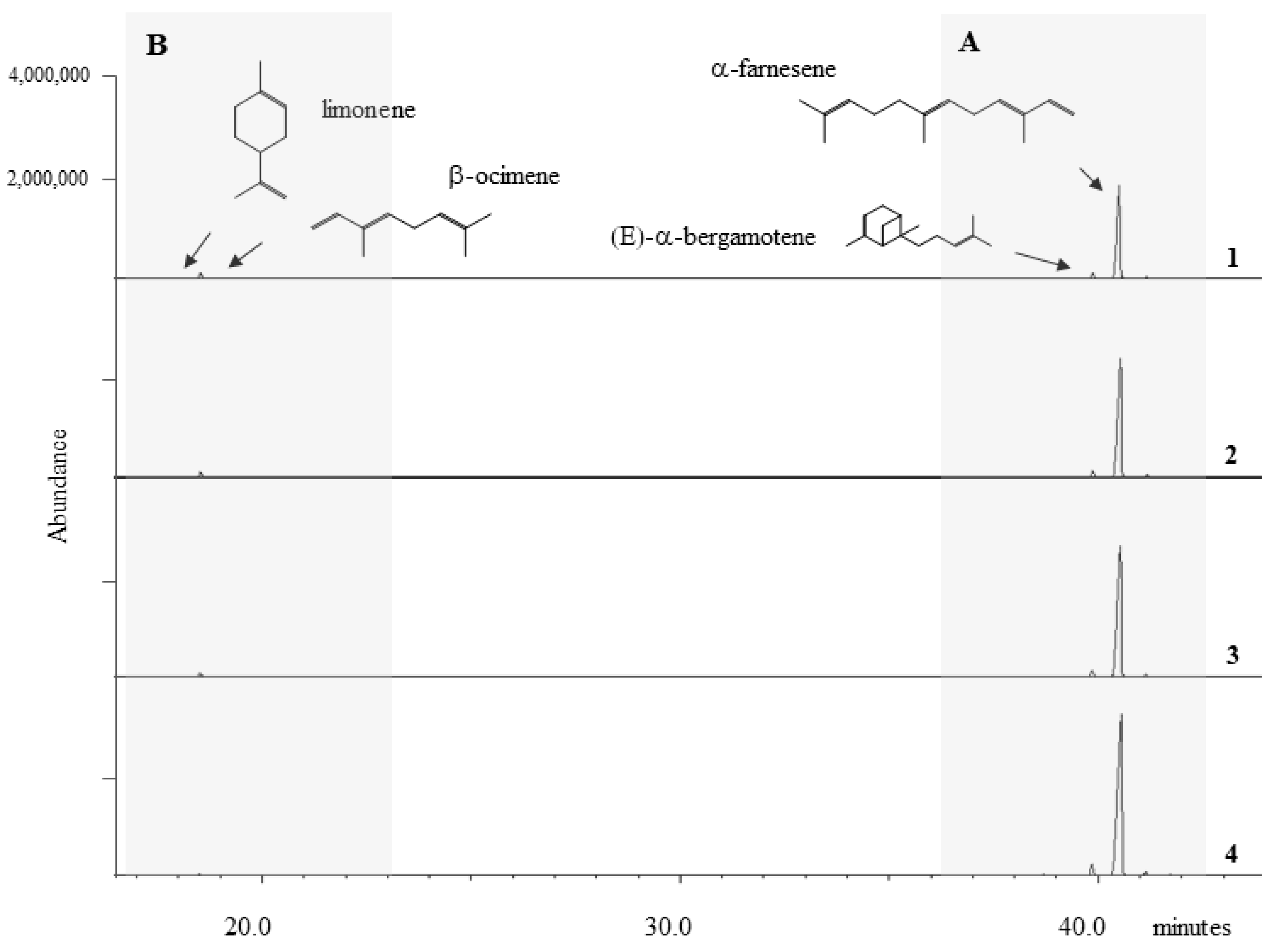
2.1. The BVOC Profile
2.1.1. Trend in Sesquiterpene Response
2.1.2. Trend in Monoterpene Response
2.2. PR1 Genes Expression
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Nematode Isolation
4.3. SPME Sampling and GC-MS Analysis
4.4. Quantitative Gene Expression Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Wine Market Observatory. European Commission, Wine Production 2015. Available online: https://ec.europa.eu/agriculture/wine/statistics_it (accessed on 22 April 2020).
