Antimicrobial Peptides Display Strong Synergy with Vancomycin Against Vancomycin-Resistant E. faecium, S. aureus, and Wild-Type E. coli
Abstract
:1. Introduction
2. Results
2.1. Antimicrobial Activity
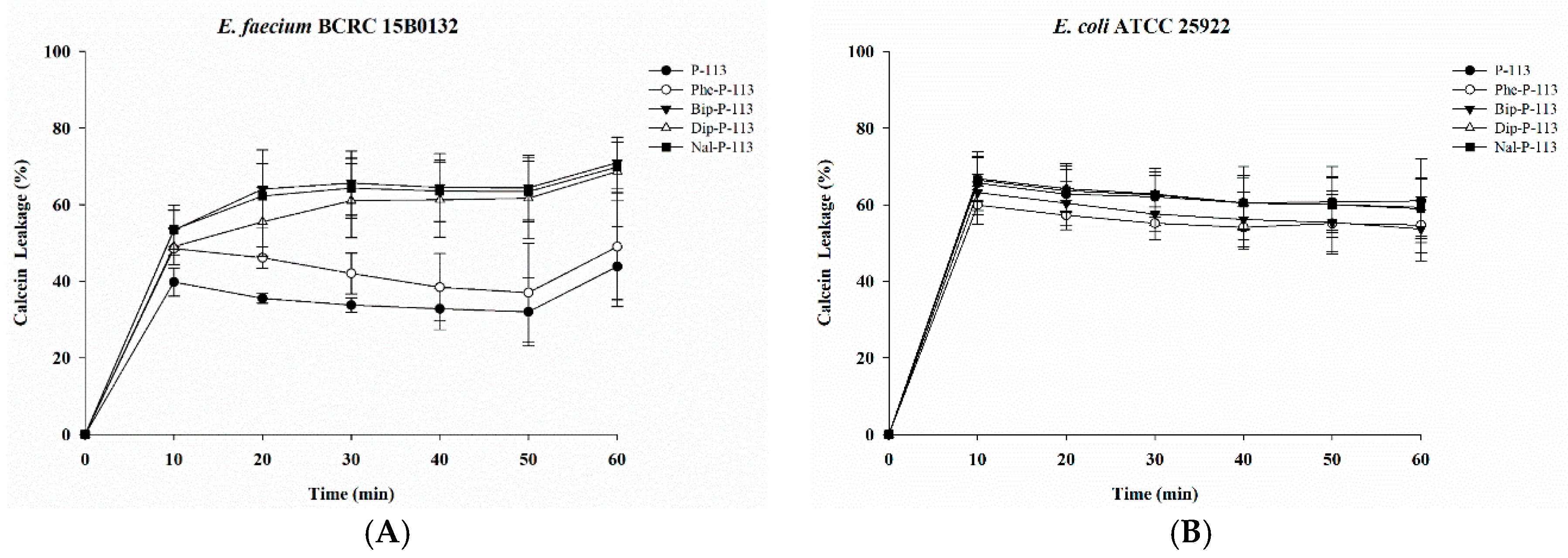
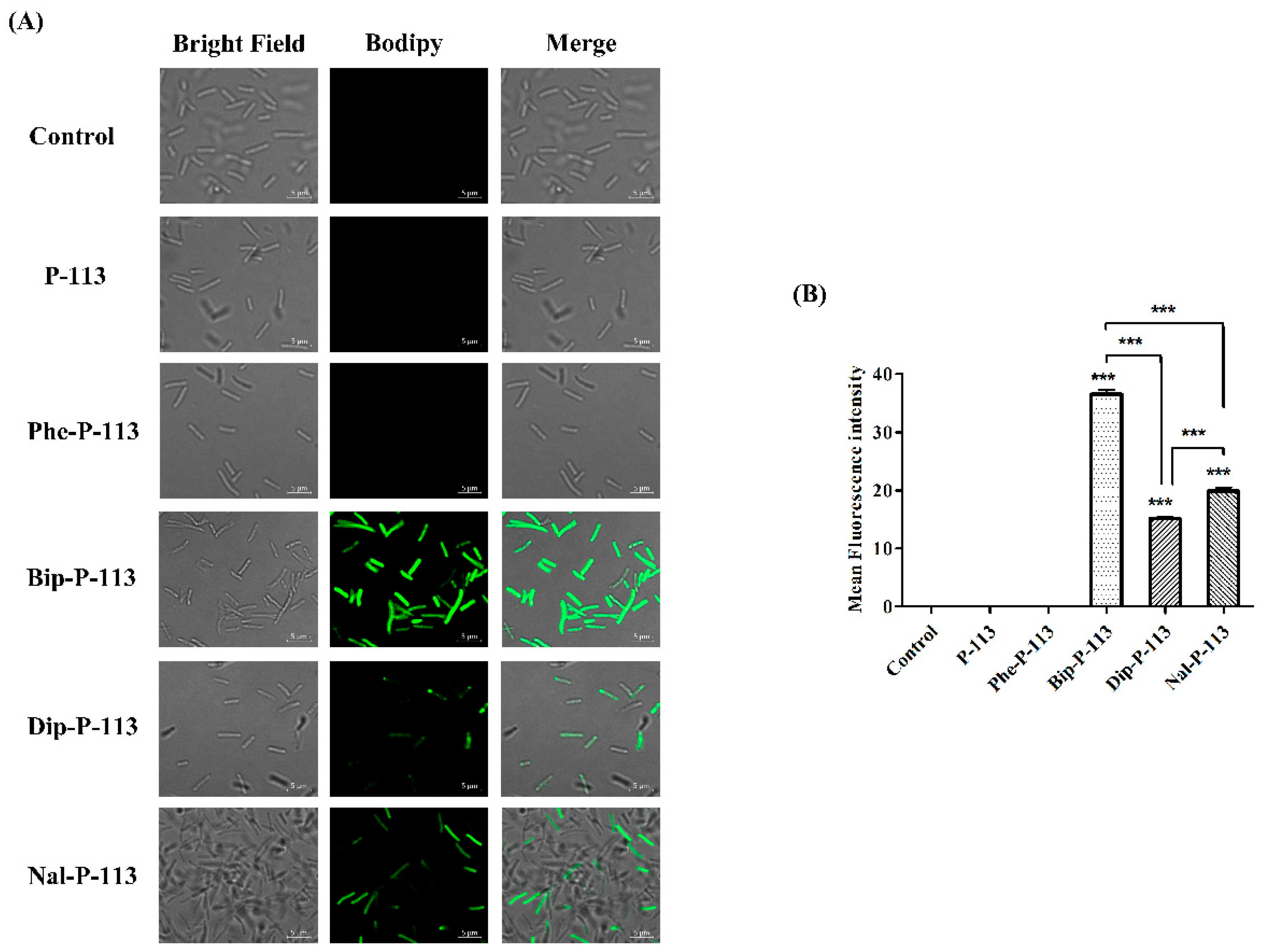
2.2. Bacterial Membrane Permeabilization
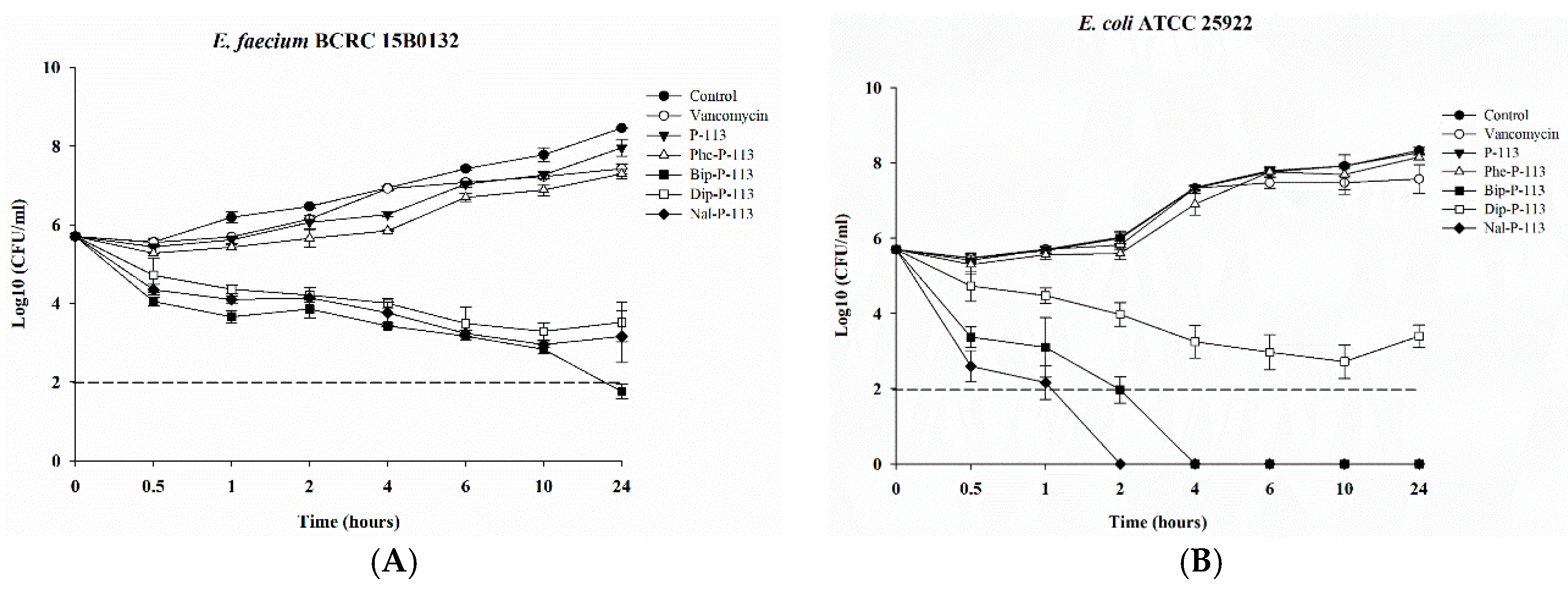
2.3. Bacterial Killing Kinetics
2.4. Synergistic Effect with Vancomycin in the Presence of a Sub-Inhibitory Concentration (¼ ×MIC) of Peptides
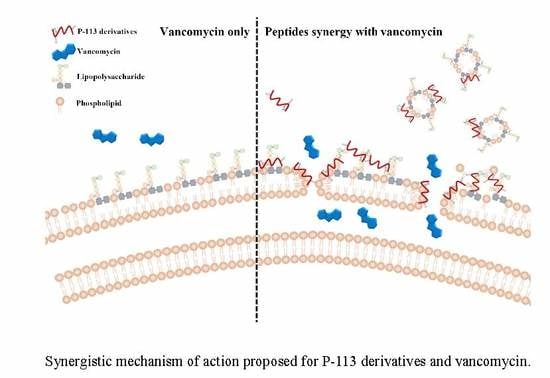
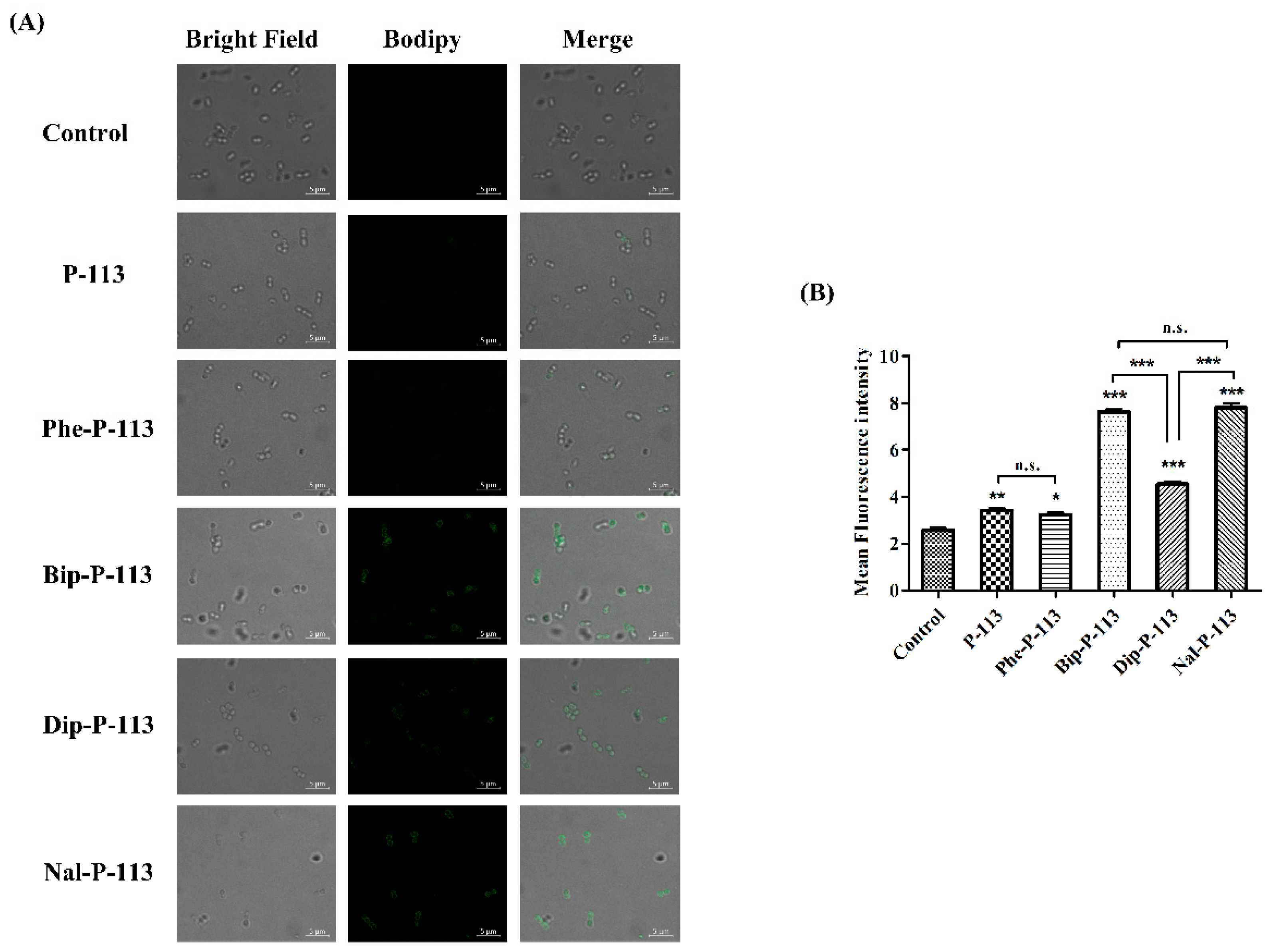
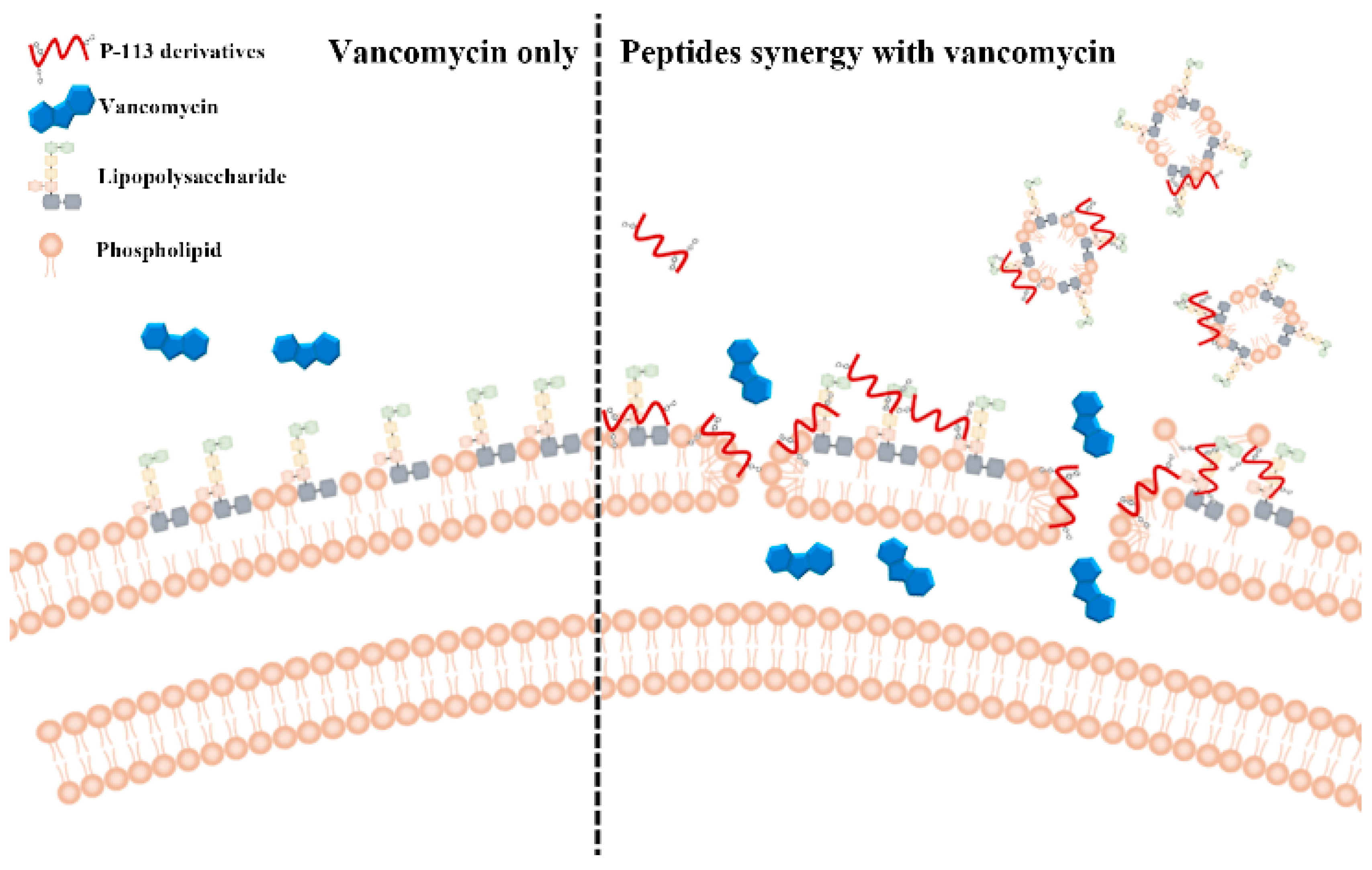
2.5. Mechanism of Resensitization of Bacteria to Vancomycin by P-113 and its Derivatives
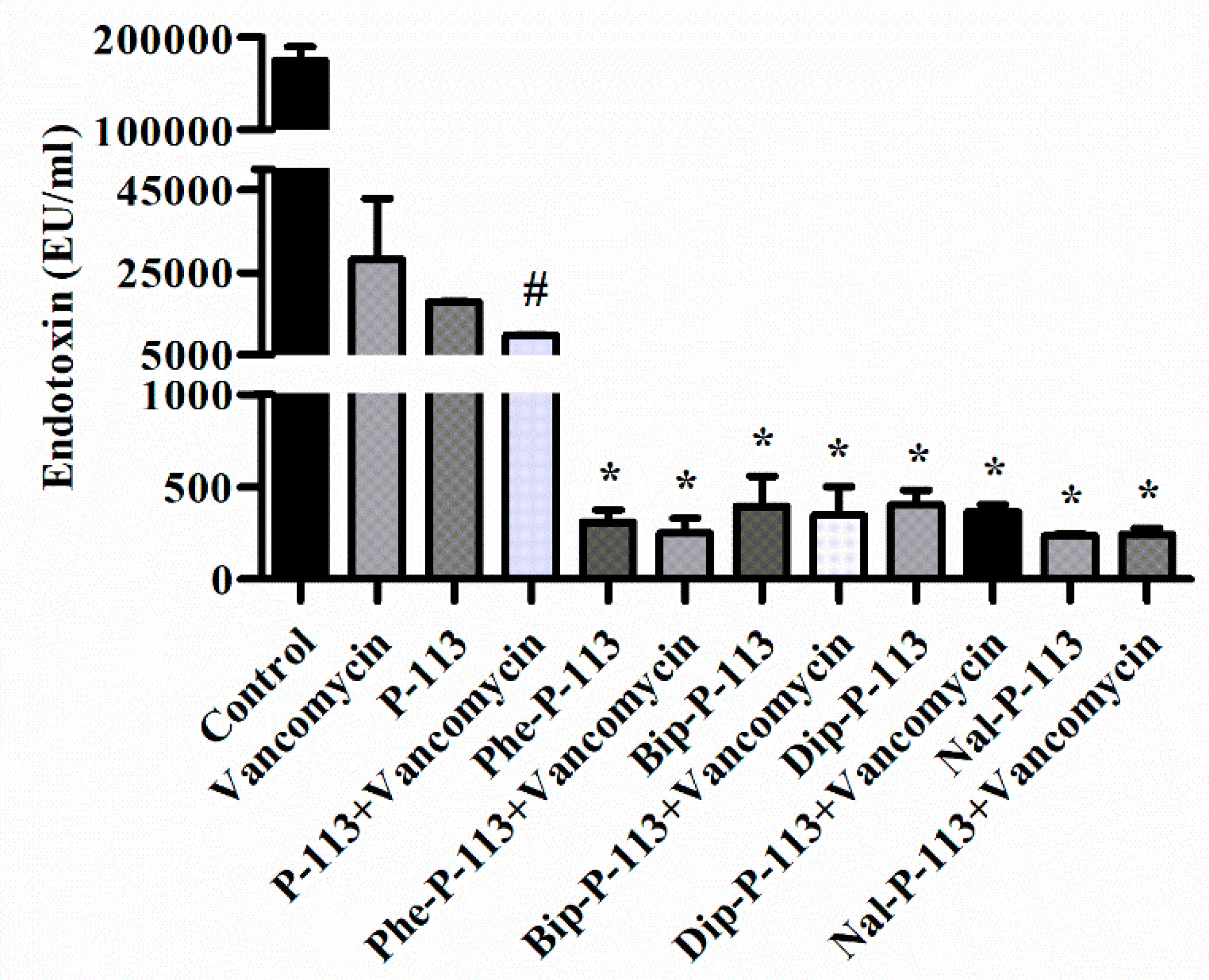
2.6. P-113 and its Derivatives Attenuate Vancomycin-Induced LPS Release
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Bacterial Strains and Culture Conditions
4.3. Serial Passage
4.4. Antimicrobial Activity Assay
4.5. Checkerboard Assay
4.6. Confocal Laser Scanning Microscopy
4.7. Calcein Leakage Assay
4.8. Time Killing Assay
4.9. Anti-Endotoxin Studies
