In Vivo Analysis of the Biocompatibility and Immune Response of Jellyfish Collagen Scaffolds and its Suitability for Bone Regeneration
Abstract
:1. Introduction
2. Results
2.1. Results of the Histological Analyses
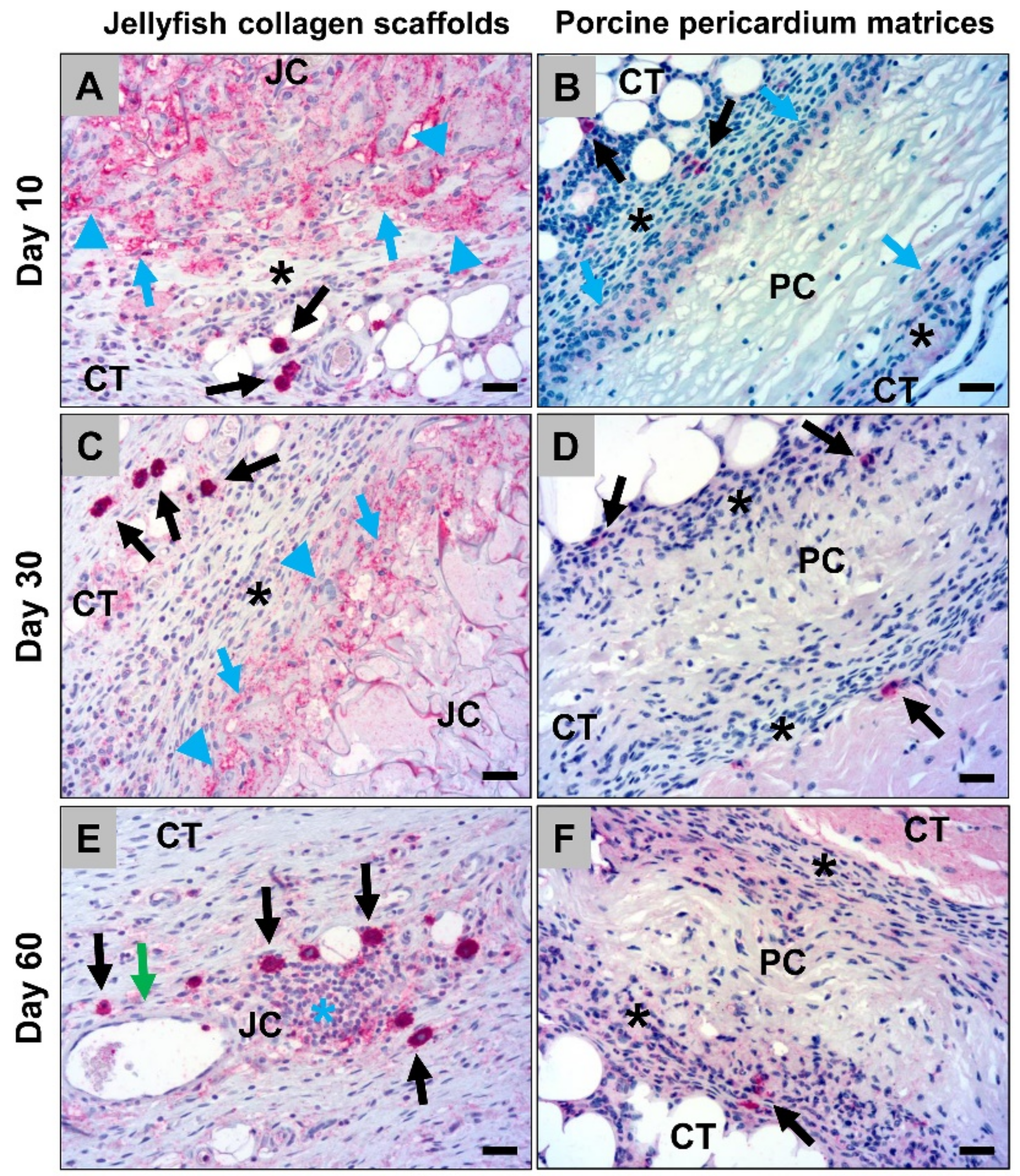
2.1.1. Histological Analysis of the Subcutaneous Implants
2.1.2. Histological Analysis of the Immune Response Within the Subcutaneous Tissue
2.1.3. Histological Analysis of the Calvarian Implantation Beds
2.2. Histomorphometrical (Quantitative) Analysis
2.2.1. Analysis of the Immune Response Within the Subcutaneous Tissue
2.2.2. Analysis of the Level of Bone Regeneration in the Calvarian Implantation Beds
2.2.3. Analysis of the Occurrence of M1- and M2-Macrophages Within the Calvarian Implantation Beds
3. Discussion
4. Materials and Methods
4.1. Biomaterials
4.1.1. Jellyfish Collagen Scaffolds
4.1.2. Porcine Pericardium-Based Collagen Matrices
4.2. In Vivo Study Design, Implantation, and Explanation Procedure
4.2.1. Subcutaneous Implantation and Explanation Procedure
4.2.2. Calvarial Implantation
4.3. Sample Preparation and Staining Procedures
4.4. Histopathological Analysis
4.5. Histomorphometrical Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.; Kerns, D.G. Mechanisms of Guided Bone Regeneration: A Review. Open Dent. J. 2014, 8, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.D.; Zhang, H.J.; Fan, H.S.; Li, W.; Zhang, X.D. Effect of Phase Composition and Microstructure of Calcium Phosphate Ceramic Particles on Protein Adsorption. Acta Biomater. 2010, 6, 1536–1541. [Google Scholar] [CrossRef]
- Yoganand, C.P.; Selvarajan, V.; Cannillo, V.; Sola, A.; Roumeli, E.; Goudouri, O.M.; Paraskevopoulos, K.M.; Rouabhia, M. Characterization and in Vitro-Bioactivity of Natural Hydroxyapatite Based Bio-Glass-Ceramics Synthesized by Thermal Plasma Processing. Ceram. Int. 2010, 36, 1757–1766. [Google Scholar] [CrossRef]
- Ni, S.; Chang, J.; Chou, L.; Zhai, W. Comparison of Osteoblast-like Cell Responses to Calcium Silicate and Tricalcium Phosphate Creamics in Vitro. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2007, 80, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Kuttappan, S.; Mathew, D.; Nair, M.B. Biomimetic Composite Scaffolds Containing Bioceramics and Collagen/Gelatin for Bone Tissue Engineering—A Mini Review. Int. J. Biol. Macromol. 2016, 93, 1390–1401. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Epple, M. Composites of Calcium Phosphate and Polymers as Bone Substitution Materials. Eur. J. Trauma 2006, 32, 125–131. [Google Scholar] [CrossRef]
