A Review on the Environmental Fate Models for Predicting the Distribution of Engineered Nanomaterials in Surface Waters
Abstract
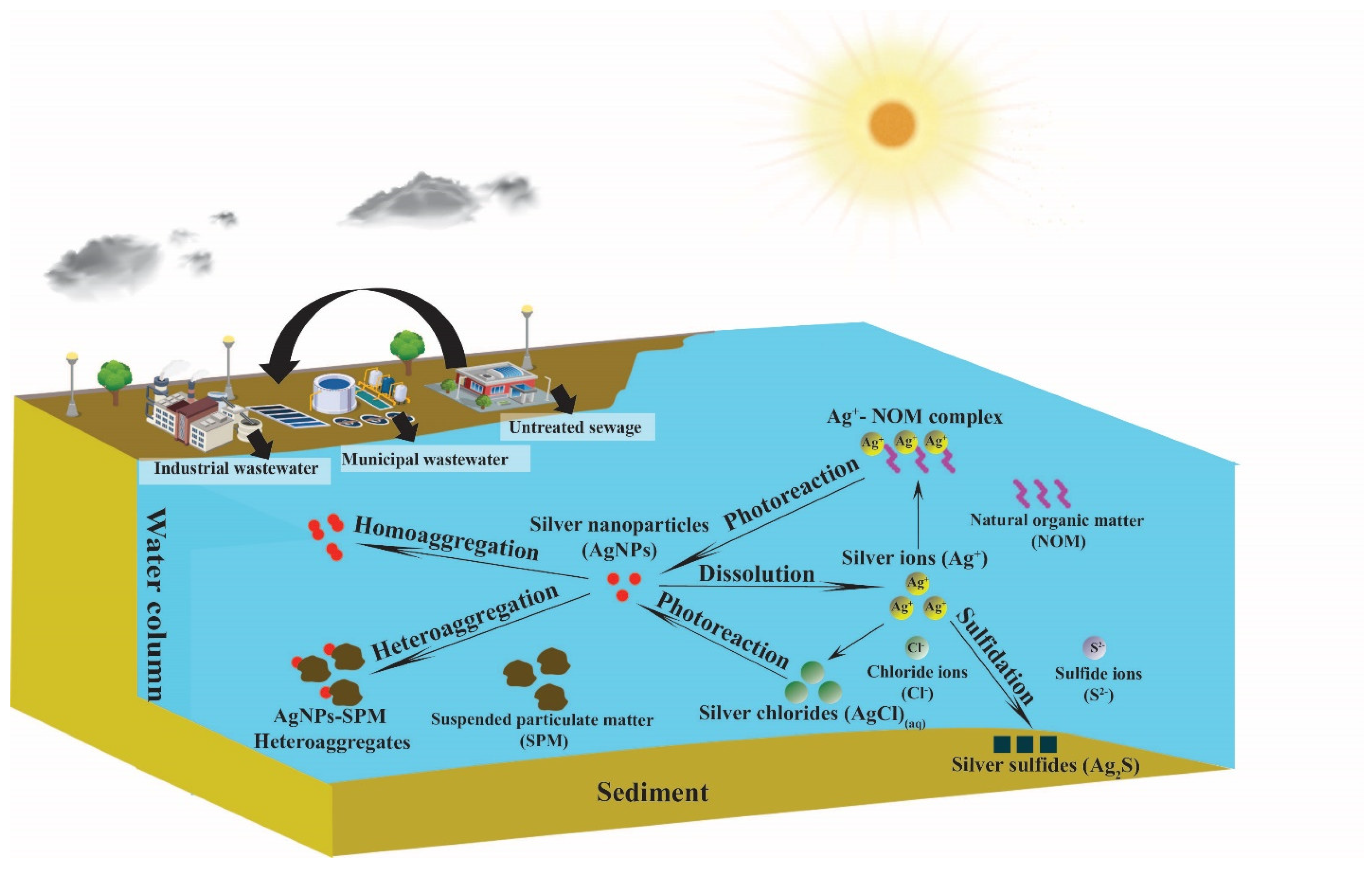
:1. Introduction
2. Exposure Models for the Prediction of Engineered Nanomaterials’ (ENMs’) Concentrations in Surface Waters
2.1. Material Flow Analysis Models (MFAMs)
| Model Classification | Model Name | Model Features | Compartments Considered | Fate Processes | References |
|---|---|---|---|---|---|
| Material flow analysis models | MFAMs | Steady state, less information required, simplified structure | Air, water, soil | - | Mueller and Nowack (2008) [47] |
| P-MFAMs | Accounting for the uncertainty of model input parameters using probabilistic distribution | Air, water, soil, sediment | - | Gotschalk et al. (2009) [61], Gotschalk et al. (2010) [48], Gotschalk et al. (2011) [62], Sun et al. (2015) [49], Liu et al. (2015) [56] | |
| DP-MFAMs | Accounting for time-dependent changes in the system behavior | Air, water, soil, sediment | - | Bornhöft et al. (2016) [50], Sun et al. (2016) [63], Wang and Nowack (2018) [51] | |
| Multimedia compartmental models | MendNano | Intermedia transport processes included partitioning ratios | Air, water, soil, sediment, biota | Homoaggregation, heteroaggregation, dissolution | Liu and Cohen (2014) [54] |
| SimpleBox4 Nano (SB4N) | Steady state environmental ENM fate processes are modeled mechanistically using first-order rate constants | Air, water, soil, sediment | Heteroaggregation, dissolution | Meesters et al. (2014) [36] | |
| RedNano | A model system which combines a P-MFAMs based release model (LearNano) and a multimedia fate model (MendNano) | Air, water, soil, sediment, biota | Homoaggregation, heteroaggregation, dissolution | Liu et al. (2015) [56] | |
| SimpleBox4 Nano (SB4N) | Steady state environmental ENMs’ fate, probabilistic distribution | Air, water, soil, sediment | Heteroaggregation, dissolution | Meesters et al. (2016) [55] | |
| nanoFate | Dynamic environmental ENMs’ fate | Air, water, soil, sediment | Heteroaggregation, dissolution | Garner et al. (2017) [57] | |
| Spatial river/watershed models | Rhine river box model | Steady state box model | Water, sediment | Heteroaggregation | Praetorius et al. (2012) [35] |
| Rhone river box model | Cluster analysis, steady state box model | Water, sediment | Heteroaggregation | Sani-Kast et al. (2015) [64] | |
| Diagenesis model | 1-D sediment diagenesis model | Freshwater sediment | Dissolution, sulfidation | Dale et al. (2013) [65] | |
| GWAVA | Gridded probability distribution | Water | Dissolution | Dumont et al. (2015) [66] | |