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marston, H.D.; Dixon, D.M.; Knisely, J.M.; Palmore, T.N.; Fauci, A.S. Antimicrobial resistance. JAMA 2016, 316, 1193–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritsche, T.R.; Rhomberg, P.R.; Sader, H.S.; Jones, R.N. In vitro activity of omiganan pentahydrochloride tested against vancomycin-tolerant -intermediate, and -resitant Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 2008, 60, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J.M.; French, S.; Ovchinnikova, O.G.; Bouwman, C.; Whitfield, C.; Brown, E.D. Cold stress makes Escherichia colisusceptible to glycopeptide antibiotics by altering outer membrane integrity. Cell Chem. Biol. 2016, 23, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Chih, Y.H.; Lin, Y.S.; Yip, B.S.; Wei, H.J.; Chu, H.L.; Yu, H.Y.; Cheng, H.T.; Chou, Y.T.; Cheng, J.W. Ultrashort antimicrobial peptides with antiendotoxin properties. Antimcrob. Agents Chemother. 2015, 59, 5052–5056. [Google Scholar] [CrossRef] [Green Version]
- Chu, H.L.; Yu, H.Y.; Yip, B.S.; Chih, Y.H.; Liang, C.W.; Cheng, H.T.; Cheng, J.W. Boosting salt resistance of short antimicrobial peptides. Antimcrob. Agents Chemother. 2013, 57, 4050–4052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.Y.; Chen, Y.A.; Yip, B.S.; Wang, S.Y.; Wei, H.J.; Chih, Y.H.; Chen, K.H.; Cheng, J.W. Role of b-naphthylalanine end-tags in the enhancement of antiendotoxin activities: Solution structure of the antimicrobial peptide S1-Nal-Nal in complex with lipopolysaccharide. Biochim. Biophys. Acta 2017, 1859, 1114–1123. [Google Scholar] [CrossRef]