- Kohli, N.; Ho, S.; Brown, S.J.; Sawadkar, P.; Sharma, V.; Snow, M.; García-Gareta, E. Bone Remodelling in Vitro: Where Are We Headed?: -A Review on the Current Understanding of Physiological Bone Remodelling and Inflammation and the Strategies for Testing Biomaterials in Vitro. Bone 2018, 110, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for Bone Tissue Regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Hoyer, B.; Bernhardt, A.; Lode, A.; Heinemann, S.; Sewing, J.; Klinger, M.; Notbohm, H.; Gelinsky, M. Jellyfish Collagen Scaffolds for Cartilage Tissue Engineering. Acta Biomater. 2014, 10, 883–892. [Google Scholar] [CrossRef]
- Song, E.; Yeon Kim, S.; Chun, T.; Byun, H.J.; Lee, Y.M. Collagen Scaffolds Derived from a Marine Source and Their Biocompatibility. Biomaterials 2006, 27, 2951–2961. [Google Scholar] [CrossRef]
- Hempel, U.; Reinstorf, A.; Poppe, M.; Fischer, U.; Gelinsky, M.; Pompe, W.; Wenzel, K.W. Proliferation and Differentiation of Osteoblasts on Biocement D Modified with Collagen Type I and Citric Acid. J. Biomed. Mater. Res. 2004, 71B, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Hesse, E.; Hefferan, T.E.; Tarara, J.E.; Haasper, C.; Meller, R.; Krettek, C.; Lu, L.; Yaszemski, M.J. Collagen Type I Hydrogel Allows Migration, Proliferation, and Osteogenic Differentiation of Rat Bone Marrow Stromal Cells. J. Biomed. Mater. Res.-Part A 2010, 94, 442–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, C.; Zhang, P.; Vulesevic, B.; Kuraitis, D.; Li, F.; Yang, A.F.; Griffith, M.; Ruel, M.; Suuronen, E.J. A Collagen-Chitosan Hydrogel for Endothelial Differentiation and Angiogenesis. Tissue Eng.-Part A 2010, 16, 3099–3109. [Google Scholar] [CrossRef] [PubMed]
- Callegari, A.; Bollini, S.; Iop, L.; Chiavegato, A.; Torregrossa, G.; Pozzobon, M.; Gerosa, G.; De Coppi, P.; Elvassore, N.; Sartore, S. Neovascularization Induced by Porous Collagen Scaffold Implanted on Intact and Cryoinjured Rat Hearts. Biomaterials 2007, 28, 5449–5461. [Google Scholar] [CrossRef]
