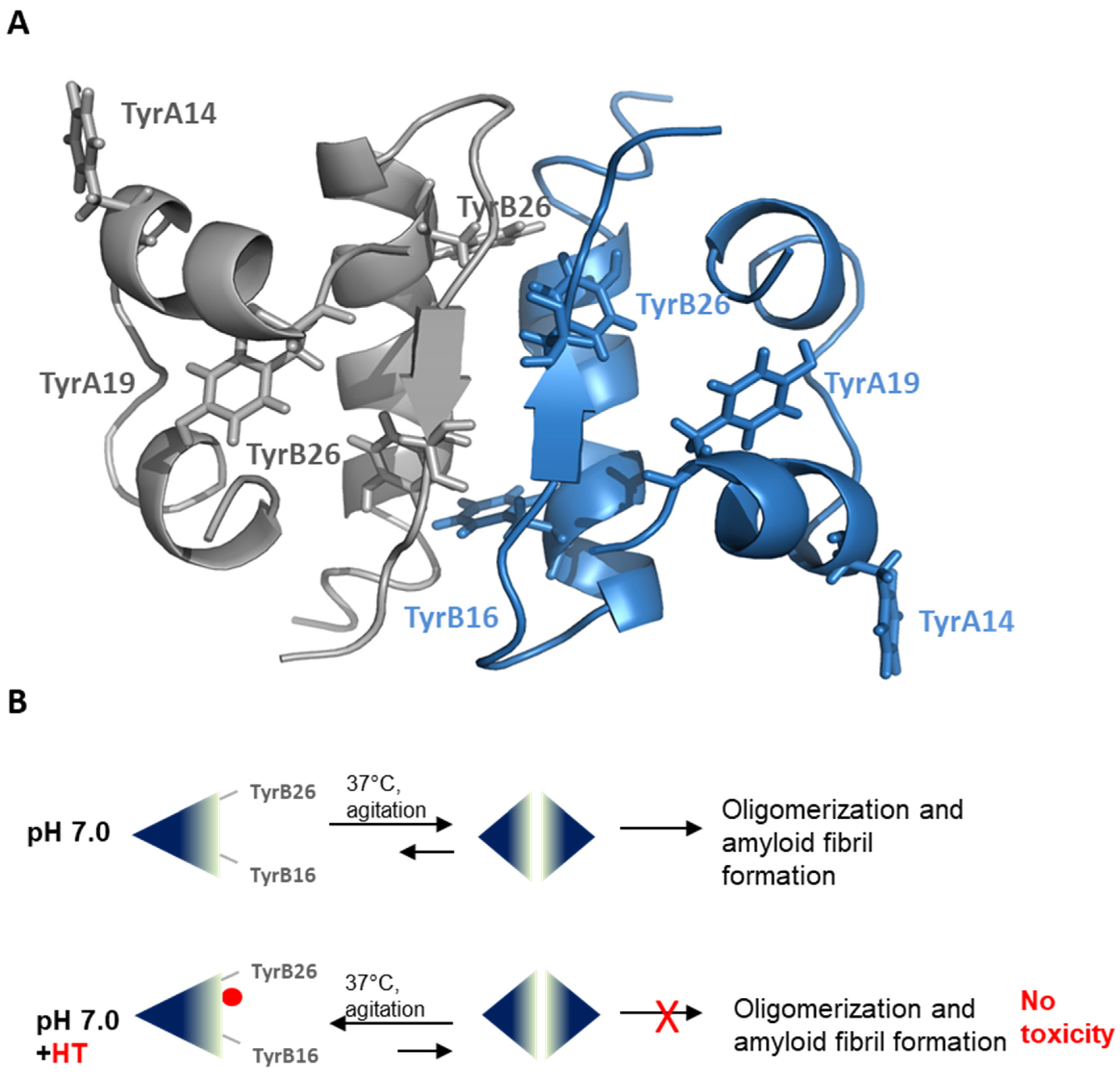
Hydroxytyrosol Inhibits Protein Oligomerization and Amyloid Aggregation in Human Insulin
Abstract
:1. Introduction
2. Results
2.1. Inhibitory Effect of HT on Insulin Amyloid Aggregation
2.2. Effect of HT on Insulin Amyloid Toxicity
2.3. Effect of HT on Insulin Oligomerization State
2.4. HT-Insulin Interaction
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Insulin Preparation and Amyloid Aggregation
4.3. Circular Dichroism Measurements
4.4. Fluorescence Measurements
4.5. Dynamic Light Scattering
4.6. Cell Cultures and Treatments
4.7. MTT Assay
4.8. Statistical Analysis
4.9. Data Availability
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chiti, F.; Dobson, C.M. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Rupasinghe, H.V. Polyphenols: Multipotent Therapeutic Agents in Neurodegenerative Diseases. Oxidative Med. Cell. Longev. 2013, 2013, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Forbes-Hernandez, T.Y.; Gasparrini, M.; Afrin, S.; Bompadre, S.; Mezzetti, B.; Quiles, J.L.; Giampieri, F.; Battino, M. The Healthy Effects of Strawberry Polyphenols: Which Strategy behind Antioxidant Capacity? Crit. Rev. Food Sci. Nutr. 2015, 56, S46–S59. [Google Scholar] [CrossRef]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, V.; Bucciantini, M.; Stefani, M. Calabrese Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [Green Version]
- Scarmeas, N.; Stern, Y.; Tang, M.-X.; Mayeux, R.; Luchsinger, J. Mediterranean diet and risk for Alzheimer’s disease. Ann. Neurol. 2006, 59, 912–921. [Google Scholar] [CrossRef] [Green Version]
- Rigacci, S.; Stefani, M. Nutraceutical Properties of Olive Oil Polyphenols. An Itinerary from Cultured Cells through Animal Models to Humans. Int. J. Mol. Sci. 2016, 17, 843. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Morató, J.; Xicota, L.; Fitó, M.; Farré, M.; Dierssen, M.; De La Torre, R. Potential Role of Olive Oil Phenolic Compounds in the Prevention of Neurodegenerative Diseases. Molecules 2015, 20, 4655–4680. [Google Scholar] [CrossRef] [Green Version]
- Robles-Almazan, M.; Pulido-Morán, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; García, C.R.; Quiles, J.L.; Tortosa, M.C.R. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- Marković, A.K.; Torić, J.; Barbarić, M.; Brala, C.J. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [Green Version]
- De La Torre, R.; Covas, M.I.; Pujadas, M.; Fitó, M.; Farré, M. Is dopamine behind the health benefits of red wine? Eur. J. Nutr. 2006, 45, 307–310. [Google Scholar] [CrossRef]
- Meiser, J.; Weindl, D.; Hiller, K. Complexity of dopamine metabolism. Cell Commun. Signal. 2013, 11, 34. [Google Scholar] [CrossRef] [Green Version]
- Rigacci, S.; Guidotti, V.; Bucciantini, M.; Parri, M.; Nediani, C.; Cerbai, E.; Stefani, M.; Berti, A. Oleuropein aglycon prevents cytotoxic amyloid aggregation of human amylin. J. Nutr. Biochem. 2010, 21, 726–735. [Google Scholar] [CrossRef]
