SGL 121 Attenuates Nonalcoholic Fatty Liver Disease through Adjusting Lipid Metabolism Through AMPK Signaling Pathway
Abstract
:1. Introduction
2. Results
2.1. Effects of SGL 121 on Body Weight and Body Fat
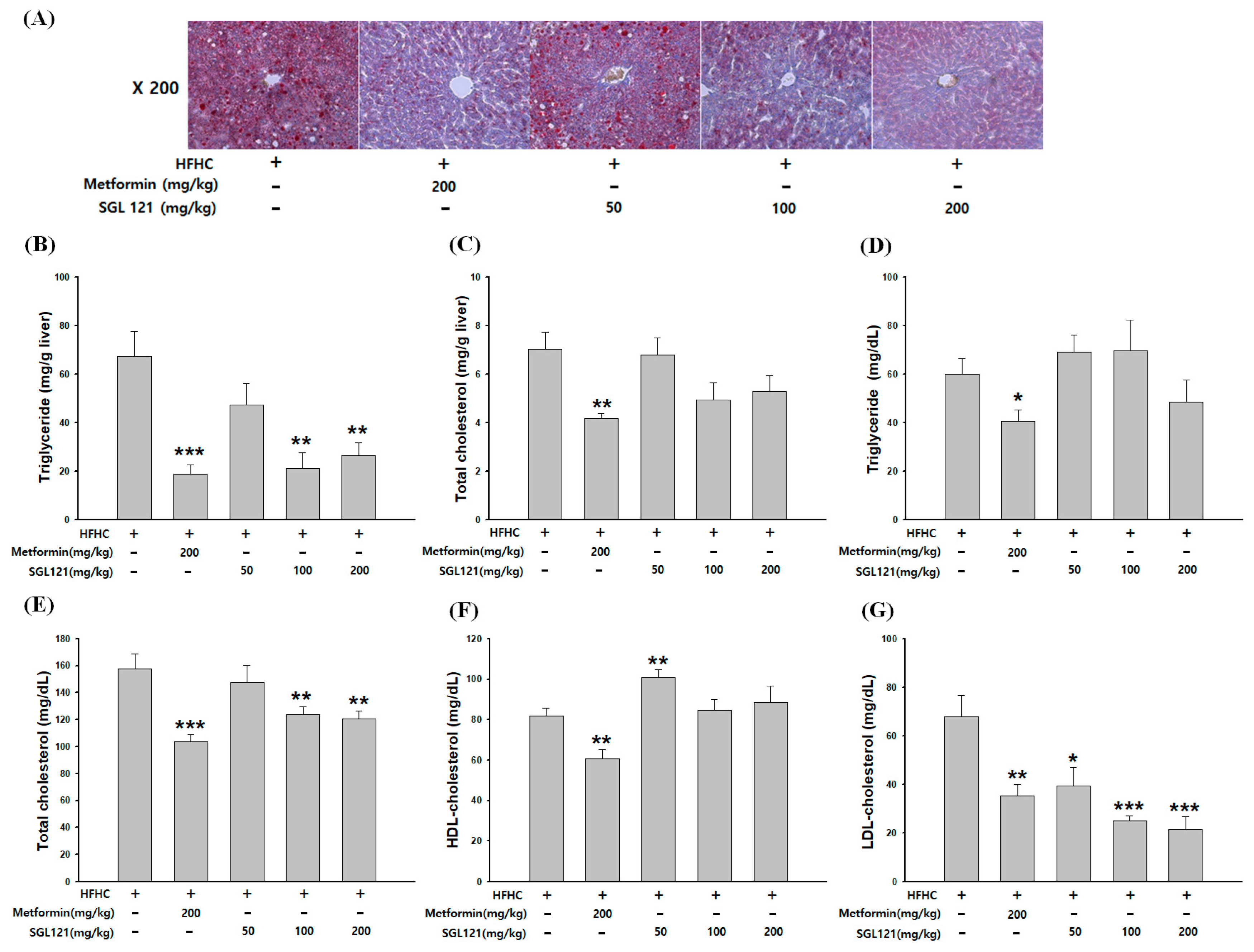
2.2. The Effect of SGL 121 on Lipid Accumulation in NAFLD-Induced Mice
2.3. Effect of SGL 121 on Liver Function Improvement in NAFLD-Induced Mice
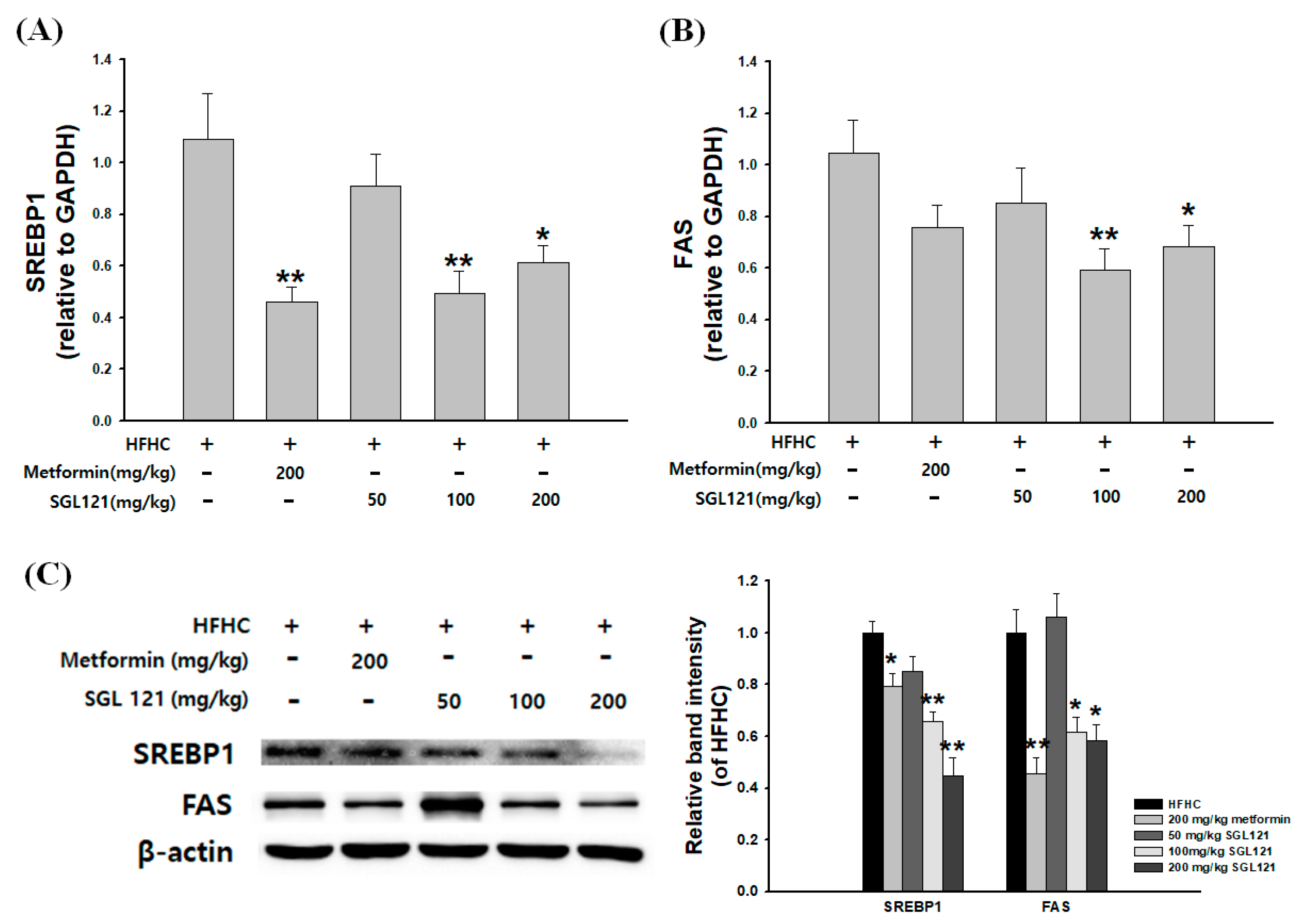
2.4. Effects of SGL 121 on the Expression of Liver Fat Metabolism-Related Genes in NAFLD-Induced Mice
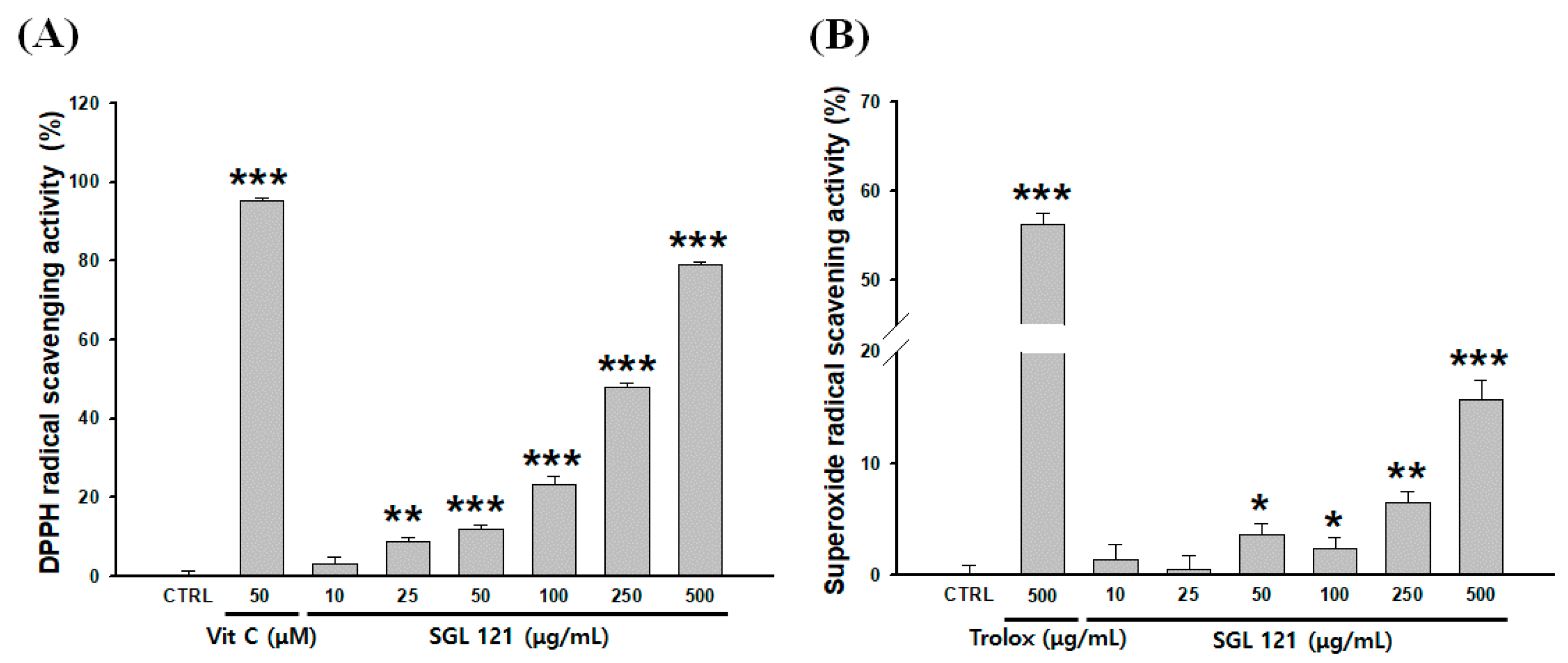
2.5. Antioxidant Activities of SGL 121 in a Cell-Free System
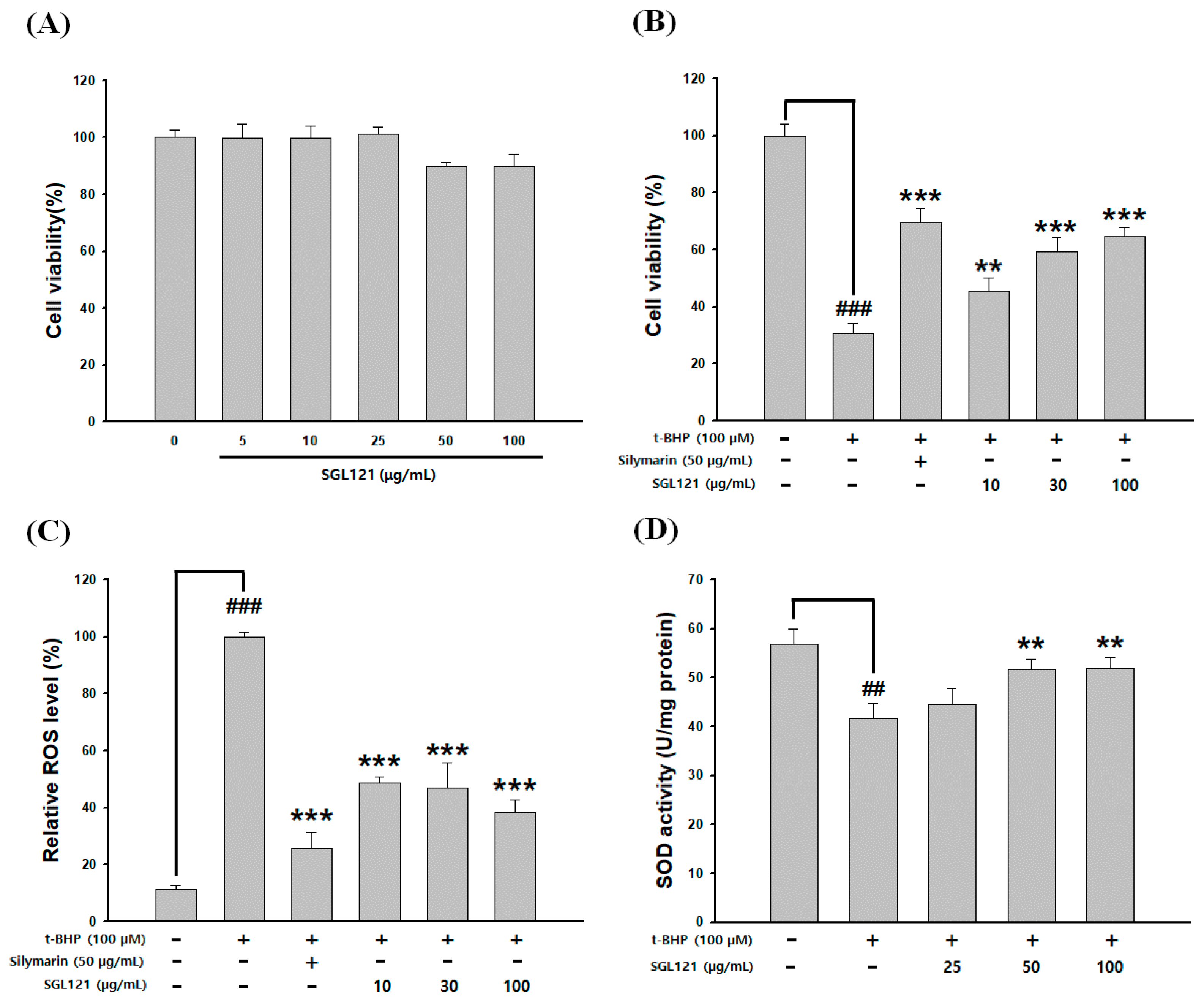
2.6. Cytotoxicity and Cytoprotective Effect of SGL 121
2.7. Effect of SGL 121 on ROS Level and Antioxidant Activity
2.8. Effects of SGL 121 on HO-1 Expression and Nrf2 Nuclear Translocation in HepG2 Cells
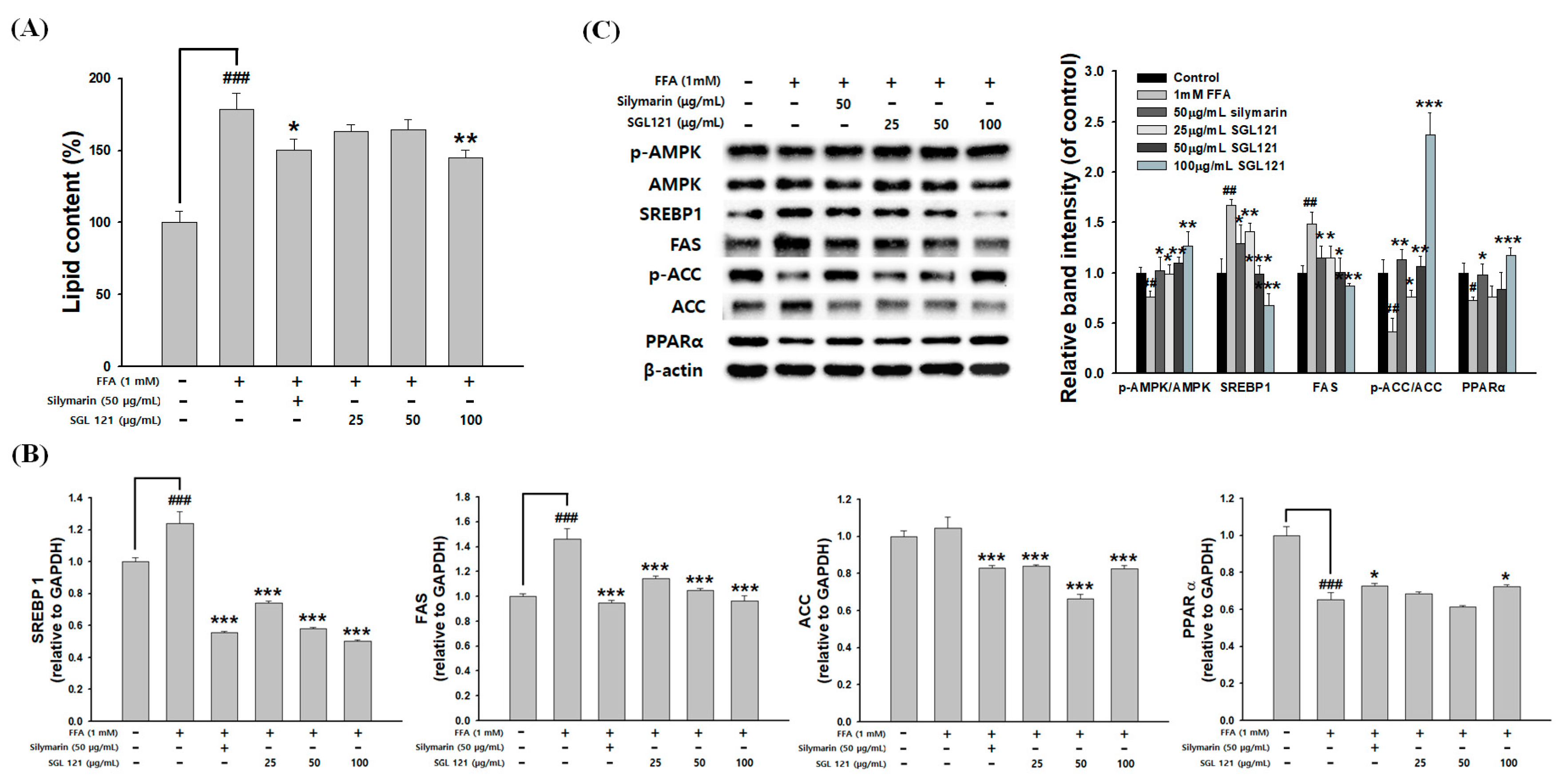
2.9. Effects of SGL 121 on Lipid Accumulation and Lipogenesis in HepG2 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of SGL 121
4.3. Animal Experiment
4.4. Biochemical Assays
4.5. Hepatic Biochemical Analysis
4.6. Body Fat Composition Analysis
4.7. Histopathology
4.8. DPPH Free Radical Scavenging Activity
4.9. Superoxide Radical Scavenging Activity
4.10. Cell Culture and Cytotoxicity Assay
4.11. Measurement of ROS Level
4.12. Measurement of Lipid Content with Oil Red O Staining
4.13. Analysis of mRNA Expression
4.14. Western Blotting
4.15. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ong, J.P.; Pitts, A.; Younossi, Z.M. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J. Hepatol. 2008, 49, 608–612. [Google Scholar] [CrossRef]
- Nassir, F.; Rector, R.S.; Hammoud, G.M.; Ibdah, J.A. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol. Hepatol. 2015, 11, 167–175. [Google Scholar]
- Veerle Bieghs, C.T. Innate immune signaling and gut-liver interactions in non-alcoholic fatty liver disease. Hepatobiliary Surg. Nutr. 2014, 3, 377. [Google Scholar]
