What Is the Evolutionary Fingerprint in Neutrophil Granulocytes?
Abstract
1. Introduction
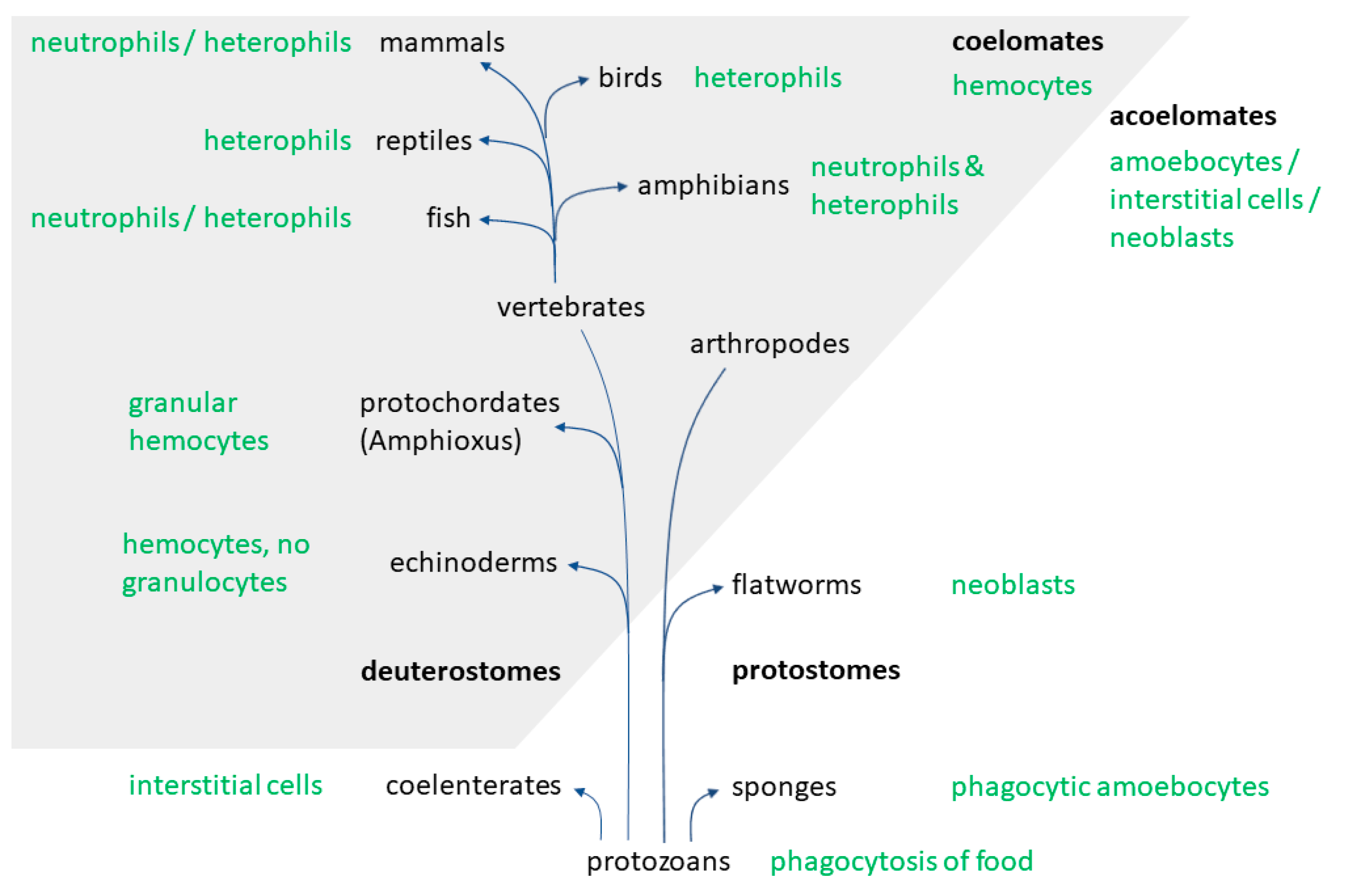
2. Evolution
3. Cell Number and Granulopoiesis
3.1. Cell Number
3.2. Granulopoiesis
4. Morphology and Composition of Neutrophils
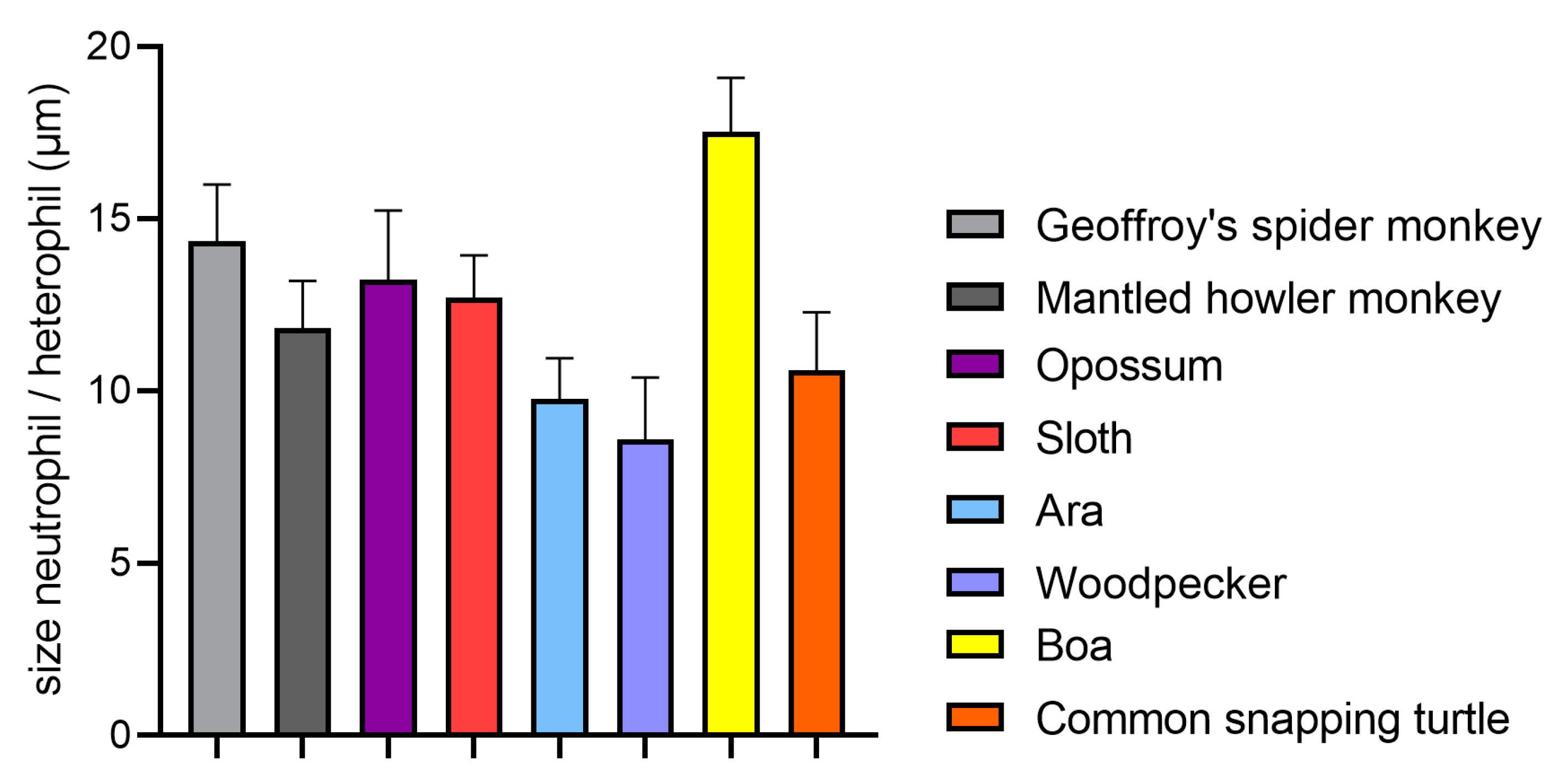
4.1. Size
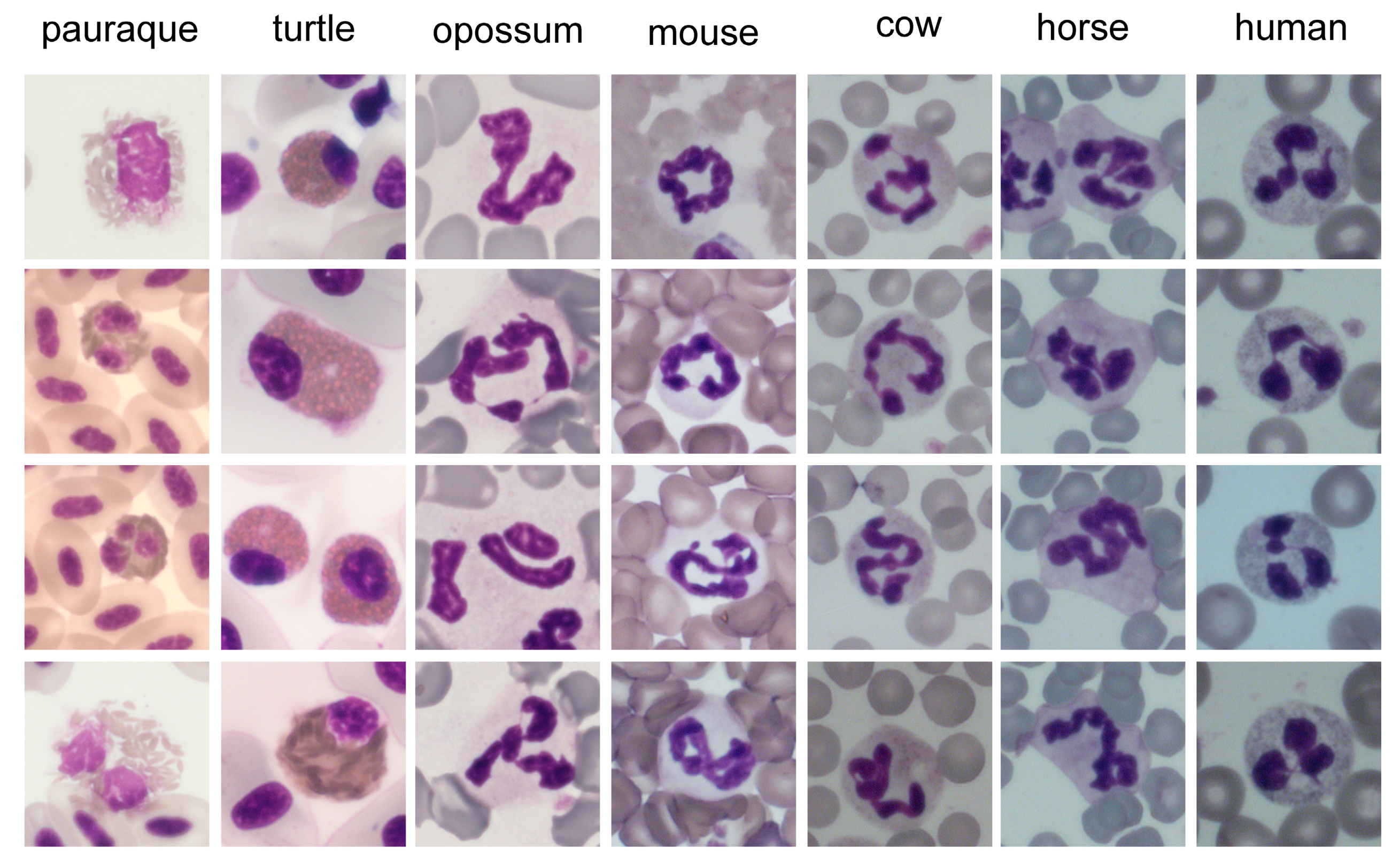
4.2. Nucleus
4.2.1. Core Shape
4.2.2. Factors for Segmentation
4.3. Cytoplasm and Granules
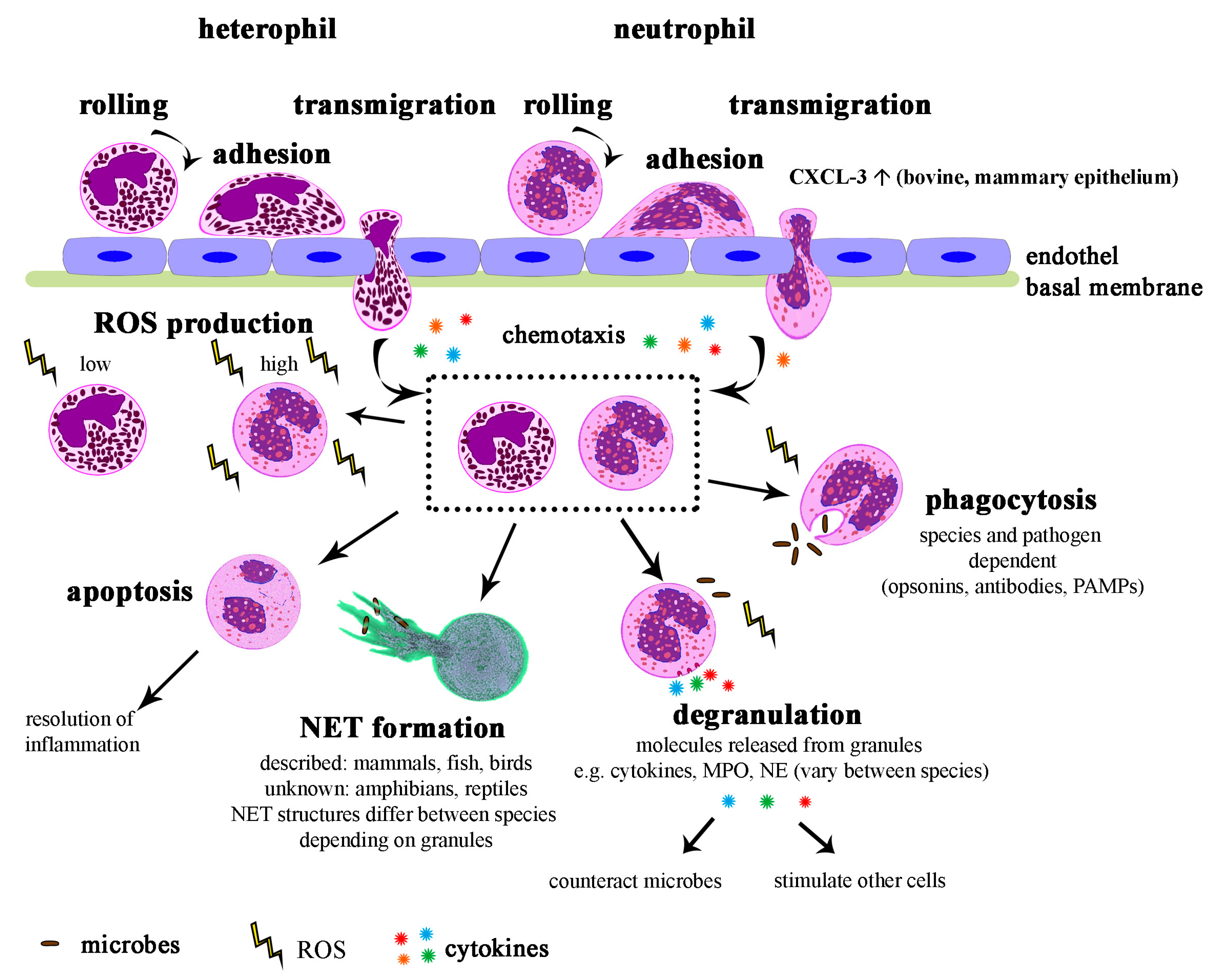
5. Function of Neutrophils
6. Diseases of Neutrophils
7. Influence of Temperature
8. Cell Culture and Storage
9. Conclusions and Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AML | Acute myeloid leukemia |
| AP | Alkaline phosphatase |
| BACH | BTB and CNC homology |
| BPI | Bactericidal permeability increasing protein |
| C/EBP | CCAAT/enhancer-binding protein |
| dbcAMP | Dibutyryl cyclic adenosine monophosphate |
| DMSO | Dimethyl sulfoxide |
| eNAP | Equine neutrophil antimicrobial peptide |
| fMLP | N-Formylmethionyl-leucyl-phenylalanine |
| G-CSF | Granulocyte colony stimulating factor |
| G-MDSC | Granulocytic myeloid-derived suppressor cell |
| HDN | High-density neutrophil |
| LBR | Lamin B receptor |
| LDN | Low-density neutrophil |
| LOX-1 | Lectin-type oxidized LDL receptor 1 |
| LPS | Lipopolysaccharide |
| MNV | Mean neutrophil volume |
| MP | Metalloproteinase |
| MPO | Myeloperoxidase |
| NE | Neutrophil elastase |
| NET | Neutrophil extracellular trap |
| NOX2 | NADPH oxidase of neutrophils |
| PAMPs | Pathogen-associated molecular patterns |
| PMN | Polymorphonuclear neutrophil |
| ROS | Reactive oxygen species |
| TAN | Tumor associated neutrophil |
| TGF-β | Transforming growth factor-β |
Appendix A
Appendix A.1. Blood Smears
Appendix A.2. DIFF-Quick Staining and Analysis
References
- Schalm, O. Schalm’s Veterinary Hematology, 6th ed.; Weiss, D.J., Wardrop, K.J., Eds.; Wiley-Blackwell: Ames, IA, USA, 2010; ISBN 978-0-8138-1798-9. [Google Scholar]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Henson, P.M.; Johnston, R.B. Tissue injury in inflammation. Oxidants, proteinases, and cationic proteins. J. Clin. Investig. 1987, 79, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.J. Role of neutrophils in systemic autoimmune diseases. Arthritis Res. Ther. 2013, 15, 219. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C. Neutrophils and immunity: Challenges and opportunities. Nat. Rev. Immunol. 2006, 6, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Christoffersson, G.; Phillipson, M. The neutrophil: One cell on many missions or many cells with different agendas? Cell Tissue Res. 2018, 371, 415–423. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; Fridlender, Z.G.; Glogauer, M.; Scapini, P. Neutrophil Diversity in Health and Disease. Trends Immunol. 2019, 40, 565–583. [Google Scholar] [CrossRef]
- Snelgrove, R.J.; Patel, D.F.; Patel, T.; Lloyd, C.M. The enigmatic role of the neutrophil in asthma: Friend, foe or indifferent? Clin. Exp. Allergy 2018, 48, 1275–1285. [Google Scholar] [CrossRef]
- Neumann, A.; Brogden, G.; von Köckritz-Blickwede, M. Extracellular Traps: An Ancient Weapon of Multiple Kingdoms. Biology (Basel) 2020, 9, 34. [Google Scholar] [CrossRef]
- Millar, D.A.; Ratcliffe, N.A. The evolution of blood cells: Facts and enigmas. Endeavour 1989, 13, 72–77. [Google Scholar] [CrossRef]
- Hartenstein, V. Blood Cells and Blood Cell Development in the Animal Kingdom. Annu. Rev. Cell Dev. Biol. 2006, 22, 677–712. [Google Scholar] [CrossRef]
- Anderson, R. Invertebrate Blood Cells, 2nd ed.; Ratcliffe, N., Rowley, A., Eds.; Academic Press: London, UK, 1981. [Google Scholar]
- Sminia, T.; van der Knaap, W.P.; Boerrigter-Barendsen, L.H. Peroxidase-positive blood cells in snails. J. Reticuloendothel. Soc. 1982, 31, 399–404. [Google Scholar] [PubMed]
- Rhodes, C.P.; Ratcliffe, N.A.; Rowley, A.F. Presence of coelomocytes in the primitive chordate amphioxus (Branchiostoma lanceolatum). Science 1982, 217, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Browne, N.; Heelan, M.; Kavanagh, K. An analysis of the structural and functional similarities of insect hemocytes and mammalian phagocytes. Virulence 2013, 4, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Pandey, J.P.; Tiwari, R.K. An Overview of Insect Hemocyte Science and its Future Application in Applied and Biomedical Fields. Am. J. Biochem. Mol. Biol. 2012, 2, 82–105. [Google Scholar]
- Salzet, M. Vertebrate innate immunity resembles a mosaic of invertebrate immune responses. Trends Immunol. 2001, 22, 285–288. [Google Scholar] [CrossRef]
- Hanson, M.A.; Lemaitre, B.; Unckless, R.L. Dynamic Evolution of Antimicrobial Peptides Underscores Trade-Offs Between Immunity and Ecological Fitness. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Belov, K.; Sanderson, C.E.; Deakin, J.E.; Wong, E.S.W.; Assange, D.; McColl, K.A.; Gout, A.; de Bono, B.; Barrow, A.D.; Speed, T.P.; et al. Characterization of the opossum immune genome provides insights into the evolution of the mammalian immune system. Genome. Res. 2007, 17, 982–991. [Google Scholar] [CrossRef]
- Cutts, J.H.; Krause, W.J. Leukocytes in the peripheral blood of the developing opossum. J. Anat. 1980, 130, 113–120. [Google Scholar]
- Basden, K.; Cooper, D.W.; Deane, E.M. Development of the lymphoid tissues of the tammar wallaby Macropus eugenii. Reprod. Fertil. Dev. 1997, 9, 243. [Google Scholar] [CrossRef]
- Yadav, M. Characteristics of blood in the pouch young of a marsupial, Setonix brachyurus. Aust. J. Zool. 1972, 20, 249. [Google Scholar] [CrossRef]
- Palis, J.; Yoder, M.C. Yolk-sac hematopoiesis: The first blood cells of mouse and man. Exp. Hematol. 2001, 29, 927–936. [Google Scholar] [CrossRef]
- Golub, R.; Cumano, A. Embryonic hematopoiesis. Blood Cells Mol. Dis. 2013, 51, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Tiedemann, K.; van Ooyen, B. Prenatal hematopoiesis and blood characteristics of the cat. Anat. Embryol. (Berl) 1978, 153, 243–267. [Google Scholar] [CrossRef] [PubMed]
- Winqvist, G. Morphology of the blood and the hemopoietic organs in cattle under normal and some experimental conditions. Acta Anat. Suppl. (Basel) 1954, 21, 1–157. [Google Scholar]
- Maheshwari, A.; Christensen, R.D. Developmental Granulocytopoiesis. In Fetal and Neonatal Physiology; Elsevier: Amsterdam, The Netherland, 2004; Volume 2–2, pp. 1388–1396. ISBN 0721696546. [Google Scholar]
- Slayton, W.B.; Li, Y.; Calhoun, D.A.; Juul, S.E.; Iturraspe, J.; Braylan, R.C.; Christensen, R.D. The first-appearance of neutrophils in the human fetal bone marrow cavity. Early Hum. Dev. 1998, 53, 129–144. [Google Scholar] [CrossRef]
- Cebulj-Kadunc, N.; Kosec, M.; Cestnik, V. The Variations of White Blood Cell Count in Lipizzan Horses. J. Vet. Med. Ser. A 2003, 50, 251–253. [Google Scholar] [CrossRef]
- Bauer, N.; Beelitz, P. Klinische Labordiagnostik in der Tiermedizin, 7th ed.; Moritz, A., Ed.; Schattauer GmbH: Stuttgart, Germany, 2014; ISBN 9783794527373. [Google Scholar]
- Manroe, B.L.; Weinberg, A.G.; Rosenfeld, C.R.; Browne, R. The neonatal blood count in health and disease. I. Reference values for neutrophilic cells. J. Pediatr. 1979, 95, 89–98. [Google Scholar] [CrossRef]
- Leiding, J.W. Neutrophil Evolution and Their Diseases in Humans. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef]
- Anderson, D.C.; Abbassi, O.; Kishimoto, T.K.; Koenig, J.M.; McIntire, L.V.; Smith, C.W. Diminished lectin-, epidermal growth factor-, complement binding domain-cell adhesion molecule-1 on neonatal neutrophils underlies their impaired CD18-independent adhesion to endothelial cells in vitro. J. Immunol. 1991, 146, 3372–3379. [Google Scholar]
- Harris, M.C.; Shalit, M.; Southwick, F.S. Diminished actin polymerization by neutrophils from newborn infants. Pediatr. Res. 1993, 33, 27–31. [Google Scholar] [CrossRef]
