Lipid Signaling in Ocular Neovascularization
Abstract
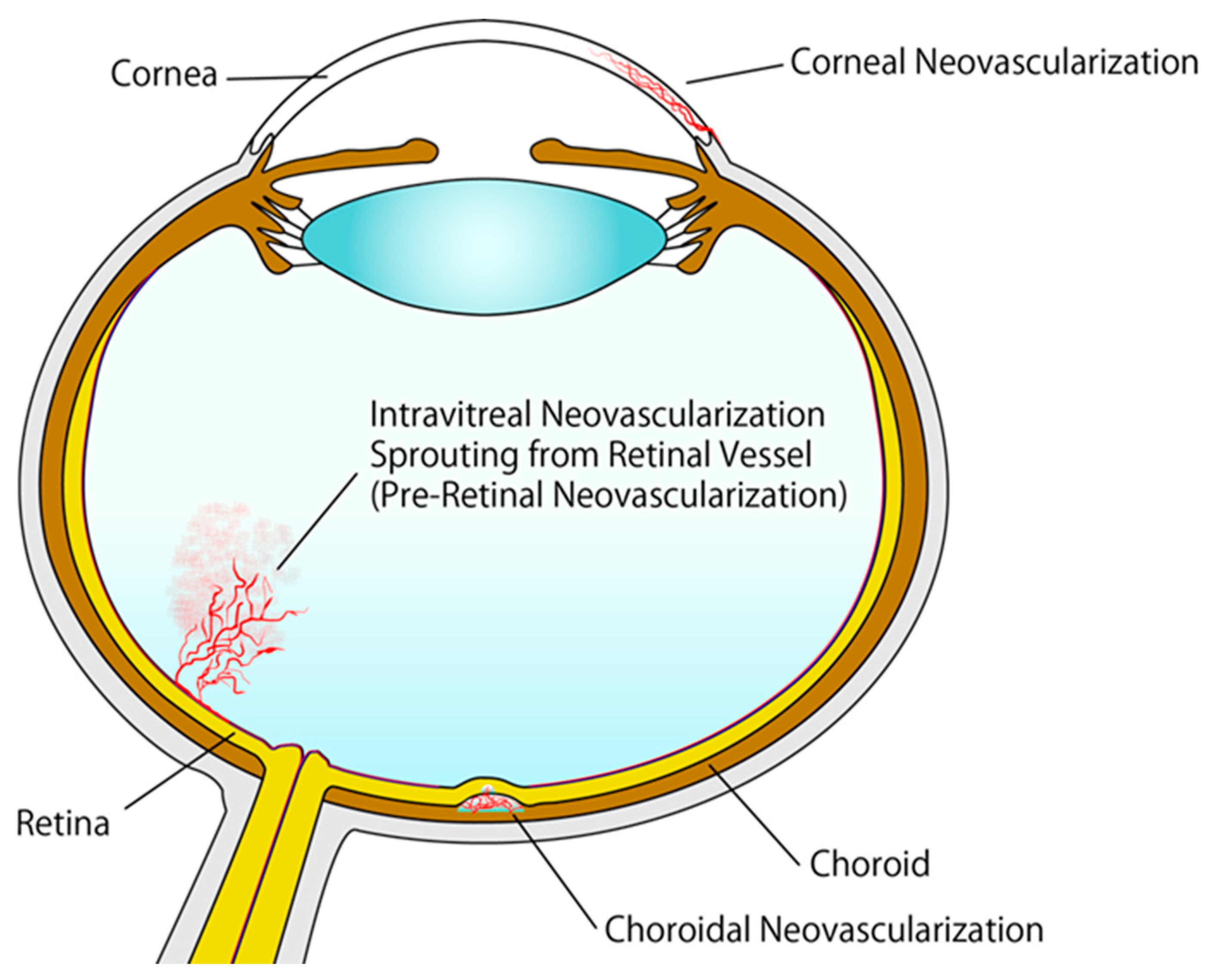
1. Introduction
Signaling in Ocular Neovascularization
2. Glycerophospholipids in Ocular Neovascularization
2.1. Lysophosphatidic Acid (LPA)
2.2. Lysophosphatidic Acid and Ocular Neovascularization
3. Sphingolipids in Ocular Neovascularization
3.1. Sphingosine 1-Phosphate
3.2. Sphingosine 1-Phosphate and Ocular Neovascularization
4. The Role of Fatty Acids and Their Metabolites in Ocular Neovascularization
4.1. ω-6 Polyunsaturated Fatty Acids
4.2. ω-3 Polyunsaturated Fatty Acids
5. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | arachidonic acid |
| ACER | alkaline ceramidase |
| AGK | acylglycerol kinase |
| Akt | protein kinase B |
| AMD | age-related macular degeneration |
| ATX | autotaxin |
| cAMP | cyclic adenosine monophosphate |
| CNV | choroidal neovascularization |
| COX | cyclooxygenase |
| DHA | docosahexaenoic acid |
| DLL4 | delta-like ligand |
| eNOS | endothelial nitric oxide synthase |
| EP | prostaglandin E2 receptor |
| EPA | eicosapentaenoic acid |
| ERK | extracellular signal-regulated kinase |
| FGF | fibroblast growth factor |
| GLUT | glucose transporter |
| GTPases | guanosine triphosphatases |
| HIF | hypoxia inducible factor |
| HSV | herpes simplex virus |
| HRE | hypoxia response elements |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| LPA | lysophosphatidic acid |
| LPC | lysophosphatidylcholine |
| LPE | lysophosphatidylethanolamine |
| LysoPS | lysophosphatidylserine |
| MAPK | mitogen-activated protein kinase |
| mTOR | mammalian target of rapamycin |
| NV | neovascularization |
| OIR | oxygen-induced retinopathy |
| PA | phosphatidic acid |
| PA-PLA | phosphatidic acid specific phospholipase A |
| PC | phosphatidyl choline |
| PDGF | platelet-derived growth factor |
| PDR | proliferative diabetic retinopathy |
| PE | phosphatidylethanolamines |
| PG | prostaglandin |
| PGI2 | prostacyclin |
| PI3K | phosphoinositide 3-kinase |
| PKC | protein kinase C |
| PLA | phospholipase A |
| PLD | phospholipase D |
| PPAR | peroxisome proliferator-activated receptor |
| PS | phosphatidylserine |
| PUFA | polyunsaturated fatty acid |
| ROCK | Rho-associated protein kinase |
| ROP | retinopathy of prematurity |
| RPE | retinal pigment epithelium |
| Rv | resolvin |
| RVO | retinal vein occlusion |
| SMase | sphingomyelinase |
| STAT | signal transducers and activator of transcription |
| SphK | sphingosine kinase |
| S1P | sphingosine 1-phosphate |
| TAZ | transcriptional co-activator with PDZ-binding motif |
| TNF | tumor Necrosis Factor |
| TX | thromboxane |
| VCAM | vascular cell adhesion molecule |
| VE | vascular endothelial |
| VEGF | vascular endothelial growth factor |
| VEGFR | vascular endothelial growth factor receptor |
| YAP | yes-associated protein |
References