- Rigacci, S.; Guidotti, V.; Bucciantini, M.; Nichino, D.; Relini, A.; Berti, A.; Stefani, M. Aβ(1-42) aggregates into non-toxic amyloid assemblies in the presence of the natural polyphenol oleuropein aglycon. Curr. Alzheimer Res. 2011, 8, 841–852. [Google Scholar] [CrossRef]
- Omar, S.H. Oleuropein in Olive and its Pharmacological Effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef] [Green Version]
- Daccache, A.; Lion, C.; Sibille, N.; Gérard, M.; Slomianny, C.; Lippens, G.; Cotelle, P. Oleuropein and derivatives from olives as Tau aggregation inhibitors. Neurochem. Int. 2011, 58, 700–707. [Google Scholar] [CrossRef]
- Wu, L.; Velander, P.; Liu, D.; Xu, B. Olive Component Oleuropein Promotes β-Cell Insulin Secretion and Protects β-Cells from Amylin Amyloid-Induced Cytotoxicity. Biochemistry 2017, 56, 5035–5039. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; Cerezo, A.B.; Troncoso, A.M.; García-Parrilla, M.C. Protective effects of hydroxytyrosol against α-synuclein toxicity on PC12 cells and fibril formation. Food Chem. Toxicol. 2018, 120, 41–49. [Google Scholar] [CrossRef]
- Orsini, F.; Ami, D.; Lascialfari, A.; Natalello, A. Inhibition of lysozyme fibrillogenesis by hydroxytyrosol and dopamine: An Atomic Force Microscopy study. Int. J. Boil. Macromol. 2018, 111, 1100–1105. [Google Scholar] [CrossRef]
- Leri, M.; Oropesa-Nuñez, R.; Canale, C.; Raimondi, S.; Giorgetti, S.; Bruzzone, E.; Bellotti, V.; Stefani, M.; Bucciantini, M. Oleuropein aglycone: A polyphenol with different targets against amyloid toxicity. Biochim. et Biophys. Acta (BBA) - Gen. Subj. 2018, 1862, 1432–1442. [Google Scholar] [CrossRef]
- Leri, M.; Natalello, A.; Bruzzone, E.; Stefani, M.; Bucciantini, M. Oleuropein aglycone and hydroxytyrosol interfere differently with toxic Aβ1-42 aggregation. Food Chem. Toxicol. 2019, 129, 1–12. [Google Scholar] [CrossRef]
- Palazzi, L.; Leri, M.; Cesaro, S.; Stefani, M.; Bucciantini, M.; De Laureto, P.P. Insight into the molecular mechanism underlying the inhibition of α-synuclein aggregation by hydroxytyrosol. Biochem. Pharmacol. 2020, 173, 113722. [Google Scholar] [CrossRef] [PubMed]
- Dische, F.E.; Wernstedt, C.; Westermark, G.T.; Westermark, P.; Pepys, M.B.; Rennie, J.A.; Gilbey, S.G.; Watkins, P.J. Insulin as an amyloid-fibril protein at sites of repeated insulin injections in a diabetic patient. Diabetologia 1988, 31, 158–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, L.; Khurana, R.; Coats, A.; Frokjaer, S.; Brange, J.; Vyas, S.; Uversky, V.N.; Fink, A.L. Effect of environmental factors on the kinetics of insulin fibril formation: elucidation of the molecular mechanism. Biochemistry 2001, 40, 6036–6046. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.; Theis, J.D.; Vrana, J.A.; Dogan, A. Pharmaceutical amyloidosis associated with subcutaneous insulin and enfuvirtide administration. Amyloid 2014, 21, 71–75. [Google Scholar] [CrossRef]