| Nano DUFLOW | 1-D unsteady flow in open-channel systems | Water, sediment | Homoaggregation, heteroaggregation, dissolution | Quik et al. (2015) [58], Klein et al. (2016) [67] | |
| SOBEK river-DELWAQ | A model system integrates open channel hydraulics and water quality models | Water | Homoaggregation, heteroaggregation, dissolution | Markus et al. (2016) [68] | |
| WASP7–HSPF | Dynamic, mass-balance, spatially resolved differential fate and transport modeling framework | Water, sediment | Dissolution, sulfidation | Dale et al. (2015) [59] | |
| WASP8 | A detailed surface water quality model with ENM fate and transport processes | Water, sediment | Dissolution, sulfidation, heteroaggregation, photoreaction | Bouchard et al. (2017) [41], Han et al. (2019) [42] | |
| SWMM-EFDC | Suitable for urban stormwater and sewage systems; coupling both surface hydrology and hydrodynamic models | Water, sediment | Heteroaggregation, dissolution | Saharia et al. (2019) [69] |
2.2. Environmental Fate Models (EFMs)
2.2.1. Multimedia Compartmental Models (MCMs)
2.2.2. Spatial River/Watershed Models (SRWMs)
3. Engineered Nanomaterial (ENM) Fate Processes in Surface Waters
3.1. Aggregation
3.2. Dissolution
3.3. Sulfidation
3.4. Photoreaction
4. Path Forward
5. Summary
Funding
Conflicts of Interest
References
- European Commission. RECOMMENDATIONS: COMMISSION RECOMMENDATION of 18 October 2011 on the Definition of Nanomaterial (2011/696/EU). Off. J. Eur. Union 2011, 38–40. [Google Scholar]
- Dolez, P.I. Nanomaterials Definitions, Classifications, and Applications. Nanoengineering 2015, 3–40. [Google Scholar] [CrossRef]
- Auffan, M.; Rose, J.; Bottero, J.-Y.; Lowry, G.V.; Jolivet, J.-P.; Wiesner, M.R. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mei, L.; Chen, X.; Wang, Q. Recent Developments in Food Packaging Based on Nanomaterials. Nanomaterials 2018, 8, 830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fytianos, G.; Rahdar, A.; Kyzas, G.Z. Nanomaterials in Cosmetics: Recent Updates. Nanomaterials 2020, 10, 979. [Google Scholar] [CrossRef] [PubMed]
- AZoNano.com. Nanotechnology and Consumer Products—Opportunities for Nanotechnology in Consumer Products. Available online: https://www.azonano.com/article.aspx?ArticleID=2364 (accessed on 13 June 2020).
- Izzyfortiz. Nano-textiles: The Fabric of the Future. Sustainable Nano. Available online: http://sustainable-nano.com/2018/11/28/nano-textiles/ (accessed on 13 June 2020).
- Says, S.W.; AZoNano.com. Sport and Nanotechnology: Are the Big Sports Looking to Go Small? Available online: https://www.azonano.com/article.aspx?ArticleID=4859 (accessed on 13 June 2020).