- Yu, H.Y.; Tu, C.H.; Yip, B.S.; Chen, H.L.; Cheng, H.T.; Huang, K.C.; Lo, H.J.; Cheng, J.W. Easy strategy to increase salt resistance of antimicrobial peptides. Antimicrob. Agents Chemother. 2011, 55, 4918–4921. [Google Scholar] [CrossRef] [Green Version]
- Chih, Y.H.; Wang, S.Y.; Yip, B.S.; Cheng, K.T.; Hsu, S.Y.; Wu, C.L.; Yu, H.Y.; Cheng, J.W. Dependence on size and shape of non-nature amino acids in the enhancement of lipopolysaccharide (LPS) neutralizing activities of antimicrobial peptides. J. Colloid Interface Sci. 2019, 533, 492–502. [Google Scholar] [CrossRef]
- Corbett, D.; Wise, A.; Langley, T.; Skinner, K.; Trimby, E.; Birchall, S.; Dorali, A.; Sandiford, S.; Williams, J.; Warn, P.; et al. Potentiation of Antibiotic Activity by a Novel Cationic Peptide: Potency and Spectrum of Activity of SPR741. Antimcrob. Agents Chemother. 2017, 61, e00200-17. [Google Scholar] [CrossRef] [Green Version]
- Naghmouchi, K.; Baah, J.; Hober, D.; Jouy, E.; Rubrecht, C.; Sane, F.; Drider, D. Synergistic effect between Colistin and Bacteriocins in controlling Gram-negative pathogens and their potential to reduce antibiotic toxicity in mammalian epithelial cells. Antimcrob. Agents Chemother. 2013, 57, 2719–2725. [Google Scholar] [CrossRef] [Green Version]