- Hellman, U.; Wernstedt, C.; Westermark, P.; O’Brien, T.; William, R.B.; Johnson, K.H. Amino acid sequence from degu islet amyloid-derived insulin shows unique sequence characteristics. Biochem. Biophys. Res. Commun. 1990, 169, 571–577. [Google Scholar] [CrossRef]
- Okamura, S.; Hayashino, Y.; Kore-Eda, S.; Tsujii, S. Localized Amyloidosis at the Site of Repeated Insulin Injection in a Patient With Type 2 Diabetes. Diabetes Care 2013, 36, e200. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, M.R. Insulin amyloid at injection sites of patients with diabetes. Amyloid 2016, 23, 1–9. [Google Scholar] [CrossRef]
- Ansari, A.M.; Osmani, L.; Matsangos, A.E.; Li, Q.K. Current insight in the localized insulin-derived amyloidosis (LIDA): clinico-pathological characteristics and differential diagnosis. Pathol. Res. Pr. 2017, 213, 1237–1241. [Google Scholar] [CrossRef]
- Iwaya, K.; Zako, T.; Fukunaga, J.; Sörgjerd, K.M.; Ogata, K.; Kogure, K.; Kosano, H.; Noritake, M.; Maeda, M.; Ando, Y.; et al. Toxicity of insulin-derived amyloidosis: a case report. BMC Endocr. Disord. 2019, 19, 61. [Google Scholar] [CrossRef] [Green Version]
- Nagase, T.; Iwaya, K.; Iwaki, Y.; Kotake, F.; Uchida, R.; Oh-I, T.; Sekine, H.; Miwa, K.; Murakami, S.; Odaka, T.; et al. Insulin-derived Amyloidosis and Poor Glycemic Control: A Case Series. Am. J. Med. 2014, 127, 450–454. [Google Scholar] [CrossRef] [Green Version]
- Arora, A.; Ha, C.; Park, C.B. Insulin amyloid fibrillation at above 100°C: New insights into protein folding under extreme temperatures. Protein Sci. 2004, 13, 2429–2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, A.; Millett, I.S.; Doniach, S.; Uversky, V.N.; Fink, A.L. Stimulation of Insulin Fibrillation by Urea-induced Intermediates. J. Boil. Chem. 2004, 279, 14999–15013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selivanova, O.M.; Galzitskaya, O.V. Structural polymorphism and possible pathways of amyloid fibril formation on the example of insulin protein. Biochem. (Moscow) 2012, 77, 1237–1247. [Google Scholar] [CrossRef]
- Iannuzzi, C.; Borriello, M.; Carafa, V.; Altucci, L.; Vitiello, M.; Balestrieri, M.L.; Ricci, G.; Irace, G.; Sirangelo, I. D-ribose-glycation of insulin prevents amyloid aggregation and produces cytotoxic adducts. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2016, 1862, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Iannuzzi, C.; Borriello, M.; Portaccio, M.; Irace, G.; Sirangelo, I. Insights into Insulin Fibril Assembly at Physiological and Acidic pH and Related Amyloid Intrinsic Fluorescence. Int. J. Mol. Sci. 2017, 18, 2551. [Google Scholar] [CrossRef] [Green Version]
- Sirangelo, I.; Borriello, M.; Irace, G.; Iannuzzi, C. Intrinsic blue-green fluorescence in amyloyd fibrils. AIMS Biophys. 2018, 5, 155–165. [Google Scholar] [CrossRef]