- Alvarez, P.J.J.; Chan, C.K.; Elimelech, M.; Halas, N.J.; Villagrán, D. Emerging opportunities for nanotechnology to enhance water security. Nat. Nanotechnol. 2018, 13, 634–641. [Google Scholar] [CrossRef]
- Westerhoff, P.; Alvarez, P.; Li, Q.; Gardea-Torresdey, J.; Zimmerman, J. Overcoming implementation barriers for nanotechnology in drinking water treatment. Environ. Sci. Nano 2016, 3, 1241–1253. [Google Scholar] [CrossRef]
- Mauter, M.S.; Zucker, I.; Perreault, F.; Werber, J.R.; Kim, J.-H.; Elimelech, M. The role of nanotechnology in tackling global water challenges. Nat. Sustain. 2018, 1, 166–175. [Google Scholar] [CrossRef]
- Luo, C.; Ren, X.; Dai, Z.; Zhang, Y.; Qi, X.; Pan, C. Present Perspectives of Advanced Characterization Techniques in TiO2-Based Photocatalysts. ACS Appl. Mater. Interfaces 2017, 9, 23265–23286. [Google Scholar] [CrossRef]
- Azzouz, I.; Habba, Y.G.; Capochichi-Gnambodoe, M.; Marty, F.; Vial, J.; Leprince-Wang, Y.; Bourouina, T. Zinc oxide nano-enabled microfluidic reactor for water purification and its applicability to volatile organic compounds. Microsyst. Nanoeng. 2018, 4, 17093. [Google Scholar] [CrossRef]
- Singh, R.; Dutta, S. A review on H2 production through photocatalytic reactions using TiO2/TiO2-assisted catalysts. Fuel 2018, 220, 607–620. [Google Scholar] [CrossRef]
- Farooq, U.; Phul, R.; Alshehri, S.M.; Ahmed, J.; Ahmad, T. Electrocatalytic and Enhanced Photocatalytic Applications of Sodium Niobate Nanoparticles Developed by Citrate Precursor Route. Sci. Rep. 2019, 9, 4488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Zeng, H.; Chen, Y. Emerging Nano Drug Delivery Systems Targeting Cancer-Associated Fibroblasts for Improved Antitumor Effect and Tumor Drug Penetration. Mol. Pharm. 2020, 17, 1028–1048. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Khan, I.; Usman, M.; Khan, A.; Shah, S.S.; Khan, A.Z.; Saeed, K.; Yaseen, M.; Ehsan, M.F.; Tahir, M.N.; et al. Hematite and Magnetite Nanostructures for Green and Sustainable Energy Harnessing and Environmental Pollution Control: A Review. Chem. Res. Toxicol. 2020, 33. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G. Antimicrobial silver nanoparticles—Regulatory situation in the European Union. Mater. Today Proc. 2017, 4, S200–S207. [Google Scholar] [CrossRef]
- Potter, P.M.; Navratilova, J.; Rogers, K.R.; Al-Abed, S.R. Transformation of silver nanoparticle consumer products during simulated usage and disposal. Environ. Sci. Nano 2019, 6, 592–598. [Google Scholar] [CrossRef]
- AZoNano.com. Metal Oxide Nanoparticles—Are they Safe? Available online: https://www.azonano.com/article.aspx?ArticleID=5444 (accessed on 13 June 2020).
- Ahsan, M.d.A.; Jabbari, V.; Imam, M.A.; Castro, E.; Kim, H.; Curry, M.L.; Valles-Rosales, D.J.; Noveron, J.C. Nanoscale nickel metal organic framework decorated over graphene oxide and carbon nanotubes for water remediation. Sci.Total Environ. 2020, 698, 134214. [Google Scholar] [CrossRef]
- Ahsan, M.A.; Jabbari, V.; El-Gendy, A.A.; Curry, M.L.; Noveron, J.C. Ultrafast catalytic reduction of environmental pollutants in water via MOF-derived magnetic Ni and Cu nanoparticles encapsulated in porous carbon. Appl. Surf. Sci. 2019, 497, 143608. [Google Scholar] [CrossRef]
- Camboni, M.; Hanlon, J.; Pérez García, R.; Floyd, P.; European Chemicals Agency. A State of Play Study of the Market for so Called “Next Generation” Nanomaterials. 2019. Available online: https://op.europa.eu/publication/manifestation_identifier/PUB_ED0219746ENN (accessed on 11 March 2020).
- Calipinar, H.; Ulas, D. Development of Nanotechnology in the World and Nanotechnology Standards in Turkey. Procedia Comput. Sci. 2019, 158, 1011–1018. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, X.; Zhang, W.; Zeng, Z.; Liu, Z.; Zhang, C.; Liu, Y.; Shao, B.; Liang, Q.; Tang, W.; et al. Advances in the application, toxicity and degradation of carbon nanomaterials in environment: A review. Environ. Int. 2020, 134, 105298. [Google Scholar] [CrossRef]
- Romeo, D.; Salieri, B.; Hischier, R.; Nowack, B.; Wick, P. An integrated pathway based on in vitro data for the human hazard assessment of nanomaterials. Environ. Int. 2020, 137, 105505. [Google Scholar] [CrossRef] [PubMed]
- Sayre, P.G.; Steinhäuser, K.G.; van Teunenbroek, T. Methods and data for regulatory risk assessment of nanomaterials: Questions for an expert consultation. NanoImpact 2017, 8, 20–27. [Google Scholar] [CrossRef]
- Nowack, B. Evaluation of environmental exposure models for engineered nanomaterials in a regulatory context. NanoImpact 2017, 8, 38–47. [Google Scholar] [CrossRef]