- Auger, F.A.; Gibot, L.; Lacroix, D. The Pivotal Role of Vascularization in Tissue Engineering. Annu. Rev. Biomed. Eng. 2013, 15, 177–200. [Google Scholar] [CrossRef]
- Kannan, R.Y.; Salacinski, H.J.; Sales, K.; Butler, P.; Seifalian, A.M. The Roles of Tissue Engineering and Vascularisation in the Development of Micro-Vascular Networks: A Review. Biomaterials 2005, 26, 1857–1875. [Google Scholar] [CrossRef]
- Subhan, F.; Ikram, M.; Shehzad, A.; Ghafoor, A. Marine Collagen: An Emerging Player in Biomedical Applications. J. Food Sci. Technol. 2015, 52, 4703–4707. [Google Scholar] [CrossRef] [Green Version]
- Widdowson, J.P.; Picton, A.J.; Vince, V.; Wright, C.J.; Mearns-Spragg, A. In Vivo Comparison of Jellyfish and Bovine Collagen Sponges as Prototype Medical Devices. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1524–1533. [Google Scholar] [CrossRef] [Green Version]
- Miyata, T.; Taira, T.; Noishiki, Y. Collagen Engineering for Biomaterial Use. Clin. Mater. 1992, 9, 139–148. [Google Scholar] [CrossRef]
- Li, S.-T. Biologic Biomaterials: Tissue-Derived Biomaterials (Collagen). In Biomaterials; Wong, J.Y., Bronzino, J.D., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Aamodt, J.M.; Grainger, D.W. Extracellular Matrix-Based Biomaterial Scaffolds and the Host Response. Biomaterials 2016, 86, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Addad, S.; Exposito, J.Y.; Faye, C.; Ricard-Blum, S.; Lethias, C. Isolation, Characterization and Biological Evaluation of Jellyfish Collagen for Use in Biomedical Applications. Mar. Drugs 2011, 9, 967–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastian, Z.; Pütz, S.; Wang, Y.J.; Kumar, S.; Fleissner, F.; Weidner, T.; Parekh, S.H. Type I Collagen from Jellyfish Catostylus Mosaicus for Biomaterial Applications. ACS Biomater. Sci. Eng. 2018, 4, 2115–2125. [Google Scholar] [CrossRef]
- Krishnan, S.; Perumal, P. Preparation and Biomedical Characterization of Jellyfish (Chrysaora Quinquecirrha) Collagen from Southeast Coast of India. Int. J. Pharm. Pharm. Sci. 2013, 5, 698–701. [Google Scholar]
- Yamada, S.; Yamamoto, K.; Ikeda, T.; Yanagiguchi, K.; Hayashi, Y. Potency of Fish Collagen as a Scaffold for Regenerative Medicine. Biomed Res. Int. 2014, 2014, 302932. [Google Scholar] [CrossRef]