- Morones-Ramirez, J.R.; Winkler, J.A.; Spina, C.S.; Collins, J.J. Silver enhances antibiotics activity against gram-negative bacteria. Sci. Transl Med. 2013, 5, 190ra81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zharkova, M.S.; Orlov, D.S.; Golubeva, O.Y.; Chakchir, O.B.; Eliseev, I.E.; Grinchuk, T.M.; Shamova, O.V. Application of antimicrobial peptides of the innate immune system in combination with conventional antibiotics-A novel way to combat antibiotic resistance. Front. Cell Infect. Microbiol. 2019, 9, 128. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.; Huang, Y.; Chen, M.; Li, G.; Chen, Y. Functional synergy of a-helical antimicrobial peptides and traditional antibiotics against Gram-negative and Gram-positive bacteria in vitro and in vivo. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 197–204. [Google Scholar] [CrossRef]
- Li, D.; Yang, Y.; Tian, Z.; Lv, J.; Sun, F.; Wang, Q.; Liu, Y.; Xia, P. Synergistic antibiotic effect of looped antimicrobial peptide CLP-19 with bactericidal and bacteriostatic agents. Oncotarget 2017, 8, 55958–55966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trautmann, M.; Heinemann, M.; Zick, R.; Moricke, A.; Seidelmann, M.; Berger, D. Antibacterial