- Gao, X.; Avellan, A.; Laughton, S.; Vaidya, R.; Rodrigues, S.M.; Casman, E.A.; Lowry, G.V. CuO Nanoparticle Dissolution and Toxicity to Wheat (Triticum aestivum) in Rhizosphere Soil. Environ. Sci. Technol. 2018, 52, 2888–2897. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.-C.; Westerhoff, P.; Posner, J.D. Biological accumulation of engineered nanomaterials: A review of current knowledge. Environ. Sci. Process. Impacts. 2013, 15, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Lead, J.R.; Batley, G.E.; Alvarez, P.J.J.; Croteau, M.-N.; Handy, R.D.; McLaughlin, M.J.; Judy, J.D.; Schirmer, K. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects-An updated review: Nanomaterials in the environment. Environ. Toxicol. Chem. 2018, 37, 2029–2063. [Google Scholar] [CrossRef]
- Johnston, L.J.; Gonzalez-Rojano, N.; Wilkinson, K.J.; Xing, B. Key challenges for evaluation of the safety of engineered nanomaterials. NanoImpact 2020, 18, 100219. [Google Scholar] [CrossRef]
- Dale, A.L.; Casman, E.A.; Lowry, G.V.; Lead, J.R.; Viparelli, E.; Baalousha, M. Modeling Nanomaterial Environmental Fate in Aquatic Systems. Environ. Sci. Technol. 2015, 49, 2587–2593. [Google Scholar] [CrossRef]
- Williams, R.J.; Harrison, S.; Keller, V.; Kuenen, J.; Lofts, S.; Praetorius, A.; Svendsen, C.; Vermeulen, L.C.; van Wijnen, J. Models for assessing engineered nanomaterial fate and behaviour in the aquatic environment. Curr. Opin. Environ. Sustain. 2019, 36, 105–115. [Google Scholar] [CrossRef]
- Praetorius, A.; Scheringer, M.; Hungerbühler, K. Development of Environmental Fate Models for Engineered Nanoparticles—A Case Study of TiO2 Nanoparticles in the Rhine River. Environ. Sci. Technol. 2012, 46, 6705–6713. [Google Scholar] [CrossRef]
- Meesters, J.A.J.; Koelmans, A.A.; Quik, J.T.K.; Hendriks, A.J.; van de Meent, D. Multimedia Modeling of Engineered Nanoparticles with SimpleBox4nano: Model Definition and Evaluation. Environ. Sci. Technol. 2014, 48, 5726–5736. [Google Scholar] [CrossRef] [PubMed]
- Lowry, G.V.; Gregory, K.B.; Apte, S.C.; Lead, J.R. Transformations of Nanomaterials in the Environment. Environ. Sci. Technol. 2012, 46, 6893–6899. [Google Scholar] [CrossRef] [PubMed]
- Praetorius, A.; Tufenkji, N.; Goss, K.-U.; Scheringer, M.; von der Kammer, F.; Elimelech, M. The road to nowhere: Equilibrium partition coefficients for nanoparticles. Environ. Sci. Nano 2014, 1, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Cornelis, G. Fate descriptors for engineered nanoparticles: The good, the bad, and the ugly. Environ. Sci. Nano 2015, 2, 19–26. [Google Scholar] [CrossRef]
- Baalousha, M.; Cornelis, G.; Kuhlbusch, T.A.J.; Lynch, I.; Nickel, C.; Peijnenburg, W.; van den Brink, N.W. Modeling nanomaterial fate and uptake in the environment: Current knowledge and future trends. Environ. Sci. Nano 2016, 3, 323–345. [Google Scholar] [CrossRef]
- Bouchard, D.; Knightes, C.; Chang, X.; Avant, B. Simulating Multiwalled Carbon Nanotube Transport in Surface Water Systems Using the Water Quality Analysis Simulation Program (WASP). Environ. Sci. Technol. 2017, 51, 11174–11184. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Knightes, C.D.; Bouchard, D.; Zepp, R.; Avant, B.; Hsieh, H.-S.; Chang, X.; Acrey, B.; Henderson, W.M.; Spear, J. Simulating graphene oxide nanomaterial phototransformation and transport in surface water. Environ. Sci. Nano 2019, 6, 180–194. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Environmental Remediation and Application of Nanoscale Zero-Valent Iron and Its Composites for the Removal of Heavy Metal Ions: A Review. Environ. Sci. Technol. 2016, 50, 7290–7304. [Google Scholar] [CrossRef]
- Krol, M.; Khan, U.T.; Asad, M.A. Factors affecting nano scale zero valent iron (nZVI) travel distance in heterogeneous groundwater aquifers: A statistical modeling approach. In Proceedings of the AGU Fall Meeting, Washington, DC, USA, 10–14 December 2018. [Google Scholar]
- Asad, M.A.; Krol, M.; Briggs, S. Nano zero valent iron (nZVI) remediation: A COMSOL modelling approach. In Proceedings of the Canadian Geotechnical Society (CGS) Conference (GeoEdmonton 2018), Edmonton, AB, Canada, 23–26 September 2018. [Google Scholar]
- Yu, Z.; Hu, L.; Lo, I.M.C. Transport of the arsenic (As)-loaded nano zero-valent iron in groundwater-saturated sand columns: Roles of surface modification and As loading. Chemosphere 2019, 216, 428–436. [Google Scholar] [CrossRef]
- Mueller, N.C.; Nowack, B. Exposure Modeling of Engineered Nanoparticles in the Environment. Environ. Sci. Technol. 2008, 42, 4447–4453. [Google Scholar] [CrossRef]
- Gottschalk, F.; Scholz, R.W.; Nowack, B. Probabilistic material flow modeling for assessing the environmental exposure to compounds: Methodology and an application to engineered nano-TiO2 particles. Environ. Model. Softw. 2010, 25, 320–332. [Google Scholar] [CrossRef]
- Sun, T.Y.; Conroy, G.; Donner, E.; Hungerbühler, K.; Lombi, E.; Nowack, B. Probabilistic modelling of engineered nanomaterial emissions to the environment: A spatio-temporal approach. Environ. Sci. Nano 2015, 2, 340–351. [Google Scholar] [CrossRef]
