Angiotensin II, Hypercholesterolemia, and Vascular Smooth Muscle Cells: A Perfect Trio for Vascular Pathology
Abstract
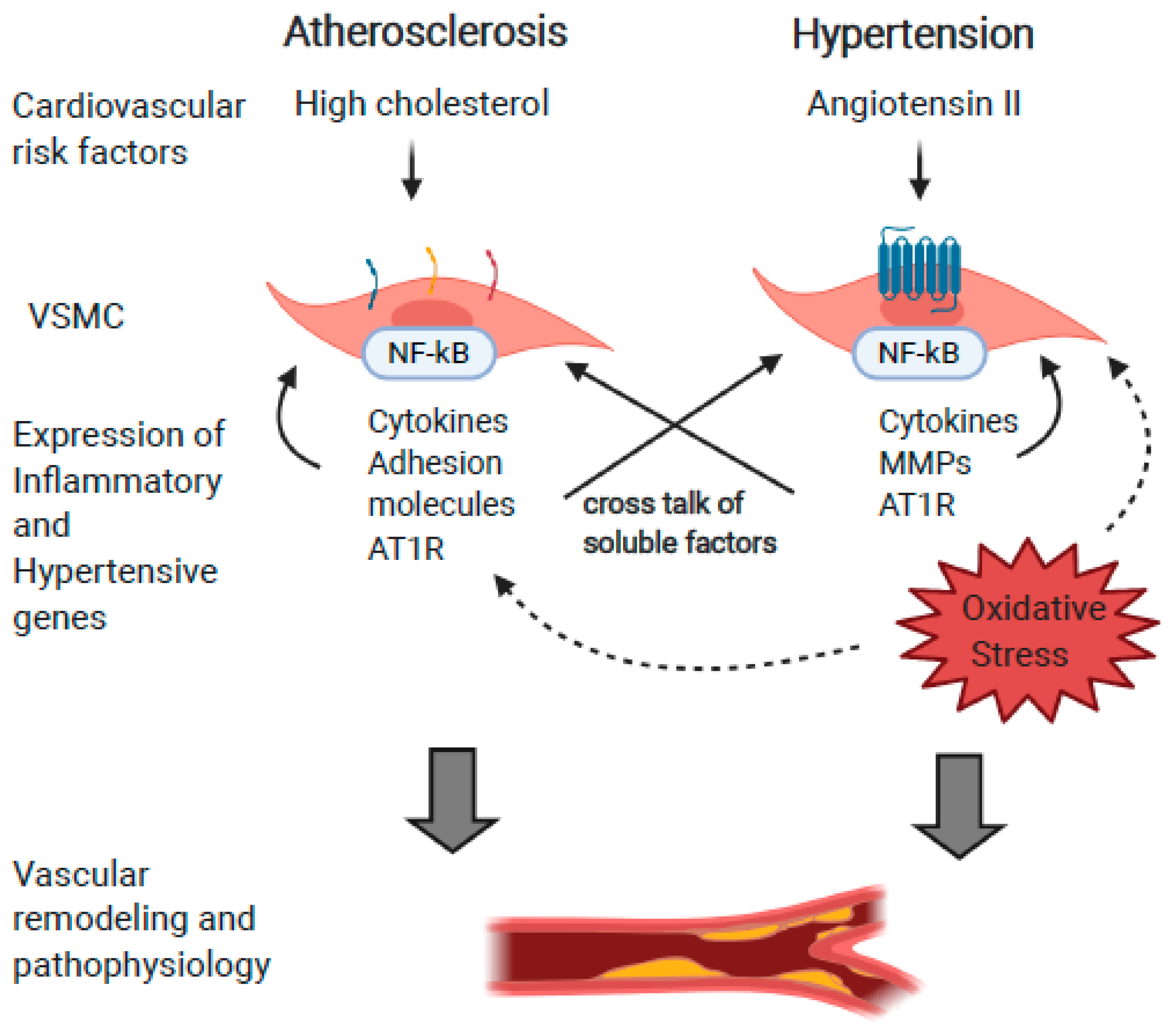
:1. Introduction
1.1. Metabolic Syndrome, Hypertension, and Atherosclerosis
1.2. Vascular Inflammation and Atherosclerosis
1.3. Hypertension and Angiotensin II
2. Relationships between RAS, Inflammation, and Hyperlipidemia
2.1. Atherogenic Stimuli Induce Ang II and Components of the RAS Pathway
2.2. Angiotensin II Induces Expression of Atherosclerotic Proteins
3. Direct Causative Relationships between Angiotensin II and Atherosclerosis
3.1. Angiotensin II Can Exacerbate Vascular Inflammation and Promote Atherosclerosis
3.2. Pharmacological Inhibitors of RAS Attenuate Atherosclerosis
3.3. Studies in Genetically Modified Mice
3.4. Clinical Studies in Humans
4. Angiotensin II, Atherosclerosis, and Vascular Aging
4.1. Aging and Angiotensin II
4.2. Aging and Atherosclerosis
5. Conclusions
Funding
Conflicts of Interest
References
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, L.; Monticone, R.E.; Lakatta, E.G. Proinflammation: The key to arterial aging. Trends Endocrinol. Metab. 2014, 25, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Liao, F.; Berliner, J.A.; Mehrabian, M.; Navab, M.; Demer, L.L.; Lusis, A.J.; Fogelman, A.M. Minimally modified low density lipoprotein is biologically active in vivo in mice. J. Clin. Investig. 1991, 87, 2253–2257. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.T.; Parthasarathy, S.; Fong, L.G.; Steinberg, D. Oxidatively modified low density lipoproteins: A potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc. Natl. Acad. Sci. USA 1987, 84, 2995–2998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinberg, D. Arterial metabolism of lipoproteins in relation to atherogenesis. Ann. N. Y. Acad. Sci. 1990, 598, 125–135. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, L.P.; Tan, S.H.; Ow, G.S.; Tang, Z.; Ching, J.; Kovalik, J.P.; Poh, S.C.; Chin, C.T.; Richards, A.M.; Martinez, E.C.; et al. Plasma Ceramides as Prognostic Biomarkers and Their Arterial and Myocardial Tissue Correlates in Acute Myocardial Infarction. JACC Basic Transl. Sci. 2018, 3, 163–175. [Google Scholar] [CrossRef]
- Ogretmen, B.; Hannun, Y.A. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat. Rev. Cancer 2004, 4, 604–616. [Google Scholar] [CrossRef]
- Gimbrone, M.A.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [Green Version]
- Eelen, G.; de Zeeuw, P.; Simons, M.; Carmeliet, P. Endothelial cell metabolism in normal and diseased vasculature. Circ. Res. 2015, 116, 1231–1244. [Google Scholar] [CrossRef]
- Basatemur, G.L.; Jørgensen, H.F.; Clarke, M.C.H.; Bennett, M.R.; Mallat, Z. Vascular smooth muscle cells in atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 727–744. [Google Scholar] [CrossRef]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef]