activity of Meropenem against Pseudomonas aeruginosa, including antibiotic-induced morphological changes and endotoxin-liberating effects. Eur. J. Clin. Microbiol. Infect. Dis. 1998, 17, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Roslansky, P.F.; Novitsky, T.J. Sensitivity of Limulus amebocyte lysate (LAL) to LAL-reactive glucans. J. Clin. Microbiol. 1991, 29, 2477–2483. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Gao, H.; Tang, M.; Gu, J.; Xia, P.; Xiao, G. Lipopolysaccharide (LPS) detoxification of analogue peptides derived from limulus anti-LPS factor. Peptides 2010, 31, 1853–1859. [Google Scholar] [CrossRef]
- Srivastava, S.; Ghosh, J.K. Introduction of a lysine residue promotes aggregation of temporin L in lipopolysaccharides and augmentation of its antiendotoxin property. Antimicrob. Agents Chemother. 2013, 57, 2457–2466. [Google Scholar] [CrossRef] [Green Version]
- Jacob, B.; Park, I.S.; Bang, J.K.; Shin, S.Y. Short KR-12 analogs designed from human cathelicidin LL-37 possessing both antimicrobial and antiendotoxic activities without mammalian cell toxicity. J. Pept. Sci. 2013, 19, 700–707. [Google Scholar] [CrossRef]
