Effect of Acupuncture on the p38 Signaling Pathway in Several Nervous System Diseases: A Systematic Review
Abstract
:1. Introduction
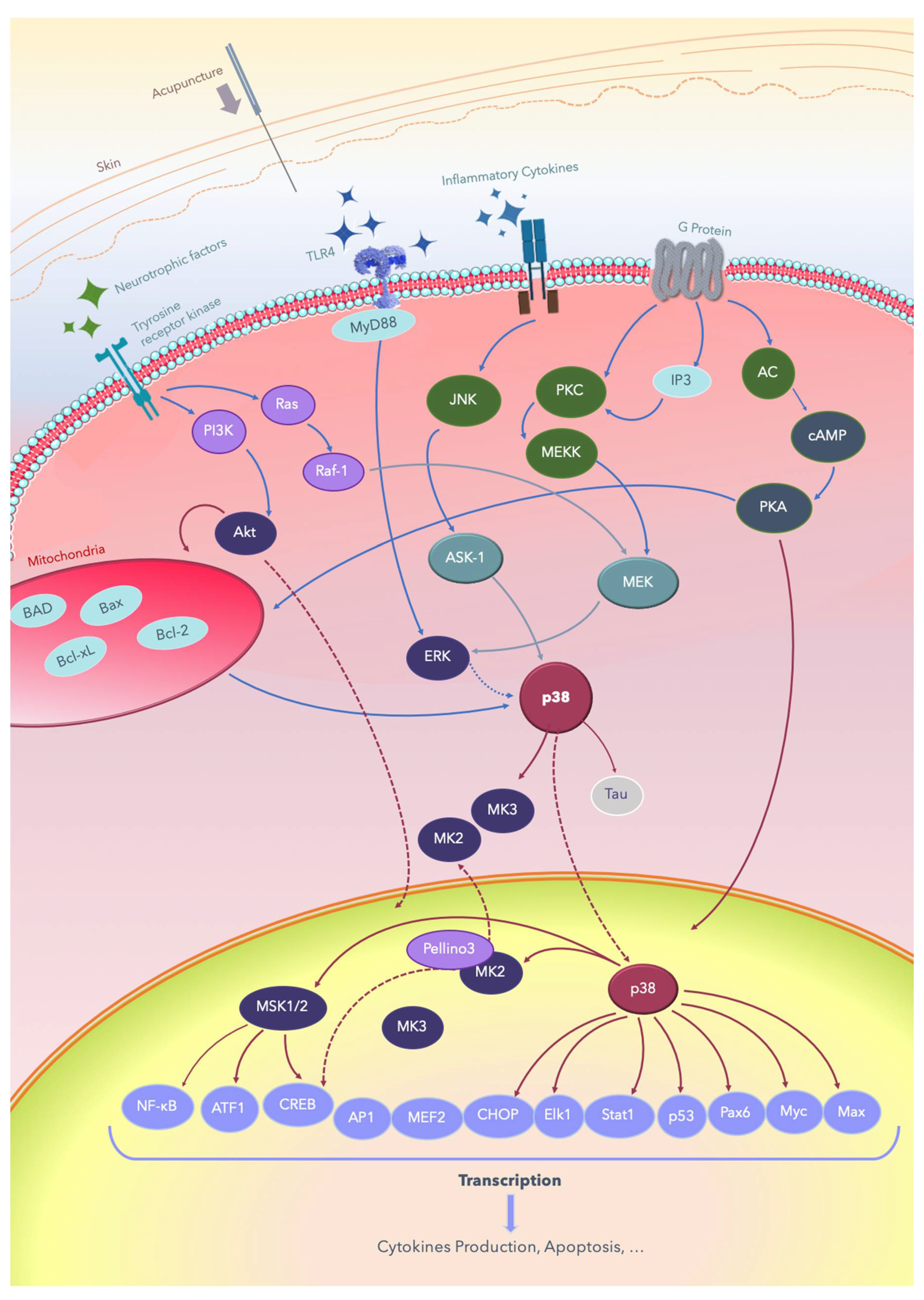
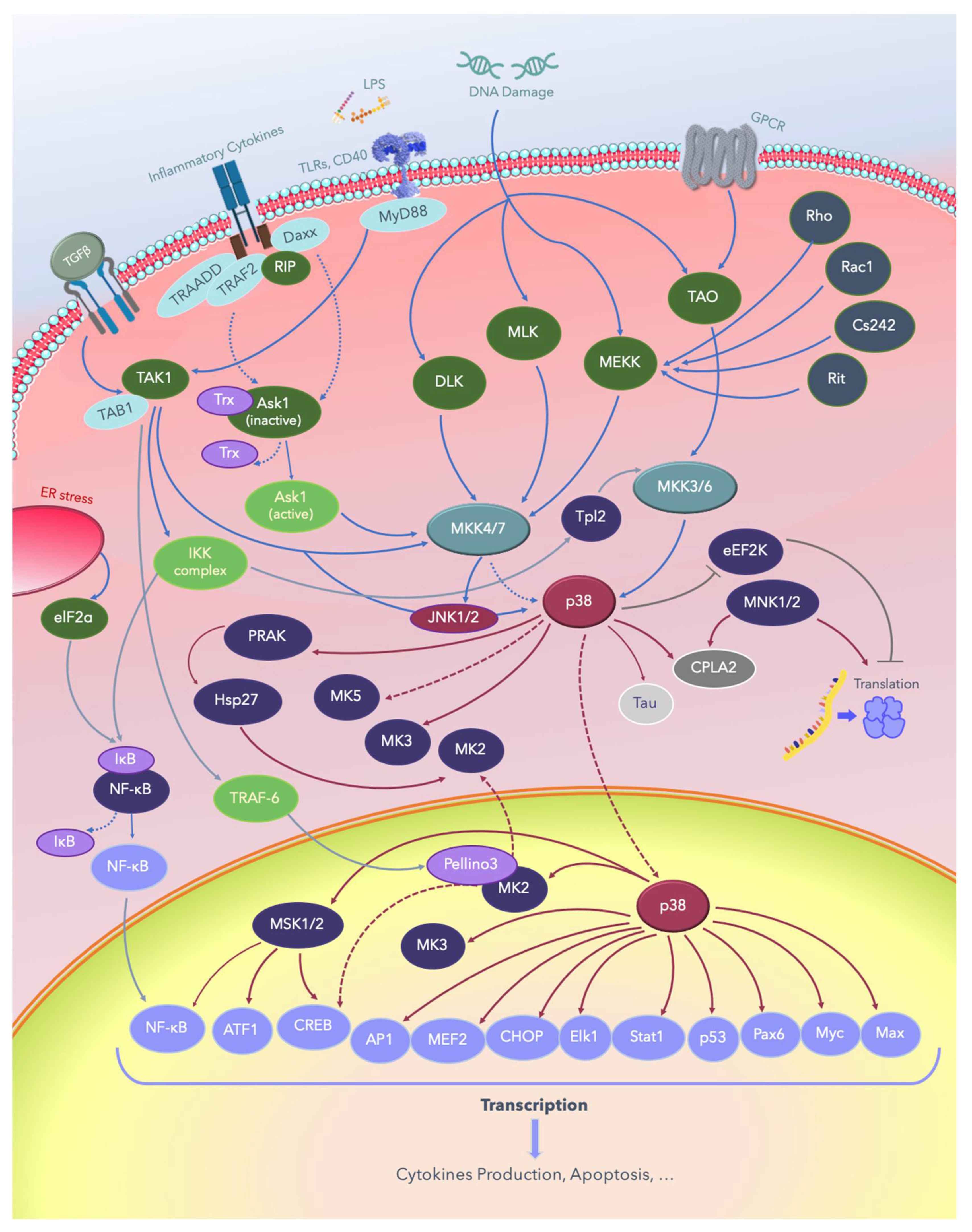
2. The p38 MAPKs
3. MAPK Substrates, Signaling Pathways, and Functions
3.1. Dual Phosphorylation by MKKs
3.2. Autophosphorylation
4. TRPV1 and the p38 Pathway
5. Brain-Derived Neurotrophic Factor and p38 Pathways
6. Acupuncture and the Effects of Electric Fields on Nerve Regeneration
7. Inflammatory and Neuropathic Pain
7.1. Inflammatory Pain
7.2. Neuropathic Pain
7.3. Post-Operation Pain
7.4. Migraine
7.5. Transcutaneous Electrical Nerve Stimulation Versus EA
7.6. Hypothesis Regarding the Long-Term Effect of Acupuncture
8. Cerebral Ischemia
9. Epilepsy and Seizure
10. Motion Sickness
11. Degenerative Nerve Diseases
11.1. Alzheimer’s Disease
11.2. Parkinson’s Disease
12. Fibromyalgia
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| A1R | adenosine A1 receptor |
| α7 nAChR | α7 nicotinic acetylcholine receptor |
| AC | adenyl cyclase |
| ACC1 | acetyl-CoA carboxylase (ACC) 1 is a biotin-dependent enzyme that catalyzes the irreversible carboxylation of acetyl-CoA to produce malonyl-CoA |
| AD | Alzheimer’s disease |
| Akt (PKB) | protein kinase B (PKB) is also known as Akt |
| Alk5 | TGFβ type I receptor kinase |
| AMP | adenosine mono-phosphate |
| AMPK | 5’ AMP-activated protein kinase |
| AP-1 | activator protein 1 |
| ARE | AU-rich elements |
| ASIC3 | acid-sensing ion channel 3 |
| ASK-1 | apoptosis signal-regulating kinase 1 |
| ATF | activating transcription factor |
| ATP | adenosine tri-phosphate |
| BACE1 | beta-secretase 1, an enzyme responsible for Aβ generation in Alzheimer’s disease |
| Bad | BCL2 associated agonist of cell death; a protein involved in initiating apoptosis. |
| Bax | Bcl-2 associated X |
| Bac-2 | B-cell lymphoma 2 |
| BDNF | brain-derived neurotrophic factor |
| CaMKII | calmodulin-dependent protein kinase II |
| CCI | chronic constriction injury |
| Cd242 | The 40- to 42-kiloDalton red cell membrane glycoprotein bearing the ICAM-4 antigen named by the LW blood system. |
| CFA-treated | Complete Freund’s adjuvant injections produced significant mechanical and thermal hyperalgesia in mice. |
| c-Fos | A proto-oncogene that is expressed within some neurons following depolarization. |
| CHOP | C/EBP homologous protein; belongs to the family of CCAAT/enhancer-binding proteins (C/EBPs) and is involved in the regulation of genes that encode proteins involved in proliferation |
| c-Jun | A proto-oncogene that with c-Fos forms the AP-1 early response transcription factor that regulates gene expression in response to extracellular stimuli. |
| CL100 | The human CL100 gene is induced in skin fibroblasts in response to oxidative/heat stress and growth factors. The CL100 gene encodes a dual specificity (Tyr/Thr) protein phosphatase that specifically inactivates MAPKs. |
| CLCA3A2 | chloride channel accessory 3A2 |
| CLIC3 | chloride intracellular channel 3 |
| Cot (Tp2) | cancer Osaka thyroid oncogene (=Tpl-2) |
| COX-2 | cyclooxygenase-2 |
| cPLA2 | cytosolic phospholipase A2 |
| CREB | cAMP response element-binding protein |
| CRF | corticotropin-releasing factor |
| CWP | components of calcium wave propagation |
| DLK (MAP3K12) | dual leucine zipper kinase, also known as MAP3K12 |
| DJ-1 | a protein deglycase |
| DLG1 (SAP97) | discs large homolog 1 scaffold protein |
| Daxx | the death-associated protein |
| DHT | dihydrotestosterone |
| DRG | dorsal root ganglion |
| EA | electroacupuncture |
| elF2a | eukaryotic translation initiation factor 2A; functions by a separate mechanism in eukaryotic translation |
| eEF2 | eukaryotic elongation factor 2 |
| eEF2K | eukaryotic elongation factor 2 kinase |
| elF2α | a subunit of the heterotrimeric eIF2 complex |
| Elk-1 | erythroblast transformation specific (ETS) like-1 protein |
| ERK | extracellular signal–regulated kinases |
| ETS | erythroblast transformation specific protein, or E26 transformation-specific, or E-twenty-six transcription factors family |
| FADD | Fas-associated protein with death domain; also called MORT1 |
| Fas pathway | Fas and Fas Ligand (FasL) are involved in the regulation of cell death. |
| GABA | gamma-aminobutyric acid |
| GFAP | glial fibrillary acidic protein; a marker of astrocytes |
| GPCRs | G-protein-coupled receptors |
| HMG-CoA Reductase | 3-hydroxy-3-methyl-glutaryl-CoA reductase or HMGR is the rate-controlling enzyme of the mevalonate pathway, responsible for cholesterol and other isoprenoid biosynthesis. |
| Hsc70 | heat shock cognate 70 |
| Hsp | heat shock proteins |
| 5-HT | 5-hydroxytryptamine |
| Iba-1 | ionized calcium–binding adapter molecule 1 |
| IκB | inhibitor of nuclear factor kappa-B |
| IκB | inhibitor of nuclear factor kappa-B kinase |
| IKKs | I-kappa-B kinases |
| IL-1β | Interleukin 1 beta is a member of the interleukin 1 family of cytokines produced by activated macrophages. |
| IRF | interferon regulatory factor |
| JNK | c-Jun amino-terminal kinases |
| LTD | long-term depression; a term in neurophysiology describing an activity-dependent reduction in the efficacy of neuronal synapses lasting hours or longer following a long patterned stimulus. |
| LTP | long-term potentiation; a persistent strengthening of synapses based on recent patterns of activity. These are patterns of synaptic activity that produce a long-lasting increase in signal transmission between two neurons. |
| MA | manual acupuncture |
| MAPK | mitogen-activated protein kinase |
| MAPKK (MAP2K, MKK) | MAPK kinase |
| MAPKKK (MAP3K) | MAPKK kinase |
| Max | a transcription factor coded by the myc-associated factor X |
| MEF | myocyte enhancing factor |
| MEK (MKK) | MAPK/ERK kinase |
| MEKK | MEK kinase |
| mGluR3 | metabotropic glutamate receptor 3 |
| MKP | MAPK-phosphatase |
| MAPKAPK-2 | mitogen-activated protein kinase-activated protein kinase 2 |
| MLK | mixed-lineage protein kinase |
| MNK | mitogen activated protein kinase–interacting protein |
| Myc | A group of transcription factors coded by a regulator genes and proto-oncogenes called Myc |
| MyD88 | myeloid differentiation primary response 88 |
| MSK | mitogen and stress activated protein kinase |
| mTOR | mammalian target of rapamycin |
| Nav | voltage-gated sodium channel |
| NFκB | nuclear factor-kappa-B |
| P105 | The 105 kD protein is a Rel protein-specific transcription inhibitor encoded by the NFKB1 gene |
| PAG | periaqueductal gray area |
| NIH3Ts cells | A cell line derived from mouse embryonic fibroblasts. |
| NMDA | N-methyl-D-aspartate, an amino acid derivative |
| PKB | Protein kinase B; also known as Akt |
| NO | nitric oxide |
| NR1 | NMDA receptor subunits includes NR1 and NR2Bi |
| Pax6 | Paired-box protein Pax-6, also known as aniridia type II protein (AN2) or oculorhombin; a “master control” gene for the development of eyes and other sensory organs |
| P2RX7 | P2X purinoceptor 7 is a protein belonging to the family of purinoceptors for ATP. |
| P2X4Rs | P2X4 receptors are a subtype of ionotropic ATP receptors |
| P2Y1, -2 | human purinergic G protein-coupled receptors |
| PD | Parkinson’s disease |
| Pellino | A group of proteins extremely well conserved during evolution; Pellinos interact with key mediators in TLR/IL-1R-induced signaling pathways. |
| PHF-tau | paired helical filament–tau protein |
| PI3K | phosphoinositide 3-kinases; also called phosphatidylinositol 3-kinases |
| PKC | protein kinase C |
| PSD | postsynaptic density protein |
| PRAK | p38-regulated and -activated kinase |
| Rac2 | Ras-related C3 botulinum toxin substrate 2 |
| RAF | rapidly accelerated fibrosarcoma-related oncogene |
| Raf1 | v-raf-1 murine leukemia viral oncogene homolog 1 |
| RAGE | The receptor for advanced glycation end products is a member of the immunoglobulin superfamily of cell surface molecules. |
| RCT study | randomized controlled trial study |
| Rel | A proto-oncogene protein encoded by the REL gene. It is a member of the NF-κB family of transcription factors and contains a Rel homology domain (RHD) at its N-terminus and two C-terminal transactivation domains. |
| Rho | Rho (ρ) factor is a protein that acts in bacterial cells to mediate termination of transcription at distinct sites, which mediates the dissociation of the RNA from the very stable ternary transcription complex. |
| Rit | Ras-like protein in tissues |
| RIP | receptor-interacting protein kinases; a class of serine/threonine protein kinases |
| ROS | reactive oxidative species |
| S100B | S100 calcium-binding protein B |
| SAP | stress-activated protein or synapse-associated protein; SAP90 [(synapse-associated protein 90 is also known as PSD-95 (postsynaptic density-95)] |
| SAPK | stress-activated protein kinase |
| Smac/DIABLO | second mitochondrial-derived activator of caspase/direct inhibitor of apoptosis protein-binding protein with low isoelectric point |
| SNpc | substantia nigra pars compacta |
| Stat1 | signal transducer and activator of transcription 1 |
| STK | serine-threonine/tyrosine kinases |
| TAES | transcutaneous acupoint electrical stimulation |
| TAK-1 | transforming growth factor-β-activated kinase 1 |
| TAO | thousand and one amino acids |
| TAB1 | TGF-beta-activated kinase |
| TGFβ | transforming growth factor-β |
| TLR4 | toll-like receptor 4 |
| TNF | tumor necrosis factor |
| Tpl2 | tumor progression locus 2, also known as COT or MAP3K8 |
| TRADD | tumor necrosis factor receptor type 1-associated DEATH domain protein |
| TRAF6 | tumor necrosis factor receptor (TNFR)-associated factor 6 |
| TrkB | tyrosine kinase receptor B |
| TRPA1 | transient receptor potential cation channel subfamily A member 1 |
| TRPV | transient receptor potential vanilloid receptors |
| 3′UTR | 3′untranslated region |
Appendix A

References
- Zhou, W.; Benharash, P. Effects and mechanisms of acupuncture based on the principle of meridians. J. Acupunct. Meridian Stud. 2014, 7, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, N.L.; Wu, A.Q. The Yellow Emperor’s Canon of Internal Medicine, 1st ed.; China Science & Technology. Press: Beijing, China, 1997; 1999, 2nd edition. [Google Scholar]