- Saliba, A.E.; Vonkova, I.; Gavin, A.C. The systematic analysis of protein-lipid interactions comes of age. Nat. Rev. Mol. Cell Biol. 2015, 16, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.T.; Yudell, B.E.; Loor, J.J. Regulation of energy metabolism by long-chain fatty acids. Prog. Lipid Res. 2014, 53, 124–144. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.B. Membranes and evolution. Curr. Biol. 2018, 28, R381–R385. [Google Scholar] [CrossRef] [PubMed]
- Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef] [PubMed]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef]
- Escribá, P.V. Membrane-lipid therapy: A new approach in molecular medicine. Trends Mol. Med. 2006, 12, 34–43. [Google Scholar] [CrossRef]
- Hui, D.Y. Intestinal phospholipid and lysophospholipid metabolism in cardiometabolic disease. Curr. Opin. Lipidol. 2016, 27, 507–512. [Google Scholar] [CrossRef]
- Nagahashi, M.; Abe, M.; Sakimura, K.; Takabe, K.; Wakai, T. The role of sphingosine-1-phosphate in inflammation and cancer progression. Cancer Sci. 2018, 109, 3671–3678. [Google Scholar] [CrossRef]
- Saito, J.; Morishige, N.; Chikama, T.; Gu, J.; Sekiguchi, K.; Nishida, T. Differential regulation of focal adhesion kinase and paxillin phosphorylation by the small GTP-binding protein Rho in human corneal epithelial cells. Jpn. J. Ophthalmol. 2004, 48, 199–207. [Google Scholar] [CrossRef]
- Solati, Z.; Ravandi, A. Lipidomics of Bioactive Lipids in Acute Coronary Syndromes. Int. J. Mol. Sci. 2019, 20, 1051. [Google Scholar] [CrossRef]
- Kermorvant-Duchemin, E.; Sennlaub, F.; Sirinyan, M.; Brault, S.; Andelfinger, G.; Kooli, A.; Germain, S.; Ong, H.; d’Orleans-Juste, P.; Gobeil, F., Jr.; et al. Trans-arachidonic acids generated during nitrative stress induce a thrombospondin-1-dependent microvascular degeneration. Nat. Med. 2005, 11, 1339–1345. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fierro, I.M.; Kutok, J.L.; Serhan, C.N. Novel lipid mediator regulators of endothelial cell proliferation and migration: Aspirin-triggered-15R-lipoxin A(4) and lipoxin A(4). J. Pharmacol. Exp. Ther. 2002, 300, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Moccia, F.; Negri, S.; Shekha, M.; Faris, P.; Guerra, G. Endothelial Ca(2+) Signaling, Angiogenesis and Vasculogenesis: Just What It Takes to Make a Blood Vessel. Int. J. Mol. Sci. 2019, 20, 3962. [Google Scholar] [CrossRef] [PubMed]
- Moriya, J.; Minamino, T. Angiogenesis, Cancer, and Vascular Aging. Front. Cardiovasc. Med. 2017, 4, 65. [Google Scholar] [CrossRef] [PubMed]
- Ratajska, A.; Jankowska-Steifer, E.; Czarnowska, E.; Olkowski, R.; Gula, G.; Niderla-Bielińska, J.; Flaht-Zabost, A.; Jasińska, A. Vasculogenesis and Its Cellular Therapeutic Applications. Cells Tissues Organs 2017, 203, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.; Shaw, L.C.; Ljubimov, A.V.; Boulton, M.E.; Segal, M.S.; Grant, M.B. Retinal and choroidal microangiopathies: Therapeutic opportunities. Microvasc. Res. 2007, 74, 131–144. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.Y. Targeting Tumor Adaption to Chronic Hypoxia: Implications for Drug Resistance, and How It Can Be Overcome. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000, 6, 389–395. [Google Scholar] [CrossRef]