- Hayashi, Y.; Yamada, S.; Yanagi Guchi, K.; Koyama, Z.; Ikeda, T. Chitosan and Fish Collagen as Biomaterials for Regenerative Medicine. In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2012; Volume 65, pp. 107–120. [Google Scholar] [CrossRef] [Green Version]
- Sridharan, R.; Cameron, A.R.; Kelly, D.J.; Kearney, C.J.; O’Brien, F.J. Biomaterial Based Modulation of Macrophage Polarization: A Review and Suggested Design Principles. Mater. Today 2015, 18, 313–325. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign Body Reaction to Biomaterials. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2008; pp. 86–100. [Google Scholar] [CrossRef] [Green Version]
- Brown, B.N.; Ratner, B.D.; Goodman, S.B.; Amar, S.; Badylak, S.F. Macrophage Polarization: An Opportunity for Improved Outcomes in Biomaterials and Regenerative Medicine. Biomaterials 2012, 33, 3792–3802. [Google Scholar] [CrossRef] [Green Version]
- Oishi, Y.; Manabe, I. Macrophages in Inflammation, Repair and Regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef]
- Korzinskas, T.; Jung, O.; Smeets, R.; Stojanovic, S.; Najman, S.; Glenske, K.; Hahn, M.; Wenisch, S.; Schnettler, R.; Barbeck, M. In Vivo Analysis of the Biocompatibility and Macrophage Response of a Non-Resorbable PTFE Membrane for Guided Bone Regeneration. Int. J. Mol. Sci. 2018, 19, 2952. [Google Scholar] [CrossRef] [Green Version]
- Rothamel, D.; Schwarz, F.; Fienitz, T.; Smeets, R.; Dreiseidler, T.; Ritter, L.; Happe, A.; Zöller, J. Biocompatibility and Biodegradation of a Native Porcine Pericardium Membrane: Results of in Vitro and in Vivo Examinations. Int. J. Oral Maxillofac. Implants 2012, 27, 146–154. [Google Scholar]
- Papagiannoulis, N.; Daum, O.; Tadic, D.; Steigmann, M.; Gbr, I. Knochenregenerationsmaterial Mit Pericardium Membran in Der Horizontalen Gesteuerten Augmentation von Alveolardefekten. Dent. Implantol. 2012, 16, 360–369. [Google Scholar]
- Rothamel, D.; Schwarz, F.; Smeets, R.; Happe, A.; Fienitz, T.; Mazor, Z.; Zöller, J. Sinusbodenelevation Mit Einem Gesinterten, Natürlichen Knochenmineral. Zeitschrift fur Zahnarztl. Implantol. 2011, 1, 60–67. [Google Scholar] [CrossRef]
- Benefits of Next Generation Collagen for lab applications—Jellagen. Available online: https://www.jellagen.co.uk/products/cell-culture-reagents/benefits-of-next-generation-collagen-for-lab-applications/ (accessed on 15 December 2019).