- Bannerman, R.H. Acupuncture: The WHO View; World Health Organization: Geneva, Switzerland, 1979; Volume 12, p. 27e8. [Google Scholar]
- Park, J.; Sohn, Y.; White, A.R.; Lee, H. The safety of acupuncture during pregnancy: A systematic review. Acupunct. Med. 2014, 32, 257–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Hao, Z.; Zhang, L.L.; Guo, Q. Efficacy and safety of acupuncture in children: An overview of systematic reviews. Pediatr. Res. 2015, 78, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.Y.; Miller, D.W.; Bolash, B.; Bauer, M.; McDonal, J.; Faggert, S.; He, H.; Li, Y.M.; Matecki, A.; Camardella, L.; et al. Acupuncture’s role in solving the opioid epidemic: Evidence, cost-effectiveness, and care availability for acupuncture as a primary, nonpharmacologic method for pain relief and management–white paper. J. Integr. Med. 2017, 15, 411–425. [Google Scholar] [CrossRef]
- Acupuncture: In Depth. National Center for Complementary and Alternative Medicine. Available online: https://nccih.nih.gov/health/acupuncture/introduction (accessed on 25 January 2020).
- Hsieh, C.L. Acupuncture as treatment for nervous system diseases. BioMed 2012, 2, 51–57. [Google Scholar] [CrossRef]
- He, X.R.; Wang, Q.; Li, P.P. Acupuncture and moxibustion for cancer-related fatigue: A systematic review and meta-analysis. Asian Pac. J. Cancer Prev. 2013, 14, 3067–3074. [Google Scholar] [CrossRef] [Green Version]
- Cox, J.; Varatharajan, S.; Côté, P.; Optima Collaboration. Effectiveness of acupuncture therapies to manage musculoskeletal disorders of the extremities: A systematic review. J. Orthop. Sports Phys. Ther. 2016, 46, 409–429. [Google Scholar] [CrossRef] [Green Version]
- Forde, J.C.; Jaffe, E.; Stone, B.V.; Te, A.E.; Espinosa, G.; Chughtai, B. The role of acupuncture in managing overactive bladder: A review of the literature. Int. Urogynecol. J. 2016, 27, 1645–1651. [Google Scholar] [CrossRef]
- Yao, Q.; Li, S.; Liu, X.; Qin, Z.; Liu, Z. The effectiveness and safety of acupuncture for patients with chronic urticaria: A systematic review. Biomed Res. Int. 2016, 2016, 5191729. [Google Scholar] [CrossRef] [Green Version]
- Bäcker, M.; Hammes, M.G.; Valet, M.; Deppe, M.; Valet, M.; Conrad, B.; Tölle, T.R.; Doboset, G. Different modes of manual acupuncture stimulation differentially modulate cerebral blood flow velocity, arterial blood pressure and heart rate in human subjects. [published correction appears in Neurosci. Lett. 2003, 6, 337, 117]. Neurosci. Lett. 2002, 333, 203–206. [Google Scholar] [CrossRef]
- Hsieh, C.L.; Chang, Q.Y.; Lin, I.H.; Lin, J.G.; Liu, C.H.; Tang, N.Y.; Lane, H.Y. The study of electroacupuncture on cerebral blood flow in rats with and without cerebral ischemia. Am. J. Chin. Med. 2006, 34, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.; Lin, Y.; Tang, N.; Cheng, C.Y.; Hsieh, C.L. Electric stimulation of the ears ameliorated learning and memory impairment in rats with cerebral ischemia-reperfusion injury. Sci. Rep. 2016, 6, 20381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachstetter, A.D.; Xing, B. Microglial p38alpha MAPK is a key regulator of proinflammatory cytokine up-regulation induced by toll-like receptor (TLR) ligands or beta-amyloid (Abeta). J. Neuroinflammation 2011, 8, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Fan, Y. Effects of sodium ferulate on amyloid-beta-induced MKK3/MKK6-p38 MAPK-Hsp27 signal pathway and apoptosis in rat hippocampus. Acta Pharm. Sin. 2006, 27, 1309–1316. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, R. Neuroprotection by dihydrotestosterone in LPS-induced neuroinflammation. Neurobiol. Dis. 2020, 104814. [Google Scholar] [CrossRef]
- Yan, S.D.; Bierhaus, A. RAGE and Alzheimer’s disease: A progression factor for amyloid-beta-induced cellular perturbation? J. Alzheimers Dis 2009, 16, 833–843. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Xu, A.; Shao, S.; Zhou, Y.; Xiong, B.; Li, Z. Electroacupuncture ameliorates cognitive impairment by inhibiting the JNK signaling pathway in a mouse model of Alzheimer’s disease. Front. Aging Neurosci. 2020, 12, 23. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Wu, H.; Wang, G.; Li, M.; Zhang, Z.; Gu, G. The effects of electroacupuncture on TH1/TH2 cytokine mRNA expression and mitogen-activated protein kinase signaling pathways in the splenic T cells of traumatized rats. Anesth Analg. 2009, 109, 1666–1673. [Google Scholar] [CrossRef]
- Cheng, K.J. Neurobiological mechanisms of acupuncture for some common illnesses: A clinician’s perspective. J. Acupunct. Meridian Stud. 2014, 7, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.; You, Y.; Tao, J.; Ye, X.; Huang, J.; Yang, S.; Lin, Z.; Hong, Z.; Peng, J.; Chen, L. Electro-acupuncture at points of Zusanli and Quchi exerts anti-apoptotic effect through the modulation of PI3K/Akt signaling pathway. Neurosci. Lett. 2014, 558, 14–19. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Liang, F.; Liu, J.; Li, J.; Lu, J.; Fu, Y.; Chen, Q.; Has, Q.; Wu, S. Effects of electroacupuncture on electrocardiogram, myocardial pathological morphology and PI3K/Akt pathway in rats with chronic myocardial ischemia. Zhongguo Zhen Jiu 2016, 36, 389–395. [Google Scholar]
- Hwang, I.K.; Chung, J.Y.; Yoo, D.Y.; Yi, S.S.; Youn, H.Y.; Seong, J.K.; Soon, Y.S. Effects of electroacupuncture at Zusanli and Baihui on brain-derived neurotrophic factor and cyclic AMP response element-binding protein in the hippocampal dentate gyrus. J. Vet. Med. Sci. 2010, 72, 1431–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Qin, F.; Liu, A.; Sun, Q.; Wang, Q.; Xie, S.; Lu, S.; Zhang, D.; Lu, Z. Electro-acupuncture attenuates the mice premature ovarian failure via mediating PI3K/AKT/mTOR pathway. Life Sci. 2019, 217, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, Q.; Hu, B.; Peng, Z.; Zhao, Y.; Ma, L.; Xiong, L.; Lu, Y.; Zhu, X.; Chen, S. Involvement of ERK 1/2 activation in electroacupuncture pretreatment via cannabinoid CB1 receptor in rats. Brain Res. 2010, 1360, 1–7. [Google Scholar] [CrossRef]
- Xie, G.; Yang, S.; Chen, A.; Lan, L.; Lin, Z.; Gao, Y.; Huang, J.; Lin, J.; Peng, J.; Tao, J.; et al. Electroacupuncture at Quchi and Zusanli treats cerebral ischemia-reperfusion injury through activation of ERK signaling. Exp. Ther. Med. 2013, 5, 1593–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Wang, J.; Chun, L.; Zhou, G.; Xu, X.; Zhang, X.; Lan, X. Effect of electroacupuncture on cell apoptosis and erk signal pathway in the hippocampus of adult rats with cerebral ischemia-reperfusion. Evid. Based Complementary Altern. Med. 2015, 414965. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Li, C.; Zhou, G.; Yang, L.; Jiang, G.; Chen, J.; Li, Q.; Zhan, Z.; Xu, X.; Zhang, X. Effects of electroacupuncture on the cortical extracellular signal regulated kinase pathway in rats with cerebral ischaemia/reperfusion. Acupunct. Med. 2017, 35, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Wu, Q.; Lin, X.; Boriongan, C.; He, Z.; Tan, J.; Cao, C.; Zhou, S. Brain-derived neurotrophic factor signaling pathway: Modulation by acupuncture in telomerase knockout mice. Altern. Health Med. 2015, 21, 36–46. [Google Scholar]
- Wang, S.J.; Ma, J.; Gong, Y.X.; Wang, Y.C.; Zeng, X.L.; Liang, Y.; Sun, G.J. Effect of electroacupuncture intervention on ERK 1/2 signaling and TNF-alpha and IL-1beta protein levels in the substantia nigra in rats with Parkinson’s disease. Zhen Ci Yan Jiu 2014, 39, 456–460. [Google Scholar]
- Lan, X.; Zhang, X.; Zhou, G.P.; Wu, C.X.; Li, C.; Xu, X.H. Electroacupuncture reduces apoptotic index and inhibits p38 mitogen-activated protein kinase signaling pathway in the hippocampus of rats with cerebral ischemia/reperfusion injury. Neural Regen. Res. 2017, 12, 409–416. [Google Scholar] [CrossRef]
- Xing, Y.; Yang, S.D.; Wang, M.M.; Dong, F.; Feng, Y.S.; Zhang, F. Electroacupuncture alleviated neuronal apoptosis following ischemic stroke in rats via midkine and ERK/JNK/p38 signaling pathway. J. Mol. Neurosci. 2018, 66, 26–36. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Yang, S.; Huang, J.; Xue, X.; Zheng, Y.; Shang, G.; Tao, J.; Chen, L. Electroacupunctre improves motor impairment via inhibition of microglia-mediated neuroinflammation in the sensorimotor cortex after ischemic stroke. Life Sci. 2016, 151, 313–322. [Google Scholar] [CrossRef]
- Xu, J.; She, Y.; Su, N.; Zhang, R.; Lao, L.; Xu, S. Effects of electroacupuncture on chronic unpredictable mild stress rats depression-like behavior and expression of p-ERK/ERK and p-P38/P38. Evid. Based Complement Altern. Med. 2015, 2015, 650729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Guo, Z.; Lu, J.; Zhao, B.; Fei, Y.; Li, J.; Jiang, H.; Sun, L.; Wang, Y.; Sun, Y.; et al. The Role of MAPK and dopaminergic synapse signaling pathways in antidepressant effect of electroacupuncture pretreatment in chronic restraint stress rats. Evid. Based Complementary Altern. Med. 2017, 2017, 2357653. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.M.; Dong, X.; Tu, Y.; Liu, P. A microarray study of chronic unpredictable mild stress rat blood serum with electro-acupuncture intervention. Neurosci. Lett. 2016, 627, 160–167. [Google Scholar] [CrossRef]
- Zhao, G.; Li, D.; Ding, X.; Li, L. Nerve growth factor pretreatment inhibits lidocaine-induced myelin damage via increasing BDNF expression and inhibiting p38 mitogen activation in the rat spinal cord. Mol. Med. Rep. 2017, 16, 4678–4684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, E.T.; Lin, Y.W.; Huang, C.P.; Tang, N.Y.; Hsieh, C.L. Electric stimulation of ear reduces the effect of Toll-like receptor 4 signaling pathway on kainic acid-induced epileptic seizures in rats. Biomed. Res. Int. 2018, 2018, 5407256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Yang, S.; Liu, J.; Huang, J.; Peng, J.; Lin, J.; Tao, J.; Chen, L. Electroacupuncture ameliorates cognitive impairment through inhibition of NF-κB-mediated neuronal cell apoptosis in cerebral ischemia-reperfusion injured rats. Mol. Med. Rep. 2013, 7, 1516–1522. [Google Scholar] [CrossRef] [Green Version]