- Rajabi, M.; Mousa, S.A. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5. [Google Scholar] [CrossRef]
- Viallard, C.; Larrivée, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Duran, C.L.; Howell, D.W.; Dave, J.M.; Smith, R.L.; Torrie, M.E.; Essner, J.J.; Bayless, K.J. Molecular Regulation of Sprouting Angiogenesis. Compr. Physiol. 2017, 8, 153–235. [Google Scholar] [CrossRef] [PubMed]
- De Bock, K.; Georgiadou, M.; Carmeliet, P. Role of endothelial cell metabolism in vessel sprouting. Cell Metab. 2013, 18, 634–647. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Ferreira, V.; Breier, G.; Pollefeyt, S.; Kieckens, L.; Gertsenstein, M.; Fahrig, M.; Vandenhoeck, A.; Harpal, K.; Eberhardt, C.; et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996, 380, 435–439. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Karaman, S.; Leppänen, V.M.; Alitalo, K. Vascular endothelial growth factor signaling in development and disease. Development 2018, 145, 151019. [Google Scholar] [CrossRef]
- Estrada, C.C.; Maldonado, A.; Mallipattu, S.K. Therapeutic Inhibition of VEGF Signaling and Associated Nephrotoxicities. J. Am. Soc. Nephrol. 2019, 30, 187–200. [Google Scholar] [CrossRef]
- Fryer, B.H.; Field, J. Rho, Rac, Pak and angiogenesis: Old roles and newly identified responsibilities in endothelial cells. Cancer Lett. 2005, 229, 13–23. [Google Scholar] [CrossRef]
- Bhattarai, D.; Xu, X.; Lee, K. Hypoxia-inducible factor-1 (HIF-1) inhibitors from the last decade (2007 to 2016): A “structure-activity relationship” perspective. Med. Res. Rev. 2018, 38, 1404–1442. [Google Scholar] [CrossRef]
- Waugh, D.J.; Wilson, C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef]
- Petreaca, M.L.; Yao, M.; Liu, Y.; Defea, K.; Martins-Green, M. Transactivation of vascular endothelial growth factor receptor-2 by interleukin-8 (IL-8/CXCL8) is required for IL-8/CXCL8-induced endothelial permeability. Mol. Biol. Cell 2007, 18, 5014–5023. [Google Scholar] [CrossRef] [PubMed]
- Wallez, Y.; Vilgrain, I.; Huber, P. Angiogenesis: The VE-cadherin switch. Trends Cardiovasc. Med. 2006, 16, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.W.; Madsen, J.R. VEGF Signaling in Neurological Disorders. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Hou, H.; Liang, H.; Weinreb, R.N.; Wang, H.; Wang, Y. Bone marrow-derived cells in ocular neovascularization: Contribution and mechanisms. Angiogenesis 2016, 19, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Wang, Z.; Sun, Y.; Chen, J. Animal models of ocular angiogenesis: From development to pathologies. FASEB J. 2017, 31, 4665–4681. [Google Scholar] [CrossRef]