- Doran, A.C.; Meller, N.; McNamara, C.A. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 812–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, T.; Owens, G.K. Molecular determinants of vascular smooth muscle cell diversity. Circ. Res. 2005, 96, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Libby, P. The immune response in atherosclerosis: A double-edged sword. Nat. Rev. Immunol. 2006, 6, 508–519. [Google Scholar] [CrossRef]
- Schmidt-Ott, K.M.; Kagiyama, S.; Phillips, M.I. The multiple actions of angiotensin II in atherosclerosis. Regul. Pept. 2000, 93, 65–77. [Google Scholar] [CrossRef]
- Touyz, R.M.; He, G.; El Mabrouk, M.; Diep, Q.; Mardigyan, V.; Schiffrin, E.L. Differential activation of extracellular signal-regulated protein kinase 1/2 and p38 mitogen activated-protein kinase by AT1 receptors in vascular smooth muscle cells from Wistar-Kyoto rats and spontaneously hypertensive rats. J. Hypertens. 2001, 19, 553–559. [Google Scholar] [CrossRef]
- Lucchesi, P.A.; Bell, J.M.; Willis, L.S.; Byron, K.L.; Corson, M.A.; Berk, B.C. Ca(2+)-dependent mitogen-activated protein kinase activation in spontaneously hypertensive rat vascular smooth muscle defines a hypertensive signal transduction phenotype. Circ. Res. 1996, 78, 962–970. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Lorenzo, O.; Rupérez, M.; Suzuki, Y.; Egido, J. Angiotensin II activates nuclear transcription factor-kappaB in aorta of normal rats and in vascular smooth muscle cells of AT1 knockout mice. Nephrol. Dial. Transplant 2001, 16, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Zafari, A.M.; Ushio-Fukai, M.; Akers, M.; Yin, Q.; Shah, A.; Harrison, D.G.; Taylor, W.R.; Griendling, K.K. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension 1998, 32, 488–495. [Google Scholar] [CrossRef] [Green Version]
- Ushio-Fukai, M.; Zafari, A.M.; Fukui, T.; Ishizaka, N.; Griendling, K.K. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J. Biol. Chem. 1996, 271, 23317–23321. [Google Scholar] [CrossRef] [Green Version]
- Griendling, K.K.; Sorescu, D.; Ushio-Fukai, M. NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ. Res. 2000, 86, 494–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubanyi, G.M.; Vanhoutte, P.M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am. J. Physiol. 1986, 250, H822–H827. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ortega, M.; Lorenzo, O.; Rupérez, M.; Esteban, V.; Suzuki, Y.; Mezzano, S.; Plaza, J.J.; Egido, J. Role of the renin-angiotensin system in vascular diseases: Expanding the field. Hypertension 2001, 38, 1382–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schieffer, B.; Schieffer, E.; Hilfiker-Kleiner, D.; Hilfiker, A.; Kovanen, P.T.; Kaartinen, M.; Nussberger, J.; Harringer, W.; Drexler, H. Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques: Potential implications for inflammation and plaque instability. Circulation 2000, 101, 1372–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.C.; Phillips, M.I.; Mohuczy, D.; Meng, H.; Shen, L.; Mehta, P.; Mehta, J.L. Increased angiotensin II type 1 receptor expression in hypercholesterolemic atherosclerosis in rabbits. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1433–1439. [Google Scholar] [CrossRef] [Green Version]