- Lan, L.; Tao, J.; Chen, A.; Xie, G.; Huang, J.; Lin, J.; Peng, J.; Chen, L. Electroacupuncture exerts anti-inflammatory effects in cerebral ischemia-reperfusion injured rats via suppression of the TLR4/NF-κB pathway. Int. J. Mol. Med. 2013, 31, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Zeng, Y.; Wang, X.; Ye, X. Effect of electroacupuncture on the expression of mTOR and eIF4E in hippocampus of rats with vascular dementia. Neurol. Sci. 2013, 34, 1093–1097. [Google Scholar] [CrossRef]
- Xu, T.; Li, W.; Liang, Y.; Yang, Z.; Liu, J.; Wang, Y.; Su, N. Neuroprotective effects of electro acupuncture on hypoxic-ischemic encephalopathy in newborn rats Ass. Pak. J. Pharm. Sci. 2014, 27 (Suppl. 6), 1991–2000. [Google Scholar] [PubMed]
- Oh, J.; Kim, Y.; Kim, S.; Lee, B.; Jang, J.; Kwon, S.; Park, H. Acupuncture modulates stress response by the mTOR signaling pathway in a rat post-traumatic stress disorder model. Sci. Rep. 2018, 8, 11864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Zhuo, P.; Li, L.; Jin, H.; Lin, B.; Zhang, Y.; Liang, S.; Wu, J.; Huang, J.; Wang, Z.; et al. Activation of brain glucose metabolism ameliorating cognitive impairment in APP/PS1 transgenic mice by electroacupuncture. Free. Radic. Biol. Med. 2017, 112, 174–190. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Kim, H.N.; Jang, J.Y.; Park, C.; Lee, J.H.; Shin, H.K.; Choi, Y.H.; Choi, B.T. Effects of electroacupuncture on apoptotic pathways in a rat model of focal cerebral ischemia. Int. J. Mol. Med. 2013, 32, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Lawan, A.; Bennett, A.M. Mitogen-activated protein kinase regulation in hepatic metabolism. Trends Endocrinol. Metab. 2017, 28, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.N.; Kim, S.T.; Doo, A.R.; Park, J.Y.; Moon, W.; Chae, Y.; Yin, C.S.; Lee, H.; Park, H.J. Phosphatidylinositol 3-kinase/Akt signaling pathway mediates acupuncture-induced dopaminergic neuron protection and motor function improvement in a mouse model of Parkinson’s disease. Int. J. Neurosci. 2011, 121, 562–569. [Google Scholar] [CrossRef]
- Kim, S.N.; Doo, A.R.; Park, J.Y.; Bae, H.; Chae, Y.; Shim, I.; Lee, H.; Moon, W.; Lee, H.; Park, H.J. Acupuncture enhances the synaptic dopamine availability to improve motor function in a mouse model of Parkinson’s disease. PLoS ONE 2011, 6, e27566. [Google Scholar] [CrossRef]
- Li, Q.Q.; Shi, G.X.; Yang, J.W.; Li, Z.H.; Zhang, Z.H.; He, T.; Wang, J.; Liu, L.Y.; Liu, C.Z. Hippocampal cAMP/PKA/CREB is required for neuroprotective effect of acupuncture. Physiol. Behav. 2015, 139, 482–490. [Google Scholar] [CrossRef]
- Lu, F.; Zhu, H.M.; Xie, J.J.; Zhou, H.H.; Chen, Y.L.; Hu, J.Y. Effects of electroacupuncture on behavior, plasma COR and expressions of PKA and PKC in hippocampus of the depression model rat. Zhongguo Zhen Jiu 2008, 28, 214–218. [Google Scholar]
- Liu, J.J.; Wu, Z.F.; Sun, J.; Jiang, L.; Jiang, S.; Fu, W.B. Role of AC-cAMP-PKA cascade in antidepressant action of electroacupuncture treatment in rats. Evid. Based Complementary Altern. Med. 2012, 2012, 932414. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.; Lin, Y.; Tao, J.; Chen, B.; You, K.; Chen, J.; Li, X.; Chen, L.D. Electroacupuncture ameliorates learning and memory in rats with cerebral ischemia-reperfusion injury by inhibiting oxidative stress and promoting p-CREB expression in the hippocampus. Mol. Med. Rep. 2015, 12, 6807–6814. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.; Kim, Y.; Kim, H.; Shin, Y.I.; Shin, H.K.; Choi, B.T. Electroacupuncture ameliorates memory impairments by enhancing oligodendrocyte regeneration in a mouse model of prolonged cerebral hypoperfusion. Sci. Rep. 2016, 6, 28646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, R.; Tao, J.; Wu, Y.; Chen, B.; You, K.; Chen, J.; Li, X.; Chen, L.D. Electroacupuncture improves cognitive ability following cerebral ischemia reperfusion injury via CaM-CaMKIV-CREB signaling in the rat hippocampus. Exp. Ther. Med. 2016, 12, 777–782. [Google Scholar] [CrossRef] [Green Version]
- Yun, Y.C.; Jang, D.; Yoon, S.B.; Kim, D.; Choi, D.H.; Kwon, O.S.; Lee, Y.M.; Youn, D. Laser acupuncture exerts neuroprotective effects via regulation of Creb, Bdnf, Bcl-2, and Bax gene expressions in the hippocampus. Evid. Based Complementary Altern. Med. 2017, 2017, 7181637. [Google Scholar] [CrossRef] [Green Version]
- Pak, M.E.; Jung, D.H.; Lee, H.J.; Shin, M.J.; Kim, S.Y.; Shin, Y.B.; Yun, Y.J.; Shin, H.K.; Choi, B.T. Combined therapy involving electroacupuncture and treadmill exercise attenuates demyelination in the corpus callosum by stimulating oligodendrogenesis in a rat model of neonatal hypoxia-ischemia. Exp. Neurol. 2018, 300, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liang, J.; Wang, J.R.; Hu, L.; Tu, Y.; Guo, J.Y. Acupuncture activates ERK-CREB pathway in rats exposed to chronic unpredictable mild stress. Evid. Based Complementary Altern. Med. 2013, 2013, 469765. [Google Scholar] [CrossRef]
- Dimaras, H.; Gallie, B.L. Retinoblastoma Protein, Biological and Clinical Functions. In Encyclopedia of Cancer; Schwab, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 4046–4050. [Google Scholar] [CrossRef]
- Duval, D.; Trouillas, M.; Thibault, C.; Dembelé, D.; Diemunsch, F.; Reinhardt, B.; Mertz, A.L.; Dierich, A.; Boeuf, H. Apoptosis and differentiation commitment: Novel insights revealed by gene profiling studies in mouse embryonic stem cells. Cell Death Differ. 2005, 13, 564–575. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jiang, H.; Meng, H.; Li, J.; Yang, X.; Zhao, B.; Sun, Y.; Bro, T. Antidepressant mechanism research of acupuncture: Insights from a genome-wide transcriptome analysis of frontal cortex in rats with chronic restraint stress. Evid. Based Complementary Altern. Med. 2017, 2017, 1676808. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, Q.; Yang, S.; Huang, J.; Feng, X.; Peng, J.; Lin, Z.; Liu, W.; Tao, W.; Chen, L. Electroacupuncture inhibits apoptosis of peri-ischemic regions via modulating p38, extracellular signal-regulated kinase (ERK1/2), and c-Jun N terminal kinases (JNK) in cerebral ischemia-reperfusion-injured rats. Med. Sci. Monit. 2018, 24, 4395–4404. [Google Scholar] [CrossRef]
- Zhu, Y.; Deng, L.; Tang, H.; Gao, X.; Wang, Y.; Guo, K.; Kong, J.; Yang, C. Electroacupuncture improves neurobehavioral function and brain injury in rat model of intracerebral hemorrhage. Brain Res. Bull. 2017, 131, 123–132. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, X.; Li, P.; Itoua, E.S.; Moukassa, D.; Ndinga Andely, F. Effects of acupuncture on mRNA levels of apoptotic factors in perihematomal brain tissue during the acute phase of cerebral hemorrhage. Med. Sci. Monit. 2017, 23, 1522–1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.Y.; Xu, L.; Liu, C.L.; Huang, L.S.; Zhu, X.Y. Electroacupuncture Intervention inhibits the decline of learning-memory ability and overexpression of cleaved Caspase-3 and Bax in hippocampus induced by isoflurane in APPswe/PS 1. Zhen Ci Yan Jiu 2016, 41, 24–30. [Google Scholar]
- Wang, T.; Liu, C.Z.; Yu, J.C.; Jiang, W.; Han, J.X. Acupuncture protected cerebral multi-infarction rats from memory impairment by regulating the expression of apoptosis related genes Bcl-2 and Bax in hippocampus. Physiol. Behav. 2009, 96, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.J.; Huang, L.N.; Wang, R.H.; An, J.M.; Zhang, M. Effects of scalp-acupuncture on astrocyte apoptosis in hippocampal CA 1 region in rats with vascular dementia. Zhen Ci Yan Jiu 2015, 40, 6–12. [Google Scholar] [PubMed]
- Lai, H.C.; Chang, Q.Y.; Hsieh, C.L. Signal transduction pathways of acupuncture for treating some nervous system diseases. Evid. Based Complement. Altern. Med. 2019, 2019, 2909632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.Q.; Zhu, S.X.; Zhang, Y.; Wang, F.; Zhu, Q.Y. Effects of transcutaneous electrostimulation of auricular points on behavior and hippocampal IL-1 β and TNF-α expression in temporal lobe epilepsy rats. Acupunct. Res. 2016, 41, 283–290. [Google Scholar]
- Ding, X.; Yu, J.; Yu, T.; Fu, Y.; Han, J. Acupuncture regulates the aging-related changes in gene profile expression of the hippocampus in senescence-accelerated mouse (SAMP10). Neurosci. Lett. 2006, 399, 11–16. [Google Scholar] [CrossRef]
- Zhang, M.; Xv, G.; Wang, W.; Meng, D.; Ji, Y. Electroacupuncture improves cognitive deficits and activates PPAR-γ in a rat model of Alzheimer’s disease. Acupunct. Med. 2017, 35, 44–51. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, X.; Yu, J.; Han, J.; Yu, T.; Shi, J.; Zhao, L.; Nie, K. Acupuncture for patients with mild to moderate Alzheimer’s disease: A randomized controlled trial. BMC Complement Altern. Med. 2017, 17, 556. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Dong, L.; He, Y.; Xiao, H. Acupuncture plus herbal medicine for Alzheimer’s disease: A systematic review and meta-analysis. Am. J. Chin. Med. 2017, 45, 1327–1344. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Wang, X.; Du, S.; Yan, C.; Yang, N.; Lin, L.; Shi, G.; Liu, C. Anti-oxidative and anti-apoptotic effects of acupuncture: Role of thioredoxin-1 in the hippocampus of vascular dementia rats. Neuroscience 2018, 379, 281–291. [Google Scholar] [CrossRef]
- Wang, S.; Fang, J.; Ma, J.; Wang, Y.; Zeng, X.; Zhou, D.; Sun, G. Influence of electroacupuncture on p38-mitogen activated protein kinase in substantia nigra cells of rats with Parkinson disease model. Zhongguo Zhen Jiu 2013, 33, 329–333. [Google Scholar] [PubMed]
- Krens, S.F.; Spaink, H.P.; Snaar-Jagalska, B. Functions of the MAPK family in vertebrate-development. FEBS Lett. 2006, 580, 4984–4990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roux, P.P.; Blenis, J. ERK and p38 MAPK-activated protein kinases: A family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 2004, 68, 320–344. [Google Scholar] [CrossRef] [Green Version]
- Ajibade, A.A.; Wang, Q. TAK1 negatively regulates NF-kappaB and p38 MAP kinase activation in Gr-1+CD11b+ neutrophils. Immunity 2012, 36, 43–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand, S. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: New immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut 2009, 58, 1152–1167. [Google Scholar] [CrossRef] [Green Version]