- Tizard, I.R. Veterinary immunology, 10th ed.; Elsevier: St. Louis, MO, USA, 2018; ISBN 9780323523493. [Google Scholar]
- Havixbeck, J.J.; Rieger, A.M.; Wong, M.E.; Hodgkinson, J.W.; Barreda, D.R. Neutrophil contributions to the induction and regulation of the acute inflammatory response in teleost fish. J. Leukoc. Biol. 2016, 99, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Prinzinger, R.; Misovic, A.; Nagel, B. Aviäre Hämatologie Das Vogelblut: Struktur, Funktion, Diagnose und Parasiten, 1st ed.; Cuvillier Verlag: Göttingen, Germany, 2012; ISBN 9783954041404. [Google Scholar]
- Breves, G. Physiologie der Haustiere, 3rd ed.; von Engelhardt, W., Ed.; Enke Verlag: Stuttgart, Germany, 2010; ISBN 9783830410782. [Google Scholar]
- Wedel, A. Ziervögel: Erkrankungen, Haltung, Fütterung, 2nd ed.; Parey Verlag: Stuttgart, Germany, 2004; ISBN 3830441584. [Google Scholar]
- Bayyari, G.; Huff, W.; Rath, N.; Balog, J.; Newberry, L.; Villines, J.; Skeeles, J.; Anthony, N.; Nestor, K. Effect of the genetic selection of turkeys for increased body weight and egg production on immune and physiological responses. Poult. Sci. 1997, 76, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Gehlen, H.; Antys-Becker, M.; Barsnick, R.; Barton, A.-K.; Birkmann, K.; Cavalleri, J.-M.; Cehak, A.; de Heus, P.; Ertelt, A.; Feichtenschlager, A.; et al. Differenzialdiagnosen Innere Medizin beim Pferd: Vom Leitsymptom zur Diagnose; Enke: Berlin, Germany, 2017; ISBN 3132212210. [Google Scholar]
- Allen, B.V.; Kane, C.E.; Powell, D.G. Leucocyte counts in the healthy English Thoroughbred in training. Equine Vet. J. 1984, 16, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Dubreuil, P.; Couture, Y.; Farmer, C.; Petitclerc, D. Hematological and biochemical changes following an acute stress in control and somatostatin-immunized pigs. Can. J. Anim. Sci. 1993, 73, 241–252. [Google Scholar] [CrossRef]
- Stockham, S.L.; Scott, M.A. Leukocytes. In Fundamentals of Veterinary Clinical Pathology; Wiley-Blackwell: Hoboken, NJ, USA, 2008; ISBN 978-0-813-80076-9. [Google Scholar]
- Donovan, D.C.; Jackson, C.A.; Colahan, P.T.; Norton, N.N.; Clapper, J.L.; Moore, J.N.; Hurley, D.J. Assessment of exercise-induced alterations in neutrophil function in horses. Am. J. Vet. Res. 2007, 68, 1198–1204. [Google Scholar] [CrossRef]
- Elektronenmikroskopischer Atlas von Zellen, Geweben und Organen. Available online: http://www.drjastrow.de/WAI/EM/EMNeutro.html (accessed on 1 April 2020).
- Fielder, S.E. MSD MANUAL Veterinary Manual Hematologic Reference Ranges. Available online: https://www.msdvetmanual.com/special-subjects/reference-guides/hematologic-reference-ranges (accessed on 31 March 2020).
- Klinische Labordiagnostik. Leitsymptome bei Papageien und Sittichen; Pees, M., Ed.; Enke Verlag: Stuttgart, Germany, 2004; ISBN 3830410239. [Google Scholar]
- Heatley, J.J.; Russell, K.E. Box Turtle (Terrapene spp.) Hematology. J. Exot. Pet. Med. 2010, 19, 160–164. [Google Scholar] [CrossRef]
- Pires, T.T.; Rostan, G.; Bittencourt, T.C.C.d.; Guimarães, J.E. Hemograma e bioquímica sérica de tartarugas cabeçudas (Caretta caretta) de vida livre e mantidas em cativeiro, no litoral norte da Bahia. Brazilian J. Vet. Res. Anim. Sci. 2009, 46, 11–18. [Google Scholar] [CrossRef][Green Version]
- Acevedo, L.M.R.; Blas, S.S.M.; Fuentes-Mascorro, G. Hemograma y características morfológicas de las células sanguíneas de tortuga golfina (Lepidochelys olivacea) de oaxaca, México. Rev. Cient. la Fac. Ciencias Vet. la Univ. del Zulia 2012, 22, 468–476. [Google Scholar]
- Vasaruchapong, T.; Disarapong, P.; Chulasugandha, P.; Khow, O.; Chanhome, L.; Chiobamroongkiat, M.; Chaiyabutr, N.; Sitprija, V. Comparative studies on hematological and plasma biochemical parameters in different types of venomous snakes in Thailand. Comp. Clin. Path. 2014, 23, 955–959. [Google Scholar] [CrossRef]
- Belic, M.; Lukac, M.; Dvojkovic, N.; Galesic, T.; Robic, M.; Turk, R. Preliminary evaluation of complete blood count and diurnal variation of hematological parameters in black rat snake (Pantherophis obsoletus). Vet. Glas. 2020, 1–10. [Google Scholar] [CrossRef]
- Wack, R.F.; Hansen, E.; Small, M.; Poppenga, R.; Bunn, D.; Johnson, C.K. Hematology and plasma biochemistry values for the giant garter snake (Thamnophis gigas) and valley garter snake (Thamnophis sirtalis fitchi) in the Central Valley of California. J. Wildl. Dis. 2012, 48, 307–313. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Opossum (Didelphis Virginiana) Blood Normals. Available online: https://opossumsocietyus.org/opossum-blood-normals/ (accessed on 31 March 2020).
- Giacometti, L.; Berntzen, A.K.; Bliss, M.L. Hematologic parameters of the opossum (Didelphis virginiana). Comp. Biochem. Physiol. Part A Comp. Physiol. 1972, 43, 287–292. [Google Scholar] [CrossRef]
- Havixbeck, J.; Barreda, D. Neutrophil Development, Migration, and Function in Teleost Fish. Biology (Basel) 2015, 4, 715–734. [Google Scholar] [CrossRef]
- Kato, H.; Igarashi, K. To be red or white: Lineage commitment and maintenance of the hematopoietic system by the “inner myeloid.”. Haematologica 2019, 104, 1919–1927. [Google Scholar] [CrossRef]
- Radomska, H.S.; Huettner, C.S.; Zhang, P.; Cheng, T.; Scadden, D.T.; Tenen, D.G. CCAAT/Enhancer Binding Protein α Is a Regulatory Switch Sufficient for Induction of Granulocytic Development from Bipotential Myeloid Progenitors. Mol. Cell Biol. 1998, 18, 4301–4314. [Google Scholar] [CrossRef] [PubMed]
- Laslo, P.; Spooner, C.J.; Warmflash, A.; Lancki, D.W.; Lee, H.-J.; Sciammas, R.; Gantner, B.N.; Dinner, A.R.; Singh, H. Multilineage Transcriptional Priming and Determination of Alternate Hematopoietic Cell Fates. Cell 2006, 126, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard, M.D.; Jurlander, J.; Klausen, P.; Borregaard, N.; Cowland, J.B. The in vivo profile of transcription factors during neutrophil differentiation in human bone marrow. Blood 2003, 101, 4322–4332. [Google Scholar] [CrossRef]
- Summers, C.; Rankin, S.M.; Condliffe, A.M.; Singh, N.; Peters, A.M.; Chilvers, E.R. Neutrophil kinetics in health and disease. Trends Immunol. 2010, 31, 318–324. [Google Scholar] [CrossRef]
- Borregaard, N. Neutrophils, from Marrow to Microbes. Immunity 2010, 33, 657–670. [Google Scholar] [CrossRef]
- Deniset, J.F.; Surewaard, B.G.; Lee, W.-Y.; Kubes, P. Splenic Ly6Ghigh mature and Ly6Gint immature neutrophils contribute to eradication of S. pneumoniae. J. Exp. Med. 2017, 214, 1333–1350. [Google Scholar] [CrossRef]
- Weisgrau, K.L.; Vosler, L.J.; Pomplun, N.L.; Hayes, J.M.; Simmons, H.A.; Friedrichs, K.R.; Rakasz, E.G. Neutrophil progenitor populations of rhesus macaques. J. Leukoc. Biol. 2019, 105, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Claver, J.A.; Quaglia, A.I.E. Comparative Morphology, Development, and Function of Blood Cells in Nonmammalian Vertebrates. J. Exot. Pet. Med. 2009, 18, 87–97. [Google Scholar] [CrossRef]
- Wright, K. Amphibian hematology. In Amphibian Medicine and Captive Husbandry, 1st ed.; Wright, K.M., Whitaker, B., Eds.; Krieger Publishing Company: Malabar, FL, USA, 2001; pp. 129–146. ISBN 0894649175. [Google Scholar]
- Niemiec, M.J.; De Samber, B.; Garrevoet, J.; Vergucht, E.; Vekemans, B.; De Rycke, R.; Björn, E.; Sandblad, L.; Wellenreuther, G.; Falkenberg, G.; et al. Trace element landscape of resting and activated human neutrophils on the sub-micrometer level. Metallomics 2015, 7, 996–1010. [Google Scholar] [CrossRef] [PubMed]
- Doerschuk, C.M.; Beyers, N.; Coxson, H.O.; Wiggs, B.; Hogg, J.C. Comparison of neutrophil and capillary diameters and their relation to neutrophil sequestration in the lung. J. Appl. Physiol. 1993, 74, 3040–3045. [Google Scholar] [CrossRef] [PubMed]