- Cheng, X.; Shao, Z.; Li, C.; Yu, L.; Raja, M.A.; Liu, C. Isolation, Characterization and Evaluation of Collagen from Jellyfish Rhopilema Esculentum Kishinouye for Use in Hemostatic Applications. PLoS ONE 2017, 12, e0169731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frangogiannis, N.G. Inflammation in Cardiac Injury, Repair and Regeneration. Curr. Opin. Cardiol. 2015, 30, 240–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.M.; Miller, K.M. Biomaterial Biocompatibility and the Macrophage. Biomaterials 1984, 5, 5–10. [Google Scholar] [CrossRef]
- Barbeck, M.; Motta, A.; Migliaresi, C.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Heterogeneity of Biomaterial-Induced Multinucleated Giant Cells: Possible Importance for the Regeneration Process? J. Biomed. Mater. Res.-Part A 2016, 104, 413–418. [Google Scholar] [CrossRef]
- Barbeck, M.; Booms, P.; Unger, R.; Hoffmann, V.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Multinucleated Giant Cells in the Implant Bed of Bone Substitutes Are Foreign Body Giant Cells—New Insights into the Material-Mediated Healing Process. J. Biomed. Mater. Res.-Part A 2017, 105, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liang, H.; Zen, K. Molecular Mechanisms That Influence the Macrophage M1-M2 Polarization Balance. Front. Immunol. 2014, 5. (NOV). [Google Scholar] [CrossRef] [Green Version]
- Puisys, A.; Zukauskas, S.; Kubilius, R.; Barbeck, M.; Razukevičius, D.; Linkevičiene, L.; Linkevičius, T. Clinical and Histologic Evaluations of Porcine-Derived Collagen Matrix Membrane Used for Vertical Soft Tissue Augmentation: A Case Series. Int. J. Periodontics Restorative Dent. 2019, 39, 341–347. [Google Scholar] [CrossRef]
- Sieger, D.; Korzinskas, T.; Jung, O.; Stojanovic, S.; Wenisch, S.; Smeets, R.; Gosau, M.; Schnettler, R.; Najman, S.; Barbeck, M. The Addition of High Doses of Hyaluronic Acid to a Biphasic Bone Substitute Decreases the Proinflammatory Tissue Response. Int. J. Mol. Sci. 2019, 20, 1969. [Google Scholar] [CrossRef] [Green Version]
- Gueldenpfennig, T.; Houshmand, A.; Najman, S.; Stojanovic, S.; Korzinskas, T.; Smeets, R.; Gosau, M.; Pissarek, J.; Emmert, S.; Jung, O.B.M. The Condensation of Collagen Leads to an Extended Standing Time and a Decreased Pro-Inflammatory Tissue Response to a Newly Developed Pericardium-Based Barrier Membrane for Guided Bone Regeneration. In Vivo (Brooklyn). 2020, 34, 985–1000. [Google Scholar] [CrossRef]
- Barbeck, M.; Jung, O.; Smeets, R.; Gosau, M.; Schnettler, R.; Rider, P.; Houshmand, A.; Korzinskas, T. Implantation of an Injectable Bone Substitute Material Enables Integration Following the Principles of Guided Bone Regeneration. In Vivo (Brooklyn). 2020, 34, 557–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanaati, S.; Barbeck, M.; Hilbig, U.; Hoffmann, C.; Unger, R.E.; Sader, R.A.; Peters, F.; Kirkpatrick, C.J. An Injectable Bone Substitute Composed of Beta-Tricalcium Phosphate Granules, Methylcellulose and Hyaluronic Acid Inhibits Connective Tissue Influx into Its Implantation Bed in Vivo. Acta Biomater. 2011, 7, 4018–4028. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, J.; Barbeck, M.; Kirkpatrick, C.; Sader, R.; Lerner, H.; Ghanaati, S. Injectable Bone Substitute Material on the Basis of β-TCP and Hyaluronan Achieves Complete Bone Regeneration While Undergoing Nearly Complete Degradation. Int. J. Oral Maxillofac. Implants 2018, 33. [Google Scholar] [CrossRef]