- Lee, C.-C.; Nayak, A.; Sethuraman, A.; Belfort, G.; McRae, G.J. A Three-Stage Kinetic Model of Amyloid Fibrillation. Biophys. J. 2007, 92, 3448–3458. [Google Scholar] [CrossRef] [Green Version]
- Grudzielanek, S.; Velkova, A.; Shukla, A.; Smirnovas, V.; Tatarek-Nossol, M.; Rehage, H.; Kapurniotu, A.; Winter, R. Cytotoxicity of Insulin within its Self-assembly and Amyloidogenic Pathways. J. Mol. Boil. 2007, 370, 372–384. [Google Scholar] [CrossRef]
- Chatani, E.; Imamura, H.; Yamamoto, N.; Kato, M. Stepwise Organization of the β-Structure Identifies Key Regions Essential for the Propagation and Cytotoxicity of Insulin Amyloid Fibrils*. J. Boil. Chem. 2014, 289, 10399–10410. [Google Scholar] [CrossRef] [Green Version]
- Hua, Q.; Weiss, M.A. Comparative 2D NMR studies of human insulin and despentapeptide insulin: sequential resonance assignment and implications for protein dynamics and receptor recognition. Biochemistry 1991, 30, 5505–5515. [Google Scholar] [CrossRef]
- Ahmad, A.; Uversky, V.N.; Hong, D.; Fink, A.L. Early Events in the Fibrillation of Monomeric Insulin. J. Boil. Chem. 2005, 280, 42669–42675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attri, A.; Fernandez, C.; Minton, A.P. pH-dependent self-association of zinc-free insulin characterized by concentration-gradient static light scattering. Biophys. Chem. 2010, 148, 28–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, Q.-X.; Weiss, M.A. Mechanism of Insulin Fibrillation. J. Boil. Chem. 2004, 279, 21449–21460. [Google Scholar] [CrossRef] [Green Version]
- Smirnovas, V.; Winter, R. Revealing Different Aggregation Pathways of Amyloidogenic Proteins by Ultrasound Velocimetry. Biophys. J. 2008, 94, 3241–3246. [Google Scholar] [CrossRef] [Green Version]
- Yoshihara, H.; Saito, J.; Tanabe, A.; Amada, T.; Asakura, T.; Kitagawa, K.; Asada, S. Characterization of Novel Insulin Fibrils That Show Strong Cytotoxicity Under Physiological pH. J. Pharm. Sci. 2016, 105, 1419–1426. [Google Scholar] [CrossRef]
- Iannuzzi, C.; Borriello, M.; Irace, G.; Cammarota, M.; Di Maro, A.; Sirangelo, I. Vanillin Affects Amyloid Aggregation and Non-Enzymatic Glycation in Human Insulin. Sci. Rep. 2017, 7, 15086. [Google Scholar] [CrossRef] [Green Version]
- Levine, H. Thioflavine T interaction with synthetic Alzheimer’s disease beta-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 2008, 2, 404–410. [Google Scholar] [CrossRef]
- Biancalana, M.; Koide, S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim. et Biophys. Acta (BBA) - Bioenerg. 2010, 1804, 1405–1412. [Google Scholar] [CrossRef] [Green Version]
- Iannuzzi, C.; Carafa, V.; Altucci, L.; Irace, G.; Borriello, M.; Vinciguerra, R.; Sirangelo, I. Glycation of Wild-Type Apomyoglobin Induces Formation of Highly Cytotoxic Oligomeric Species. J. Cell. Physiol. 2015, 230, 2807–2820. [Google Scholar] [CrossRef]