- Campochiaro, P.A. Ocular neovascularization. J. Mol. Med. (Berl) 2013, 91, 311–321. [Google Scholar] [CrossRef]
- Aiello, L.P.; Avery, R.L.; Arrigg, P.G.; Keyt, B.A.; Jampel, H.D.; Shah, S.T.; Pasquale, L.R.; Thieme, H.; Iwamoto, M.A.; Park, J.E.; et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994, 331, 1480–1487. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Clark, W.L.; Boyer, D.S.; Heier, J.S.; Brown, D.M.; Vitti, R.; Kazmi, H.; Berliner, A.J.; Erickson, K.; Chu, K.W.; et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: The 24-week results of the VIBRANT study. Ophthalmology 2015, 122, 538–544. [Google Scholar] [CrossRef]
- Brown, D.M.; Schmidt-Erfurth, U.; Do, D.V.; Holz, F.G.; Boyer, D.S.; Midena, E.; Heier, J.S.; Terasaki, H.; Kaiser, P.K.; Marcus, D.M.; et al. Intravitreal Aflibercept for Diabetic Macular Edema: 100-Week Results From the VISTA and VIVID Studies. Ophthalmology 2015, 122, 2044–2052. [Google Scholar] [CrossRef]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Brown, D.M.; Kaiser, P.K.; Michels, M.; Soubrane, G.; Heier, J.S.; Kim, R.Y.; Sy, J.P.; Schneider, S. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Illingworth, C. Treatments for corneal neovascularization: A review. Cornea 2011, 30, 927–938. [Google Scholar] [CrossRef]
- Yaylali, V.; Ohta, T.; Kaufman, S.C.; Maitchouk, D.Y.; Beuerman, R.W. In vivo confocal imaging of corneal neovascularization. Cornea 1998, 17, 646–653. [Google Scholar] [CrossRef]
- Scroggs, M.W.; Proia, A.D.; Smith, C.F.; Halperin, E.C.; Klintworth, G.K. The effect of total-body irradiation on corneal neovascularization in the Fischer 344 rat after chemical cauterization. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2105–2111. [Google Scholar]
- Hamill, C.E.; Bozorg, S.; Peggy Chang, H.Y.; Lee, H.; Sayegh, R.R.; Shukla, A.N.; Chodosh, J. Corneal alkali burns: A review of the literature and proposed protocol for evaluation and treatment. Int. Ophthalmol. Clin. 2013, 53, 185–194. [Google Scholar] [CrossRef]
- Safvati, A.; Cole, N.; Hume, E.; Willcox, M. Mediators of neovascularization and the hypoxic cornea. Curr. Eye Res. 2009, 34, 501–514. [Google Scholar] [CrossRef]
- Roshandel, D.; Eslani, M.; Baradaran-Rafii, A.; Cheung, A.Y.; Kurji, K.; Jabbehdari, S.; Maiz, A.; Jalali, S.; Djalilian, A.R.; Holland, E.J. Current and emerging therapies for corneal neovascularization. Ocul. Surf. 2018, 16, 398–414. [Google Scholar] [CrossRef]
- Frisardi, V.; Panza, F.; Seripa, D.; Farooqui, T.; Farooqui, A.A. Glycerophospholipids and glycerophospholipid-derived lipid mediators: A complex meshwork in Alzheimer’s disease pathology. Prog. Lipid Res. 2011, 50, 313–330. [Google Scholar] [CrossRef]