- Van Hout, G.P.J.; van Solinge, W.W.; Gijsberts, C.M.; Teuben, M.P.J.; Leliefeld, P.H.C.; Heeres, M.; Nijhoff, F.; de Jong, S.; Bosch, L.; de Jager, S.C.A.; et al. Elevated mean neutrophil volume represents altered neutrophil composition and reflects damage after myocardial infarction. Basic Res. Cardiol. 2015, 110, 58. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.J.; Evanson, O.A. Evaluation of lipopolysaccharide-induced activation of equine neutrophils. Am. J. Vet. Res. 2002, 63, 811–815. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, J.T.; Kreutzer, D.L.; Ward, P.A. Neutrophil aggregation and swelling induced by chemotactic agents. J. Immunol. 1977, 119, 232–239. [Google Scholar]
- Lilliehöök, I.; Tvedten, H.W.; Bröjer, J.; Edner, A.; Nostell, K. Time-related changes in equine neutrophils after experimental endotoxemia: Myeloperoxidase staining, size, and numbers. Vet. Clin. Pathol. 2016, 45, 66–72. [Google Scholar] [CrossRef]
- D’Onofrio, G.; Mancini, S.; Tamburrini, E.; Mango, G.; Ortona, L. Giant neutrophils with increased peroxidase activity. Another evidence of dysgranulopoiesis in AIDS. Am. J. Clin. Pathol. 1987, 87, 584–591. [Google Scholar] [CrossRef]
- Downey, G.P.; Doherty, D.E.; Schwab, B.; Elson, E.L.; Henson, P.M.; Worthen, G.S. Retention of leukocytes in capillaries: Role of cell size and deformability. J. Appl. Physiol. 1990, 69, 1767–1778. [Google Scholar] [CrossRef]
- Brahimi, M.; Adda, A.; Lazreg, H.; Beliali, H.; Osmani, S.; Bekadja, M.A. Can Sex Be Determined from a Blood Smear? Turkish J. Hematol. 2013, 30, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Cadar, M. Cytological estamination of sexual dimorphism by microscopic examination of peripheral blood in some laboratory animals. Bull. USAMV-CN 2007, 63–64. [Google Scholar] [CrossRef]
- Lashari, M.H.; Farooq, U.; Arshad, A.; Tahir, M.N.; Idris, M.; Khurshid, A.; Rehman, Z.U. Sex determination through Barr Bodies of Neutrophils in cattle and buffalo. Biologia Pakistan 2019, 65, 1–5. [Google Scholar]
- Sanchez, J.A.; Karni, R.J.; Wangh, L.J. Fluorescent in situ hybridization (FISH) analysis of the relationship between chromosome location and nuclear morphology in human neutrophils. Chromosoma 1997, 106, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-K.; Tsai, M.-H.; Huang, D.-C.; Zheng, Z.-H.; Hung, K.-D. Leukocyte Nucleus Segmentation and Nucleus Lobe Counting. BMC Bioinform. 2010, 11, 558. [Google Scholar] [CrossRef]
- Hoffmann, K.; Sperling, K.; Olins, A.L.; Olins, D.E. The granulocyte nucleus and lamin B receptor: Avoiding the ovoid. Chromosoma 2007, 116, 227–235. [Google Scholar] [CrossRef]
- Tavasoli, B.; Tabibian, S.; Majid, G.; Souri, S.; Shams, M.; Rashidpanah, J.; Firoozkohi, F.; Dorgalaleh, A. Extensive hematoma in a patient with hereditary hypersegmentation of neutrophils. J. Cell. Mol. Anesth. 2016, 1, 109–114. [Google Scholar]
- Tvedten, H.; Riihimäki, M. Hypersegmentation of equine neutrophils. Vet. Clin. Pathol. 2007, 36, 4–5. [Google Scholar] [CrossRef]
- Mohanty, T.; Sørensen, O.E.; Nordenfelt, P. NETQUANT: Automated Quantification of Neutrophil Extracellular Traps. Front. Immunol. 2017, 8, 1999. [Google Scholar] [CrossRef]
- Zhu, Y.; Gong, K.; Denholtz, M.; Chandra, V.; Kamps, M.P.; Alber, F.; Murre, C. Comprehensive characterization of neutrophil genome topology. Genes Dev. 2017, 31, 141–153. [Google Scholar] [CrossRef]
- Carvalho, L.O.; Aquino, E.N.; Neves, A.C.D.; Fontes, W. The Neutrophil Nucleus and Its Role in Neutrophilic Function. J. Cell. Biochem. 2015, 116, 1831–1836. [Google Scholar] [CrossRef] [PubMed]
- Canfield, P. Comparative cell morphology in the peripheral blood film from exotic and native animals. Aust. Vet. J. 1998, 76, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Manley, H.R.; Keightley, M.C.; Lieschke, G.J. The Neutrophil Nucleus: An Important Influence on Neutrophil Migration and Function. Front. Immunol. 2018, 9, 2867. [Google Scholar] [CrossRef] [PubMed]
- Gaines, P.; Tien, C.W.; Olins, A.L.; Olins, D.E.; Shultz, L.D.; Carney, L.; Berliner, N. Mouse neutrophils lacking lamin B-receptor expression exhibit aberrant development and lack critical functional responses. Exp. Hematol. 2008, 36, 965–976. [Google Scholar] [CrossRef]
- Friedl, P.; Weigelin, B. Interstitial leukocyte migration and immune function. Nat. Immunol. 2008, 9, 960–969. [Google Scholar] [CrossRef]
- Rowat, A.C.; Jaalouk, D.E.; Zwerger, M.; Ung, W.L.; Eydelnant, I.A.; Olins, D.E.; Olins, A.L.; Herrmann, H.; Weitz, D.A.; Lammerding, J. Nuclear Envelope Composition Determines the Ability of Neutrophil-type Cells to Passage through Micron-scale Constrictions. J. Biol. Chem. 2013, 288, 8610–8618. [Google Scholar] [CrossRef]
- Hoffmann, K.; Dreger, C.K.; Olins, A.L.; Olins, D.E.; Shultz, L.D.; Lucke, B.; Karl, H.; Kaps, R.; Müller, D.; Vayá, A.; et al. Mutations in the gene encoding the lamin B receptor produce an altered nuclear morphology in granulocytes (Pelger–Huët anomaly). Nat. Genet. 2002, 31, 410–414. [Google Scholar] [CrossRef]
- Olins, A.L.; Zwerger, M.; Herrmann, H.; Zentgraf, H.; Simon, A.J.; Monestier, M.; Olins, D.E. The human granulocyte nucleus: Unusual nuclear envelope and heterochromatin composition. Eur. J. Cell Biol. 2008, 87, 279–290. [Google Scholar] [CrossRef]
- Faurschou, M.; Borregaard, N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003, 5, 1317–1327. [Google Scholar] [CrossRef]
- Borregaard, N.; Sørensen, O.E.; Theilgaard-Mönch, K. Neutrophil granules: A library of innate immunity proteins. Trends Immunol. 2007, 28, 340–345. [Google Scholar] [CrossRef]
- Borregaard, N.; Sehested, M.; Nielsen, B.; Sengelov, H.; Kjeldsen, L. Biosynthesis of granule proteins in normal human bone marrow cells. Gelatinase is a marker of terminal neutrophil differentiation. Blood 1995, 85, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, L.; Bainton, D.; Sengelov, H.; Borregaard, N. Structural and functional heterogeneity among peroxidase-negative granules in human neutrophils: Identification of a distinct gelatinase- containing granule subset by combined immunocytochemistry and subcellular fractionation. Blood 1993, 82, 3183–3191. [Google Scholar] [CrossRef] [PubMed]
- Rørvig, S.; Honore, C.; Larsson, L.-I.; Ohlsson, S.; Pedersen, C.C.; Jacobsen, L.C.; Cowland, J.B.; Garred, P.; Borregaard, N. Ficolin-1 is present in a highly mobilizable subset of human neutrophil granules and associates with the cell surface after stimulation with fMLP. J. Leukoc. Biol. 2009, 86, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Gennaro, R.; Dewald, B.; Horisberger, U.; Gubler, H.U.; Baggiolini, M. A novel type of cytoplasmic granule in bovine neutrophils. J. Cell Biol. 1983, 96, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Styrt, B. Species Variation in Neutrophil Biochemistry and Function. J. Leukoc. Biol. 1989, 46, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Rausch, P.G.; Moore, T.G. Granule enzymes of polymorphonuclear neutrophils: A phylogenetic comparison. Blood 1975, 46, 913–919. [Google Scholar] [CrossRef]
- Palić, D.; Beck, L.S.; Palić, J.; Andreasen, C.B. Use of rapid cytochemical staining to characterize fish blood granulocytes in species of special concern and determine potential for function testing. Fish Shellfish Immunol. 2011, 30, 646–652. [Google Scholar] [CrossRef]
- Lollike, K.; Kjeldsen, L.; Sengeløv, H.; Borregaard, N. Lysozyme in human neutrophils and plasma. A parameter of myelopoietic activity. Leukemia 1995, 9, 159–164. [Google Scholar]
- Barton, J.C.; Parmley, R.T.; Butler, T.W.; Williamson, S.; MacKenzie, S.; Chandler, D.B.; Blackburn, W.; Heck, L.W. Neutrophil lactoferrin content: Variation among mammals. Anat. Rec. 1988, 221, 567–575. [Google Scholar] [CrossRef]