- Parkinson, J.; Mitreva, M.; Whitton, C.; Thomson, M.; Daub, J.; Martin, J.; Schmid, R.; Hall, N.; Bar-rell, B.; Waterston, R.H.; et al. A transcriptomic analysis of the phylum Nematoda. Nat. Genet. 2004, 36, 1259. [Google Scholar] [CrossRef]
- Pastorelli, R.; Irdani, T. Metagenomica Nella Rizosfera: Il Ruolo dei Nematodi; Accademia dei Georgofili: Florence, Italy, 2010. [Google Scholar]
- Davies, L.J.; Elling, A.A. Resistance genes against plant-parasitic nematodes: A durable control strategy? Nematology 2015, 17, 249–263. [Google Scholar] [CrossRef]
- Singh, S.K.; Hodda, M.; Ash, G.J. Plant-parasitic nematodes of potential phytosanitary importance, their main hosts and reported yield losses. Bull. OEPP 2013, 43, 334–374. [Google Scholar] [CrossRef]
- Nicol, J.M.; Stirling, G.R.; Rose, B.J.; May, P.; Van Heeswijck, R. Impact of nematodes on grapevine growth and productivity: Current knowledge and future directions, with special reference to Australian viticulture. Aust. J. Grape Wine Res. 1999, 5, 109–127. [Google Scholar] [CrossRef]
- Andret-Link, P.; Marmonier, A.; Belval, L.; Hleibieh, K.; Ritzenthaler, C.; Demangeat, G. Ectoparasitic nematode vectors of grapevine viruses. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 505–529. [Google Scholar]
- Bonkowski, M.; Villenave, C.; Griffiths, B. Rhizosphere fauna: The functional and structural diversity of intimate interactions of soil fauna with plant roots. Plant Soil 2009, 321, 213–233. [Google Scholar] [CrossRef]
- Nguyen, V.C.; Villate, L.; Gutierrez-Gutierrez, C.; Castillo, P.; Van Ghelder, C.; Plantard, O.; Esmenjaud, D. Phylogeography of the soil-borne vector nematode Xiphinema index highly suggests Eastern origin and dissemination with domesticated grapevine. Sci. Rep. 2019, 9, 7313. [Google Scholar] [CrossRef] [PubMed]
- Andret-Link, P.; Laporte, C.; Valat, L.; Ritzenthaler, C.; Demangeat, G.; Vigne, E.; Laval, V.; Pfeiffer, P.; Stussi-Garaud, C.; Fuchs, M. Grapevine fanleaf virus: Still a major threat to the grape-vine industry. J. Plant Pathol. 2004, 86, 183–195. [Google Scholar]
- Groen, S.C.; Wamonje, F.O.; Murphy, A.M.; Carr, J.P. Engineering resistance to virus transmission. Curr. Opin. Virol. 2017, 26, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.E.; Raski, D.J. On the transmission of grape fanleaf by Xiphinema index. Nematologica 1964, 10, 489–495. [Google Scholar] [CrossRef]
- Oliver, J.E.; Fuchs, M.F. Fanleaf Degeneration/Decline Disease of Grapevines. Integrated Pest Management; New York State IPM Program: New York, NY, USA, 2011; pp. 1–3. [Google Scholar]
- Bovey, R.; Gärtel, W.; Hewitt, W.B.; Martelli, G.P.; Vuittenez, A. Soil-borne viruses transmitted by nematodes. In Virus and Virus-Like Diseases of Grapevines; Bovey, R., Gartel, W., Hewitt, W.B., Martelli, G.P., Vuittenez, A., Eds.; Payot: Lausanne, Switzerland, 1980; pp. 46–50. [Google Scholar]
- Andret-Link, P.; Schmitt-Keichinger, C.; Demangeat, G.; Komar, V.; Fuchs, M. The specific transmission of Grapevine fanleaf virus by its nematode vector Xiphinema index is solely determined by the viral coat protein. Virology 2004, 320, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Coppola, M.; Cascone, P.; Madonna, V.; Di Lelio, I.; Esposito, F.; Avitabile, C.; Romanelli, A.; Guerrieri, E.; Vitiello, A.; Pennacchio, F.; et al. Plant-to-plant communication triggered by systemin primes anti-herbivore resistance in tomato. Sci. Rep. 2017, 7, 15522. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.E.; Bever, J.D.; Bowers, M.D. Arbuscular mycorrhizal fungal species suppress inducible plant responses and alter defensive strategies following herbivory. Oecologia 2009, 160, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Pontier, D.; Balagué, C.; Roby, D. The hypersensitive response. A programmed cell death associated with plant resistance. Comptes Rendus Acad. Sci. III Sci. Vie 1998, 321, 721–734. [Google Scholar] [CrossRef]
- Morel, J.B.; Dangl, J.L. The hypersensitive response and the induction of cell death in plants. Cell Death Differ. 1997, 4, 671–683. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R.; Shulaev, V.; Seskar, M.; Lam, E. Inhibition of programmed cell death in tobacco plants during a pathogen-induced hypersensitive response at low oxygen pressure. Plant Cell 1996, 8, 1991–2001. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Van Strien, E.A. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- Pečenková, T.; Pleskot, R.; Žárský, V. Subcellular localization of Arabidopsis pathogenesis-related 1 (PR1) protein. Int. J. Mol. Sci. 2017, 18, 825. [Google Scholar] [CrossRef] [Green Version]
- Mitsuhara, I.; Iwai, T.; Seo, S.; Yanagawa, Y.; Kawahigasi, H.; Hirose, S.; Ohkawa, Y.; Ohashi, Y. Characteristic expression of twelve rice PR1 family genes in response to pathogen infection, wounding, and defense-related signal compounds (121/180). Mol. Genet. Genom. 2008, 279, 415–427. [Google Scholar] [CrossRef] [Green Version]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [Green Version]
- Mejias, J.; Truong, N.M.; Abad, P.; Favery, B.; Quentin, M. Plant proteins and processes targeted by parasitic nematode effectors. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Wang, D.; Chen, X.; Köllner, T.G.; Mazarei, M.; Guo, H.; Pantalone, V.R.; Arelli, P.; Stewart, C.N., Jr.; Wang, N.; et al. An (E,E)-α-farnesene synthase gene of soybean has a role in defence against nematodes and is involved in synthesizing insect-induced volatiles. Plant Biotechnol. J. 2017, 15, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ro, D.K.; Petri, J.; Gershenzon, J.; Bohlmann, J.; Pichersky, E.; Tholl, D. Characterization of a root-specific Arabidopsis terpene synthase responsible for the formation of the volatile monoterpene 1,8-cineole. Plant Physiol. 2004, 135, 1956–1966. [Google Scholar] [CrossRef] [Green Version]
- Rostás, M.; Cripps, M.G.; Silcock, P. Aboveground endophyte affects root volatile emission and host plant selection of a belowground insect. Oecologia 2015, 177, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Unsicker, S.B.; Kunert, G.; Gershenzon, J. Protective perfumes: The role of vegetative volatiles in plant defense against herbivores. Curr. Opin. Plant Biol. 2009, 12, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Owen, S.M.; Peñuelas, J. Volatile organic compounds in the roots and rhizosphere of Pinus spp. Soil Biol. Biochem. 2007, 39, 951–960. [Google Scholar] [CrossRef]
- Laznik, Ž.; Trdan, S. An investigation on the chemotactic responses of different entomopathogenic nematode strains to mechanically damaged maize root volatile compounds. Exp. Parasitol. 2013, 134, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, J.; Hiltpold, I.; Köllner, T.G.; Frey, M.; Gierl, A.; Gershenzon, J.; Hibbard, B.E.; Ellersieck, M.R.; Turlings, T.C. Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. Proc. Natl. Acad. Sci. USA 2009, 106, 13213–13218. [Google Scholar] [CrossRef] [Green Version]