- Bornhöft, N.A.; Sun, T.Y.; Hilty, L.M.; Nowack, B. A dynamic probabilistic material flow modeling method. Environ. Model. Softw. 2016, 76, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Nowack, B. Dynamic probabilistic material flow analysis of nano-SiO2, nano iron oxides, nano-CeO2, nano-Al2O3, and quantum dots in seven European regions. Environ. Pollut. 2018, 235, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Scheringer, M.; Praetorius, A.; Goldberg, E.S. Environmental Fate and Exposure Modeling of Nanomaterials. Front. Nanosci. 2014, 7, 89–125. [Google Scholar] [CrossRef]
- Di Guardo, A.; Gouin, T.; MacLeod, M.; Scheringer, M. Environmental fate and exposure models: Advances and challenges in 21st century chemical risk assessment. Environ. Sci. Process. Impacts 2018, 20, 58–71. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.H.; Cohen, Y. Multimedia Environmental Distribution of Engineered Nanomaterials. Environ. Sci. Technol. 2014, 48, 3281–3292. [Google Scholar] [CrossRef]
- Meesters, J.A.J.; Quik, J.T.K.; Koelmans, A.A.; Hendriks, A.J.; van de Meent, D. Multimedia environmental fate and speciation of engineered nanoparticles: A probabilistic modeling approach. Environ. Sci. Nano 2016, 3, 715–727. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.H.; Bilal, M.; Lazareva, A.; Keller, A.; Cohen, Y. Simulation tool for assessing the release and environmental distribution of nanomaterials. Beilstein J. Nanotechnol. 2015, 6, 938–951. [Google Scholar] [CrossRef] [Green Version]
- Garner, K.L.; Suh, S.; Keller, A.A. Assessing the Risk of Engineered Nanomaterials in the Environment: Development and Application of the nanoFate Model. Environ. Sci. Technol. 2017, 51, 5541–5551. [Google Scholar] [CrossRef] [Green Version]
- Quik, J.T.K.; de Klein, J.J.M.; Koelmans, A.A. Spatially explicit fate modelling of nanomaterials in natural waters. Water Res. 2015, 80, 200–208. [Google Scholar] [CrossRef]
- Dale, A.L.; Lowry, G.V.; Casman, E.A. Stream Dynamics and Chemical Transformations Control the Environmental Fate of Silver and Zinc Oxide Nanoparticles in a Watershed-Scale Model. Environ. Sci. Technol. 2015, 49, 7285–7293. [Google Scholar] [CrossRef] [PubMed]
- ECHA. Guidance on Information Requirements and Chemical Safety Assessment Chapter R.16: Environmental Exposure Assessment, Version 3.0—February 2016; ISBN 978-92-9247-775-2. European Chemicals Agency (ECHA): Helsinki, Finland, 2016. [Google Scholar]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Modeled Environmental Concentrations of Engineered Nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for Different Regions. Environ. Sci. Technol. 2009, 43, 9216–9222. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, F.; Ort, C.; Scholz, R.W.; Nowack, B. Engineered nanomaterials in rivers—Exposure scenarios for Switzerland at high spatial and temporal resolution. Environ. Pollut. 2011, 159, 3439–3445. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.Y.; Bornhöft, N.A.; Hungerbühler, K.; Nowack, B. Dynamic Probabilistic Modeling of Environmental Emissions of Engineered Nanomaterials. Environ. Sci. Technol. 2016, 50, 4701–4711. [Google Scholar] [CrossRef]
- Sani-Kast, N.; Scheringer, M.; Slomberg, D.; Labille, J.; Praetorius, A.; Ollivier, P.; Hungerbühler, K. Addressing the complexity of water chemistry in environmental fate modeling for engineered nanoparticles. Sci. Total. Environ. 2015, 535, 150–159. [Google Scholar] [CrossRef]
- Dale, A.L.; Lowry, G.V.; Casman, E.A. Modeling Nanosilver Transformations in Freshwater Sediments. Environ. Sci. Technol. 2013, 47, 12920–12928. [Google Scholar] [CrossRef]
- Dumont, E.; Johnson, A.C.; Keller, V.D.J.; Williams, R.J. Nano silver and nano zinc-oxide in surface waters —Exposure estimation for Europe at high spatial and temporal resolution. Environ. Pollut. 2015, 196, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Klein, J.J.M.; de Quik, J.T.K.; Bäuerlein, P.S.; Koelmans, A.A. Towards validation of the NanoDUFLOW nanoparticle fate model for the river Dommel, The Netherlands. Environ. Sci. Nano 2016, 3, 434–441. [Google Scholar] [CrossRef]
- Markus, A.A.; Parsons, J.R.; Roex, E.W.M.; de Voogt, P.; Laane, R.W.P.M. Modelling the transport of engineered metallic nanoparticles in the river Rhine. Water Res. 2016, 91, 214–224. [Google Scholar] [CrossRef]
- Saharia, A.M.; Zhu, Z.; Aich, N.; Baalousha, M.; Atkinson, J.F. Modeling the transport of titanium dioxide nanomaterials from combined sewer overflows in an urban river. Sci. Total Environ. 2019, 696, 133904. [Google Scholar] [CrossRef]
- Shoemaker, L.; Dai, T.; Koenig, J. TMDL Model Evaluation and Research Needs; EPA/600/R-05/149; National Risk Management Research Laboratory, Office Of Research And Development, U.S. Environmental Protection Agency: Cincinnati, OH, USA, 2005.