- Chibber, R.; Castle, A.G. Biochemical characterisation of porcine polymorphonuclear leucocytes: Comparison with human polymorphonuclear leucocytes. Comp. Biochem. Physiol. Part B Comp. Biochem. 1983, 75, 335–340. [Google Scholar] [CrossRef]
- De Buhr, N.; Bonilla, M.C.; Jimenez-Soto, M.; von Köckritz-Blickwede, M.; Dolz, G. Extracellular Trap Formation in Response to Trypanosoma cruzi Infection in Granulocytes Isolated From Dogs and Common Opossums, Natural Reservoir Hosts. Front. Microbiol. 2018, 9, 966. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.M.; Biegel, J.M.; Stewart, C.-B. Evolution of the mammalian lysozyme gene family. BMC Evol. Biol. 2011, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Mateo, M.R.; Roberts, E.D.; Enright, F.M. Morphologic, cytochemical, and functional studies of peripheral blood cells of young healthy American alligators (Alligator mississippiensis). Am. J. Vet. Res. 1984, 45, 1046–1053. [Google Scholar] [PubMed]
- Alleman, A.R.; Jacobson, E.R.; Raskin, R.E. Morphologic, cytochemical staining, and ultrastructural characteristics of blood cells from eastern diamondback rattlesnakes (Crotalus adamanteus). Am. J. Vet. Res. 1999, 60, 507–514. [Google Scholar]
- Ciuraszkiewicz, J.; Biczycki, M.; Maluta, A.; Martin, S.; Wątorek, W.; Olczak, M. Reptilian transferrins: Evolution of disulphide bridges and conservation of iron-binding center. Gene 2007, 396, 28–38. [Google Scholar] [CrossRef]
- Salakij, C.; Salakij, J.; Apibal, S.; Narkkong, N.-A.; Chanhome, L.; Rochanapat, N. Hematology, Morphology, Cytochemical Staining, and Ultrastructural Characteristics of Blood Cells in King Cobras (Ophiophagus hannah). Vet. Clin. Pathol. 2002, 31, 116–126. [Google Scholar] [CrossRef]
- Salakij, C.; Salakij, J.; Prihirunkit, K.; Narkkong, N.-A.; Sanyathitiseree, P.; Kranjanapitukkul, K. Quantitative and qualitative morphologic, cytochemical, and ultrastructural characteristics of blood cells in captive Asian water monitors. Vet. Clin. Pathol. 2014, 43, 538–546. [Google Scholar] [CrossRef]
- Petrie-Hanson, L.; Ainsworth, A.J. Differential cytochemical staining characteristics of channel catfish leukocytes identify cell populations in lymphoid organs. Vet. Immunol. Immunopathol. 2000, 73, 129–144. [Google Scholar] [CrossRef]
- Shigdar, S.; Harford, A.; Ward, A.C. Cytochemical characterisation of the leucocytes and thrombocytes from Murray cod (Maccullochella peelii peelii, Mitchell). Fish Shellfish Immunol. 2009, 26, 731–736. [Google Scholar] [CrossRef]
- Casadei, E.; Wang, T.; Zou, J.; González Vecino, J.L.; Wadsworth, S.; Secombes, C.J. Characterization of three novel β-defensin antimicrobial peptides in rainbow trout (Oncorhynchus mykiss). Mol. Immunol. 2009, 46, 3358–3366. [Google Scholar] [CrossRef]
- Van Hoek, M. Antimicrobial Peptides in Reptiles. Pharmaceuticals 2014, 7, 723–753. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, O.; Grötzinger, J.; Cascorbi, I.; Jung, S. Antimicrobial peptides and proteins of the horse - insights into a well-armed organism. Vet. Res. 2011, 42, 98. [Google Scholar] [CrossRef] [PubMed]
- Linde, A.; Ross, C.R.; Davis, E.G.; Dib, L.; Blecha, F.; Melgarejo, T. Innate Immunity and Host Defense Peptides in Veterinary Medicine. J. Vet. Intern. Med. 2008, 22, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Boman, H.G. Antibacterial peptides: Basic facts and emerging concepts. J. Intern. Med. 2003, 254, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Yount, N.Y.; Yuan, J.; Tarver, A.; Castro, T.; Diamond, G.; Tran, P.A.; Levy, J.N.; McCullough, C.; Cullor, J.S.; Bevins, C.L.; et al. Cloning and Expression of Bovine Neutrophil β-Defensins. J. Biol. Chem. 1999, 274, 26249–26258. [Google Scholar] [CrossRef] [PubMed]
- Selsted, M.E.; Ouellette, A.J. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 2005, 6, 551–557. [Google Scholar] [CrossRef]
- Scapinello, S.; Brooks, A.S.; MacInnes, J.I.; Hammermueller, J.; Clark, M.E.; Caswell, J.L. Bactericidal activity of porcine neutrophil secretions. Vet. Immunol. Immunopathol. 2011, 139, 113–118. [Google Scholar] [CrossRef]
- Leonard, B.C.; Affolter, V.K.; Bevins, C.L. Antimicrobial peptides: Agents of border protection for companion animals. Vet. Dermatol. 2012, 23, 177-e36. [Google Scholar] [CrossRef]
- Lennartsson, A.; Pieters, K.; Vidovic, K.; Gullberg, U. A murine antibacterial ortholog to human bactericidal/permeability-increasing protein (BPI) is expressed in testis, epididymis, and bone marrow. J. Leukoc. Biol. 2005, 77, 369–377. [Google Scholar] [CrossRef]
- Puttabyatappa, M.; Jacot, T.A.; Al-Alem, L.F.; Rosewell, K.L.; Duffy, D.M.; Brännström, M.; Curry, T.E. Ovarian Membrane-Type Matrix Metalloproteinases: Induction of MMP14 and MMP16 During the Periovulatory Period in the Rat, Macaque, and Human1. Biol. Reprod. 2014, 91, 1–12. [Google Scholar] [CrossRef]
- Clegg, P.D.; Burke, R.M.; Coughlan, A.R.; Riggs, C.M.; Carter, S.D. Characterisation of equine matrix metalloproteinase 2 and 9; and identification of the cellular sources of these enzymes in joints. Equine Vet. J. 1997, 29, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, X.; Ma, S. Secretion of 92kDa gelatinase (MMP-9) by bovine neutrophils. Vet. Immunol. Immunopathol. 1999, 67, 247–258. [Google Scholar] [CrossRef]
- Murphy, G.; Ward, R.; Hembry, R.M.; Reynolds, J.J.; Kühn, K.; Tryggvason, K. Characterization of gelatinase from pig polymorphonuclear leucocytes. A metalloproteinase resembling tumour type IV collagenase. Biochem. J. 1989, 258, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Fermín, M.L.; Gaitán, S.; Fragío, C.; Léon, L.G.; Ostronoff, L.K.; Kremmer, E.; Kolb, H.J.; Tejero, C. Canine Long-Term Bone Marrow Culture Neutrophil Production and Functionality. Acta Haematol. 2004, 111, 196–204. [Google Scholar] [CrossRef]
- Rath, N.; Huff, G.; Balog, J.; Huff, W. Fluorescein isothiocyanate staining and characterization of avian heterophils. Vet. Immunol. Immunopathol. 1998, 64, 83–95. [Google Scholar] [CrossRef]
- Pijanowski, L.; Golbach, L.; Kolaczkowska, E.; Scheer, M.; Verburg-van Kemenade, B.M.L.; Chadzinska, M. Carp neutrophilic granulocytes form extracellular traps via ROS-dependent and independent pathways. Fish. Shellfish Immunol. 2013, 34, 1244–1252. [Google Scholar] [CrossRef]
- Lippolis, J.D.; Reinhardt, T.A. Proteomic survey of bovine neutrophils. Vet. Immunol. Immunopathol. 2005, 103, 53–65. [Google Scholar] [CrossRef]
- Barliya, T.; Dardik, R.; Nisgav, Y.; Dachbash, M.; Gaton, D.; Kenet, G.; Ehrlich, R.; Weinberger, D.; Livnat, T. Possible involvement of NETosis in inflammatory processes in the eye: Evidence from a small cohort of patients. Mol. Vis. 2017, 23, 922–932. [Google Scholar]
- Boettcher, M.; Meier, D.; Jiménez-Alcázar, M.; Eschenburg, G.; Mietzsch, S.; Vincent, D.; Klinke, M.; Trochimiuk, M.; Appl, B.; Tiemann, B.; et al. Degradation of Extracellular DNA by DNase1 Significantly Reduces Testicular Damage after Testicular Torsion in Rats. Urology 2017, 109, 223.e1–223.e7. [Google Scholar] [CrossRef]
- De la Rebière de Pouyade, G.; Serteyn, D.; Deby-Dupont, G.; Franck, T. Method for co-purification of equine neutrophil elastase and myeloperoxidase from a limited blood volume. Res. Vet. Sci. 2009, 87, 358–363. [Google Scholar] [CrossRef]
- Maunder, C.L.; Reynolds, Z.F.; Peacock, L.; Hall, E.J.; Day, M.J.; Cogan, T.A. Campylobacter Species and Neutrophilic Inflammatory Bowel Disease in Cats. J. Vet. Intern. Med. 2016, 30, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Chuammitri, P.; Ostojić, J.; Andreasen, C.B.; Redmond, S.B.; Lamont, S.J.; Palić, D. Chicken heterophil extracellular traps (HETs): Novel defense mechanism of chicken heterophils. Vet. Immunol. Immunopathol. 2009, 129, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Wernersson, S.; Reimer, J.M.; Poorafshar, M.; Karlson, U.; Wermenstam, N.; Bengtén, E.; Wilson, M.; Pilström, L.; Hellman, L. Granzyme-like sequences in bony fish shed light on the emergence of hematopoietic serine proteases during vertebrate evolution. Dev. Comp. Immunol. 2006, 30, 901–918. [Google Scholar] [CrossRef] [PubMed]