- Hannigan, M.; Zhan, L. The role of p38 MAP kinase in TGF-beta1-induced signal transduction in human neutrophils. Biochem. Biophys. Res. Commun. 1998, 346, 55–58. [Google Scholar] [CrossRef]
- Heineke, J.; Molkentin, J.D. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat. Rev. Mol. Cell Biol. 2006, 77, 589–600. [Google Scholar] [CrossRef]
- Shiojima, I.; Walsh, K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006, 20, 3347–3365. [Google Scholar] [CrossRef] [Green Version]
- Shioi, T.; Kang, P.M. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J. 2000, 19, 2537–2548. [Google Scholar] [CrossRef] [Green Version]
- Tanike, M.; Yamaguchi, O. Apoptosis signal-regulating kinase 1/p38 signaling pathway negatively regulates physiological hypertrophy. Circulation 2008, 117, 545–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solinas, G.; Becattini, B. JNK at the crossroad of obesity, insulin resistance, and cell stress response. Mol. Metab. 2017, 6, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Davis, R.J. Cell Signaling and Stress Responses. Cold Spring Harb. Perspect Biol. 2016, 8. [Google Scholar] [CrossRef] [Green Version]
- Ning, C.; Wang, X. Chicory inulin ameliorates type 2 diabetes mellitus and suppresses JNK and MAPK pathways in vivo and in vitro. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Koh, A.; Molinaro, A. Microbially produced imidazole propionate impairs insulin signaling through mTORC1. Cell 2018, 175, 947–961.e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eagleton, M.J. Inflammation in abdominal aortic aneurysms: Cellular infiltrate and cytokine profiles. Vascular 2012, 20, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Hussien, H.; Soekhoe, R.G. Collagen degradation in the abdominal aneurysm: A conspiracy of matrixmetalloproteinase and cysteine collagenases. Am. J. Pathol. 2007, 170, 809–817. [Google Scholar] [CrossRef] [Green Version]
- Saratzis, A.; Abbas, A.A. Abdominal aortic aneurysm: A review of the genetic basis. Angiology 2011, 62, 18–32. [Google Scholar] [CrossRef]
- Saratzis, A.; Kitas, G.D. Can statins suppress the development of abdominal aortic aneurysms? A review of the current evidence. Angiology 2010, 61, 137–144. [Google Scholar] [CrossRef]
- Papalambros, E.; Sigala, F. Immunohistochemical expression of metalloproteinases MMP-2 and MMP-9 in abdominal aortic aneurysms: Correlation with symptoms and aortic diameter. Int. J. Mol. Med. 2003, 12, 965–968. [Google Scholar] [CrossRef]
- Petersen, E.; Gineitis, A. Activity of matrix metalloproteinase-2 and -9 in abdominal aortic aneurysms. Relation to size and rupture. Eur. J. Vasc. Endovasc. Surg. 2000, 20, 457–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.Q.; Li, W. MCP-1 Stimulates MMP-9 expression via ERK 1/2 and p38 MAPK signaling pathways in human aortic smooth muscle cells. Cell Physiol. Biochem. 2014, 34, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ma, C. Metabolism: A Novel Shared Link between Diabetes Mellitus and Alzheimer’s Disease. J. Diabetes Res. 2020, 2020, 4981814. [Google Scholar] [CrossRef]
- Kyriskis, J.M.; Avruch, J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Am. J. Physiol. 2001, 81, 808–869. [Google Scholar] [CrossRef] [Green Version]
- Lang, E.; Bissinger, R.; Qadri, S.M.; Lang, F. Suicidal death of erythrocytes in cancer and its chemotherapy: A potential target in the treatment of tumor-associated anemia. Int. J. Cancer 2017, 141, 1522–1528. [Google Scholar] [CrossRef] [Green Version]
- Brancho, D.; Tanaka, N.; Jaeschke, A.; Ventura, J.-J.; Kelkar, N.; Tanaka, Y.; Kyuuma, M.; Takeshita, T.; Flavell, R.A.; Davis, R.J. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 2003, 17, 1969–1978. [Google Scholar] [CrossRef] [Green Version]
- Krishna, M.; Narang, H. The complexity of mitogen-activated protein kinases (MAPKs) made simple. Cell Mol. Life Sci. 2008, 65, 3525–3544. [Google Scholar] [CrossRef] [PubMed]
- Bardwell, L. Mechanisms of MAPK signalling specificity. Biochem. Soc. Trans. 2006, 34, 837–841. [Google Scholar] [CrossRef] [Green Version]
- Akella, R.; Moon, T.M.; Goldsmith, E.J. Unique MAP kinase binding sites. Biochim. Biophys. Acta 2008, 1784, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Cuadrado, A.; Nebreda, A.R. Mechanisms and functions of p38 MAPK signalling. Biochim. J. 2010, 429, 403–417. [Google Scholar] [CrossRef] [Green Version]
- Bonney, E.A. Mapping out p38MAPK. Am. J. Reporod. Immunol. 2017, 77. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Ding, L.; Ji, H.; Xu, Z.; Liu, Q.; Zheng, Y. The role of p38 MAPK in the development of diabetic cardiomyopathy. Int. J. Mol. Sci. 2016, 17, 1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.I.; Xu, B.E.; Cobb, M.H.; Goldsmith, E.J. Crystal structures of MAP kinase p38 complexed to the docking sites on its nuclear substrate MEF2A and activator MKK3b. Mol. Cell 2002, 9, 1241–1249. [Google Scholar] [CrossRef]
- O’Callaghan, C.; Fanning, L.J.; Barry, O.P. p38δ MAPK: Emerging Roles of a Neglected Isoform. Int. J. Cell Biol. 2014, 2014, 272689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabio, G.; Arthur, J.S.C.; Kuma, Y.; Peggie, M.; Carr, J.; Murray-Tait, V.; Centeno, F.; Goedert, M.; Morrice, N.A.; Cuenda, A. p38γ regulates the localisation of SAP97 in the cytoskeleton by modulating its interaction with GKAP. EMBO J. 2005, 24, 1134–1145. [Google Scholar] [CrossRef]
- Parker, C.G.; Hunt, J.; Diener, K.; McGinley, M.; Soriano, B.; Keesler, G.A.; Bray, J.; Yao, Z.; Wang, X.S.; Kohno, T.; et al. Identification of stathmin as a novel substrate for p38 Delta. Biochem. Biophys. Res. Commun. 1998, 249, 791–796. [Google Scholar] [CrossRef]
- Rubin, C.I.; Atweh, G.F. The role of stathmin in the regulation of the cell cycle. J. Cell Biochem. 2004, 93, 242–250. [Google Scholar] [CrossRef]
- Knebel, A.; Morrice, N.; Cohen, P. A novel method to identify protein kinase substrates: eEF2 kinase is phosphorylated and inhibited by SAPK4/p38δ. EMBO J. 2001, 20, 4360–4369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goedert, M.; Hasegawa, M.; Jakes, R.; Lawler, S.; Cuenda, A.; Cohen, P. Phosphorylation of microtubule-associated protein tau by stress-activated protein kinases. FEBS Lett. 1997, 409, 57–62. [Google Scholar] [CrossRef]
- Masuda, K.; Shima, H.; Watanabe, M.; Kikuchi, K. MKP-7, a novel mitogen-activated protein kinase phosphatase, functions as a shuttle protein. J. Biol. Chem. 2001, 276, 39002–39011. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.S.; Clarkson, M.W.; Kunze, M.B.A.; Granata, D.; Wand, A.J.; Lindorff-Larsen, K.; Page, R.; Peti, W. Dynamic activation and regulation of the mitogen-activated protein kinase p38. Proc. Natl. Acad. Sci. USA 2018, 115, 4655–4660. [Google Scholar] [CrossRef] [Green Version]
- Cuenda, A.; Rousseau, S. p38 MAP-Kinases pathway regulation, function and role in human disease. Biochim. Biophys. Acta 2007, 1773, 1358–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; McGowan, C.H.; He, L.; Downey, J.S.; Fearns, C.; Wang, Y.; Huang, S.; Han, J. Involvement of the MKK6-p38gamma cascade in gamma-radiation-induced cell cycle arrest. Mol. Cell Biol. 2000, 20, 4543–4552. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.Q.; Zhang, S.J. Involvement of ATM/ATR-p38 MAPK cascade in MNNG induced G1-S arrest. World J. Gastroenterol. 2003, 9, 2073–2077. [Google Scholar] [CrossRef]
- Tang, J.; Qi, X.; Mercola, D.; Han, J.; Chen, G. Essential role of p38gamma in K-Ras transformation independent of phosphorylation. J. Biol. Chem. 2005, 280, 23910–23917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puri, P.L.; Wu, Z.; Zhang, P.; Wood, L.D.; Bhakta, K.S.; Han, J.; Feramisco, J.R.; Karin, M.; Wang, J.Y. Induction of terminal differentiation by constitutive activation of p38 MAP kinase in human rhabdomyosarcoma cells. Genes Dev. 2000, 14, 574–584. [Google Scholar] [PubMed]
- Campbell, R.M.; Anderson, B.D.; Brooks, N.A.; Brooks, H.B.; Chan, E.M.; De Dios, A.; Gilmour, R.; Graff, J.R.; Jambrina, E.; Mader, M.; et al. Characterization of LY2228820 dimesylate, a potent and selective inhibitor of p38 MAPK with antitumor activity. Mol. Cancer 2014, 13, 364–374. [Google Scholar] [CrossRef] [Green Version]
- Maimon, A.; Mogilevsky, M.; Shilo, A.; Golan-Gerstl, R.; Obiedat, A.; Ben-Hur, V.; Lebenthal-Loinger, I.; Stein, I.; Reich, R.; Beenstock, J.; et al. Mnk2 alternative splicing modulates the p38-MAPK pathway and impacts Ras-induced transformation. Cell Rep. 2014, 7, 501–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azijli, K.; Yuvaraj, S.; van Roosmalen, I.; Flach, K.; Giovannetti, E.; Peters, G.J.; de Jong, S.; Kruyt, F.A. MAPK p38 and JNK have opposing activities on TRAIL-induced apoptosis activation in NSCLC H460 cells that involves RIP1 and caspase-8 and is mediated by Mcl-1. Apoptosis 2013, 18, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Rozengurt, E. PKD, PKD2, and p38 MAPK mediate Hsp27 Serine-82 phosphorylation induced by neurotensin in pancreatic cancer PANC-1 cells. J. Cell Biochem. 2008, 103, 648–662. [Google Scholar] [CrossRef]
- Cánovas, B.; Igea, A.; Sartori, A.A.; Gomis, R.R.; Paull, T.T.; Isoda, M.; Pérez-Montoyo, H.; Serra, V.; González-Suárez, E.; Stracker, T.H.; et al. Targeting p38alpha increases DNA damage, chromosome instability, and the anti-tumoral response to taxanes in breast cancer cells. Cancer Cell 2018, 33, 1094–1110 e1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junttila, M.R.; Ala-aho, R.; Jokilehto, T.; Peltonen, J.; Kallajoki, M.; Grenman, R.; Jaakkola, P.; Westermarck, J.; Kähäri, V.-M. p38α and p38δ mitogen-activated protein kinase isoforms regulate invasion and growth of head and neck squamous carcinoma cells. Oncogene 2007, 26, 5267–5279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ringshausen, I.; Dechow, T.; Schneller, F.; Weick, K.; Oelsner, M.; Peschel, C.; Decker, T. Constitutive activation of the MAPkinase p38 is critical for MMP-9 production and survival of B-CLL cells on bone marrow stromal cells. Leukemia 2004, 18, 1964–1970. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Jiang, B.H.; Leonard, S.S.; Corum, L.; Zhang, Z.; Roberts, J.R.; Antonini, J.; Zheng, J.Z.; Flynn, D.C.; Castranova, V.; et al. p38 signaling-mediated hypoxia-inducible factor 1alpha and vascular endothelial growth factor induction by Cr(VI) in DU145 human prostate carcinoma cells. J. Biol. Chem. 2002, 277, 45041–45048. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.S.; Sohn, Y.W.; Moon, A. TGF-beta-induced transcriptional activation of MMP-2 is mediated by activating transcription factor (ATF)2 in human breast epithelial cells. Cancer Lett. 2007, 252, 147–156. [Google Scholar] [CrossRef]