- Rasmann, S.; Köllner, T.G.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T.C. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005, 434, 732. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.G.; Tang, M. Effect of arbuscular mycorrhizal fungi inoculation on root traits and root volatile organic compound emissions of Sorghum bicolor. S. Afr. J. Bot. 2013, 88, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Zitzelsberger, C.; Buchbauer, G. Essential oils as “a cry for help”. A review. Nat. Prod. Commun. 2015, 10, 1127–1138. [Google Scholar] [CrossRef] [Green Version]
- Šimpraga, M.; Takabayashi, J.; Holopainen, J.K. Language of plants: Where is the word? J. Integr. Plant Biol. 2016, 58, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Kesselmeier, J.; Staudt, M. Biogenic volatile organic compounds (VOC): An overview on emission, physiology and ecology. J. Atmos. Chem. 1999, 33, 23–88. [Google Scholar] [CrossRef]
- Paré, P.W.; Tumlinson, J.H. De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol. 1997, 114, 1161–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imbiscuso, G.; Trotta, A.; Maffei, M.; Bossi, S. Herbivory induces a ROS burst and the release of volatile organic compounds in the fern Pteris vittata L. J. Plant Interact. 2009, 4, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Trowbridge, A.M.; Stoy, P.C. BVOC-mediated plant-herbivore interactions. In Biology, Controls and Models of Tree Volatile Organic Compound Emissions; Niinemets, Ü., Monson Russell, K., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 21–46. [Google Scholar]
- Cheng, A.X.; Lou, Y.G.; Mao, Y.B.; Lu, S.; Wang, L.J.; Chen, X.Y. Plant terpenoids: Biosynthesis and ecological functions. J. Integr. Plant Biol. 2007, 49, 179–186. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Pierpoint, W.S.; Boller, T.H.; Conejero, V. Recommendations for naming plant pathogenesis-related proteins. Plant Mol. Biol. Rep. 1994, 12, 245–264. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kännaste, A.; Copolovici, L. Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Front. Plant Sci. 2013, 4, 262. [Google Scholar] [CrossRef] [Green Version]
- Röse, U.S.; Manukian, A.; Heath, R.R.; Tumlinson, J.H. Volatile semiochemicals released from undamaged cotton leaves (a systemic response of living plants to caterpillar damage). Plant Physiol. 1996, 111, 487–495. [Google Scholar] [CrossRef] [Green Version]
- Loughrin, J.H.; Manukian, A.R.A.; Heath, R.R.; Turlings, T.C.; Tumlinson, J.H. Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plant. Proc. Natl. Acad. Sci. USA 1994, 91, 11836–11840. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Kügler, A.; McGale, E.; Haverkamp, A.; Knaden, M.; Guo, H.; Beran, F.; Yon, F.; Li, R.; Lackus, N.; et al. Tissue-specific emission of (E)-α-bergamotene helps resolve the dilemma when pollinators are also herbivores. Current Biol. 2017, 27, 1336–1341. [Google Scholar] [CrossRef] [Green Version]
- Farré-Armengol, G.; Filella, I.; Llusià, J.; Peñuelas, J. β-Ocimene, a key floral and foliar volatile involved in multiple interactions between plants and other organisms. Molecules 2017, 22, 1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llusia, J.; Peñuelas, J.; Asensio, D.; Munné-Bosch, S. Airborne limonene confers limited thermotolerance to Quercus ilex. Physiol. Plant. 2005, 123, 40–48. [Google Scholar] [CrossRef]