- Bagheri, M.; Keller, S.; Dathe, M. Interaction of W-Substituted Analogs of Cyclo-RRRWFW with Bacterial Lipopolysaccharides: The Role of the Aromatic Cluster in Antimicrobial Activity. Antimcrob. Agents Chemother. 2011, 55, 788–797. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.L. Antimicrobial peptides stage a come back. Nat. Biotech. 2013, 31, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Bisht, G.S.; Rawat, D.S.; Kumar, A.; Kumar, R.; Maiti, S.; Pasha, S. Interaction studies of novel cell selective antimicrobial peptides with model membranes and E. coli ATCC 11775. Biochim. Biophys. Acta 2010, 1798, 1864–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Name | Chemical Structure a | Sequence b | Molecular Weight (Da) |
|---|---|---|---|
| P-113 |  | Ac- A K R His His G Y K R K F His -NH2 | 1605.86 |
| Phe-P-113 |  | Ac- A K R Phe Phe G Y K R K F Phe -NH2 | 1635.99 |
| Bip-P-113 |  | Ac- A K R Bip Bip G Y K R K F Bip -NH2 | 1864.97 |
| Dip-P-113 |  | Ac- A K R Dip Dip G Y K R K F Dip -NH2 | 1864.06 |
| Nal-P-113 |  | Ac- A K R Nal Nal G Y K R K F Nal -NH2 | 1786.03 |
| Bacterial Strains | MIC (μg/mL) | |||||
|---|---|---|---|---|---|---|
| Vancomycin | P-113 | Phe- P-113 | Bip- P-113 | Dip- P-113 | Nal- P-113 | |
| E. faecium BCRC 15B0132 (VRE) | 64 | >64 | >64 | 4 | 4 | 4 |