- Guo, Y.; Xiong, Y.; Sheng, Q.; Zhao, S.; Wattacheril, J.; Flynn, C.R. A micro-RNA expression signature for human NAFLD progression. J. Gastroenterol. 2016, 51, 1022–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, J.F.; Shi, H.B.; Liu, L.R.; Jiang, P.; Liang, L.; Wang, C.L.; Liu, X.Y. Non-alcoholic fatty liver disease: An early mediator predicting metabolic syndrome in obese children? World J. Gastroenterol. 2011, 17, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.R.D.A.; Diniz, M.D.F.F.D.; Medeiros-Filho, J.E.M.D.; Araújo, M.S.T.D. Metabolic syndrome and risk factors for non-alcoholic fatty liver disease. Arq. De Gastroenterol. 2012, 49, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Boursier, J.; Diehl, A.M. Implication of Gut Microbiota in Nonalcoholic Fatty Liver Disease. PLoS Pathog. 2015, 11, e1004559. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.; Taskinen, M.R.; Borén, J. Crosstalk between nonalcoholic fatty liver disease and cardiometabolic syndrome. Obes. Rev. 2019, 20, 599–611. [Google Scholar] [CrossRef] [Green Version]
- Laursen, T.L.; Hagemann, C.A.; Wei, C.; Kazankov, K.; Thomsen, K.L.; Knop, F.K.; Grønbæk, H. Bariatric surgery in patients with non-alcoholic fatty liver disease - from pathophysiology to clinical effects. World J. Hepatol. 2019, 11, 138–149. [Google Scholar] [CrossRef]
- Lau, J.K.C.; Zhang, X.; Yu, J. Animal models of non-alcoholic fatty liver disease: Current perspectives and recent advances. J. Pathol. 2016, 241, 36–44. [Google Scholar] [CrossRef]
- Higgins, V.; Adeli, K. Pediatric Metabolic Syndrome: Pathophysiology and Laboratory Assessment. Ejifcc 2017, 28, 25–42. [Google Scholar]
- Divella, R.; Mazzocca, A.; Daniele, A.; Sabbà, C.; Paradiso, A. Obesity, Nonalcoholic Fatty Liver Disease and Adipocytokines Network in Promotion of Cancer. Int. J. Biol. Sci. 2019, 15, 610–616. [Google Scholar] [CrossRef] [Green Version]
- Salomone, F.; Godos, J.; Zelber-Sagi, S. Natural antioxidants for non-alcoholic fatty liver disease: Molecular targets and clinical perspectives. Liver Int. 2015, 36, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Chen, S.C.; Ou, T.T.; Chyau, C.C.; Chang, Y.C.; Wang, C.J. Mulberry leaf polyphenol extracts reduced hepatic lipid accumulation involving regulation of adenosine monophosphate activated protein kinase and lipogenic enzymes. J. Funct. Foods 2013, 5, 1620–1632. [Google Scholar] [CrossRef]