- Arimura, G.I.; Ozawa, R.; Kugimiya, S.; Takabayashi, J.; Bohlmann, J. Herbivore-induced defense response in a model legume. Two-spotted spider mites induce emission of (E)-β-ocimene and transcript accumulation of (E)-β-ocimene synthase in Lotus japonicus. Plant Physiol. 2004, 135, 1976–1983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cascone, P.; Iodice, L.; Maffei, M.E.; Bossi, S.; Arimura, G.I.; Guerrieri, E. Tobacco over-expressing β-ocimene induces direct and indirect responses against aphids in receiver tomato plants. J. Plant Physiol. 2015, 173, 28–32. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Kainulainen, P.; Aflatuni, A.; Tiilikkala, K.; Holopainen, J.K. Insecticidal, repellent, antimicrobial activity and phytotoxicity of essential oils: With special reference to limonene and its suitability for control of insect pests. Agr. Food Sci. Finl. 2001, 10, 243–259. [Google Scholar] [CrossRef]
- Lincoln, J.E.; Sanchez, J.P.; Zumstein, K.; Gilchrist, D.G. Plant and animal PR1 family members inhibit programmed cell death and suppress bacterial pathogens in plant tissues. Mol. Plant Pathol. 2018, 19, 2111–2123. [Google Scholar] [CrossRef] [Green Version]
- Hussain, R.M.; Sheikh, A.H.; Haider, I.; Quareshy, M.; Linthorst, H.J. Arabidopsis WRKY50 and TGA transcription factors synergistically activate expression of PR1. Front. Plant Sci. 2018, 9, 930. [Google Scholar] [CrossRef] [PubMed]
- Wielgoss, A.; Kortekamp, A. Comparison of PR1 expression in grapevine cultures after inoculation with a host-and a non-host pathogen. Vitis 2006, 45, 9. [Google Scholar]
- Hamamouch, N.; Li, C.; Seo, P.J.; Park, C.M.; Davis, E.L. Expression of Arabidopsis pathogenesis-related genes during nematode infection. Mol. Plant Pathol. 2011, 12, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Azeem, F.; Li, H.; Bohlmann, H. Smart parasitic nematodes use multifaceted strategies to parasitize plants. Front. Plant Sci. 2017, 8, 1699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villate, L.; Morin, E.; Demangeat, G.; Van Helden, M.; Esmenjaud, D. Control of Xiphinema index populations by fallow plants under greenhouse and field conditions. Phytopathology 2012, 102, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Raski, D.J.; Goheen, A.C.; Lider, L.A.; Meredith, C.P. Strategies against Grapevine fanleaf virus. Plant Dis. 1983, 67, 335. [Google Scholar] [CrossRef]
- Ferris, H.; Zheng, L.; Walker, M.A. Resistance of grape rootstocks to plant-parasitic nematodes. J. Nematol. 2012, 44, 377. [Google Scholar] [PubMed]
- Esmenjaud, D.; Bouquet, A. Selection and application of resistant germplasm for grapevine nematodes management. In Integrated Management of Fruit Crops Nematodes; Ciancio, A., Mukerji, K., Eds.; Springer: Dordrecht, The Netherlands, 2009; Volume 4, pp. 195–214. [Google Scholar]
- Esmenjaud, D.; Walter, B.; Valentin, G.; Guo, Z.T.; Cluzeau, D.; Minot, J.C.; Voisin, R.; Cornuet, P. Vertical distribution and infectious potential of Xiphinema index (Thorne et Allen, 1950) (Nematoda: Longidoridae) in fields affected by grapevine fanleaf virus in vineyards in the Champagne region of France. Agronomie 1992, 12, 395–399. [Google Scholar] [CrossRef]