- Borregaard, N.; Cowland, J.B. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 1997, 89, 3503–3521. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Horiuchi, Y.; Tezuka, T. Presence of bactericidal/permeability-increasing protein in human and rat skin. Exp. Dermatol. 2004, 13, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, L. A Teleost Bactericidal Permeability-Increasing Protein Kills Gram-Negative Bacteria, Modulates Innate Immune Response, and Enhances Resistance against Bacterial and Viral Infection. PLoS ONE 2016, 11, e0154045. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zheng, J.; Xiao, G. Effect of porcine bactericidal/permeability-increasing protein on Gram-negative bacteria. Chin. J. Infect. Dis. 1992, 17, 47–48. [Google Scholar]
- VGNC Vertebrate Gene Nomenclature Committee. Available online: https://vertebrate.genenames.org/data/gene-symbol-report/#!/vgnc_id/VGNC:49265 (accessed on 18 May 2020).
- Chiang, S.-C.; Veldhuizen, E.J.A.; Barnes, F.A.; Craven, C.J.; Haagsman, H.P.; Bingle, C.D. Identification and characterisation of the BPI/LBP/PLUNC-like gene repertoire in chickens reveals the absence of a LBP gene. Dev. Comp. Immunol. 2011, 35, 285–295. [Google Scholar] [CrossRef]
- Azevedo, A.; Lunardi, L.O. Cytochemical characterization of eosinophilic leukocytes circulating in the blood of the turtle (Chrysemys dorbignih). Acta Histochem. 2003, 105, 99–105. [Google Scholar] [CrossRef]
- Brandley, M.C.; Young, R.L.; Warren, D.L.; Thompson, M.B.; Wagner, G.P. Uterine Gene Expression in the Live-Bearing Lizard, Chalcides ocellatus, Reveals Convergence of Squamate Reptile and Mammalian Pregnancy Mechanisms. Genome Biol. Evol. 2012, 4, 394–411. [Google Scholar] [CrossRef]
- Leonard, B.C.; Chu, H.; Johns, J.L.; Gallo, R.L.; Moore, P.F.; Marks, S.L.; Bevins, C.L. Expression and Activity of a Novel Cathelicidin from Domestic Cats. PLoS ONE 2011, 6, e18756. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M. The role of cathelicidins in the innate host defenses of mammals. Curr. Issues Mol. Biol. 2005, 7, 179–196. [Google Scholar] [PubMed]
- Chang, C.-I.; Pleguezuelos, O.; Zhang, Y.-A.; Zou, J.; Secombes, C.J. Identification of a Novel Cathelicidin Gene in the Rainbow Trout, Oncorhynchus mykiss. Infect. Immun. 2005, 73, 5053–5064. [Google Scholar] [CrossRef] [PubMed]
- Skerlavaj, B.; Scocchi, M.; Gennaro, R.; Risso, A.; Zanetti, M. Structural and Functional Analysis of Horse Cathelicidin Peptides. Antimicrob. Agents Chemother. 2001, 45, 715–722. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xie, F.; Zan, Y.; Zhang, X.; Zhang, H.; Jin, M.; Zhang, W.; Zhang, Y.; Liu, S. Differential Abilities of Mammalian Cathelicidins to Inhibit Bacterial Biofilm Formation and Promote Multifaceted Immune Functions of Neutrophils. Int. J. Mol. Sci. 2020, 21, 1871. [Google Scholar] [CrossRef]
- Bassel, L.L.; Caswell, J.L. Bovine neutrophils in health and disease. Cell Tissue Res. 2018, 371, 617–637. [Google Scholar] [CrossRef]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014, 9, 181–218. [Google Scholar] [CrossRef]
- Ribeiro, C.; Brehélin, M. Insect haemocytes: What type of cell is that? J. Insect Physiol. 2006, 52, 417–429. [Google Scholar] [CrossRef]
- DeVries, M.E.; Kelvin, A.A.; Xu, L.; Ran, L.; Robinson, J.; Kelvin, D.J. Defining the origins and evolution of the chemokine/chemokine receptor system. J. Immunol. 2006, 176, 401–415. [Google Scholar] [CrossRef]
- Van der Linden, M.; Meyaard, L. Fine-tuning neutrophil activation: Strategies and consequences. Immunol. Lett. 2016, 178, 3–9. [Google Scholar] [CrossRef]
- Latimer, K.S.; Tang, K.N.; Goodwin, M.A.; Steffens, W.L.; Brown, J. Leukocyte changes associated with acute inflammation in chickens. Avian Dis. 1988, 32, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Harmon, B. Avian heterophils in inflammation and disease resistance. Poult. Sci. 1998, 77, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Manual of Exotic Pet. Practice, 1st ed.; Mitchell, M.A., Tully, T.N.J., Eds.; Elsevier: St. Louis, MO, USA, 2009; ISBN 9781416001195. [Google Scholar]
- Edwards, S.W. Biochemistry and Physiology of the Neutrophil, 1st ed.; Cambridge University Press: New York, NY, USA, 1994; ISBN 0 52141698 1. [Google Scholar]
- Rosales, C. Neutrophils at the crossroads of innate and adaptive immunity. J. Leukoc. Biol. 2020, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rainard, P.; Riollet, C.; Berthon, P.; Cunha, P.; Fromageau, A.; Rossignol, C.; Gilbert, F.B. The chemokine CXCL3 is responsible for the constitutive chemotactic activity of bovine milk for neutrophils. Mol. Immunol. 2008, 45, 4020–4027. [Google Scholar] [CrossRef]
- Brooks, R.L.; Bounous, D.I.; Andreasen, C.B. Functional comparison of avian heterophils with human and canine neutrophils. Comp. Haematol. Int. 1996, 6, 153–159. [Google Scholar] [CrossRef]
- Guido, D.; Demaurex, N.; Nunes, P. Junctate boosts phagocytosis by recruiting endoplasmic reticulum Ca2+ stores near phagosomes. J. Cell Sci. 2015, 128, 4074–4082. [Google Scholar] [CrossRef]
- Paape, M.J.; Bannerman, D.D.; Zhao, X.; Lee, J.-W. The bovine neutrophil: Structure and function in blood and milk. Vet. Res. 2004, 34, 597–627. [Google Scholar]
- Hakkim, A.; Furnrohr, B.G.; Amann, K.; Laube, B.; Abed, U.A.; Brinkmann, V.; Herrmann, M.; Voll, R.E.; Zychlinsky, A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl. Acad. Sci. USA 2010, 107, 9813–9818. [Google Scholar] [CrossRef]
- Giaglis, S.; Hahn, S.; Hasler, P. “The NET Outcome”: Are Neutrophil Extracellular Traps of Any Relevance to the Pathophysiology of Autoimmune Disorders in Childhood? Front. Pediatr. 2016, 4, 1–8. [Google Scholar] [CrossRef]
- Stacy, N.I.; Fredholm, D.V.; Rodriguez, C.; Castro, L.; Harvey, J.W. Whip-like heterophil projections in consecutive blood films from an injured gopher tortoise (Gopherus polyphemus) with systemic inflammation. Vet. Q. 2017, 37, 162–165. [Google Scholar] [CrossRef][Green Version]
- DeCoursey, T.E.; Ligeti, E. Regulation and termination of NADPH oxidase activity. Cell. Mol. Life Sci. 2005, 62, 2173–2193. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive Oxygen Species and Neutrophil Function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.; Dragunow, M.; Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J. Leukoc. Biol. 2012, 92, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.; Winterbourn, C.C. Reactive oxidants and myeloperoxidase and their involvement in neutrophil extracellular traps. Front. Immunol. 2012, 3, 424. [Google Scholar] [CrossRef]
- Penniall, R.; Spitznagel, J.K. Chicken neutrophils: Oxidative metabolism in phagocytic cells devoid of myeloperoxidase. Proc. Natl. Acad. Sci. USA 1975, 72, 5012–5015. [Google Scholar] [CrossRef]
- Stabler, J.G.; McCormick, T.W.; Powell, K.C.; Kogut, M.H. Avian heterophils and monocytes: Phagocytic and bactericidal activities against Salmonella enteritidis. Vet. Microbiol. 1994, 38, 293–305. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef]
- Tung, J.-P.; Fraser, J.F.; Wood, P.; Fung, Y. Respiratory burst function of ovine neutrophils. BMC Immunol. 2009, 10, 25. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A. Neutrophils: Cinderella of innate immune system. Int. Immunopharmacol. 2010, 10, 1325–1334. [Google Scholar] [CrossRef]
- Tsuda, Y.; Takahashi, H.; Kobayashi, M.; Hanafusa, T.; Herndon, D.N.; Suzuki, F. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity 2004, 21, 215–226. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Q.; Chertov, O.; Oppenheim, J.J. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J. Leukoc. Biol. 2000, 68, 9–14. [Google Scholar] [PubMed]