- Barbeck, M.; Hoffmann, C.; Sader, R.; Peters, F.; Hübner, W.D.; Kirkpatrick, C.J.; Ghanaati, S. Injectable Bone Substitute Based on β-TCP Combined with a Hyaluronan-Containing Hydrogel Contributes to Regeneration of a Critical Bone Size Defect towards Restitutio Ad Integrum. J. Oral Implantol. 2016, 42, 127–137. [Google Scholar] [CrossRef]
- Powell, H.M.; Boyce, S.T. EDC Cross-Linking Improves Skin Substitute Strength and Stability. Biomaterials 2006, 27, 5821–5827. [Google Scholar] [CrossRef]
- Nam, K.; Sakai, Y.; Funamoto, S.; Kimura, T.; Kishida, A. Engineering a Collagen Matrix That Replicates the Biological Properties of Native Extracellular Matrix. J. Biomater. Sci. Polym. Ed. 2011, 22, 1963–1982. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Panigrahi, S.; Zhang, C. Collagen as Biomaterial for Medical Application--Drug Delivery and Scaffolds for Tissue Regeneration: A Review. Biol. Eng. 2010, 2, 63–88. [Google Scholar] [CrossRef]
- Bauer, A.J.P.; Liu, J.; Windsor, L.J.; Song, F.; Li, B. Current Development of Collagen-Based Biomaterials for Tissue Repair and Regeneration. Soft Mater. 2014, 12, 359–370. [Google Scholar] [CrossRef]
- Delgado, L.M.; Bayon, Y.; Pandit, A.; Zeugolis, D.I. To Cross-Link or Not to Cross-Link? Cross-Linking Associated Foreign Body Response of Collagen-Based Devices. Tissue Eng. Part B. Rev. 2015, 21, 298–313. [Google Scholar] [CrossRef] [Green Version]
- De Castro Brás, L.E.; Proffitt, J.L.; Bloor, S.; Sibbons, P.D. Effect of Crosslinking on the Performance of a Collagen-Derived Biomaterial as an Implant for Soft Tissue Repair: A Rodent Model. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2010, 95B, 239–249. [Google Scholar] [CrossRef]
- Yahyouche, A.; Zhidao, X.; Czernuszka, J.T.; Clover, A.J.P. Macrophage-Mediated Degradation of Crosslinked Collagen Scaffolds. Acta Biomater. 2011, 7, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Nagarajan, S.; Bechelany, M.; Kalkura, S.N. Collagen Based Biomaterials for Tissue Engineering Applications: A Review. In Lecture Notes in Earth System Sciences; Springer International Publishing: Cham, Switzerland, 2020; pp. 3–22. [Google Scholar] [CrossRef]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Raynal, N.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Control of Crosslinking for Tailoring Collagen-Based Scaffolds Stability and Mechanics. Acta Biomater. 2015, 25, 131–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, M.H.; Silva, R.M.; Dumont, V.C.; Neves, J.S.; Mansur, H.S.; Heneine, L.G.D. Extraction and Characterization of Highly Purified Collagen from Bovine Pericardium for Potential Bioengineering Applications. Mater. Sci. Eng. C 2013, 33, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Whelan, A.; Duffy, J.; Gaul, R.T.; O’Reilly, D.; Nolan, D.R.; Gunning, P.; Lally, C.; Murphy, B.P. Collagen Fibre Orientation and Dispersion Govern Ultimate Tensile Strength, Stiffness and the Fatigue Performance of Bovine Pericardium. J. Mech. Behav. Biomed. Mater. 2019, 90, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Kayed, H.R.; Sizeland, K.H.; Kirby, N.; Hawley, A.; Mudie, S.T.; Haverkamp, R.G. Collagen Cross Linking and Fibril Alignment in Pericardium. RSC Adv. 2015, 5, 3611–3618. [Google Scholar] [CrossRef]
- Filova, E.; Burdikova, Z.; Stankova, L.; Hadraba, D.; Svindrych, Z.; Schornik, D.; Bacakova, L.; Chlup, H.; Gultova, E.; Vesely, J.; et al. Collagen Structures in Pericardium and Aortic Heart Valves and Their Significance for Tissue Engineering. In Proceedings of the 2013 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 21–23 November 2013. [Google Scholar] [CrossRef]
- Vignery, A. Macrophage Fusion: The Making of Osteoclasts and Giant Cells. J. Exp. Med. 2005, 202, 337–340. [Google Scholar] [CrossRef] [Green Version]