- Park, H.; Liu, Y.; Kim, H.S.; Shin, J.H. Chokeberry attenuates the expression of genes related to de novo lipogenesis in the hepatocytes of mice with nonalcoholic fatty liver disease. Nutr. Res. 2016, 36, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Kim, D.; Jo, K.; Hwang, J.K. Nordihydroguaiaretic acid protects against high-fat diet-induced fatty liver by activating AMP-activated protein kinase in obese mice. Biochem. Biophys. Res. Commun. 2010, 401, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, H.X.; Pan, W.S.; Khan, F.U.; Qian, C.; Qi-Li, F.R.; Xu, X. Novel hepatoprotective role of Leonurine hydrochloride against experimental non-alcoholic steatohepatitis mediated via AMPK/SREBP1 signaling pathway. Biomed. Pharmacother. 2019, 110, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Liu, Y.; Kim, H.S. Hawthorn Fruit Extract Elevates Expression of Nrf2/HO-1 and Improves Lipid Profiles in Ovariectomized Rats. Nutrients 2016, 8, 283. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Tang, K.; Chen, R.; Nie, H.; Liang, S.; Zhang, J.; Zhang, Y.; Yang, Q. Berberine attenuates hepatic oxidative stress in rats with non-alcoholic fatty liver disease via the Nrf2/ARE signalling pathway. Exp. Ther. Med. 2019, 17, 2091–2098. [Google Scholar] [CrossRef] [Green Version]
- Le, T.A.; Loomba, R. Management of Non-alcoholic Fatty Liver Disease and Steatohepatitis. J. Clin. Exp. Hepatol. 2012, 2, 156–173. [Google Scholar] [CrossRef] [Green Version]
- Ozturk, Z.A.; Kadayifci, A. Insulin sensitizers for the treatment of non-alcoholic fatty liver disease. World J. Hepatol. 2014, 6, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Neuschwander-Tetri, B.A. Pharmacologic Management of Non-Alcoholic Fatty Liver Disease. Clin. Liver Dis. 2004, 8, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Kaser, S.; Ebenbichler, C.F.; Tilg, H. Pharmacological and non-pharmacological treatment of non-alcoholic fatty liver disease. Int. J. Clin. Pract. 2010, 64, 968–983. [Google Scholar] [CrossRef] [PubMed]