| Vancomycin-intermediate S. aureus 01 (VISA01) | 4 | >64 | >64 | 16 | 16 | 8 |
| Vancomycin-intermediate S. aureus 02 (VISA02) | 4 | >64 | >64 | 16 | 16 | 8 |
| Vancomycin-intermediate S. aureus 03 (VISA03) | 4 | >64 | >64 | 8 | 16 | 8 |
| Vancomycin-resistant S. aureus 01 (VRSA01) | 32 | >64 | >64 | 16 | 32 | 8 |
| Vancomycin-resistant S. aureus 02 (VRSA02) | 32 | >64 | >64 | 16 | 32 | 8 |
| Vancomycin-resistant S. aureus 03 (VRSA03) | 32 | >64 | >64 | 8 | 32 | 16 |
| E. coli ATCC 25922 | >64 | >64 | >64 | 32 | 64 | 32 |
| Strains | AMP (μg/mL) (¼ ×MIC) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P-113 | Phe-P-113 | Bip-P-113 | Dip-P-113 | Nal-P-113 | ||||||
| VAN a | FICI b | VAN | FICI | VAN | FICI | VAN | FICI | VAN | FICI | |
| VRE | 32 | 0.75 | 32 | 0.75 | 8 | 0.38 | 16 | 0.50 | 16 | 0.50 |
| VISA 01 | 4 | 1.25 | 4 | 1.25 | 1 | 0.50 | 4 | 1.25 | 2 | 0.75 |
| VISA 02 | 4 | 1.25 | 4 | 1.25 | 1 | 0.50 | 4 | 1.25 | 2 | 0.75 |
| VISA 03 | 4 | 1.25 | 4 | 1.25 | 1 | 0.50 | 2 | 0.75 | 1 | 0.50 |
| VRSA 01 | 32 | 1.25 | 32 | 1.25 | 2 | 0.31 | 16 | 0.75 | 8 | 0.50 |
| VRSA 02 | 32 | 1.25 | 32 | 1.25 | 2 | 0.31 | 8 | 0.50 | 8 | 0.50 |
| VRSA 03 | 32 | 1.25 | 32 | 1.25 | 4 | 0.38 | 16 | 0.75 | 4 | 0.38 |