- Di Toro, D.M.; Mahony, J.D.; Hansen, D.J.; Berry, W.J. A model of the oxidation of iron and cadmium sulfide in sediments. Environ. Toxicol. Chem. 1996, 15, 2168–2186. [Google Scholar] [CrossRef]
- Hou, W.-C.; Stuart, B.; Howes, R.; Zepp, R.G. Sunlight-Driven Reduction of Silver Ions by Natural Organic Matter: Formation and Transformation of Silver Nanoparticles. Environ. Sci. Technol. 2013, 47, 7713–7721. [Google Scholar] [CrossRef]
- Singh, A.; Hou, W.-C.; Lin, T.-F.; Zepp, R.G. Roles of Silver–Chloride Complexations in Sunlight-Driven Formation of Silver Nanoparticles. Environ. Sci. Technol. 2019, 53, 11162–11169. [Google Scholar] [CrossRef] [PubMed]
- Elimelech, M.; Gregory, J.; Jia, X.; Williams, R.A. Particle Deposition and Aggregation: Measurement, Modelling and Simulation; Butterworth-Heinemann: Oxford, UK, 1998. [Google Scholar]
- Petosa, A.R.; Jaisi, D.P.; Quevedo, I.R.; Elimelech, M.; Tufenkji, N. Aggregation and Deposition of Engineered Nanomaterials in Aquatic Environments: Role of Physicochemical Interactions. Environ. Sci. Technol. 2010, 44, 6532–6549. [Google Scholar] [CrossRef] [Green Version]
- Quik, J.T.K.; Velzeboer, I.; Wouterse, M.; Koelmans, A.A.; van de Meent, D. Heteroaggregation and sedimentation rates for nanomaterials in natural waters. Water Res. 2014, 48, 269–279. [Google Scholar] [CrossRef]
- Velzeboer, I.; Quik, J.T.K.; van de Meent, D.; Koelmans, A.A. Rapid settling of nanoparticles due to heteroaggregation with suspended sediment: Nanoparticle settling due to heteroaggregation with sediment. Environ. Toxicol. Chem. 2014, 33, 1766–1773. [Google Scholar] [CrossRef]
- Bouchard, D.; Chang, X.; Chowdhury, I. Heteroaggregation of multiwalled carbon nanotubes with sediments. Environ. Nanotechnol. Monit. Manag. 2015, 4, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Clavier, A.; Praetorius, A.; Stoll, S. Determination of nanoparticle heteroaggregation attachment efficiencies and rates in presence of natural organic matter monomers. Monte Carlo modelling. Sci. Total Environ. 2019, 650, 530–540. [Google Scholar] [CrossRef] [Green Version]
- Praetorius, A.; Badetti, E.; Brunelli, A.; Clavier, A.; Gallego-Urrea, J.A.; Gondikas, A.; Hassellöv, M.; Hofmann, T.; Mackevica, A.; Marcomini, A.; et al. Strategies for determining heteroaggregation attachment efficiencies of engineered nanoparticles in aquatic environments. Environ. Sci. Nano 2020, 7, 351–367. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Dai, Y.; Wang, Z.; Ren, W.; Wei, Y.; Cao, X.; Xing, B. Toxicity of GO to Freshwater Algae in the Presence of Al2 O3 Particles with Different Morphologies: Importance of Heteroaggregation. Environ. Sci. Technol. 2018, 52, 13448–13456. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Dang, F.; Liu, C.; Wang, D.-J.; Cui, P.-X.; Yan, H.-J.; Zhou, D.-M. Heteroaggregation and dissolution of silver nanoparticles by iron oxide colloids under environmentally relevant conditions. Environ. Sci. Nano 2019, 6, 195–206. [Google Scholar] [CrossRef]
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative Toxicity of Nanoparticulate ZnO, Bulk ZnO, and ZnCl2 to a Freshwater Microalga (Pseudokirchneriella subcapitata): The Importance of Particle Solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef]
- Liu, J.; Hurt, R.H. Ion Release Kinetics and Particle Persistence in Aqueous Nano-Silver Colloids. Environ. Sci. Technol. 2010, 44, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.-W.; Mudunkotuwa, I.A.; Rupasinghe, T.; Grassian, V.H. Aggregation and Dissolution of 4 nm ZnO Nanoparticles in Aqueous Environments: Influence of pH, Ionic Strength, Size, and Adsorption of Humic Acid. Langmuir 2011, 27, 6059–6068. [Google Scholar] [CrossRef] [PubMed]
- Peretyazhko, T.S.; Zhang, Q.; Colvin, V.L. Size-Controlled Dissolution of Silver Nanoparticles at Neutral and Acidic pH Conditions: Kinetics and Size Changes. Environ. Sci. Technol. 2014, 48, 11954–11961. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, J.; Blomberg, E.; Odnevall Wallinder, I. In the Search for Nanospecific Effects of Dissolution of Metallic Nanoparticles at Freshwater-Like Conditions: A Critical Review. Environ. Sci. Technol. 2019, 53, 4030–4044. [Google Scholar] [CrossRef]
- Petersen, E.J.; Huang, Q.; Weber, W.J. Relevance of octanol-water distribution measurements to the potential ecological uptake of multi-walled carbon nanotubes. Environ. Toxicol. Chem. 2010, 29, 1106–1112. [Google Scholar] [CrossRef] [Green Version]