- Mangione, M.R.; Piccionello, A.P.; Marino, C.; Ortore, M.G.; Picone, P.; Vilasi, S.; Di Carlo, M.; Buscemi, S.; Bulone, D.; Biagio, P.L.S. Photo-inhibition of Aβ fibrillation mediated by a newly designed fluorinated oxadiazole. RSC Adv. 2015, 5, 16540–16548. [Google Scholar] [CrossRef]
- Nettleton, E.J.; Tito, P.; Sunde, M.; Bouchard, M.; Dobson, C.M.; Robinson, C.V. Characterization of the oligomeric states of insulin in self-assembly and amyloid fibril formation by mass spectrometry. Biophys. J. 2000, 79, 1053–1065. [Google Scholar] [CrossRef] [Green Version]
- Šneideris, T.; Darguzis, D.; Botyriute, A.; Grigaliunas, M.; Winter, R.; Smirnovas, V. pH-Driven Polymorphism of Insulin Amyloid-Like Fibrils. PLoS ONE 2015, 10, e0136602. [Google Scholar] [CrossRef]
- Ziaunys, M.; Sneideris, T.; Smirnovas, V. Self-inhibition of insulin amyloid-like aggregation. Phys. Chem. Chem. Phys. 2018, 20, 27638–27645. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, S.I.; Chang, X.; Abseher, R.; Nilges, M.; Led, J.J. Unraveling the symmetry ambiguity in a hexamer: calculation of the R6 human insulin structure. J. Biomol. NMR 2000, 16, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Strickland, E.H.; Mercola, D. Near-ultraviolet tyrosyl circular dichroism of pig insulin monomers, dimers, and hexamers. Dipole-dipole coupling calculations in the monopole approximation. Biochemistry 1976, 15, 3875–3884. [Google Scholar] [CrossRef]
- Uversky, V.N.; Garriques, L.N.; Millett, I.S.; Frokjaer, S.; Brange, J.; Doniach, S.; Fink, A.L. Prediction of the association state of insulin using spectral parameters. J. Pharm. Sci. 2003, 92, 847–858. [Google Scholar] [CrossRef]
- Hvidt, S. Insulin association in neutral solutions studied by light scattering. Biophys. Chem. 1991, 39, 205–213. [Google Scholar] [CrossRef]
- Bastianetto, S.; Quirion, R. Natural antioxidants and neurodegenerative diseases. Front. Biosci. 2004, 9, 3447. [Google Scholar] [CrossRef]
- Stefani, M.; Rigacci, S. Protein Folding and Aggregation into Amyloid: The Interference by Natural Phenolic Compounds. Int. J. Mol. Sci. 2013, 14, 12411–12457. [Google Scholar] [CrossRef] [Green Version]
- Nardiello, P.; Pantano, D.; Lapucci, A.; Stefani, M.; Casamenti, F. Diet Supplementation with Hydroxytyrosol Ameliorates Brain Pathology and Restores Cognitive Functions in a Mouse Model of Amyloid-β Deposition. J. Alzheimer’s Dis. 2018, 63, 1161–1172. [Google Scholar] [CrossRef]
- Casamenti, F.; Stefani, M. Olive polyphenols: new promising agents to combat aging-associated neurodegeneration. Expert Rev. Neurother. 2016, 17, 345–358. [Google Scholar] [CrossRef]
- Brunetti, G.; Di Rosa, G.; Scuto, M.; Leri, M.; Stefani, M.; Schmitz-Linneweber, C.; Calabrese, V.; Saul, N. Healthspan Maintenance and Prevention of Parkinson’s-like Phenotypes with Hydroxytyrosol and Oleuropein Aglycone in C. elegans. Int. J. Mol. Sci. 2020, 21, 2588. [Google Scholar] [CrossRef] [Green Version]