- Nickenig, G.; Jung, O.; Strehlow, K.; Zolk, O.; Linz, W.; Schölkens, B.A.; Böhm, M. Hypercholesterolemia is associated with enhanced angiotensin AT1-receptor expression. Am. J. Physiol. 1997, 272, 2701–2707. [Google Scholar] [CrossRef]
- Chen, X.; Lu, H.; Zhao, M.; Tashiro, K.; Cassis, L.A.; Daugherty, A. Contributions of leukocyte angiotensin-converting enzyme to development of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2075–2080. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Saldeen, T.; Romeo, F.; Mehta, J.L. Oxidized LDL upregulates angiotensin II type 1 receptor expression in cultured human coronary artery endothelial cells: The potential role of transcription factor NF-kappaB. Circulation 2000, 102, 1970–1976. [Google Scholar] [CrossRef]
- Wongsurawat, T.; Woo, C.C.; Giannakakis, A.; Lin, X.Y.; Cheow, E.S.H.; Lee, C.N.; Richards, M.; Sze, S.K.; Nookaew, I.; Kuznetsov, V.A.; et al. Distinctive molecular signature and activated signaling pathways in aortic smooth muscle cells of patients with myocardial infarction. Atherosclerosis 2018, 271, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Sorokin, V.; Woo, C.C. Role of Serpina3 in vascular biology. Int. J. Cardiol. 2020, 304, 154–155. [Google Scholar] [CrossRef]
- Jenkinson, S.E.; Brown, L.J.; Ombor, J.; Milburn, J.A.; Smulders-Srinivasan, T.; Veuger, S.; Edwards, D.R.; Bass, R. Identification of novel peptide motifs in the serpin maspin that affect vascular smooth muscle cell function. Biochim. Biophys. Acta. Mol. Cell Res. 2017, 1864, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Nickenig, G.; Harrison, D.G. The AT(1)-type angiotensin receptor in oxidative stress and atherogenesis: Part II: AT(1) receptor regulation. Circulation 2002, 105, 530–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keidar, S. Angiotensin, LDL peroxidation and atherosclerosis. Life Sci. 1998, 63, 1–11. [Google Scholar] [CrossRef]
- Keidar, S.; Kaplan, M.; Aviram, M. Angiotensin II-modified LDL is taken up by macrophages via the scavenger receptor, leading to cellular cholesterol accumulation. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 97–105. [Google Scholar] [CrossRef]
- Sato, A.; Ueda, C.; Kimura, R.; Kobayashi, C.; Yamazaki, Y.; Ebina, K. Angiotensin II induces the aggregation of native and oxidized low-density lipoprotein. Eur. Biophys J. 2018, 47, 1–9. [Google Scholar] [CrossRef]
- Sendra, J.; Llorente-Cortés, V.; Costales, P.; Huesca-Gómez, C.; Badimon, L. Angiotensin II upregulates LDL receptor-related protein (LRP1) expression in the vascular wall: A new pro-atherogenic mechanism of hypertension. Cardiovasc. Res. 2008, 78, 581–589. [Google Scholar] [CrossRef]
- Morawietz, H.; Rueckschloss, U.; Niemann, B.; Duerrschmidt, N.; Galle, J.; Hakim, K.; Zerkowski, H.R.; Sawamura, T.; Holtz, J. Angiotensin II induces LOX-1, the human endothelial receptor for oxidized low-density lipoprotein. Circulation 1999, 100, 899–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.Y.; Zhang, Y.C.; Philips, M.I.; Sawamura, T.; Mehta, J.L. Upregulation of endothelial receptor for oxidized low-density lipoprotein (LOX-1) in cultured human coronary artery endothelial cells by angiotensin II type 1 receptor activation. Circ. Res. 1999, 84, 1043–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajagopalan, S.; Kurz, S.; Münzel, T.; Tarpey, M.; Freeman, B.A.; Griendling, K.K.; Harrison, D.G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Investig. 