- Ecker, J.; Liebisch, G. Application of stable isotopes to investigate the metabolism of fatty acids, glycerophospholipid and sphingolipid species. Prog. Lipid Res. 2014, 54, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Hermansson, M.; Hokynar, K.; Somerharju, P. Mechanisms of glycerophospholipid homeostasis in mammalian cells. Prog. Lipid Res. 2011, 50, 240–257. [Google Scholar] [CrossRef] [PubMed]
- Croset, M.; Brossard, N.; Polette, A.; Lagarde, M. Characterization of plasma unsaturated lysophosphatidylcholines in human and rat. Biochem. J. 2000, 345 Pt 1, 61–67. [Google Scholar] [CrossRef]
- D’Arrigo, P.; Servi, S. Synthesis of lysophospholipids. Molecules 2010, 15, 1354–1377. [Google Scholar] [CrossRef] [PubMed]
- Moolenaar, W.H.; van Meeteren, L.A.; Giepmans, B.N. The ins and outs of lysophosphatidic acid signaling. Bioessays 2004, 26, 870–881. [Google Scholar] [CrossRef]
- Vukotic, M.; Nolte, H.; König, T.; Saita, S.; Ananjew, M.; Krüger, M.; Tatsuta, T.; Langer, T. Acylglycerol Kinase Mutated in Sengers Syndrome Is a Subunit of the TIM22 Protein Translocase in Mitochondria. Mol. Cell 2017, 67, 471–483.e7. [Google Scholar] [CrossRef]
- Zhao, Y.; Natarajan, V. Lysophosphatidic acid signaling in airway epithelium: Role in airway inflammation and remodeling. Cell. Signal. 2009, 21, 367–377. [Google Scholar] [CrossRef]
- Choi, J.W.; Herr, D.R.; Noguchi, K.; Yung, Y.C.; Lee, C.W.; Mutoh, T.; Lin, M.E.; Teo, S.T.; Park, K.E.; Mosley, A.N.; et al. LPA receptors: Subtypes and biological actions. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 157–186. [Google Scholar] [CrossRef]
- Lei, L.; Su, J.; Chen, J.; Chen, W.; Chen, X.; Peng, C. The role of lysophosphatidic acid in the physiology and pathology of the skin. Life Sci. 2019, 220, 194–200. [Google Scholar] [CrossRef]
- Palis, J.; Robertson, S.; Kennedy, M.; Wall, C.; Keller, G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development 1999, 126, 5073–5084. [Google Scholar]
- Aoki, J. Mechanisms of lysophosphatidic acid production. Semin. Cell Dev. Biol. 2004, 15, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, N.; Ishii, I.; Contos, J.J.; Weiner, J.A.; Chun, J. Lysophospholipid receptors. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 507–534. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.W.; Clair, T.; Kim, Y.S.; McMarlin, A.; Schiffmann, E.; Liotta, L.A.; Stracke, M.L. Autotaxin (NPP-2), a metastasis-enhancing motogen, is an angiogenic factor. Cancer Res. 2001, 61, 6938–6944. [Google Scholar] [PubMed]
- Fukushima, N.; Chun, J. The LPA receptors. Prostaglandins Other Lipid Mediat. 2001, 64, 21–32. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.; Oh, Y.S.; Jun, H.S. Lysophosphatidic Acid Signaling in Diabetic Nephropathy. Int. J. Mol. Sci. 2019, 20, 2850. [Google Scholar] [CrossRef]
- Park, F.; Miller, D.D. Role of lysophosphatidic acid and its receptors in the kidney. Physiol. Genomics 2017, 49, 659–666. [Google Scholar] [CrossRef]