- Bagherniya, M.; Nobili, V.; Blesso, C.N.; Sahebkar, A. Medicinal plants and bioactive natural compounds in the treatment of non-alcoholic fatty liver disease: A clinical review. Pharmacol. Res. 2018, 130, 213–240. [Google Scholar] [CrossRef]
- Sahin, E.; Bagci, R.; Bektur Aykanat, N.E.; Kacar, S.; Sahinturk, V. Silymarin attenuated nonalcoholic fatty liver disease through the regulation of endoplasmic reticulum stress proteins GRP78 and XBP-1 in mice. J. Food Biochem. 2020, 18, e13194. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sodrul, I.M.; Wang, C.; Chen, X.; Du, J.; Sun, H. Role of ginsenosides in reactive oxygen species-mediated anticancer therapy. Oncotarget 2018, 9, 2931–2950. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.M.; Cho, O.S.; Kim, S.J.; Im, B.O.; Cho, S.H.; Lee, S.; Kim, M.G.; Kim, K.T.; Leem, K.H.; Ko, S.K. Inhibitory Effects of Ginsenoside Re Isolated from Ginseng Berry on Histamine and Cytokine Release in Human Mast Cells and Human Alveolar Epithelial Cells. J. Ginseng Res. 2012, 36, 369–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Jin, Y.; Yin, C.; Bai, L. Co-transformation of Panax major ginsenosides Rb1 and Rg1 to minor ginsenosides C–K and F1 by Cladosporium cladosporioides. J. Ind. Microbiol. Biotechnol. 2012, 39, 521–527. [Google Scholar] [CrossRef]
- Liu, C.Y.; Zhou, R.X.; Sun, C.K.; Jin, Y.H.; Yu, H.S.; Zhang, T.Y.; Xu, L.Q.; Jin, F.X. Preparation of minor ginsenosides C-Mc, C-Y, F2, and C-K from American ginseng PPD-ginsenoside using special ginsenosidase type-I from Aspergillus niger g.848. J. Ginseng Res. 2014, 39, 221–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, C.; Jeon, B.M.; Fu, Y.; Im, W.T.; Kim, S.C. High-density immobilization of a ginsenoside-transforming β-glucosidase for enhanced food-grade production of minor ginsenosides. Appl. Microbiol. Biotechnol. 2019, 103, 7003–7015. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.Y.; Lee, J.M.; Shin, H.S.; Park, S.Y.; Yang, J.E.; Cho, S.K.; Yi, T.H. Anti-Cancer Effect of Ginsenoside F2 against Glioblastoma Multiforme in Xenograft Model in SD Rats. J. Ginseng Res. 2012, 36, 86–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siraj, F.M.; SathishKumar, N.; Kim, Y.J.; Kim, S.Y.; Yang, D.C. Ginsenoside F2 possesses anti-obesity activity via binding with PPARγ and inhibiting adipocyte differentiation in the 3T3-L1 cell line. J. Enzym. Inhib. Med. Chem. 2015, 30, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, C.H.; Kim, J.K.; Kim, S.C.; Im, W.T. Characterization of a Ginsenoside-Transforming β-glucosidase from Paenibacillus mucilaginosus and Its Application for Enhanced Production of Minor Ginsenoside F2. PLoS ONE 2014, 9, e85727. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.C.; Hung, H.F.; Lu, C.W.; Chang, H.H.; Lee, L.T.; Huang, K.C. Association of Non-alcoholic Fatty Liver Disease with Metabolic Syndrome Independently of Central Obesity and Insulin Resistance. Sci. Rep. 2016, 6, 27034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miele, L.; Gasbarrini, G.; Giorgio, V.; Gasbarrini, A.; Grieco, A. Nonalcoholic fatty liver disease as trigger of cardiovascular and metabolic complication in metabolic syndrome. Intern. Emerg. Med. 2015, 11, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, K.W.; Wong, A.S.T. Pharmacology of ginsenosides: A literature review. Chin. Med. 2010, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- Shin, K.C.; Oh, D.K. Classification of glycosidases that hydrolyze the specific positions and types of sugar moieties in ginsenosides. Crit. Rev. Biotechnol. 2016, 36, 1036–1049. [Google Scholar] [CrossRef]
- Cui, L.; Wu, S.Q.; Zhao, C.A.; Yin, C.R. Microbial conversion of major ginsenosides in ginseng total saponins by Platycodon grandiflorum endophytes. J. Ginseng Res. 2016, 40, 366–374. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.J.; Liu, W.J.; Wen, M.L.; Liang, H.; Wu, S.M.; Zhu, Y.Z.; Zhao, J.Y.; Dong, X.Q.; Li, M.G.; Bian, L.; et al. Ameliorative effects of Compound K and ginsenoside Rh1 on non-alcoholic fatty liver disease in rats. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Shen, L.; Xiong, Y.; Wang, D.Q.; Howles, P.; Basford, J.E.; Wang, J.; Xiong, Y.Q.; Hui, D.Y.; Woods, S.C.; Liu, M. Ginsenoside Rb1 reduces fatty liver by activating AMP-activated protein kinase in obese rats. J. Lipid Res. 2013, 54, 1430–4138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.D.; Yang, Y.Y.; Ouyang, D.S.; Yang, G.P. A review of biotransformation and pharmacology of ginsenoside compound K. Fitoterapia 2015, 100, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Mai, T.T.; Moon, J.; Song, Y.; Viet, P.Q.; Van Phuc, P.; Lee, J.M.; Yi, T.H.; Cho, M.; Cho, S.K. Ginsenoside F2 induces apoptosis accompanied by protective autophagy in breast cancer stem cells. Cancer Lett. 2012, 321, 144–153. [Google Scholar] [CrossRef]
- Shin, H.S.; Park, S.Y.; Hwang, E.S.; Lee, D.G.; Song, H.G.; Mavlonov, G.T.; Yi, T.H. The inductive effect of ginsenoside F2 on hair growth by altering the WNT signal pathway in telogen mouse skin. Eur. J. Pharmacol. 2014, 730, 82–89. [Google Scholar] [CrossRef]
- Ekstedt, M.; Franzén, L.E.; Mathiesen, U.L.; Holmqvist, M.; Bodemar, G.; Kechagias, S. Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: A histopathological follow-up study. J. Hepatol. 2007, 47, 135–141. [Google Scholar] [CrossRef]
- Filippatos, T.D.; Gazi, I.F.; Liberopoulos, E.N.; Athyros, V.G.; Elisaf, M.S.; Tselepis, A.D.; Kiortsis, D.N. The effect of orlistat and fenofibrate, alone or in combination, on small dense LDL and lipoprotein-associated phospholipase A2 in obese patients with metabolic syndrome. Atherosclerosis 2007, 193, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Kostapanos, M.S.; Mikhailidis, D.P.; Elisaf, M.S. Adding ezetimibe to statin treatment: Is LDL-C lowering the only benefit? Future Cardiol. 2012, 8, 813–817. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, J.; Liu, W.; Kimura, Y.; Zheng, Y. Anti-Obesity effects of protopanaxdiol types of Ginsenosides isolated from the leaves of American ginseng (Panax quinquefolius L.) in mice fed with a high-fat diet. Fitoterapia 2010, 81, 1079–1087. [Google Scholar] [CrossRef]