1996, 97, 1916–1923. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, H.; Suzuki, H.; Ohtsu, H.; Chao, J.Y.; Utsunomiya, H.; Frank, G.D.; Eguchi, S. Angiotensin II regulates vascular and endothelial dysfunction: Recent topics of Angiotensin II type-1 receptor signaling in the vasculature. Curr. Vasc. Pharmacol. 2006, 4, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Seto, S.W.; Krishna, S.M.; Yu, H.; Liu, D.; Khosla, S.; Golledge, J. Impaired acetylcholine-induced endothelium-dependent aortic relaxation by caveolin-1 in angiotensin II-infused apolipoprotein-E (ApoE-/-) knockout mice. PLoS ONE 2013, 8, e58481. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Bai, X.; Chen, X. Angiotensin II induces endothelial cell senescence via the activation of mitogen-activated protein kinases. Cell Biochem. Funct. 2008, 26, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Pueyo, M.E.; Gonzalez, W.; Nicoletti, A.; Savoie, F.; Arnal, J.F.; Michel, J.B. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 645–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, H.; Frank, G.D.; Utsunomiya, H.; Higuchi, S.; Eguchi, S. Current understanding of the mechanism and role of ROS in angiotensin II signal transduction. Curr. Pharm. Biotechnol. 2006, 7, 81–86. [Google Scholar] [CrossRef]
- Chen, X.L.; Tummala, P.E.; Olbrych, M.T.; Alexander, R.W.; Medford, R.M. Angiotensin II induces monocyte chemoattractant protein-1 gene expression in rat vascular smooth muscle cells. Circ. Res. 1998, 83, 952–959. [Google Scholar] [CrossRef] [Green Version]
- Sahar, S.; Dwarakanath, R.S.; Reddy, M.A.; Lanting, L.; Todorov, I.; Natarajan, R. Angiotensin II enhances interleukin-18 mediated inflammatory gene expression in vascular smooth muscle cells: A novel cross-talk in the pathogenesis of atherosclerosis. Circ. Res. 2005, 96, 1064–1071. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Hu, Y.; Xu, Q.; Ye, S. Different effects of angiotensin II and angiotensin-(1-7) on vascular smooth muscle cell proliferation and migration. PLoS ONE 2010, 5, e12323. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, D.E.; Lazos, S.A.; Tong, K. Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells. A potential link between the renin-angiotensin system and thrombosis. J. Clin. Investig. 1995, 95, 995–1001. [Google Scholar] [CrossRef] [Green Version]
- Aono, J.; Suzuki, J.; Iwai, M.; Horiuchi, M.; Nagai, T.; Nishimura, K.; Inoue, K.; Ogimoto, A.; Okayama, H.; Higaki, J. Deletion of the angiotensin II type 1a receptor prevents atherosclerotic plaque rupture in apolipoprotein E-/- mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1453–1459. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Tempel, D.; van Haperen, R.; van Damme, L.; Algür, M.; Krams, R.; de Crom, R. Activation of MMP8 and MMP13 by angiotensin II correlates to severe intra-plaque hemorrhages and collagen breakdown in atherosclerotic lesions with a vulnerable phenotype. Atherosclerosis 2009, 204, 26–33. [Google Scholar] [CrossRef]