- Hong, I.K.; Kim, Y.M.; Jeoung, D.I.; Kim, K.C.; Lee, H. Tetraspanin CD9 induces MMP-2 expression by activating p38 MAPK, JNK and c-Jun pathways in human melanoma cells. Exp. Mol. Med. 2005, 37, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Villa-Diaz, L.G.; Miyano, T. Activation of p38 MAPK during porcine oocyte maturation. Biol. Reprod. 2004, 71, 691–696. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.T.; Yang, D.D.; Wysk, M.; Gatti, E.; Mellman, I.; Davis, R.J.; Flavell, R.A. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J. 1999, 18, 1845–1857. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, N.; Kamanaka, M.; Enslen, H.; Dong, C.; Wysk, M.; Davis, R.J.; Flavelle, R.A. Differential involvement of p38 mitogen-activated protein kinase kinases MKK3 and MKK6 in T-cell apoptosis. EMBO Rep. 2002, 3, 785–791. [Google Scholar] [CrossRef] [Green Version]
- Menon, M.B.; Gaestel, M. TPL2 meets p38MAPK: Emergence of a novel positive feedback loop in inflammation. Biochem. J. 2016, 473, 2995–2999. [Google Scholar] [CrossRef]
- Xu, D.; Matsumoto, M.L.; MacKenzie, B.S.; Zarrin, A.A. TPL2 kinase action and control of inflammation. Pharm. Res. 2018, 129, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, L.; Li, B.; Yang, J.; Huen, M.S.Y.; Pan, X.; Tsao, S.W.; Cheung, A.L.M. F-box only protein 31 (FBXO31) negatively regulates p38 mitogen-activated protein kinase (MAPK) signaling by mediating lysine 48-linked ubiquitination and degradation of mitogen-activated protein kinase kinase 6 (MKK6). J. Biol. Chem. 2014, 289, 21508–21518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanno, M.; Bassi, R. Diverse mechanisms of myocardial p38 mitogen-activated protein kinase activation: Evidence for MKK-independent activation by a TAB1-associated mechanism contributing to injury during myocardial ischemia. Circ. Res. 2003, 93, 254–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amantini, C.; Mosca, M.; Nabissi, M.; Lucciarini, R.; Caprodossi, S.; Arcella, A.; Giangaspero, F.; Santoni, G. Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. J. Neurochem. 2007, 102, 977–990. [Google Scholar] [CrossRef] [Green Version]
- Marrone, M.C.; Morabito, A.; Giustizieri, M.; Chiurchiù, V.; Leuti, A.; Mattioli, M.; Marinelli, S.; Riganti, L.; Lombardi, M.; Murana, E.; et al. TRPV1 channels are critical brain inflammation detectors and neuropathic pain biomarkers in mice. Nat. Commun. 2017, 8, 15292. [Google Scholar] [CrossRef]
- Surowy, C.S.; Kym, P.R.; Reilly, R.M. Biochemical Pharmacology of TRPV1: Molecular intergrator of pain signals. In Vanilloid Receptor TRPV1 in Drug Discovery: Targeting Pain and other Pathological Disorders, 2nd ed.; Gomtsyan, A., Faltynek, C.R., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, (published simultaneously in Canada); 2010; pp. 119–120. [Google Scholar]
- Bianco, F.; Perrotta, C.; Novellino, L.; Francolini, M.; Riganti, L.; Menna, E.; Saglietti, L.; Schuchman, E.H.; Furlan, R.; Clementi, E.; et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 2009, 28, 1043–1054. [Google Scholar] [CrossRef] [Green Version]
- Suter, M.R.; Berta, T.; Gao, Y.J.; Decosterd, I.; Ji, R.R. Large A-Fiber activity is required for microglial proliferation and p38 MAPK activation in the spinal cord: Different effects of resiniferatoxin and bupivacaine on spinal microglial changes after spared nerve injury. Mol. Pain 2009, 5, 53. [Google Scholar] [CrossRef] [Green Version]
- Liao, H.Y.; Hsieh, C.L.; Huang, C.P. Electroacupuncture Attenuates CFA-induced Inflammatory Pain by suppressing Nav1.8 through S100B, TRPV1, Opioid, and Adenosine Pathways in Mice. Sci. Rep. 2017, 13, 42531. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.T.; Wu, J.R.; Chen, Z.Y.; Liu, Z.X.; Miao, B. Effects of dexmedetomidine on P2X4Rs, p38-MAPK and BDNF in spinal microglia in rats with spared nerve injury. Brain Res. 2014, 1568, 21–30. [Google Scholar] [CrossRef]
- Keller, A.F.; Beggs, S.; Salter, M.W.; De Koninck, Y. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Mol. Pain. 2007, 3, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.; Deng, G.; Huang, H. The activation of BDNF reduced inflammation in a spinal cord injury model by TrkB/p38 MAPK signaling. Exp. Med. 2019, 17, 1688–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.Y.; Lin, J.G.; Su, S.Y.; Tang, N.Y.; Kao, S.T.; Hsieh, C.L. Electroacupuncture-like stimulation at Baihui and Dazhui acupoints exerts neuroprotective effects through activation of the brain-derived neurotrophic factor-mediated MEK1/2/ERK1/2/p90RSK/bad signaling pathway in mild transient focal cerebral ischemia in rats. BMC Complement Altern. Med. 2014, 14, 92. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, C.E.; Shastri, V.R.; Vacanti, J.P.; Langer, R. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc. Natl. Acad. Sci. USA 1997, 94, 8948–8953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaffe, L.F.; Poo, M.M. Neurites grow faster towards the cathode than the anode in a steady field. J. Exp. Zool. 1979, 209, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Poo, M.M. Orientation of neurite growth by extracellular electric fields. J. Neurosci. 1982, 2, 483–496. [Google Scholar] [CrossRef]
- Kerns, J.M.; Fakhouri, A.J.; Weinrib, H.P.; Freeman, J.A. Electrical stimulation of nerve regeneration in the rat: The early effects evaluated by a vibrating probe and electron microscopy. Neuroscience 1991, 40, 93–107. [Google Scholar] [CrossRef]
- Kerns, J.M.; Lucchinetti, C. Electrical field effects on crushed nerve regeneration. Exp. Neurol. 1991, 117, 71–80. [Google Scholar] [CrossRef]
- Todorov, A.T.; Yogev, D.; Qi, P.; Fendler, J.H.; Rodziewicz, G.S. Electric-field-induced reconnection of severed axons. Brain. Res. 1992, 582, 329–334. [Google Scholar] [CrossRef]
- Pomeranz, B.; Campbell, J.J. Weak electric current accelerates motoneuron regeneration in the sciatic nerve of ten-month-old rats. Brain. Res. 1993, 603, 271–278. [Google Scholar] [CrossRef]
- Chen, Y.S.; Hu, C.L.; Hsieh, C.L.; Lin, J.G.; Tsai, C.C.; Chen, T.H.; Yao, C.H. Effects of percutaneous electrical stimulation on peripheral nerve regeneration using silicone rubber chambers. J. Biomed. Mater. Res. 2001, 57, 541–549. [Google Scholar] [CrossRef]
- Chen, Y.S.; Hsieh, C.L.; Tsai, C.C.; Chen, T.H.; Cheng, W.C.; Hu, C.L.; Yao, C.H. Increased success of peripheral nerve regeneration using silicone rubber chambers filled with an extracellular gel containing collagen, laminin and fibronectin. Biomaterials 2000, 21, 1541–1547. [Google Scholar] [CrossRef]
- Greer, R.; Daniel, J.; Uemura, E.; Kudej, R.; Chen, Y.S.; Chung, C.H. Use of a multiple lumen cuff for nerve regeneration. Mat. Res. Soc. Symp. Proc. 1994, 331, 3–11. [Google Scholar] [CrossRef]
- Chen, Y.S.; Miller, C.; Greer, M.H.; Quinones, M.; Greer, R.T. Development of a multiple-lumen nerve cuff utilizing growth stimulant patterns for controlled regeneration. Mat. Res. Soc. Symp. Proc. 1999, 550, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Lao, L.; Ren, K.; Berman, B.M. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology 2014, 120, 482–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, J.R.T.; Silva, M.L.; Prado, W.A. Analgesia induced by 2- or 100-Hz electroacupuncture in the rat tail-flick test depends on the activation of different descending pain inhibitory mechanisms. J. Pain 2011, 12, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, F.; Li, X.; Yang, Q.; Li, X.; Xu, N.; Huang, Y.; Zhang, Q.; Gou, X.; Chen, S.; et al. Electroacupuncture pretreatment attenuates cerebral ischemic injury through α7 nicotinic acetylcholine receptor-mediated inhibition of high-mobility group box 1 release in rats. J. Neuroinflammation 2012, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.Y.; Lin, J.G.; Tang, N.Y.; Kao, S.T.; Hsieh, C.L. Electroacupuncture-like stimulation at the Baihui (GV20) and Dazhui (GV14) acupoints protects rats against subacute-phase cerebral ischemia-reperfusion injuries by reducing S100B-mediated neurotoxicity. PLoS ONE 2014, 9, e91426. [Google Scholar] [CrossRef]
- Chen, H.C.; Chen, M.Y.; Hsieh, C.L.; Wu, S.Y.; Hsu, H.C.; Lin, Y.W. TRPV1 is a responding channel for acupuncture manipulation in mice peripheral and central nerve system. Cell. Physiol. Biochem. 2018, 49, 1813–1824. [Google Scholar] [CrossRef]
- Ashwell, J. The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nat. Rev. Immunol. 2006, 6, 532–540. [Google Scholar] [CrossRef]
- Zhang, C.N.; Rahimnejad, S. Molecular characterization of p38 MAPK from blunt snout bream (Megalobrama amblycephala) and its expression after ammonia stress, and lipoplysaccaride and bacterial challenge. Fish Shellfish Immunol. 2019, 84, 848–856. [Google Scholar] [CrossRef]
- Sreekanth, G.P.; Chuncharunee, A. SB203580 modulates p38 MAPK signaling and Dengue virus-induced liver injury by reducing MAPKAPK2, HSP27, and ATF2 phosporylation. PLoS ONE 2016, 11, e0149486. [Google Scholar] [CrossRef] [PubMed]
- Beardmore, V.A.; Hinton, H.J. Generation and characterization of p38beta (MAPK11) gene-targeted mice. Mol. Cell Biol. 2005, 25, 10454–10464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.; Ji, R. Intracellular Signaling in Primary Sensory Neurons and Persistent Pain. Neurochem. Res. 2008, 33, 1970–1978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizukoshi, K.; Sasakia, M.; Izumia, Y.; Miuraa, M.; Watanabe, M.; Amaya, F. Activation of p38 mitogen-activated protein kinase in the dorsal root ganglion contributes to pain hypersensitivity after plantar incision. Neuroscience 2013, 234, 77–87. [Google Scholar] [CrossRef]