- Milde, R.; Ritter, J.; Tennent, G.A.; Loesch, A.; Martinez, F.O.; Gordon, S.; Pepys, M.B.; Verschoor, A.; Helming, L. Multinucleated Giant Cells Are Specialized for Complement-Mediated Phagocytosis and Large Target Destruction. Cell Rep. 2015, 13, 1937–1948. [Google Scholar] [CrossRef] [Green Version]
- Nicosia, R.F.; Belser, P.; Bonanno, E.; Diven, J. Regulation of Angiogenesis in Vitro by Collagen Metabolism. Vitr. Cell. Dev. Biol.-Anim. 1991, 27, 961–966. [Google Scholar] [CrossRef]
- Copes, F.; Pien, N.; Van Vlierberghe, S.; Boccafoschi, F.; Mantovani, D. Collagen-Based Tissue Engineering Strategies for Vascular Medicine. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Mercado-Pagán, Á.E.; Stahl, A.M.; Shanjani, Y.; Yang, Y. Vascularization in Bone Tissue Engineering Constructs. Ann. Biomed. Eng. 2015, 43, 718–729. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, T.M.; Opperman, L.A.; Persing, J.A.; Ogle, R.C. Repair of Critical Size Rat Calvarial Defects Using Extracellular Matrix Protein Gels. J. Neurosurg. 1995, 83, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Kato, E.; Lemler, J.; Sakurai, K.; Yamada, M. Biodegradation Property of Beta-Tricalcium Phosphate-Collagen Composite in Accordance with Bone Formation: A Comparative Study with Bio-Oss Collagen® in a Rat Critical-Size Defect Model. Clin. Implant Dent. Relat. Res. 2014, 16, 202–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mian, M.; Beghe, F.; Mian, E. Collagen as a Pharmacological Approach in Wound Healing. Int. J. Tissue React. 1992, 14, 1–9. [Google Scholar] [PubMed]
- Ghanaati, S.; Barbeck, M.; Orth, C.; Willershausen, I.; Thimm, B.W.; Hoffmann, C.; Rasic, A.; Sader, R.A.; Unger, R.E.; Peters, F.; et al. Influence of β-Tricalcium Phosphate Granule Size and Morphology on Tissue Reaction in Vivo. Acta Biomater. 2010, 6, 4476–4487. [Google Scholar] [CrossRef]
- Barbeck, M.; Lorenz, J.; Holthaus, M.G.; Raetscho, N.; Kubesch, A.; Booms, P.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Porcine Dermis and Pericardium-Based, Non Cross-Linked Materials Induce Multinucleated Giant Cells after Their in Vivo Implantation: A Physiological Reaction? J. Oral Implantol. 2015, 41, e267–e280. [Google Scholar] [CrossRef]
- Barbeck, M.; Unger, R.E.; Booms, P.; Dohle, E.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. Monocyte Preseeding Leads to an Increased Implant Bed Vascularization of Biphasic Calcium Phosphate Bone Substitutes via Vessel Maturation. J. Biomed. Mater. Res.-Part A 2016, 104, 2928–2935. [Google Scholar] [CrossRef]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef] [Green Version]
- Al-Maawi, S.; Vorakulpipat, C.; Orlowska, A.; Zrnc, T.A.; Sader, R.A.; James Kirkpatrick, C.; Ghanaati, S. In Vivo Implantation of a Bovine-Derived Collagen Membrane Leads to Changes in the Physiological Cellular Pattern of Wound Healing by the Induction of Multinucleated Giant Cells: An Adverse Reaction? Front. Bioeng. Biotechnol. 2018, 6. [Google Scholar] [CrossRef]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular Matrix Assembly: A Multiscale Deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Miki, A.; Inaba, S.; Baba, T.; Kihira, K.; Fukada, H.; Oda, M. Structural and Physical Properties of Collagen Extracted from Moon Jellyfish under Neutral PH Conditions. Biosci. Biotechnol. Biochem. 2015, 79, 1603–1607. [Google Scholar] [CrossRef]