- Jafvert, C.T.; Kulkarni, P.P. Buckminsterfullerene’s (C60) Octanol−Water Partition Coefficient (Kow) and Aqueous Solubility. Environ. Sci. Technol. 2008, 42, 5945–5950. [Google Scholar] [CrossRef]
- Kaegi, R.; Voegelin, A.; Sinnet, B.; Zuleeg, S.; Hagendorfer, H.; Burkhardt, M.; Siegrist, H. Behavior of Metallic Silver Nanoparticles in a Pilot Wastewater Treatment Plant. Environ. Sci. Technol. 2011, 45, 3902–3908. [Google Scholar] [CrossRef]
- Kaegi, R.; Voegelin, A.; Ort, C.; Sinnet, B.; Thalmann, B.; Krismer, J.; Hagendorfer, H.; Elumelu, M.; Mueller, E. Fate and transformation of silver nanoparticles in urban wastewater systems. Water Res. 2013, 47, 3866–3877. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Levard, C.; Judy, J.D.; Unrine, J.M.; Durenkamp, M.; Martin, B.; Jefferson, B.; Lowry, G.V. Fate of Zinc Oxide and Silver Nanoparticles in a Pilot Wastewater Treatment Plant and in Processed Biosolids. Environ. Sci. Technol. 2014, 48, 104–112. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, F.; Allen, A.J.; Johnston-Peck, A.C.; Pettibone, J.M. Comparing sulfidation kinetics of silver nanoparticles in simulated media using direct and indirect measurement methods. Nanoscale 2018, 10, 22270–22279. [Google Scholar] [CrossRef]
- Liu, J.; Pennell, K.G.; Hurt, R.H. Kinetics and Mechanisms of Nanosilver Oxysulfidation. Environ. Sci. Technol. 2011, 45, 7345–7353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Guo, W.; Li, Q.; Wang, Z.; Liu, S. The effects and the potential mechanism of environmental transformation of metal nanoparticles on their toxicity in organisms. Environ. Sci. Nano 2018, 5, 2482–2499. [Google Scholar] [CrossRef]
- He, D.; Garg, S.; Wang, Z.; Li, L.; Rong, H.; Ma, X.; Li, G.; An, T.; Waite, T.D. Silver sulfide nanoparticles in aqueous environments: Formation, transformation and toxicity. Environ. Sci. Nano 2019, 6, 1674–1687. [Google Scholar] [CrossRef]
- Hou, W.-C.; Jafvert, C.T. Photochemical Transformation of Aqueous C60 Clusters in Sunlight. Environ. Sci. Technol. 2009, 43, 362–367. [Google Scholar] [CrossRef]
- Kong, L.; Tedrow, O.; Chan, Y.F.; Zepp, R.G. Light-Initiated Transformations of Fullerenol in Aqueous Media. Environ. Sci. Technol. 2009, 43, 9155–9160. [Google Scholar] [CrossRef]
- Isaacson, C.W.; Bouchard, D. Asymmetric flow field flow fractionation of aqueous C60 nanoparticles with size determination by dynamic light scattering and quantification by liquid chromatography atmospheric pressure photo-ionization mass spectrometry. J. Chromatogr. A 2010, 1217, 1506–1512. [Google Scholar] [CrossRef]
- Hou, W.-C.; Huang, S.-H. Photochemical reactivity of aqueous fullerene clusters: C60 versus C70. J. Hazard. Mater. 2017, 322, 310–317. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.J.; Li, Q. Photochemical Transformation of Carboxylated Multiwalled Carbon Nanotubes: Role of Reactive Oxygen Species. Environ. Sci. Technol. 2013, 47, 14080–14088. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.-C.; BeigzadehMilani, S.; Jafvert, C.T.; Zepp, R.G. Photoreactivity of Unfunctionalized Single-Wall Carbon Nanotubes Involving Hydroxyl Radical: Chiral Dependency and Surface Coating Effect. Environ. Sci. Technol. 2014, 48, 3875–3882. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Zepp, R.G. Probing Photosensitization by Functionalized Carbon Nanotubes. Environ. Sci. Technol. 2015, 49, 13835–13843. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.-C.; Chowdhury, I.; Goodwin, D.G.; Henderson, W.M.; Fairbrother, D.H.; Bouchard, D.; Zepp, R.G. Photochemical Transformation of Graphene Oxide in Sunlight. Environ. Sci. Technol. 2015, 49, 3435–3443. [Google Scholar] [CrossRef]
- Hou, W.-C.; Henderson, W.M.; Chowdhury, I.; Goodwin, D.G., Jr.; Chang, X.; Martin, S.; Fairbrother, D.H.; Bouchard, D.; Zepp, R.G. The contribution of indirect photolysis to the degradation of graphene oxide in sunlight. Carbon 2016, 110, 426–437. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, J.; Jiang, G. Sunlight-Induced Reduction of Ionic Ag and Au to Metallic Nanoparticles by Dissolved Organic Matter. ACS Nano 2012, 6, 7910–7919. [Google Scholar] [CrossRef]
- Lee, T.-W.; Chen, C.-C.; Chen, C. Chemical Stability and Transformation of Molybdenum Disulfide Nanosheets in Environmental Media. Environ. Sci. Technol. 2019, 53, 6282–6291. [Google Scholar] [CrossRef]
- Hou, W.-C.; Kong, L.; Wepasnick, K.A.; Zepp, R.G.; Fairbrother, D.H.; Jafvert, C.T. Photochemistry of Aqueous C60 Clusters: Wavelength Dependency and Product Characterization. Environ. Sci. Technol. 2010, 44, 8121–8127. [Google Scholar] [CrossRef]