- Cassatella, M.A. The production of cytokines by polymorphonuclear neutrophils. Immunol. Today 1995, 16, 21–26. [Google Scholar] [CrossRef]
- Tazzyman, S.; Niaz, H.; Murdoch, C. Neutrophil-mediated tumour angiogenesis: Subversion of immune responses to promote tumour growth. Semin. Cancer Biol. 2013, 23, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Tecchio, C.; Cassatella, M.A. Neutrophil-derived cytokines involved in physiological and pathological angiogenesis. Chem. Immunol. Allergy 2014, 99, 123–137. [Google Scholar] [PubMed]
- Nomiyama, H.; Osada, N.; Yoshie, O. The evolution of mammalian chemokine genes. Cytokine Growth Factor Rev. 2010, 21, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Tecchio, C.; Cassatella, M.A. Neutrophil-derived chemokines on the road to immunity. Semin. Immunol. 2016, 28, 119–128. [Google Scholar] [CrossRef]
- Nomiyama, H.; Osada, N.; Yoshie, O. Systematic classification of vertebrate chemokines based on conserved synteny and evolutionary history. Genes to Cells 2013, 18, 1–16. [Google Scholar] [CrossRef]
- Nomiyama, H.; Osada, N.; Yoshie, O. A family tree of vertebrate chemokine receptors for a unified nomenclature. Dev. Comp. Immunol. 2011, 35, 705–715. [Google Scholar] [CrossRef]
- Lahoz-Beneytez, J.; Elemans, M.; Zhang, Y.; Ahmed, R.; Salam, A.; Block, M.; Niederalt, C.; Asquith, B.; Macallan, D. Human neutrophil kinetics: Modeling of stable isotope labeling data supports short blood neutrophil half-lives. Blood 2016, 127, 3431–3438. [Google Scholar] [CrossRef]
- Tak, T.; Tesselaar, K.; Pillay, J.; Borghans, J.A.M.; Koenderman, L. Whatˈs your age again? Determination of human neutrophil half-lives revisited. J. Leukoc. Biol. 2013, 94, 595–601. [Google Scholar] [CrossRef]
- Pillay, J.; den Braber, I.; Vrisekoop, N.; Kwast, L.M.; de Boer, R.J.; Borghans, J.A.M.; Tesselaar, K.; Koenderman, L. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 2010, 116, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Tofts, P.S.; Chevassut, T.; Cutajar, M.; Dowell, N.G.; Peters, A.M. Doubts concerning the recently reported human neutrophil lifespan of 5.4 days. Blood 2011, 117, 6050–6052. [Google Scholar] [CrossRef] [PubMed]
- Liew, P.X.; Kubes, P. The Neutrophil’s Role During Health and Disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef] [PubMed]
- Carlyon, J.A.; Fikrig, E. Mechanisms of evasion of neutrophil killing by Anaplasma phagocytophilum. Curr. Opin. Hematol. 2006, 13, 28–33. [Google Scholar] [CrossRef]
- Greenlee-Wacker, M.C. Clearance of apoptotic neutrophils and resolution of inflammation. Immunol. Rev. 2016, 273, 357–370. [Google Scholar] [CrossRef]
- Mathias, J.R.; Perrin, B.J.; Liu, T.; Kanki, J.; Look, A.T.; Huttenlocher, A. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J. Leukoc. Biol. 2006, 80, 1281–1288. [Google Scholar] [CrossRef]
- Montali, R.J. Comparative pathology of inflammation in the higher vertebrates (reptiles, birds and mammals). J. Comp. Pathol. 1988, 99, 1–26. [Google Scholar] [CrossRef]
- Van Rees, D.J.; Szilagyi, K.; Kuijpers, T.W.; Matlung, H.L.; van den Berg, T.K. Immunoreceptors on neutrophils. Semin. Immunol. 2016, 28, 94–108. [Google Scholar] [CrossRef]
- Dumler, J.S.; Barbet, A.F.; Bekker, C.P.J.; Dasch, G.A.; Palmer, G.H.; Ray, S.C.; Rikihisa, Y.; Rurangirwa, F.R. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combi. Int. J. Syst. Evol. Microbiol. 2001, 51, 2145–2165. [Google Scholar] [CrossRef]
- Woldehiwet, Z. The natural history of Anaplasma phagocytophilum. Vet. Parasitol. 2010, 167, 108–122. [Google Scholar] [CrossRef]
- Dumler, J.S.; Choi, K.-S.; Garcia-Garcia, J.C.; Barat, N.S.; Scorpio, D.G.; Garyu, J.W.; Grab, D.J.; Bakken, J.S. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg. Infect. Dis. 2005, 11, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Rajeeve, K.; Das, S.; Prusty, B.K.; Rudel, T. Chlamydia trachomatis paralyses neutrophils to evade the host innate immune response. Nat. Microbiol. 2018, 3, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Ammon, A.; Autenrieth, I. Medizinische Mikrobiologie und Infektiologie, 7th ed.; Suerbaum, S., Hahn, H., Burchard, G.-D., Kaufmann, S.H.E., Schulz, T.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-24166-6. [Google Scholar]
- Laskay, T.; van Zandbergen, G.; Solbach, W. Neutrophil granulocytes as host cells and transport vehicles for intracellular pathogens: Apoptosis as infection-promoting factor. Immunobiology 2008, 213, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Jiménez, C.; Mora-Cartín, R.; Altamirano-Silva, P.; Chacón-Díaz, C.; Chaves-Olarte, E.; Moreno, E.; Barquero-Calvo, E. Neutrophils as Trojan Horse Vehicles for Brucella abortus Macrophage Infection. Front. Immunol. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Sipes, K.M.; Edens, H.A.; Kehrli, M.E.; Miettinen, H.M.; Cutler, J.E.; Jutila, M.A.; Quinn, M.T. Analysis of surface antigen expression and host defense function in leukocytes from calves heterozygous or homozygous for bovine leukocyte adhesion deficiency. Am. J. Vet. Res. 1999, 60, 1255–1261. [Google Scholar]
- Kehrli, M.E.; Schmalstieg, F.C.; Anderson, D.C.; Van der Maaten, M.J.; Hughes, B.J.; Ackermann, M.R.; Wilhelmsen, C.L.; Brown, G.B.; Stevens, M.G.; Whetstone, C.A. Molecular definition of the bovine granulocytopathy syndrome: Identification of deficiency of the Mac-1 (CD11b/CD18) glycoprotein. Am. J. Vet. Res. 1990, 51, 1826–1836. [Google Scholar]
- Masucci, M.T.; Minopoli, M.; Carriero, M.V. Tumor Associated Neutrophils. Their Role in Tumorigenesis, Metastasis, Prognosis and Therapy. Front. Oncol. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Sagiv, J.Y.; Michaeli, J.; Assi, S.; Mishalian, I.; Kisos, H.; Levy, L.; Damti, P.; Lumbroso, D.; Polyansky, L.; Sionov, R.V.; et al. Phenotypic Diversity and Plasticity in Circulating Neutrophil Subpopulations in Cancer. Cell Rep. 2015, 10, 562–573. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Romano, A.; Parrinello, N.L.; Simeon, V.; Puglisi, F.; La Cava, P.; Bellofiore, C.; Giallongo, C.; Camiolo, G.; D’Auria, F.; Grieco, V.; et al. High-density neutrophils in MGUS and multiple myeloma are dysfunctional and immune-suppressive due to increased STAT3 downstream signaling. Sci. Rep. 2020, 10, 1983. [Google Scholar] [CrossRef]
- Condamine, T.; Dominguez, G.A.; Youn, J.-I.; Kossenkov, A.V.; Mony, S.; Alicea-Torres, K.; Tcyganov, E.; Hashimoto, A.; Nefedova, Y.; Lin, C.; et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci. Immunol. 2016, 1, aaf8943. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.; Botta, C.; Zabaleta, A.; Puig, N.; Cedena, M.-T.; Goicoechea, I.; Alameda, D.; San-José Enériz, E.; Merino, J.; Rodriguez-Otero, P.; et al. Immunogenomic identification and characterization of granulocytic myeloid derived suppressor cells in multiple myeloma. Blood 2020. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-F.; Chen, D.-P.; Ouyang, F.-Z.; Chen, M.-M.; Wu, Y.; Kuang, D.-M.; Zheng, L. Increased autophagy sustains the survival and pro-tumourigenic effects of neutrophils in human hepatocellular carcinoma. J. Hepatol. 2015, 62, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, F.; Parrinello, N.L.; Giallongo, C.; Cambria, D.; Camiolo, G.; Bellofiore, C.; Conticello, C.; Del Fabro, V.; Leotta, V.; Markovic, U.; et al. Plasticity of High-Density Neutrophils in Multiple Myeloma is Associated with Increased Autophagy via STAT3. Int. J. Mol. Sci. 2019, 20, 3548. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of Tumor-Associated Neutrophil Phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Knaapen, A.M. Neutrophils and respiratory tract DNA damage and mutagenesis: A review. Mutagenesis 2006, 21, 225–236. [Google Scholar] [CrossRef]