- Mazzolai, L.; Duchosal, M.A.; Korber, M.; Bouzourene, K.; Aubert, J.F.; Hao, H.; Vallet, V.; Brunner, H.R.; Nussberger, J.; Gabbiani, G.; et al. Endogenous angiotensin II induces atherosclerotic plaque vulnerability and elicits a Th1 response in ApoE-/- mice. Hypertension 2004, 44, 277–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.; Powell-Braxton, L.; Ogaoawara, A.K.; Dybdal, N.; Bunting, S.; Ohneda, O.; Jin, H. Hypertension and endothelial dysfunction in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2762–2768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, D.; Kools, J.J.; Taylor, W.R. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in apoE-deficient mice. Circulation 2001, 103, 448–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daugherty, A.; Manning, M.W.; Cassis, L.A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J. Clin. Investig. 2000, 105, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, T.; Wilson, P.G.; Thompson, J.C.; Nelson, C.; Yoder, M.H.; Tannock, L.R. Prevention of TGFβ induction attenuates angII-stimulated vascular biglycan and atherosclerosis in Ldlr-/- mice. J. Lipid. Res. 2013, 54, 2255–2264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbariga, M.; Curnis, F.; Spitaleri, A.; Andolfo, A.; Zucchelli, C.; Lazzaro, M.; Magnani, G.; Musco, G.; Corti, A.; Alessio, M. Oxidation-induced structural changes of ceruloplasmin foster NGR motif deamidation that promotes integrin binding and signaling. J. Biol. Chem. 2014, 289, 3736–3748. [Google Scholar] [CrossRef] [Green Version]
- Dutta, B.; Park, J.E.; Kumar, S.; Hao, P.; Gallart-Palau, X.; Serra, A.; Ren, Y.; Sorokin, V.; Lee, C.N.; Ho, H.H.; et al. Monocyte adhesion to atherosclerotic matrix proteins is enhanced by Asn-Gly-Arg deamidation. Sci. Rep. 2017, 7, 5765. [Google Scholar] [CrossRef]
- Chuang, C.Y.; Degendorfer, G.; Hammer, A.; Whitelock, J.M.; Malle, E.; Davies, M.J. Oxidation modifies the structure and function of the extracellular matrix generated by human coronary artery endothelial cells. Biochem. J. 2014, 459, 313–322. [Google Scholar] [CrossRef]
- Keidar, S.; Attias, J.; Smith, J.; Breslow, J.L.; Hayek, T. The angiotensin-II receptor antagonist, losartan, inhibits LDL lipid peroxidation and atherosclerosis in apolipoprotein E-deficient mice. Biochem. Biophys. Res. Commun. 1997, 236, 622–625. [Google Scholar] [CrossRef]
- Kowala, M.C.; Grove, R.I.; Aberg, G. Inhibitors of angiotensin converting enzyme decrease early atherosclerosis in hyperlipidemic hamsters. Fosinopril reduces plasma cholesterol and captopril inhibits macrophage-foam cell accumulation independently of blood pressure and plasma lipids. Atherosclerosis 1994, 108, 61–72. [Google Scholar] [CrossRef]