- Han, J.Y.; Lee, S.; Yang, J.H.; Kim, S.; Sim, J.; Kim, M.G.; Jeong, T.C.; Ku, S.K.; Cho, I.J.; Ki, S.H. Korean Red Ginseng attenuates ethanol-induced steatosis and oxidative stress via AMPK/Sirt1 activation. J. Ginseng Res. 2015, 39, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Ji, G.E. Ginseng and obesity. J. Ginseng Res. 2018, 42, 1–8. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.J.; et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Zang, Y.; Qu, J.; Tang, M.; Zhang, T. The Toxicity of Metallic Nanoparticles on Liver: The Subcellular Damages, Mechanisms, And Outcomes. Int. J. Nanomed. 2019, 14, 8787–8804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.; Moin, S.; Khan, A.Q.; Zafar, A.; Fatima, N. Increased reactive oxygen species formation and oxidative stress in rheumatoid arthritis. PLoS ONE 2016, 11, e0152925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubiolo, J.A.; Mithieux, G.; Vega, F.V. Resveratrol protects primary rat hepatocytes against oxidative stress damage: Activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur. J. Pharmacol. 2008, 591, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Chambel, S.S.; Santos-Gonçalves, A.; Duarte, T.L. The Dual Role of Nrf2 in Nonalcoholic Fatty Liver Disease: Regulation of Antioxidant Defenses and Hepatic Lipid Metabolism. BioMed Res. Int. 2015. [Google Scholar] [CrossRef] [Green Version]
- Klaassen, C.D.; Reisman, S.A. Nrf2 the rescue: Effects of the antioxidative/electrophilic response on the liver. Toxicol. Appl. Pharmacol. 2010, 244, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Soejima, Y.; Fukusato, T. Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 2012, 18, 2300–2308. [Google Scholar] [CrossRef]
- Liu, H.; Zhong, H.; Yin, Y.; Jiang, Z. Genistein has beneficial effects on hepatic steatosis in high fat-high sucrose diet-treated rats. Biomed. Pharmacother. 2017, 91, 964–969. [Google Scholar] [CrossRef]