- Hou, W.-C.; Lee, P.-L.; Chou, Y.-C.; Wang, Y.-S. Antibacterial property of graphene oxide: The role of phototransformation. Environ. Sci. Nano 2017, 4, 647–657. [Google Scholar] [CrossRef]
- Chowdhury, I.; Hou, W.-C.; Goodwin, D.; Henderson, M.; Zepp, R.G.; Bouchard, D. Sunlight affects aggregation and deposition of graphene oxide in the aquatic environment. Water Res. 2015, 78, 37–46. [Google Scholar] [CrossRef]
- Fanourakis, S.K.; Peña-Bahamonde, J.; Bandara, P.C.; Rodrigues, D.F. Nano-based adsorbent and photocatalyst use for pharmaceutical contaminant removal during indirect potable water reuse. NPJ Clean Water 2020, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Meesters, J.A.J.; Peijnenburg, W.J.G.M.; Hendriks, A.J.; Van de Meent, D.; Quik, J.T.K. A model sensitivity analysis to determine the most important physicochemical properties driving environmental fate and exposure of engineered nanoparticles. Environ. Sci. Nano 2019, 6, 2049–2060. [Google Scholar] [CrossRef]
- Nowack, B.; David, R.M.; Fissan, H.; Morris, H.; Shatkin, J.A.; Stintz, M.; Zepp, R.; Brouwer, D. Potential release scenarios for carbon nanotubes used in composites. Environ. Int. 2013, 59, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Harper, S.; Wohlleben, W.; Doa, M.; Nowack, B.; Clancy, S.; Canady, R.; Maynard, A. Measuring Nanomaterial Release from Carbon Nanotube Composites: Review of the State of the Science. J. Phys. Conf. Ser. 2015, 617, 012026. [Google Scholar] [CrossRef] [Green Version]
- Wohlleben, W.; Neubauer, N. Quantitative rates of release from weathered nanocomposites are determined across 5 orders of magnitude by the matrix, modulated by the embedded nanomaterial. NanoImpact 2016, 1, 39–45. [Google Scholar] [CrossRef]
- Wohlleben, W.; Kingston, C.; Carter, J.; Sahle-Demessie, E.; Vázquez-Campose, S.; Acrey, B.; Chen, C.-Y.; Walton, E.; Egenolf, H.; Müller, P.; et al. NanoRelease: Pilot interlaboratory comparison of a weathering protocol applied to resilient and labile polymers with and without embedded carbon nanotubes. Carbon 2017, 113, 346–360. [Google Scholar] [CrossRef]
- Praetorius, A.; Arvidsson, R.; Molander, S.; Scheringer, M. Facing complexity through informed simplifications: A research agenda for aquatic exposure assessment of nanoparticles. Env. Sci. Process. Impacts 2013, 15, 161–168. [Google Scholar] [CrossRef]
- Geitner, N.K.; Bossa, N.; Wiesner, M.R. Formulation and Validation of a Functional Assay-Driven Model of Nanoparticle Aquatic Transport. Environ. Sci. Technol. 2019, 53, 3104–3109. [Google Scholar] [CrossRef]
- Stegemeier, J.P.; Avellan, A.; Lowry, G.V. Effect of Initial Speciation of Copper- and Silver-Based Nanoparticles on Their Long-Term Fate and Phytoavailability in Freshwater Wetland Mesocosms. Environ. Sci. Technol. 2017, 51, 12114–12122. [Google Scholar] [CrossRef]
- Ambrose, B.; Avant, B.; Han, Y.; Knightes, C.; Wool, T. Water Quality Assessment Simulation Program (WASP8): Upgrades to the Advanced Toxicant Module for Simulating Dissolved Chemicals, Nanomaterials, and Solids; EPA/600/R-17/326; Office of Research and Development, National Exposure Research Laboratory, U.S. Environmental Protection Agency: Washington, DC, USA, 2017.
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhendra, E.; Chang, C.-H.; Hou, W.-C.; Hsieh, Y.-C. A Review on the Environmental Fate Models for Predicting the Distribution of Engineered Nanomaterials in Surface Waters. Int. J. Mol. Sci. 2020, 21, 4554. https://doi.org/10.3390/ijms21124554
Suhendra E, Chang C-H, Hou W-C, Hsieh Y-C. A Review on the Environmental Fate Models for Predicting the Distribution of Engineered Nanomaterials in Surface Waters. International Journal of Molecular Sciences. 2020; 21(12):4554. https://doi.org/10.3390/ijms21124554
Chicago/Turabian StyleSuhendra, Edward, Chih-Hua Chang, Wen-Che Hou, and Yi-Chin Hsieh. 2020. "A Review on the Environmental Fate Models for Predicting the Distribution of Engineered Nanomaterials in Surface Waters" International Journal of Molecular Sciences 21, no. 12: 4554. https://doi.org/10.3390/ijms21124554
APA StyleSuhendra, E., Chang, C.-H., Hou, W.-C., & Hsieh, Y.-C. (2020). A Review on the Environmental Fate Models for Predicting the Distribution of Engineered Nanomaterials in Surface Waters. International Journal of Molecular Sciences, 21(12), 4554. https://doi.org/10.3390/ijms21124554