- Tsuda, M. Microglia in the spinal cord and neuropathic pain. J. Diabetes Investig. 2016, 7, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N.; Hasegawa-Moriyama, M. Lidocaine attenuates the development of diabetic-induced tactile allodynia by inhibiting microglial activation. Anesth. Analg. 2011, 113, 941–946. [Google Scholar] [CrossRef]
- Cheng, K.I.; Wang, H.C. Persistent mechanical allodynia positively correlates with an increase in activated microglia and increased P-p38 mitogen-activated protein kinase activation in streptozotocin-induced diabetic rats. Eur. J. Pain 2014, 18, 162–173. [Google Scholar] [CrossRef]
- Hsu, H.C.; Yang, N.Y.; Lin, Y.W.; Ki, T.C.; Liu, H.J.; Hsieh, C.L. Effect of electroacupuncture on rats with chronic constriction injury-Induced neuropathic pain. Sci. World J. 2014, 2014, 129875. [Google Scholar] [CrossRef]
- Jiang, S.W.; Lin, Y.W.; Hsieh, C.L. Electroacupuncture at Hua Tuo Jia Ji acupoints reduced neuropathic pain and increased GABAA receptors in rat spinal cord. Evid. Based Complement Altern. Med. 2018, 2018, 8041820. [Google Scholar] [CrossRef]
- Huang, C.P.; Lin, Y.W.; Lee, D.Y.; Hsieh, C.L. Electroacupuncture relieves CCI-induced neuropathic pain involving excitatory and inhibitory neurotransmitters. Evid. Based Complement Altern. Med. 2019, 2019, 6784735. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.G.; Lo, M.W.; Wen, Y.R.; Hsieh, C.L.; Tsai, S.K.; Sun, W.Z. The effect of high and low frequency electroacupuncture in pain after lower abdominal surgery. Pain 2002, 99, 509–514. [Google Scholar] [CrossRef]
- Wang, B.; Tang, J.; White, P.F.; Naruse, R.; Sloninsky, A.; Kariger, R.; Gold, J.; Wender, R.H. Effect of the intensity of transcutaneous acupoint electrical stimulation on the postoperative analgesic requirement. Anesth. Analg. 1997, 85, 406–413. [Google Scholar] [PubMed]
- Chen, W.H.; Hsieh, C.L.; Huang, C.P.; Lin, T.J.; Tzen, J.T.C.; Ho, T.Y.; Lin, Y.W. Acid-sensing ion channel 3 mediates peripheral anti-hyperalgesia effects of acupuncture in mice inflammatory pain. J. Biomed. Sci. 2011, 18, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.H.; Tzen, J.T.C.; Hsieh, C.L.; Chen, Y.H.; Lin, T.J.; Chen, S.Y.; Lin, Y.W. Attenuation of TRPV1 and TRPV4 expression and function in mouse inflammatory pain models using electroacupuncture. J. Biomed. Sci. 2012, 2012. [Google Scholar] [CrossRef]
- Huang, C.P.; Chen, H.N.; Su, H.L.; Hsieh, C.L.; Chen, W.H.; Lai, Z.R.; Lin, Y.W. Electroacupuncture reduces carrageenan- and CFA-induced inflammatory pain accompanied by changing the expression of Nav1.7 and Nav1.8, rather than Nav1.9, in mice dorsal root ganglia. Evid. Based Complement Altern. Med. 2013, 2013, 312184. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.Y.; Chen, W.H.; Hsueh, C.H.; Lin, Y.W. Abundant expression and functional participation of TRPV1 at Zusanli acupoint (ST36) in mice: Mechanosensitive TRPV1 as an acupuncture-responding channel. BMC Complement Altern. Med. 2014, 14, 96. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.W.; Hsu, C.K.; Hsieh, C.L.; Yang, J.; Lin, Y.W. Probing the effects and mechanisms of electroacupuncture at ipsilateral or contralateral ST36-ST37 acupoints on CFA-induced inflammatory pain. Sci. Rep. 2016, 24, 22123. [Google Scholar] [CrossRef]
- Liao, H.Y.; Hsieh, C.L.; Huang, C.P.; Lin, Y.W. Electroacupuncture attenuates induction of inflammatory pain by regulating opioid and adenosine pathways in mice. Sci. Rep. 2017, 7, 15679. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Hsieh, C.L.; Lin, Y.W. Role of transient receptor potential vanilloid 1 in electroacupuncture analgesia on chronic inflammatory pain in mice. Biomed Res. Int. 2017, 2017, 5068347. [Google Scholar] [CrossRef] [Green Version]
- Yen, C.M.; Wu, T.C.; Hsieh, C.L.; Huang, Y.W.; Lin, Y.W. Distal electroacupuncture at the LI4 acupoint reduces CFA-induced inflammatory pain via the brain TRPV1 signaling pathway. Int. J. Mol. Sci. 2019, 20, 4471. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.C.; Hsieh, C.L.; Wu, S.Y.; Lin, Y.W. Toll-like receptor 2 plays an essential role in electroacupuncture analgesia in a mouse model of inflammatory pain. Acupunct. Med. 2019, 37, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Hsieh, C.L.; Wang, N.H.; Li, T.C.; Hwang, K.L.; Yu, S.C.; Chang, M.H. Acupuncture in patients with carpal tunnel syndrome: A randomized controlled trial. Clin. J. Pain. 2009, 25, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Wang, N.H.; Li, T.C.; Hsieh, C.L.; Chang, H.H.; Hwang, K.L.; Ko, W.S.; Chang, M.H. A randomized clinical trial of acupuncture versus oral steroids for carpal tunnel syndrome: A long-term follow-up. J. Pain 2011, 12, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Chang, M.H.; Liu, P.E.; Li, T.C.; Hsieh, C.L.; Hwang, K.L.; Chang, H.H. Acupuncture versus topiramate in chronic migraine prophylaxis: A randomized clinical trial. Cephalalgia 2011, 31, 1510–1521. [Google Scholar] [CrossRef]
- Walder, R.Y.; Rasmussen, L.A.; Rainier, J.D.; Light, A.R.; Wemmie, J.A.; Sluka, K.A. ASIC1 and ASIC3 play different roles in the development of hyperalgesia after inflammatory muscle injury. J. Pain 2010, 11, 210–218. [Google Scholar] [CrossRef] [Green Version]
- Black, J.A.; Liu, S.; Tanaka, M.; Cummins, T.R.; Waxman, S.G. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain 2004, 108, 237–247. [Google Scholar] [CrossRef]
- Yu, L.; Yang, F.; Luo, H.; Liu, F.Y.; Han, J.S.; Xing, G.G.; Wan, Y. The role of TRPV1 in different subtypes of dorsal root ganglion neurons in rat chronic inflammatory nociception induced by complete Freund’s adjuvant. Mol. Pain 2008, 4, 61. [Google Scholar] [CrossRef] [Green Version]
- Goldman, N.; Chen, M.; Fujita, T.; Xu, Q.; Peng, W.; Liu, W.; Jensen, T.; Pei, Y.; Wang, F.; Han, X.; et al. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat. Neurosci. 2010, 13, 883–888. [Google Scholar] [CrossRef] [Green Version]
- Silberstein, S.D. Migraine pathophysiology and its clinical implications. Cephalalgia 2004, 24 (Suppl. 2), 2–7. [Google Scholar] [CrossRef]
- Burstein, R.; Noseda, R.; Borsook, D. Migraine: Multiple processes, complex pathophysiology. J. Neurosci. 2015, 35, 6619–6629. [Google Scholar] [CrossRef]
- Sabino, G.S.; Santos, C.M.; Francischi, J.N.; de Resende, M.A. Release of endogenous opioids following transcutaneous electric nerve stimulation in an experimental model of acute inflammatory pain. J. Pain 2008, 9, 157–163. [Google Scholar] [CrossRef] [PubMed]
- King, E.W.; Audette, K.; Athman, G.A.; Nguyen, H.O.; Sluka, K.A.; Fairbanks, C.A. Transcutaneous electrical nerve stimulation activates peripherally located alpha-2A adrenergic receptors. Pain 2005, 115, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, L.; Budelier, K.; Clinesmith, M.; Fiedler, A.; Landstrom, R.; Leeper, B.J.; Moeller, L.; Mutch, S.; O’Dell., K.; Ross, J.; et al. Transcutaneous electrical nerve stimulation (TENS) reduces chronic hyperalgesia induced by muscle inflammation. Pain 2006, 120, 182–187. [Google Scholar] [CrossRef]
- Sekido, R.; Ishimaru, K.; Sakita, M. Differences of electroacupuncture-induced analgesic effect in normal and inflammatory conditions in rats. Am. J. Chin. Med. 2003, 31, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, M.; Kirkwood, K.L. p38alpha stabilizes interleukin-6 mRNA via multiple AU-rich elements. J. Biol. Chem. 2008, 283, 1778–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tudor, C.; Marchese, F.P. p38 mitogen-activated protein kinase inhibits tristetraprolin-directed decay of the mRNA of the anti-inflammatory cytokine interleukin-10. FEBS Lett. 2009, 583, 1933–1938. [Google Scholar] [CrossRef] [Green Version]
- Berger, C.; von Kummer, R. Does NO regulate the cerebral blood flow response in hypoxia? Acta Neurol. Scand. 2009, 97, 118–125. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Lin, J.G.; Tang, N.Y.; Kao, S.T.; Hsieh, C.L. Electroacupuncture at different frequencies (5Hz and 25Hz) ameliorates cerebral ischemia-reperfusion injury in rats: Possible involvement of p38 MAPK-mediated anti-apoptotic signaling pathways. BMC Complement Altern. Med. 2015, 15, 241. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, S.C.; Delacey, M.; Ganote, C.E. Phosphorylation state of hsp27 and p38 MAPK during preconditioning and protein phosphatase inhibitor protection of rabbit cardiomyocytes. J. Mol. Cell Cardiol. 1999, 31, 555–567. [Google Scholar] [CrossRef]
- Marais, E.; Genade, S.; Salie, R.; Huisamen, B.; Maritz, S.; Moolman, J.A.; Lochner, A. The temporal relationship between p38 MAPK and HSP 27 activation in ischaemic and pharmacological preconditioning. Basic Res. Cardiol. 2005, 100, 35–47. [Google Scholar] [CrossRef]
- Fan, W.; Gao, X.K. Hsp70 interacts with mitogen-activated protein kinase (MAPK)-activated protein kinase 2 to regulate p38MAPK stability and myoblast differentiation during skeletal muscle regeneration. Mol. Cell Biol. 2018, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Q.Y.; Lin, Y.W.; Hsieh, C.L. Acupuncture and neuroregeneration in ischemic stroke. Neural Regen. Res. 2018, 13, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Sun, H.; Chen, S.H.; Zhang, Y.M.; Piao, Y.L.; Gao, Y. Effects of acupuncture at Baihui (DU20) and Zusanli (ST36) on the expression of heat shock protein 70 and tumor necrosis factor α in the peripheral serum of cerebral ischemia-reperfusion-injured rats. Chin. J. Integr. Med. 2014, 20, 369–374. [Google Scholar] [CrossRef]
- Dayalan Naidu, S.; Sutherland, C.; Zhang, Y.; Risco, A.; de la Vega, L.; Caunt, C.J.; Hastie, C.J.; Lamont, D.J.; Torrente, L.; Chowdhry, S.; et al. Heat Shock Factor 1 is a substrate for p38 mitogen-activated protein kinase. J. Mol. Cell Biol. 2016, 36, 2403–2417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, X.; Qiao, A.; Moskophidis, D.; Mivechi, N.F. Modulation of Heat Shock Factor 1 Activity through Silencing of Ser303/Ser307 Phosphorylation Supports a Metabolic Program Leading to Age-Related Obesity and Insulin Resistance. Mol. Cell Biol. 2018, 38, e00095-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, H.; Wang, X.S. MAPKAPK5, a novel mitogen-activated protein kinase (MAPK)-activated protein kinase, is a substrate of the extracellular-regulated kinase (ERK) and p38 kinase. Biochem. Biophys. Res. Comm. 1998, 243, 492–496. [Google Scholar] [CrossRef]