- Rigano, D.; Sirignano, C.; Taglialatela-Scafati, O. The potential of natural products for targeting PPARα. Acta Pharm. Sin. B 2017, 7, 427–438. [Google Scholar] [CrossRef]
- Pisonero-Vaquero, S.; Martínez-Ferreras, Á.; García-Mediavilla, M.V.; Martínez-Flórez, S.; Fernández, A.; Benet, M.; Olcoz, J.L.; Jover, R.; González-Gallego, J.; Sánchez-Campos, S. Quercetin ameliorates dysregulation of lipid metabolism genes via the PI3K/AKT pathway in a diet-induced mouse model of nonalcoholic fatty liver disease. Mol. Nutr. Food Res. 2015, 59, 879–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, D.; Liao, L.; Wang, H.; Zhang, W.; Wang, T.; Xu, Z. Canagliflozin ameliorates obesity by improving mitochondrial function and fatty acid oxidation via PPARα in vivo and in vitro. Life Sci. 2020, 247, 117414. [Google Scholar] [CrossRef] [PubMed]
- Kohli, R.; Kirby, M.; Xanthakos, S.A.; Softic, S.; Feldstein, A.E.; Saxena, V.; Tang, P.H.; Miles, L.; Miles, M.V.; Balistreri, W.F.; et al. High-Fructose Medium-Chain-Trans-Fat Diet Induces Liver Fibrosis & Elevates Plasma Coenzyme Q9 in a Novel Murine Model of Obesity and NASH. Hepatology 2010, 52, 934–944. [Google Scholar] [PubMed] [Green Version]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.E.; Chang, B.Y.; Jeon, B.M.; Baek, J.I.; Kim, S.C.; Kim, S.Y. SGL 121 Attenuates Nonalcoholic Fatty Liver Disease through Adjusting Lipid Metabolism Through AMPK Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 4534. https://doi.org/10.3390/ijms21124534
Kim DE, Chang BY, Jeon BM, Baek JI, Kim SC, Kim SY. SGL 121 Attenuates Nonalcoholic Fatty Liver Disease through Adjusting Lipid Metabolism Through AMPK Signaling Pathway. International Journal of Molecular Sciences. 2020; 21(12):4534. https://doi.org/10.3390/ijms21124534
Chicago/Turabian StyleKim, Da Eun, Bo Yoon Chang, Byeong Min Jeon, Jong In Baek, Sun Chang Kim, and Sung Yeon Kim. 2020. "SGL 121 Attenuates Nonalcoholic Fatty Liver Disease through Adjusting Lipid Metabolism Through AMPK Signaling Pathway" International Journal of Molecular Sciences 21, no. 12: 4534. https://doi.org/10.3390/ijms21124534