| E. coli ATCC 25922 | >64 | 1.25 | 64 | 0.75 | 16 | 0.38 | 32 | 0.50 | 16 | 0.38 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-L.; Hsueh, J.-Y.; Yip, B.-S.; Chih, Y.-H.; Peng, K.-L.; Cheng, J.-W. Antimicrobial Peptides Display Strong Synergy with Vancomycin Against Vancomycin-Resistant E. faecium, S. aureus, and Wild-Type E. coli. Int. J. Mol. Sci. 2020, 21, 4578. https://doi.org/10.3390/ijms21134578
Wu C-L, Hsueh J-Y, Yip B-S, Chih Y-H, Peng K-L, Cheng J-W. Antimicrobial Peptides Display Strong Synergy with Vancomycin Against Vancomycin-Resistant E. faecium, S. aureus, and Wild-Type E. coli. International Journal of Molecular Sciences. 2020; 21(13):4578. https://doi.org/10.3390/ijms21134578
Chicago/Turabian StyleWu, Chih-Lung, Ju-Yun Hsueh, Bak-Sau Yip, Ya-Han Chih, Kuang-Li Peng, and Jya-Wei Cheng. 2020. "Antimicrobial Peptides Display Strong Synergy with Vancomycin Against Vancomycin-Resistant E. faecium, S. aureus, and Wild-Type E. coli" International Journal of Molecular Sciences 21, no. 13: 4578. https://doi.org/10.3390/ijms21134578
APA StyleWu, C.-L., Hsueh, J.-Y., Yip, B.-S., Chih, Y.-H., Peng, K.-L., & Cheng, J.-W. (2020). Antimicrobial Peptides Display Strong Synergy with Vancomycin Against Vancomycin-Resistant E. faecium, S. aureus, and Wild-Type E. coli. International Journal of Molecular Sciences, 21(13), 4578. https://doi.org/10.3390/ijms21134578