- Hu, W.J.; Eaton, J.W.; Tang, L. Molecular Basis of Biomaterial-Mediated Foreign Body Reactions. Blood 2001, 98, 1231–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latour, R. Biomaterials: Protein–Surface Interactions. In Encyclopedia of Biomaterials and Biomedical Engineering, Second Edition—Four Volume Set; CRC Press: Boca Raton, FL, USA, 2008; pp. 270–284. [Google Scholar] [CrossRef]
- Schaefer, S.; Detsch, R.; Uhl, F.; Deisinger, U.; Ziegler, G. How Degradation of Calcium Phosphate Bone Substitute Materials Is Influenced by Phase Composition and Porosity. Adv. Eng. Mater. 2011, 13, 342–350. [Google Scholar] [CrossRef]
- Barbeck, M.; Dard, M.; Kokkinopoulou, M.; Markl, J.; Booms, P.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. Small-Sized Granules of Biphasic Bone Substitutes Support Fast Implant Bed Vascularization. Biomatter 2015, 5, e1056943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbeck, M.; Serra, T.; Booms, P.; Stojanovic, S.; Najman, S.; Engel, E.; Sader, R.; Kirkpatrick, C.J.; Navarro, M.; Ghanaati, S. Analysis of the in Vitro Degradation and the in Vivo Tissue Response to Bi-Layered 3D-Printed Scaffolds Combining PLA and Biphasic PLA/Bioglass Components – Guidance of the Inflammatory Response as Basis for Osteochondral Regeneration. Bioact. Mater. 2017, 2, 208–223. [Google Scholar] [CrossRef]
- Ghanaati, S.; Kirkpatrick, C.; Kubesch, A.; Lorenz, J.; Sader, R.; Udeabor, S.; Barbeck, M.; Choukroun, J. Induction of Multinucleated Giant Cells in Response to Small Sized Bovine Bone Substitute (Bio-Oss TM ) Results in an Enhanced Early Implantation Bed Vascularization. Ann. Maxillofac. Surg. 2014, 4, 150. [Google Scholar] [CrossRef] [Green Version]
- Barbeck, M.; Jung, O.; Xiong, X.; Krastev, R.; Korzinskas, T.; Najman, S.; Radenkovic, M.; Wegner, N.; Knyazeva, M.; Walther, F. Balancing Purification and Ultrastructure of Naturally Derived Bone Blocks for Bone Regeneration: Report of the Purification Effort of Two Bone Blocks. Materials 2019, 12, 3224. [Google Scholar] [CrossRef] [Green Version]
- Tawil, G.; Barbeck, M.; Unger, R.; Tawil, P.; Witte, F. Sinus Floor Elevation Using the Lateral Approach and Window Repositioning and a Xenogeneic Bone Substitute as a Grafting Material: A Histologic, Histomorphometric, and Radiographic Analysis. Int. J. Oral Maxillofac. Implants 2018, 33, 1089–1096. [Google Scholar] [CrossRef]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flaig, I.; Radenković, M.; Najman, S.; Pröhl, A.; Jung, O.; Barbeck, M. In Vivo Analysis of the Biocompatibility and Immune Response of Jellyfish Collagen Scaffolds and its Suitability for Bone Regeneration. Int. J. Mol. Sci. 2020, 21, 4518. https://doi.org/10.3390/ijms21124518
Flaig I, Radenković M, Najman S, Pröhl A, Jung O, Barbeck M. In Vivo Analysis of the Biocompatibility and Immune Response of Jellyfish Collagen Scaffolds and its Suitability for Bone Regeneration. International Journal of Molecular Sciences. 2020; 21(12):4518. https://doi.org/10.3390/ijms21124518
Chicago/Turabian StyleFlaig, Iris, Milena Radenković, Stevo Najman, Annica Pröhl, Ole Jung, and Mike Barbeck. 2020. "In Vivo Analysis of the Biocompatibility and Immune Response of Jellyfish Collagen Scaffolds and its Suitability for Bone Regeneration" International Journal of Molecular Sciences 21, no. 12: 4518. https://doi.org/10.3390/ijms21124518
APA StyleFlaig, I., Radenković, M., Najman, S., Pröhl, A., Jung, O., & Barbeck, M. (2020). In Vivo Analysis of the Biocompatibility and Immune Response of Jellyfish Collagen Scaffolds and its Suitability for Bone Regeneration. International Journal of Molecular Sciences, 21(12), 4518. https://doi.org/10.3390/ijms21124518