- Sahadevan, P.; Allen, B.G. MK5: A novel regulator of cardiac fibroblast function? Iubmb Life 2017, 69, 785–794. [Google Scholar] [CrossRef] [Green Version]
- Shiryaev, A.; Moens, U. Mitogen-activated protein kinase p38 and MK2, MK3 and MK5: Ménage à trois or ménage à quatre. Cell Signal. 2010, 22, 1185–1192. [Google Scholar] [CrossRef]
- Liu, C.H.; Lin, Y.W.; Tang, N.Y.; Liu, H.J.; Hsieh, C.L. Neuroprotective Effect of Uncaria rhynchophylla in kainic acid-induced epileptic seizures by modulating hippocampal mossy fiber sprouting, neuron survival, astrocyte proliferation, and S100B expression. Evid. Based Complement Altern. Med. 2012, 2012, 194790. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.T.; Jeon, S.; Park, H.J.; Hong, M.S.; Jeong, W.B.; Kim, J.H.; Kim, Y.; Lee, H.J.; Park, H.J.; Chung, J.H. Acupuncture inhibits kainic acid-induced hippocampal cell death in mice. J. Physiol. Sci. 2008, 58, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.T.; Doo, A.R.; Kim, S.N.; Kim, S.Y.; Kim, Y.Y.; Kim, J.H.; Lee, H.; Yin, C.S.; Park, H.J. Acupuncture suppresses kainic acid-induced neuronal death and inflammatory events in mouse hippocampus. J. Physiol. Sci. 2012, 62, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Bae, C.H.; Kim, D.S.; Jun, Y.L.; Kwon, S.; Park, H.J.; Hahm, D.H.; Lee, H.; Kim, S.T. Proteomic analysis of the effect of acupuncture on the suppression of kainic acid-induced neuronal destruction in mouse hippocampus. Evid. Based Complement Altern. Med. 2013, 2013, 436315. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Lin, Y.W.; Hsu, H.C.; Liu, H.J.; Lin, W.J.; Hsieh, C.L. Electroacupuncture at ST36-ST37 and at ear ameliorates hippocampal mossy fiber sprouting in kainic acid-induced epileptic seizure rats. Biomed Res. Int. 2014, 2014, 756019. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.C.; Tang, N.Y.; Liu, C.H.; Hsieh, C.L. Antiepileptic Effect of Uncaria rhynchophylla and Rhynchophylline involved in the initiation of c-Jun N-terminal kinase phosphorylation of MAPK signal pathways in acute seizures of kainic acid-treated rats. Evid. Based Complement Altern. Med. 2013, 2013, 961289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.W.; Hsieh, C.L. Auricular electroacupuncture reduced inflammation-related epilepsy accompanied by altered TRPA1, pPKCα, pPKCε, and pERk1/2 signaling pathways in kainic acid-treated rats. Mediat. Inflamm. 2014, 2014, 493480. [Google Scholar] [CrossRef]
- Liao, E.T.; Tang, N.Y.; Lin, Y.W.; Hsieh, C.L. Long-term electrical stimulation at ear and electro-acupuncture at ST36-ST37 attenuated COX-2 in the CA1 of hippocampus in kainic acid-induced epileptic seizure rats. Sci. Rep. 2017, 7, 472. [Google Scholar] [CrossRef] [Green Version]
- Inprasit, C.; Lin, Y.W.; Huang, C.P.; Wu, S.Y.; Hsieh, C.L. Targeting TRPV1 to relieve motion sickness symptoms in mice by electroacupuncture and gene deletion. Sci. Rep. 2018, 8, 10365. [Google Scholar] [CrossRef] [PubMed]
- Maione, S.; Cristino, L.; Migliozzi, A.; Georgiou, A.; Starowicz, K.; Salt, T.; Marzo, V. TRPV1 channels control synaptic plasticity in the developing superior colliculus. J. Physiol. 2009, 587, 2521–2535. [Google Scholar] [CrossRef]
- Puig, B.; Gomez-Isla, T. Expression of stress-activated kinases c-Jun N-terminal kinase (SAPK/JNK-P) and p38 kinase (p38-P), and tau hyperphosphorylation in neurites surrounding betaA plaques in APP Tg2576 mice. Neuropathol. Appl. Neurobiol. 2004, 30, 491–502. [Google Scholar] [CrossRef]
- Risco, A.; Cuenda, A. New Insights into the p38γ and p38δ MAPK Pathways. J. Signal Transduct. 2012, 2012, 520289. [Google Scholar] [CrossRef] [Green Version]
- Escós, A.; Risco, A. p38γ and p38δ Mitogen Activated Protein Kinases (MAPKs), New Stars in the MAPK Galaxy. Front. Cell Dev. Biol. 2016, 4, 31. [Google Scholar] [CrossRef] [Green Version]
- Maphis, N.; Jiang, S. Selective suppression of the alpha isoform of p38 MAPK rescues late-stage tau pathology. Alzheimers Res. 2016, 8, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.W.; Hsieh, C.L. Electroacupuncture at Baihui acupoint (GV20) reverses behavior deficit and long-term potentiation through N-methyl-d-aspartate and transient receptor potential vanilloid subtype 1 receptors in middle cerebral artery occlusion rats. J. Integr. Neurosci. 2010, 9, 269–282. [Google Scholar] [CrossRef]
- Dong, W.; Guo, W.; Zheng, X.; Wang, F.; Chen, Y.; Zhang, W.; Shi, H. Electroacupuncture improves cognitive deficits associated with AMPK activation in SAMP8 mice. Metab. Brain Dis. 2015, 30, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Lee, Y.J. Role of the ASK1-SEK1-JNK1- HIPK1 signal in Daxx trafficking and ASK1 oligomerization. J. Biol. Chem. 2003, 278, 47245–47252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.Y.; Nishitoh, H.; Yang, X.; Ichijo, H.; Baltimore, D. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science 1988, 281, 1860–1863. [Google Scholar] [CrossRef] [PubMed]
- Akterin, S.; Cowburn, R.F.; Miranda-Vizuete, A.; Jimenez, A.; Bogdanovic, N.; Winblad, B.; Cedazo-Minguez, A. Involvement of glutaredoxin-1 and thioredoxin-1 in amyloid toxicity and Alzheimer’s disease. Cell Death Differ. 2006, 13, 1454–1465. [Google Scholar] [CrossRef] [Green Version]
- Takahashi-Niki, K.; Niki, T.; Taira, T.; Iguchi-Ariga, S.M.M.; Ariga, H. Reduced anti-oxidative stress activities of DJ-1 mutants found in Parkinson’s disease patients. Biochem. Biophys. Res. Commun. 2004, 320, 389–397. [Google Scholar] [CrossRef]
- Junn, E.; Taniguchi, H.; Jeong, B.S.; Zhao, X.; Ichijo, H.; Mouradian, M.M. Interaction of DJ-1 with Daxx inhibits apoptosis signal-regulating kinase 1 activity and cell death. Proc. Natl. Acad. Sci. USA 2005, 102, 9691–9696. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Liu, J.; Li, X.; Zhong, Y.; Zhong, T.; Liu, Y.; Wang, J.H.; Jiang, Y. PRAK Interacts with DJ-1 and prevents oxidative stress-induced cell death. Oxid. Med. Cell. Longev. 2014, 735618. [Google Scholar] [CrossRef]
- Karunakaran, S.; Diwakar, L. Activation of apoptosis signal regulating kinase 1 (ASK1) and translocation of death-associated protein, Daxx, in substantia nigra pars compacta in a mouse model of Parkinson’s disease: Protection by alpha-lipoic acid. FASEB J. 2007, 21, 2226–2236. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.W.; Yang, J.; Hsieh, C.L.; Hsu, Y.C.; Lin, Y.W. Electroacupuncture restores spatial learning and downregulates phosphorylated N-methyl-D-aspartate receptors in a mouse model of Parkinson’s disease. Acupunct. Med. 2017, 35, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.G.; Hsieh, C.L.; Lin, Y.W. Analgesic effect of electroacupuncture in a mouse fibromyalgia model: Roles of TRPV1, TRPV4, and pERK. PLoS ONE 2015, 10, e0128037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, K.W.; Hsieh, C.L.; Yang, J.; Lin, Y.W. Effects of electroacupuncture in a mouse model of fibromyalgia: Role of N-methyl-D-aspartate receptors and related mechanisms. Acupunct. Med. 2017, 35, 59–68. [Google Scholar] [CrossRef] [Green Version]




| p38 Subfamily | Other Names | Upstream | Location/Function | Dysfunction and Diseases |
|---|---|---|---|---|
| p38α | MAPK14, SAPK2a, CSBP | MKK3, MKK4, MKK6, MKK7 | Ubiquitously expressed at significant levels in most cell types. Involved in the regulation of cell proliferation, differentiation, development, and response to stress [77,78,84,99]. | Defective placental angiogenesis causing embryo death (mouse), symmetric synchronous cell cleavage (zebrafish), reduction in erythropoietin (Epo) production [99], leading to anemia, the impairment of glucogenesis (mice), and lipid-induced insulin-resistance (rat) [77,97]. |
| p38β | MAPK 11 | MKK3, MKK4, MKK6 | Ubiquitously expressed; upregulated in the CNS and lungs, downregulated in the healthy heart [104,105,106]. | No phenotype found [77]. |
| p38γ | MAPK 13, ERK6, SAPK3 1 | MKK3, MKK4, MKK6MKK7 | Myoblast and skeletal muscle./(1) Under stress conditions, they act on scaffold proteins targeting the plasma membrane cytoskeleton at sites of neuromuscular junctions and gap junctions [107]. (2) Recent studies indicate that the microbial metabolite imidazole propionate may contribute to the pathogenesis of type 2 diabetes via the activation of p38γ/p62/ mTORC1 [89]. | No phenotype found (mouse) [77]. Meiotic G2/M progression of oocytes (xenopus) [77,104,105]. |
| p38δ | MAPK 12, SAPK4 | MKK3, MKK4, MKK6, MKK7 | Only expressed in the lungs, kidney, testis, spleen, pancreas, and small intestine in humans, rats, and mice, but not in other vertebrates; enriched in endocrine glands [107,108]./(1) Regulates cytoplasmic microtubule dynamics, including tau protein [107]. (2) Upregulated in the liver in obese patients with NAFLD [89]. | No phenotype found [77]. |
| Study | Model | Intervention | Acupoints | Evaluation | Result |
|---|---|---|---|---|---|
| Hsu et al., (2014) [173] | SD rats | CCI-induced neuropathic pain; EA, 2- and 15-Hz, 20 min | Ipsilateral ST36-ST37 of the affected limb | Behavioral responses to stimuli; expression of TRPV1/4 in the cerebral cortex and lumbar spinal cord | EA relieved neuropathic pain; downregulation of cerebral TRPV4 expression. |
| Jiang et al., (2018) [174] | SD rats | CCI-induced neuropathic pain; EA, 2- and 15-Hz, 20 min | Bilateral L4-L6 Hua Tuo Jia Ji (EX-B2) | GABAA, A1R, TRPV1/4, and mGluR3 in the DRG | EA reduced the pain response, upregulating the GABAA receptor in the spinal cord. |
| Huang et al., (2019) [175] | SD rats | CCI-induced neuropathic pain; EA, 2-, 15- and 50-Hz, 20 min | GV20, GV14 | Expression of the GABAA receptor and the level of glutamate in the hippocampus and periaqueductal gray (PAG) area. | EA reduced the pain response; suppressed hippocampal GABAA receptors; decreased thalamic glutamate levels. |