- Cools-Lartigue, J.; Spicer, J.; McDonald, B.; Gowing, S.; Chow, S.; Giannias, B.; Bourdeau, F.; Kubes, P.; Ferri, L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Investig. 2013, 123, 3446–3458. [Google Scholar] [CrossRef]
- Nolan, E.; Malanchi, I. Neutrophil ‘safety net’ causes cancer cells to metastasize and proliferate. Nature 2020. [Google Scholar] [CrossRef]
- Thålin, C.; Demers, M.; Blomgren, B.; Wong, S.L.; von Arbin, M.; von Heijne, A.; Laska, A.C.; Wallén, H.; Wagner, D.D.; Aspberg, S. NETosis promotes cancer-associated arterial microthrombosis presenting as ischemic stroke with troponin elevation. Thromb. Res. 2016, 139, 56–64. [Google Scholar] [CrossRef]
- Demers, M.; Krause, D.S.; Schatzberg, D.; Martinod, K.; Voorhees, J.R.; Fuchs, T.A.; Scadden, D.T.; Wagner, D.D. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl. Acad. Sci. USA 2012, 109, 13076–13081. [Google Scholar] [CrossRef]
- Calì, B.; Molon, B.; Viola, A. Tuning cancer fate: The unremitting role of host immunity. Open Biol. 2017, 7, 170006. [Google Scholar] [CrossRef] [PubMed]
- Michaeli, J.; Shaul, M.E.; Mishalian, I.; Hovav, A.-H.; Levy, L.; Zolotriov, L.; Granot, Z.; Fridlender, Z.G. Tumor-associated neutrophils induce apoptosis of non-activated CD8 T-cells in a TNFα and NO-dependent mechanism, promoting a tumor-supportive environment. Oncoimmunology 2017, 6, e1356965. [Google Scholar] [CrossRef] [PubMed]
- Ponzetta, A.; Carriero, R.; Carnevale, S.; Barbagallo, M.; Molgora, M.; Perucchini, C.; Magrini, E.; Gianni, F.; Kunderfranco, P.; Polentarutti, N.; et al. Neutrophils Driving Unconventional T Cells Mediate Resistance against Murine Sarcomas and Selected Human Tumors. Cell 2019, 178, 346–360.e24. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.M.; Hutton, S.; Myers, G.S.A.; Brunham, R.; Timms, P. Chlamydia pneumoniae Is Genetically Diverse in Animals and Appears to Have Crossed the Host Barrier to Humans on (At Least) Two Occasions. PLoS Pathog. 2010, 6, e1000903. [Google Scholar] [CrossRef] [PubMed]
- Hostetter, S.J. Neutrophil Function in Small Animals. Vet. Clin. N. Am. Small Anim. Pract. 2012, 42, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Alroy, J.; Freden, G.O.; Goyal, V.; Raghavan, S.S.; Schunk, K.L. Morphology of Leukocytes from Cats Affected with α-Mannosidosis and Mucopolysaccharidosis VI (MPS VI). Vet. Pathol. 1989, 26, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Chusid, M.J.; Bujak, J.S.; Dale, D.C. Defective polymorphonuclear leukocyte metabolism and function in canine cyclic neutropenia. Blood 1975, 46, 921–930. [Google Scholar] [CrossRef]
- Horwitz, M.; Benson, K.F.; Duan, Z.; Li, F.-Q.; Person, R.E. Hereditary neutropenia: Dogs explain human neutrophil elastase mutations. Trends Mol. Med. 2004, 10, 163–170. [Google Scholar] [CrossRef]
- Torikoshi, Y.; Yokota, A.; Kamio, N.; Sato, A.; Shouji, T.; Kashiwagi, T.; Miura, Y.; Maekawa, T.; Hirai, H. Impact of Hypothermia on Differentiation and Maturation of Neutrophils. Blood 2018, 132, 2393. [Google Scholar] [CrossRef]
- Capitano, M.L.; Nemeth, M.J.; Mace, T.A.; Salisbury-Ruf, C.; Segal, B.H.; McCarthy, P.L.; Repasky, E.A. Elevating body temperature enhances hematopoiesis and neutrophil recovery after total body irradiation in an IL-1–, IL-17–, and G-CSF–dependent manner. Blood 2012, 120, 2600–2609. [Google Scholar] [CrossRef]
- Pryde, J.G.; Walker, A.; Rossi, A.G.; Hannah, S.; Haslett, C. Temperature-dependent Arrest of Neutrophil Apoptosis. J. Biol. Chem. 2000, 275, 33574–33584. [Google Scholar] [CrossRef] [PubMed]
- Díaz, F.E.; Dantas, E.; Cabrera, M.; Benítez, C.A.; Delpino, M.V.; Duette, G.; Rubione, J.; Sanjuan, N.; Trevani, A.S.; Geffner, J. Fever-range hyperthermia improves the anti-apoptotic effect induced by low pH on human neutrophils promoting a proangiogenic profile. Cell Death Dis. 2016, 7, e2437. [Google Scholar] [CrossRef] [PubMed]
- Salanova, B.; Choi, M.; Rolle, S.; Wellner, M.; Scheidereit, C.; Luft, F.C.; Kettritz, R. The effect of fever-like temperatures on neutrophil signaling. FASEB J. 2005, 19, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Rosenspire, A.J.; Kindzelskii, A.L.; Petty, H.R. Cutting Edge: Fever-Associated Temperatures Enhance Neutrophil Responses to Lipopolysaccharide: A Potential Mechanism Involving Cell Metabolism. J. Immunol. 2002, 169, 5396–5400. [Google Scholar] [CrossRef] [PubMed]
- Kawata, J.; Kikuchi, M.; Saitoh, H. Genomic DNAs in a human leukemia cell line unfold after cold shock, with formation of neutrophil extracellular trap-like structures. Biotechnol. Lett. 2014, 36, 241–250. [Google Scholar] [CrossRef]
- Neubert, E.; Meyer, D.; Rocca, F.; Günay, G.; Kwaczala-Tessmann, A.; Grandke, J.; Senger-Sander, S.; Geisler, C.; Egner, A.; Schön, M.P.; et al. Chromatin swelling drives neutrophil extracellular trap release. Nat. Commun. 2018, 9, 3767. [Google Scholar] [CrossRef]
- Bouma, H.R.; Dugbartey, G.J.; Boerema, A.S.; Talaei, F.; Herwig, A.; Goris, M.; van Buiten, A.; Strijkstra, A.M.; Carey, H.V.; Henning, R.H.; et al. Reduction of body temperature governs neutrophil retention in hibernating and nonhibernating animals by margination. J. Leukoc. Biol. 2013, 94, 431–437. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Bjørløw, I.; Hagland, T.J.; Wergeland, H.I. Effect of seawater temperature on leucocyte populations in Atlantic salmon post-smolts. Vet. Immunol. Immunopathol. 2005, 106, 65–76. [Google Scholar] [CrossRef]
- Engelsma, M. Multiple acute temperature stress affects leucocyte populations and antibody responses in common carp, Cyprinus carpio L. Fish. Shellfish Immunol. 2003, 15, 397–410. [Google Scholar] [CrossRef]
- Langston, A.L.; Hoare, R.; Stefansson, M.; Fitzgerald, R.; Wergeland, H.; Mulcahy, M. The effect of temperature on non-specific defence parameters of three strains of juvenile Atlantic halibut (Hippoglossus hippoglossus L.). Fish. Shellfish Immunol. 2002, 12, 61–76. [Google Scholar] [CrossRef]
- Dalton, W.T.; Ahearn, M.J.; McCredie, K.B.; Freireich, E.J.; Stass, S.A.; Trujillo, J.M. HL-60 cell line was derived from a patient with FAB-M2 and not FAB-M3. Blood 1988, 71, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Parmley, R.T.; Akin, D.T.; Barton, J.C.; Gilbert, C.S.; Kinkade, J.M. Cytochemistry and ultrastructural morphometry of cultured HL60 myeloid leukemia cells. Cancer Res. 1987, 47, 4932–4940. [Google Scholar] [PubMed]
- Collins, S. The HL-60 promyelocytic leukemia cell line: Proliferation, differentiation, and cellular oncogene expression. Blood 1987, 70, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Rincón, E.; Rocha-Gregg, B.L.; Collins, S.R. A map of gene expression in neutrophil-like cell lines. BMC Genomics 2018, 19, 1–17. [Google Scholar] [CrossRef]
- Gupta, D.; Shah, H.P.; Malu, K.; Berliner, N.; Gaines, P. Differentiation and Characterization of Myeloid Cells. Curr. Protoc. Immunol. 2014, 104, 1–28. [Google Scholar] [CrossRef]
- Gaines, P.; Chi, J.; Berliner, N. Heterogeneity of functional responses in differentiated myeloid cell lines reveals EPRO cells as a valid model of murine neutrophil functional activation. J. Leukoc. Biol. 2005, 77, 669–679. [Google Scholar] [CrossRef]
- Yaseen, R.; Blodkamp, S.; Lüthje, P.; Reuner, F.; Völlger, L.; Naim, H.Y.; von Köckritz-Blickwede, M. Antimicrobial activity of HL-60 cells compared to primary blood-derived neutrophils against Staphylococcus aureus. J. Negat. Results Biomed. 2017, 16, 2. [Google Scholar] [CrossRef]
- American Type Culture Collection (ATCC). Available online: https://www.lgcstandards-atcc.org/products/all/CCL-240.aspx?geo_country=de#generalinformation (accessed on 9 June 2020).
- DSMZ-German Collection of Microorganisms and Cell Cultures GmbH. Available online: https://www.dsmz.de/collection/catalogue/human-and-animal-cell-lines/catalogue (accessed on 9 June 2020).