- da Cunha, V.; Tham, D.M.; Martin-McNulty, B.; Deng, G.; Ho, J.J.; Wilson, D.W.; Rutledge, J.C.; Vergona, R.; Sullivan, M.E.; Wang, Y.X. Enalapril attenuates angiotensin II-induced atherosclerosis and vascular inflammation. Atherosclerosis 2005, 178, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Lee, S.J.; Thuy, N.V.P.; Kim, J.S.; Lee, J.J.; Lee, O.H.; Kim, C.K.; Oh, J.; Park, S.; Kim, S.H.; et al. Synergistic protective effects of a statin and an angiotensin receptor blocker for initiation and progression of atherosclerosis. PLoS ONE 2019, 14, e0215604. [Google Scholar] [CrossRef] [PubMed]
- Eto, H.; Miyata, M.; Shirasawa, T.; Akasaki, Y.; Hamada, N.; Nagaki, A.; Orihara, K.; Biro, S.; Tei, C. The long-term effect of angiotensin II type 1a receptor deficiency on hypercholesterolemia-induced atherosclerosis. Hypertens. Res. 2008, 31, 1631–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wassmann, S.; Czech, T.; van Eickels, M.; Fleming, I.; Böhm, M.; Nickenig, G. Inhibition of diet-induced atherosclerosis and endothelial dysfunction in apolipoprotein E/angiotensin II type 1A receptor double-knockout mice. Circulation 2004, 110, 3062–3067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwai, M.; Chen, R.; Li, Z.; Shiuchi, T.; Suzuki, J.; Ide, A.; Tsuda, M.; Okumura, M.; Min, L.J.; Mogi, M.; et al. Deletion of angiotensin II type 2 receptor exaggerated atherosclerosis in apolipoprotein E-null mice. Circulation 2005, 112, 1636–1643. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Dandapat, A.; Chen, J.; Liu, Y.; Hermonat, P.L.; Carey, R.M.; Mehta, J.L. Over-expression of angiotensin II type 2 receptor (agtr2) reduces atherogenesis and modulates LOX-1, endothelial nitric oxide synthase and heme-oxygenase-1 expression. Atherosclerosis 2008, 199, 288–294. [Google Scholar] [CrossRef]
- MacMahon, S.; Peto, R.; Cutler, J.; Collins, R.; Sorlie, P.; Neaton, J.; Abbott, R.; Godwin, J.; Dyer, A.; Stamler, J. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: Prospective observational studies corrected for the regression dilution bias. Lancet 1990, 335, 765–774. [Google Scholar] [CrossRef]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.J.; Hill, M.M.; Chigurupati, S.; Du, G.; Parton, R.G.; Hancock, J.F. Therapeutic levels of the hydroxmethylglutaryl-coenzyme A reductase inhibitor lovastatin activate ras signaling via phospholipase D2. Mol. Cell Biol. 2011, 31, 1110–1120. [Google Scholar] [CrossRef] [Green Version]
- Borghi, C.; Prandin, M.G.; Costa, F.V.; Bacchelli, S.; Degli Esposti, D.; Ambrosioni, E. Use of statins and blood pressure control in treated hypertensive patients with hypercholesterolemia. J. Cardiovasc. Pharmacol. 2000, 35, 549–555. [Google Scholar] [CrossRef]
- Spósito, A.C.; Mansur, A.P.; Coelho, O.R.; Nicolau, J.C.; Ramires, J.A. Additional reduction in blood pressure after cholesterol-lowering treatment by statins (lovastatin or pravastatin) in hypercholesterolemic patients using angiotensin-converting enzyme inhibitors (enalapril or lisinopril). Am. J. Cardiol. 1999, 83, 1497–1499. [Google Scholar] [CrossRef]