- Groza, M.; Lazarova, S.; Costache, C.; Luca, F.D.; Rosca, I.; Fanelli, E.; Peneva, V. Morpho-logical characterisation and diagnostics of Xiphinema non-americanum group species (Nematoda: Longidoridae) from Romania using mutiplex PCR. Helminthologia 2013, 50, 215–231. [Google Scholar] [CrossRef] [Green Version]
- Meza, P.; Aballay, E.; Hinrichsen, P. Morphological and molecular characterisation of Xiphinema index Thorne and Allen, 1950 (Nematoda: Longidoridae) isolates from Chile. Nematropica 2012, 42, 41–47. [Google Scholar]
- Van Bezooijen, J. Methods and Techniques for Nematology; Wageningen University: Wageningen, The Netherlands, 2006; p. 20. [Google Scholar]
- Bononi, M.; Tateo, F. Preliminary data on volatile composition of olive fruits of cv. “Simona” and possible relationship to resistance to fly oviposition. Ital. J. Food Sci. 2017, 29, 582–590. [Google Scholar]
- Gambino, G.; Perrone, I.; Gribaudo, I. A rapid and effective method for RNA extraction from different tissues of grapevine and other woody plants. Phytochem. Anal. 2008, 19, 520–525. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Baccari, C.; Antonova, E.; Lindow, S. Biological control of pierce’s disease of grape by an endophytic bacterium. Phytopathology 2018, 109, 248–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, F.; Zhang, P.; Guo, M.; Yu, W.; Chen, K. Burdock fructooligosaccharide induces fungal resistance in postharvest Kyoho grapes by activating the salicylic acid-dependent pathway and inhibiting browning. Food Chem. 2013, 138, 539–546. [Google Scholar] [CrossRef] [PubMed]



| WW1 | WW2 | WW3 | NW1 | NW2 | NW3 | NW4 | NW5 | NW6 | |
|---|---|---|---|---|---|---|---|---|---|
| Inoculation N° | - | - | - | 80 | 80 | 50 | 50 | 80 | 80 |
| Final N° | <20 | <20 | <20 | ~320 | ~320 | ~200 | ~138 | ~184 | ~110 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castorina, G.; Grassi, F.; Consonni, G.; Vitalini, S.; Oberti, R.; Calcante, A.; Ferrari, E.; Bononi, M.; Iriti, M. Characterization of the Biogenic Volatile Organic Compounds (BVOCs) and Analysis of the PR1 Molecular Marker in Vitis vinifera L. Inoculated with the Nematode Xiphinema index. Int. J. Mol. Sci. 2020, 21, 4485. https://doi.org/10.3390/ijms21124485
Castorina G, Grassi F, Consonni G, Vitalini S, Oberti R, Calcante A, Ferrari E, Bononi M, Iriti M. Characterization of the Biogenic Volatile Organic Compounds (BVOCs) and Analysis of the PR1 Molecular Marker in Vitis vinifera L. Inoculated with the Nematode Xiphinema index. International Journal of Molecular Sciences. 2020; 21(12):4485. https://doi.org/10.3390/ijms21124485
Chicago/Turabian StyleCastorina, Giulia, Flaminia Grassi, Gabriella Consonni, Sara Vitalini, Roberto Oberti, Aldo Calcante, Enrico Ferrari, Monica Bononi, and Marcello Iriti. 2020. "Characterization of the Biogenic Volatile Organic Compounds (BVOCs) and Analysis of the PR1 Molecular Marker in Vitis vinifera L. Inoculated with the Nematode Xiphinema index" International Journal of Molecular Sciences 21, no. 12: 4485. https://doi.org/10.3390/ijms21124485
APA StyleCastorina, G., Grassi, F., Consonni, G., Vitalini, S., Oberti, R., Calcante, A., Ferrari, E., Bononi, M., & Iriti, M. (2020). Characterization of the Biogenic Volatile Organic Compounds (BVOCs) and Analysis of the PR1 Molecular Marker in Vitis vinifera L. Inoculated with the Nematode Xiphinema index. International Journal of Molecular Sciences, 21(12), 4485. https://doi.org/10.3390/ijms21124485