- Di Rosa, G.; Brunetti, G.; Scuto, M.; Salinaro, A.T.; Calabrese, E.J.; Crea, R.; Schmitz-Linneweber, C.; Calabrese, V.; Saul, N. Healthspan Enhancement by Olive Polyphenols in C. elegans Wild Type and Parkinson’s Models. Int. J. Mol. Sci. 2020, 21, 3893. [Google Scholar] [CrossRef]
- Miquel, S.; Champ, C.; Day, J.; Aarts, E.; Bahr, B.A.; Bakker, M.; Bánáti, D.; Calabrese, V.; Cederholm, T.; Cryan, J.; et al. Poor cognitive ageing: Vulnerabilities, mechanisms and the impact of nutritional interventions. Ageing Res. Rev. 2018, 42, 40–55. [Google Scholar] [CrossRef] [Green Version]
- Pilipenko, V.; Narbute, K.; Amara, I.; Trovato, A.; Scuto, M.; Pupure, J.; Jansone, B.; Poikans, J.; Bisenieks, E.; Klusa, V.; et al. GABA-containing compound gammapyrone protects against brain impairments in Alzheimer’s disease model male rats and prevents mitochondrial dysfunction in cell culture. J. Neurosci. Res. 2019, 97, 708–726. [Google Scholar] [CrossRef]
- Peters, V.; Calabrese, V.; Forsberg, E.; Volk, N.; Fleming, T.; Baelde, H.J.; Weigand, T.; Thiel, C.; Salinaro, A.T.; Scuto, M.; et al. Protective Actions of Anserine Under Diabetic Conditions. Int. J. Mol. Sci. 2018, 19, 2751. [Google Scholar] [CrossRef] [Green Version]
- Vissers, M.N.; Zock, P.; Roodenburg, A.J.C.; Leenen, R.; Katan, M.B. Olive Oil Phenols Are Absorbed in Humans. J. Nutr. 2002, 132, 409–417. [Google Scholar] [CrossRef]
- Pastor, A.; Rodríguez-Morató, J.; Olesti, E.; Pujadas, M.; Pérez-Mañá, C.; Khymenets, O.; Fitó, M.; Covas, M.-I.; Solà, R.; Motilva, M.-J.; et al. Analysis of free hydroxytyrosol in human plasma following the administration of olive oil. J. Chromatogr. A 2016, 1437, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Borriello, M.; Iannuzzi, C.; Sirangelo, I. Pinocembrin Protects from AGE-Induced Cytotoxicity and Inhibits Non-Enzymatic Glycation in Human Insulin. Cells 2019, 8, 385. [Google Scholar] [CrossRef] [Green Version]
- Iannuzzi, C.; Borriello, M.; D’Agostino, A.; Cimini, D.; Schiraldi, C.; Sirangelo, I. Protective effect of extractive and biotechnological chondroitin in insulin amyloid and advanced glycation end product-induced toxicity. J. Cell. Physiol. 2018, 234, 3814–3828. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirangelo, I.; Borriello, M.; Vilasi, S.; Iannuzzi, C. Hydroxytyrosol Inhibits Protein Oligomerization and Amyloid Aggregation in Human Insulin. Int. J. Mol. Sci. 2020, 21, 4636. https://doi.org/10.3390/ijms21134636
Sirangelo I, Borriello M, Vilasi S, Iannuzzi C. Hydroxytyrosol Inhibits Protein Oligomerization and Amyloid Aggregation in Human Insulin. International Journal of Molecular Sciences. 2020; 21(13):4636. https://doi.org/10.3390/ijms21134636
Chicago/Turabian StyleSirangelo, Ivana, Margherita Borriello, Silvia Vilasi, and Clara Iannuzzi. 2020. "Hydroxytyrosol Inhibits Protein Oligomerization and Amyloid Aggregation in Human Insulin" International Journal of Molecular Sciences 21, no. 13: 4636. https://doi.org/10.3390/ijms21134636