- Morgado, M.; Rolo, S.; Macedo, A.F.; Castelo-Branco, M. Association of statin therapy with blood pressure control in hypertensive hypercholesterolemic outpatients in clinical practice. J. Cardiovasc. Dis. Res. 2011, 2, 44–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seki, S.; Hashimoto, K.; Taniguchi, I.; Yoshimura, M.; Takeda, N. Effect of rosuvastatin on systemic blood pressure in patients with hypercholesterolemia. Exp. Clin. Cardiol. 2012, 17, 221–225. [Google Scholar] [PubMed]
- Rosei, E.A.; Rizzoni, D.; Muiesan, M.L.; Sleiman, I.; Salvetti, M.; Monteduro, C.; Porteri, E.; CENTRO (CandEsartaN on aTherosclerotic Risk factors) study investigators. Effects of candesartan cilexetil and enalapril on inflammatory markers of atherosclerosis in hypertensive patients with non-insulin-dependent diabetes mellitus. J. Hypertens. 2005, 23, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Golomb, B.A.; Dimsdale, J.E.; White, H.L.; Ritchie, J.B.; Criqui, M.H. Reduction in blood pressure with statins: Results from the UCSD Statin Study, a randomized trial. Arch. Intern. Med. 2008, 168, 721–727. [Google Scholar] [CrossRef]
- Simon, A.; Dézsi, C.A. Treatment of Hypertensive and Hypercholesterolaemic Patients with the Triple Fixed Combination of Atorvastatin, Perindopril and Amlodipine: The Results of the CORAL Study. Adv. Ther. 2019, 36, 2010–2020. [Google Scholar] [CrossRef]
- Diehl, K.J.; Stauffer, B.L.; Greiner, J.J.; Weil, B.R.; DeSouza, C.A. Nitric oxide-mediated endothlium-dependent vasodilation is impaired with borderline high-LDL cholesterol. Clin. Transl. Sci. 2012, 5, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Laufs, U.; La Fata, V.; Plutzky, J.; Liao, J.K. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation 1998, 97, 1129–1135. [Google Scholar] [CrossRef]
- Wang, M.; Monticone, R.E.; Lakatta, E.G. Proinflammation of aging central arteries: A mini-review. Gerontology 2014, 60, 519–529. [Google Scholar] [CrossRef] [Green Version]
- Asai, K.; Kudej, R.K.; Shen, Y.T.; Yang, G.P.; Takagi, G.; Kudej, A.B.; Geng, Y.J.; Sato, N.; Nazareno, J.B.; Vatner, D.E.; et al. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1493–1499. [Google Scholar] [CrossRef] [Green Version]
- Brandes, R.P.; Fleming, I.; Busse, R. Endothelial aging. Cardiovasc. Res. 2005, 66, 286–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbert, K.E.; Mistry, Y.; Hastings, R.; Poolman, T.; Niklason, L.; Williams, B. Angiotensin II-mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere-dependent and independent pathways. Circ. Res. 2008, 102, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Gepner, A.D.; Korcarz, C.E.; Colangelo, L.A.; Hom, E.K.; Tattersall, M.C.; Astor, B.C.; Kaufman, J.D.; Liu, K.; Stein, J.H. Longitudinal effects of a decade of aging on carotid artery stiffness: The multiethnic study of atherosclerosis. Stroke 2014, 45, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zhang, J.; Spinetti, G.; Jiang, L.Q.; Monticone, R.; Zhao, D.; Cheng, L.; Krawczyk, M.; Talan, M.; Pintus, G.; et al. Angiotensin II activates matrix metalloproteinase type II and mimics age-associated carotid arterial remodeling in young rats. Am. J. Pathol. 2005, 167, 1429–1442. [Google Scholar] [CrossRef] [Green Version]