| Lin et al., (2002) [176] | Human | Preoperative EA, 2- (low) or 100- (high) Hz, 20 min | Bilateral ST36 | Postoperative pain and opioid-related side effects | Both low- and high-frequency EA reduced postoperative analgesic requirements and associated side effects. |
| Wang et al., (1997) [177] | Human | Postoperative TAES, 2- (low) or 100- (high) Hz, 30 min | Bilateral LI4 | Postoperative pain and opioid-related side effects | Both low- and high-frequency EA reduced postoperative analgesic requirements and associated side effects. |
| Chen et al. (2011) [178] | CD1 mice | EA, 2-Hz, 20 min | Bilateral ST36 | Behavioral responses in the paw and ASIC3 overexpression in DRG neurons. | Rescued mechanical hyperalgesia and an ASIC3 downregulation. |
| Chen et al. (2012) [179] | ICR mice | EA, 2-Hz, 15 min | Bilateral ST36 | Behavioral responses in the paw and TRPV1/4 overexpression in DRG neurons. | TRPV1 and TRPV4 upregulation in DRG neurons was attenuated by EA. |
| Huang et al. (2013) [180] | ICR mice | EA, 2-Hz, 15 min | Bilateral ST36 | Behavioral responses in the paw and the overexpression of Nav1 in DRG neurons. | EA attenuated inflammatory pain by suppressing Nav1 overexpression. |
| Wu et al. (2014) [181] | ICR mice | MA, 60 min | Ipsilateral ST36 of the inflamed limb | Behavioral responses in paw; the overexpression of TRPV1/4, ASIC3, and CWP components in the anatomical layers of ST36. | MA induced analgesia, with high TRPV1 and CWP overexpression at ST36 upon MA. |
| Lu et al. (2016) [182] | C57/B6 mice | EA, 2-Hz, 15 min | Ipsilateral and contralateral ST36-ST37 of the inflamed limb | Behavioral responses in the paw; Nav and TRPV1 overexpression in DRG neurons. | Hyperalgesia was suppressed through ipsilateral and contralateral EA. Nav and TRPV1 were suppressed through EA. |
| Liao et al. (2017) [143] | C57/B6 mice | EA, 2-Hz, 15 min | Bilateral ST36 | Behavioral responses in the paw and the expression of Nav, GFAP, Iba-1, S100B, RAGE, and TRPV1 in DRG neurons. | EA attenuated inflammatory pain by suppressing Nav1.8 through S100B, TRPV1, opioid, and adenosine pathways. |
| Liao et al. (2017) [183] | C57/B6 mice | EA, 2-Hz, 15 min | Bilateral ST36 | Behavioral responses in the paw and the expression of GFAP, S100B, RAGE, PKCε, ERK, NF-κB, and COX-2 in DRG neurons. | EA attenuated inflammatory pain by suppressing opioid and adenosine pathways. |
| Yang et al. (2017) [184] | C57/B6 mice | EA, 2-Hz, 15 min | Bilateral ST36 | Behavioral responses in the paw and the expression of TRPV1, PKA, PKC, PI3K, ERK1/2, p38, JNK, Akt, mTOR, CREB, NF-κB, Nav1.7/1.8, GFAP, S100B, and RAGE in DRG neurons. | EA significantly reduced chronic inflammatory pain by downregulating the TRPV1 pathway from the peripheral DRG neurons to the central spinal cord. |
| Yen et al. (2019) [185] | C57/B6 mice | EA, 2-Hz, 15 min | Bilateral LI4 | Behavioral responses in the paw and the expression of TRPV1 and ERK1/2 in the prefrontal cortex, the hypothalamus, the PAG area, and DRG neurons. | Pain alleviation immediately after EA; the expression of TRPV1-associated molecules was attenuated by EA in the prefrontal cortex, the hypothalamus, the PAG area, and DRG. |
| Hsu et al. (2019) [186] | C57/B6 mice | EA, 2-Hz, 15 min | Bilateral ST36 | Behavioral responses in the paw and the expression of TLR2, PI3K, ERK1/2, p38, JNK, Akt, mTOR, CREB, NF-κB, and Nav1.7/1.8 in the thalamus. | EA attenuated inflammatory pain via TLR2 signaling. |
| Yang et al. (2009) [187] | Patients with CTS | MA, 30 min/session, 2 session a week, 8 session in total | Affected side(s), PC6, PC7 | Motor and sensory NCS; designed symptomatic questionnaire. | Short-term acupuncture was as effective as short-term low-dose steroid for mild-to-moderate CTS. |
| Yang et al. (2011) [188] | Patients with CTS | MA, 30 min/session, 2 session a week, 8 session in total | Affected side(s), PC6, PC7 | NCS; global symptom score. | Acupuncture had superior efficacy to steroid treatment not only in terms of objective changes in nerve conduction but also in terms of subjective symptom assessment in long-term follow-up. |
| Yang et al. (2011) [189] | Patients with chronic migraine (CM) | MA, 30 min/session, 2 session a week, 24 session in total | Bilateral BL2, GB20, EX-HN5, EX-HN3 (acupoints relate to the trigeminal and cervical dermatomes) | Changes in headache events, MIDAS scores, HADS scores, BDI-II scores, reduction of medication. | Acupuncture was similarly effective or more effective than prophylactic drug treatment with less side effects in migraine. |
| Study | Model | Intervention | Acupoints | Evaluation | Result |
|---|---|---|---|---|---|
| Bäcker et al., (2003) [13] | Healthy human | MA; manipulation as either high frequency (4–8 Hz) and low amplitude (Hf–La) or low frequency (1–2 Hz) and high amplitude (Lf–Ha). | Right LI4 | Cerebral blood flow velocity (CBFV) in both middle cerebral arteries, arterial blood pressure (BP), heart rate (HR). | (1) Lf–Ha stimulation was perceived as more intense and markedly increased the CBFV in the right hemisphere; (2) Hf–La stimulation slightly decreased BP and HR; (3) Lf–Ha stimulation induced an initial pressor response (increase of BP, decrease of HR) and a more marked long-term BP reduction. |
| Hsieh et al., (2006) [14] | SD rats | EA, 2-Hz, 15 min | Both ST36 | The levels of nitric oxide in the peripheral blood and amounts of calcitonin gene-related peptide (CGRP) in the cerebral cortex and thalamus. L-N (G)-nitro arginine methyl ester (L-NAME) was used to measure the changes in CBF. | Both 2- and 15-Hz EA increased CBF in rats with and without CI. |
| Cheng et al. (2014) [147] | SD rats | EA, 2-Hz, 25 min once daily for 2 consecutive days. | GV20, GV14 | Cerebral infarct area, caspase-3, BDNF, pRaf-1, MEK1/2, ERK1/2, p90RSK, and Bad. | EA significantly reduced the cerebral infarct area, caspase-3 protein expression levels, and apoptosis in the ischemic cortex. BDNF, phospho-Raf-1 (pRaf-1), phospho-MEK1/2 (pMEK1/2), phospho-ERK1/2 (pERK1/2), phospho-90 kDa ribosomal S6 kinase (pp90RSK), and phospho-Bad (pBad) were markedly upregulated, and neuronal nuclear antigen (NeuN) expression was restored. |
| Cheng et al. (2014) [161] | SD rats | EA, 2-Hz, 15 min once daily for 6 consecutive days. | GV20, GV14 | Cerebral infarct area, GFAP, S100B, NF-κB, p50, p38 MAPK, TNF-α, and iNOS. | EA significantly reduced the cerebral infarct area and downregulated astrocytic S100B expression and decreased p-p38 NF-kB. |
| Cheng et al. (2015) [203] | SD rats | EA, 5- or 25-Hz, 30 min once daily for 7 consecutive days. | GV20, GV16 | Cerebral infarct area, GFAP, Bax, Bcl-xL, Smac/DIABLO, p-p38, and CREB. | Both 5- and 25-Hz EA effectively downregulated reactive astrocytosis to exert neuroprotective effects against cerebral infarction, most likely by activating the p38 MAPK/CREB signaling pathway. |
| Xu et al. (2014) [208] | SD rats | EA, 2-Hz, 20 min once a day. | GV20, ST36 | Hsp70 and TNF-α peripheral serum. | Lowered peak levels of adrenocorticotrophic hormone and Hsp70. |
| Kuo et al. (2016) [15] | SD rats | Electrostimulation, 2-Hz, 20 min once daily for 7 consecutive days. | Both ears | Brain nicotinic acetylcholine receptors. | Two-hertz ES for ameliorated learning and memory impairment. |
| Study | Model | Intervention | Acupoints | Evaluation | Result |
|---|---|---|---|---|---|
| Kim et al., (2008) [215] | ICR mice | MA, 20 min/day, for 2 days | Bilateral HT8 | Hippocampal expression of c-Fos, c-Jun, and GAD-67 (CA1 and CA3 areas). | Reduced severity of epileptic seizures and the rate of neuronal death; downregulation of c-Fos and c-Jun; upregulation of GAD-67. |
| Kim et al. (2012) [216] | C57BL/6 mice | MA, 20 min/day, for 2 days | Bilateral HT8 | Neuronal survival, microglial and astrocyte activation, and hippocampal mRNA expression of IL-1β and TNF-α. | Inhibition of hippocampal cell death and suppression of KA-induced inflammatory events. |
| Bae et al. (2013) [217] | C57BL/6 mice | MA, 20 min/day, for 3 days | Bilateral HT8 | Neuronal survival and hippocampal astrocyte activation. | Acupuncture altered hippocampal protein expression to promote neuronal survival. |
| Liu et al. (2014) [218] | SD rats | EA, 2 Hz, 30 min/day for 7 consecutive days. | Bilateral ST-36-ST37 and ears | Changes in mossy fibers sprouting in the hippocampus. | Amelioration of mossy fibers sprouting in the hippocampus. |
| Lin et al., (2014) [220] | SD rats | EA, 2 Hz, 20 min/day, 3 days/week for 6 weeks | Bilateral ears, ST36, ST37 | EEG and EMG changes; hippocampal TRPA1, TRPV4, PKCα, PKCε, and pERK1/2 expression. | EA reduced hippocampal hyperactivity accompanied by alterations in the TRPA1, PKCε, PKCα, and pERK1/2 signaling pathways. |
| Liao et al., (2017) [221] | SD rats | EA, 2 Hz, 20 min/day, 3 days/week for 6 weeks | Bilateral ears, ST36, ST37 | EEG and EMG changes; hippocampal COX-2 levels. | Attenuated COX-2 and COX-2 immunoreactive cells in the hippocampal CA1 region after epileptic seizures. |
| Liao et al. (2018) [40] | SD rats | EA, 2 Hz, 20 min/day, 3 days/week for 6 weeks | Bilateral ears | Brain TLR4, CaMKIIα, ERK, JNK, and NF-κB expression. | Auricular EA controlled epileptic seizures by regulating the TLR4 signaling pathway. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, T.-H.; Hsieh, C.-L. Effect of Acupuncture on the p38 Signaling Pathway in Several Nervous System Diseases: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 4693. https://doi.org/10.3390/ijms21134693
Wei T-H, Hsieh C-L. Effect of Acupuncture on the p38 Signaling Pathway in Several Nervous System Diseases: A Systematic Review. International Journal of Molecular Sciences. 2020; 21(13):4693. https://doi.org/10.3390/ijms21134693
Chicago/Turabian StyleWei, Tzu-Hsuan, and Ching-Liang Hsieh. 2020. "Effect of Acupuncture on the p38 Signaling Pathway in Several Nervous System Diseases: A Systematic Review" International Journal of Molecular Sciences 21, no. 13: 4693. https://doi.org/10.3390/ijms21134693
APA StyleWei, T.-H., & Hsieh, C.-L. (2020). Effect of Acupuncture on the p38 Signaling Pathway in Several Nervous System Diseases: A Systematic Review. International Journal of Molecular Sciences, 21(13), 4693. https://doi.org/10.3390/ijms21134693