- An, L.-L.; Ma, X.-T.; Yang, Y.-H.; Lin, Y.-M.; Song, Y.-H.; Wu, K.-F. Marked Reduction of LL-37/hCAP-18, an Antimicrobial Peptide, in Patients with Acute Myeloid Leukemia. Int. J. Hematol. 2005, 81, 45–47. [Google Scholar] [CrossRef]
- Rennoll-Bankert, K.E.; Sinclair, S.H.; Lichay, M.A.; Dumler, J.S. Comparison and characterization of granulocyte cell models for Anaplasma phagocytophilum infection. Pathog. Dis. 2014, 71, 55–64. [Google Scholar] [CrossRef][Green Version]
- Lanotte, M.; Martin-Thouvenin, V.; Najman, S.; Balerini, P.; Valensi, F.; Berger, R. NB4, a maturation inducible cell line with t(15;17) marker isolated from a human acute promyelocytic leukemia (M3). Blood 1991, 77, 1080–1086. [Google Scholar] [CrossRef]
- Tucker, K.A.; Lilly, M.B.; Heck, L.; Rado, T.A. Characterization of a new human diploid myeloid leukemia cell line (PLB-985) with granulocytic and monocytic differentiating capacity. Blood 1987, 70, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Pedruzzi, E.; Fay, M.; Elbim, C.; Gaudry, M.; Gougerot-Pocidalo, M.-A. Differentiation of PLB-985 myeloid cells into mature neutrophils, shown by degranulation of terminally differentiated compartments in response to N-formyl peptide and priming of superoxide anion production by granulocyte-macrophage colony-stimulating fact. Br. J. Haematol. 2002, 117, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Skoge, M.; Wong, E.; Hamza, B.; Bae, A.; Martel, J.; Kataria, R.; Keizer-Gunnink, I.; Kortholt, A.; Van Haastert, P.J.M.; Charras, G.; et al. A Worldwide Competition to Compare the Speed and Chemotactic Accuracy of Neutrophil-Like Cells. PLoS ONE 2016, 11, e0154491. [Google Scholar] [CrossRef] [PubMed]
- Graham-Pole, J.; Davie, M.; Willoughby, M.L.N. Cryopreservation of human granulocytes in liquid nitrogen. J. Clin. Pathol. 1977, 30, 758–762. [Google Scholar] [CrossRef]
- Hill, R.S.; Mackinder, C.; Challinor, S.; Postlewaight, B. Recovery of functional capacity of human granulocytes after freeze-preservation with dimethyl sulphoxide. Cryobiology 1979, 16, 105–111. [Google Scholar] [CrossRef]
- Frim, J.; Mazur, P. Approaches to the preservation of human granulocytes by freezing. Cryobiology 1980, 17, 282–286. [Google Scholar] [CrossRef][Green Version]
- Svedentsov, E.P.; Stepanova, E.S.; Tumanova, T.V.; Shcheglova, O.O.; Devetyarova, O.N.; Utyomov, S.V. Effect of cryohypobiosis on conservation of human blood leukocytes. Cryo Letters 2005, 26, 387–394. [Google Scholar]
- Zaitseva, O.O.; Polezhaeva, T.V.; Khudyakov, A.N.; Solomina, O.N.; Laptev, D.S.; Svedentsov, E.P.; Utemov, S.V.; Kostyaev, A.A. Influence of pectins on NADPH oxidase and phagocytic activity of neutrophils during cryopreservation. Cryo Letters 2013, 34, 544–548. [Google Scholar]
- Zerbe, H.; Castilho, L.F.F.; Engelke, F.; Mattos, R.C.; Schuberth, H.-J.; Klug, E.; Leibold, W. Isolation and Cryopreservation of Functionally Competent Equine Leucocytes. J. Vet. Med. Ser. A 2003, 50, 179–184. [Google Scholar] [CrossRef]
- Price, T.H.; Dale, D.C. Neutrophil preservation: The effect of short-term storage on in vivo kinetics. J. Clin. Investig. 1977, 59, 475–480. [Google Scholar] [CrossRef]
- Voetman, A.A.; Bot, A.A.; Roos, D. Cryopreservation of enucleated human neutrophils (PMN cytoplasts). Blood 1984, 63, 234–237. [Google Scholar] [CrossRef] [PubMed]


| Species | Leukocytes 1 | Neutrophils/Heterophils 1 | Neutrophils/Heterophils 2 | Band Neutrophils/Heterophils 1 | Segmented Neutrophils/Heterophils 1 | References |
|---|---|---|---|---|---|---|
| Human | 3–11 | 1.71–8.25 | 57–75 | 0.5–0.77 | 1.35–8.1 | [38,46] |
| Mouse | 2–12 | 0.4–3.6 | 20–30 | − | − | [1,38] |
| Rat | 2–25 | 2.4–9.5 | 12–38 | − | − | [1,38] |
| Horse | 3.5–12.1 | 1.58–8.47 | 45–70 | <0.6 | 1.6–8.5 | [38,41,47] |
| Cattle | 4–13.3 | 0.6–6.65 | 15–50 | 0–0.2 | 0.6–6 | [1,38,47] |
| Pig | 10–22 | 1–10.34 | 10–47 | 0–0.88 | 2–15 | [1,38,47] |
| Dog | 5–17 | 2.75–14.45 | 55–85 | 0–0.45 | 2.9–12 | [1,38,47] |
| Cat | 5.5–19.5 | 2.48–15.21 | 45–78 | 0–0.3 | 2.5–12.5 | [1,38,47] |
| Chicken | 20–30 | 5–15 | 25–50 | − | − | [37,38] |
| Budgerigar | 3–8 | 1.29–5.92 | 43–74 | 0 | 1.3–5.9 | [48] |
| Turtle | 1–14 | 0.21–10.36 | 21–74 | − | − | [30,49,50,51] |
| Snake | 1–50 | 0.02–21 | 2–42 | − | − | [30,52,53,54] |
| Opossum | 3.9–12.6 | 0.55–6.3 | 14–50 | Rare | 0.5–6.3 | [55,56] |
| Fish | 30–100 | 0.9–10 | 3–10 | − | − | [30] |
| Human | Mouse | Rat | Horse | Cattle | Pig | Dog | Cat | Birds | Reptiles | Opossum | Fish | References | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MPO 1 | + | + | + | + | + | + | + | + | − | − | + | +/− | [1,66,99,101,102,106] |
| Lysozyme | + | + | + | + | − | + | + | − | + | + | (+) | (+) | [1,101,107] |
| AP 2 | + | − | + | + | + | + | + | − | − | +/− | (0) | +/− | [1,101,102,108,109] |
| Lactoferrin | + | + | + | + | + | + | + | + | − | − | + | − | [104,110] |
| β-Glucuronidase | + | + | + | + | + | + | + | + | + | + | (0) | +/- | [94,101,105,111,112,113,114] |
| Defensin | + | − | + | − | + | (+) | (+) | (+) | + | +/− | (+) | (+) | [19,94,115,116,117,118,119,120,121,122,123] |
| MP 3 | + | + | + | + | + | + | + | (0) | + | (0) | (0) | + | [94,124,125,126,127,128,129,130,131] |
| Elastase | + | + | + | + | + | + | + | + | + | (0) | + | + | [94,106,122,132,133,134,135,136,137,138] |
| BPI 4 | + | + | + | (+) | + | + | (+) | (+) | (+) | (+) | (0) | + | [124,132,139,140,141,142,143,144,145,146] |
| Cathelicidin | + | + | + | + | + | + | + | + | + | + | (+) | (+) | [19,94,116,117,122,132,147,148,149] |
| Disease | Pathogen/Mutation | Effect on Neutrophil | Clinical Signs | Affected Species | Ref. |
|---|---|---|---|---|---|
| Anaplasmosis | Anaplasma phagocytophilum | Restricted movement, phagocytosis, superoxide production, adherence, transmigration, apoptosis | Unspecific, fever, impaired consciousness, lameness, arthritis, organ and lymph node swelling | Dogs, humans, ruminants, horses, wildlife, rodents, etc. | [192,198,199] |
| Chlamydiosis | Chlamydia trachomatis | Paralysis: no activation, NETosis oxidative burst, reduced cell death | Often asymptomatic, conjunctivitis, genital infection, infertility | Humans | [200,223] |
| Leishmaniosis/Brucellosis/Chlamydiosis | Leishmania major/Brucella abortus/Chlamydia pneumoniae | Trojan horse: induce apoptosis to reach macrophages | Cutaneous nodules and ulcers/abortion, inflammation/pneumonia, arthritis | Humans, rodents, birds, dogs/cattle, humans, other mammals, birds/humans, horses, reptiles, amphibians, marsupials | [104,105,106,109,223] |
| Leukocyte adhesion deficiency | CD18 | Impaired adhesion and phagocytosis, normal morphology | Recurrent infections, sepsis, impaired wound healing, severe neutrophilia, often lethal | Humans, cattle (Holstein), dogs (Irish Setter), mice | [107,108,110] |
| Chédiak-Higashi syndrome | LYST (lysosomal trafficking regulator) | Disturbed formation of phagolysosome, fusion of granules (giant and pink) | Infections, partial oculocutaneous albinism, hemorrhage | Humans, cattle, cats (Persian), mice (beige), arctic foxes, mink, killer whale | [1,32,100,224] |
| Mucopoly-saccharidosis | Enzymes in mucopolysaccharide catabolism | Normal function, accumulation of metabolic byproducts → large azurophilic granules | Bone and cartilage defects, hepatomegaly | Humans, cattle, cats, dogs | [1,225] |
| Chronic granulomatous disease | NADPH oxidase | Defects in respiratory burst | Recurrent severe but not fatal infections, granulomas | Humans, dogs (Doberman Pinscher) | [1,32] |
| MPO deficiency | Myeloperoxidase | Delayed killing | Few clinical signs, recurrent fungal infection | Humans, dogs (Gray Collie) | [32,226] |
| Cyclic hematopoiesis | Neutrophil elastase (human), adaptor protein complex 3 (dog) | Mis-trafficking of neutrophil elastase | Severe cyclic neutropenia, bleeding, recurrent infections, coat color dilution, amyloidosis, often lethal | Humans, dogs | [224,227] |
| Pelger–Huët anomaly | Lamin B receptor | Hypolobulated nucleus | Heterozygote no signs, homozygote: skeletal deformation, susceptible to infections, usually lethal | Humans, horses, dogs, cats, rabbits, mice | [81,92] |
| HL-60 | NB4 | PLB-985 | |
|---|---|---|---|
| Donor | 36-year-old woman | 23-year-old woman | Subclone of HL-60, with some differences in gene expression |
| Disease | Acute myeloblastic leukemia (AML-M2) | Acute promyelocytic leukemia in second relapse (AML-M3) | Acute myeloblastic leukemia (AML-M2) |
| Tissue origin | Peripheral blood | Bone marrow | HL-60 |
| Cell type | Myeloblast | Promyelocyte | Myeloblast |
| Inducible cell types | Monocytes, macrophages, eosinophils, neutrophils | Macrophages, neutrophils | Monocytes, granulocytes |
| Differentiation towards neutrophils with (e.g.) | All-trans retinoic acid, DMSO, dibutyryl cyclic adenosine monophosphate (dbcAMP), hypoxanthine, Nutridoma | All-trans retinoic acid, DMSO, dbcAMP | All-trans retinoic acid, DMSO, dbcAMP, Nutridoma |
| Doubling time | 40 h | 35–45 h | 30 h |
| Cytogenetics | t(8;21) | t(15;17) | − |
| Cathelicidin expression (LL-37) | No | No | Not known |
| Advantages | Inducible respiratory burst, phagocytosis and NET formation (however, all less than in primary neutrophils) | Similar characteristics to HL-60, expression of secondary granules inducible | |
| Disadvantages | No secondary granules, failure in chemotaxis, deficient expression of late neutrophil-specific genes | Similar characteristics to HL-60 | |
| Pathogen interaction (e.g., A. phagocytophilum) | Infection level, reduction in defense gene transcription and oxidative burst similar to PMNs | No sufficient infection | Low infection level, minimal change in defense gene transcription and oxidative burst |
| References | [240,242,243,244,245,247,248,249,250] | [244,245,248,249,250,251] | [243,248,250,252,253] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fingerhut, L.; Dolz, G.; de Buhr, N. What Is the Evolutionary Fingerprint in Neutrophil Granulocytes? Int. J. Mol. Sci. 2020, 21, 4523. https://doi.org/10.3390/ijms21124523
Fingerhut L, Dolz G, de Buhr N. What Is the Evolutionary Fingerprint in Neutrophil Granulocytes? International Journal of Molecular Sciences. 2020; 21(12):4523. https://doi.org/10.3390/ijms21124523
Chicago/Turabian StyleFingerhut, Leonie, Gaby Dolz, and Nicole de Buhr. 2020. "What Is the Evolutionary Fingerprint in Neutrophil Granulocytes?" International Journal of Molecular Sciences 21, no. 12: 4523. https://doi.org/10.3390/ijms21124523
APA StyleFingerhut, L., Dolz, G., & de Buhr, N. (2020). What Is the Evolutionary Fingerprint in Neutrophil Granulocytes? International Journal of Molecular Sciences, 21(12), 4523. https://doi.org/10.3390/ijms21124523