- Bruno, R.M.; Duranti, E.; Ippolito, C.; Segnani, C.; Bernardini, N.; Di Candio, G.; Chiarugi, M.; Taddei, S.; Virdis, A. Different Impact of Essential Hypertension on Structural and Functional Age-Related Vascular Changes. Hypertension 2017, 69, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Marteijn, J.A.; Lans, H.; Vermeulen, W.; Hoeijmakers, J.H. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat. Rev. Mol. Cell Biol. 2014, 15, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Benigni, A.; Corna, D.; Zoja, C.; Sonzogni, A.; Latini, R.; Salio, M.; Conti, S.; Rottoli, D.; Longaretti, L.; Cassis, P.; et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. J. Clin. Invest. 2009, 119, 524–530. [Google Scholar] [CrossRef]
- Min, L.J.; Mogi, M.; Iwanami, J.; Jing, F.; Tsukuda, K.; Ohshima, K.; Horiuchi, M. Angiotensin II type 2 receptor-interacting protein prevents vascular senescence. J. Am. Soc. Hypertens. 2012, 6, 179–184. [Google Scholar] [CrossRef]
- Shan, H.Y.; Bai, X.J.; Chen, X.M. Apoptosis is involved in the senescence of endothelial cells induced by angiotensin II. Cell Biol. Int. 2008, 32, 264–270. [Google Scholar] [CrossRef]
- Weiss, D.; Sorescu, D.; Taylor, W.R. Angiotensin II and atherosclerosis. Am. J. Cardiol. 2001, 87, 25–32. [Google Scholar] [CrossRef]
- Tyrrell, D.J.; Blin, M.G.; Song, J.; Wood, S.C.; Zhang, M.; Beard, D.A.; Goldstein, D.R. Age-Associated Mitochondrial Dysfunction Accelerates Atherogenesis. Circ. Res. 2020, 126, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Shen, H.; Schenten, D.; Shan, P.; Lee, P.J.; Goldstein, D.R. Aging enhances the basal production of IL-6 and CCL2 in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 103–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, C.; Gorenne, I.; Scott, S.; Figg, N.; Kirkpatrick, P.; Ritchie, A.; Goddard, M.; Bennett, M. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: Effects of telomerase and oxidative stress. Circ. Res. 2006, 99, 156–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
St. Paul, A.; Corbett, C.B.; Okune, R.; Autieri, M.V. Angiotensin II, Hypercholesterolemia, and Vascular Smooth Muscle Cells: A Perfect Trio for Vascular Pathology. Int. J. Mol. Sci. 2020, 21, 4525. https://doi.org/10.3390/ijms21124525
St. Paul A, Corbett CB, Okune R, Autieri MV. Angiotensin II, Hypercholesterolemia, and Vascular Smooth Muscle Cells: A Perfect Trio for Vascular Pathology. International Journal of Molecular Sciences. 2020; 21(12):4525. https://doi.org/10.3390/ijms21124525
Chicago/Turabian StyleSt. Paul, Amanda, Cali B. Corbett, Rachael Okune, and Michael V. Autieri. 2020. "Angiotensin II, Hypercholesterolemia, and Vascular Smooth Muscle Cells: A Perfect Trio for Vascular Pathology" International Journal of Molecular Sciences 21, no. 12: 4525. https://doi.org/10.3390/ijms21124525
APA StyleSt. Paul, A., Corbett, C. B., Okune, R., & Autieri, M. V. (2020). Angiotensin II, Hypercholesterolemia, and Vascular Smooth Muscle Cells: A Perfect Trio for Vascular Pathology. International Journal of Molecular Sciences, 21(12), 4525. https://doi.org/10.3390/ijms21124525