- Lin, K.H.; Chiang, J.C.; Ho, Y.H.; Yao, C.L.; Lee, H. Lysophosphatidic Acid and Hematopoiesis: From Microenvironmental Effects to Intracellular Signaling. Int. J. Mol. Sci. 2020, 21, 2015. [Google Scholar] [CrossRef]
- Yun, C.C. Lysophosphatidic Acid and Autotaxin-associated Effects on the Initiation and Progression of Colorectal Cancer. Cancers (Basel) 2019, 11, 958. [Google Scholar] [CrossRef]
- Yung, Y.C.; Stoddard, N.C.; Chun, J. LPA receptor signaling: Pharmacology, physiology, and pathophysiology. J. Lipid Res. 2014, 55, 1192–1214. [Google Scholar] [CrossRef]
- Teo, S.T.; Yung, Y.C.; Herr, D.R.; Chun, J. Lysophosphatidic acid in vascular development and disease. IUBMB Life 2009, 61, 791–799. [Google Scholar] [CrossRef]
- Tanaka, M.; Okudaira, S.; Kishi, Y.; Ohkawa, R.; Iseki, S.; Ota, M.; Noji, S.; Yatomi, Y.; Aoki, J.; Arai, H. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J. Biol. Chem. 2006, 281, 25822–25830. [Google Scholar] [CrossRef] [PubMed]
- Offermanns, S.; Mancino, V.; Revel, J.P.; Simon, M.I. Vascular system defects and impaired cell chemokinesis as a result of Galpha13 deficiency. Science 1997, 275, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Yukiura, H.; Hama, K.; Nakanaga, K.; Tanaka, M.; Asaoka, Y.; Okudaira, S.; Arima, N.; Inoue, A.; Hashimoto, T.; Arai, H.; et al. Autotaxin regulates vascular development via multiple lysophosphatidic acid (LPA) receptors in zebrafish. J. Biol. Chem. 2011, 286, 43972–43983. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, H.; Matsumura, Y.; Thumkeo, D.; Koike, S.; Masu, M.; Shimizu, Y.; Ishizaki, T.; Narumiya, S. Impaired vascular remodeling in the yolk sac of embryos deficient in ROCK-I and ROCK-II. Genes Cells 2011, 16, 1012–1021. [Google Scholar] [CrossRef]
- Chuang, Y.W.; Chang, W.M.; Chen, K.H.; Hong, C.Z.; Chang, P.J.; Hsu, H.C. Lysophosphatidic acid enhanced the angiogenic capability of human chondrocytes by regulating Gi/NF-kB-dependent angiogenic factor expression. PLoS ONE 2014, 9, e95180. [Google Scholar] [CrossRef]
- Wu, P.Y.; Lin, Y.C.; Lan, S.Y.; Huang, Y.L.; Lee, H. Aromatic hydrocarbon receptor inhibits lysophosphatidic acid-induced vascular endothelial growth factor-A expression in PC-3 prostate cancer cells. Biochem. Biophys. Res. Commun. 2013, 437, 440–445. [Google Scholar] [CrossRef]
- Wei, H.; Wang, F.; Wang, X.; Yang, J.; Li, Z.; Cong, X.; Chen, X. Lysophosphatidic acid promotes secretion of VEGF by increasing expression of 150-kD Oxygen-regulated protein (ORP150) in mesenchymal stem cells. Biochim. Biophys. Acta 2013, 1831, 1426–1434. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.Y.; Lee, E.K.; Park, C.G.; Chung, H.C.; Rha, S.Y.; Kim, Y.K.; Bae, G.U.; Kim, B.K.; Han, J.W.; et al. Activation of hypoxia-inducible factor-1alpha is necessary for lysophosphatidic acid-induced vascular endothelial growth factor expression. Clin. Cancer Res. 2006, 12, 6351–6358. [Google Scholar] [CrossRef]
- Kim, K.S.; Sengupta, S.; Berk, M.; Kwak, Y.G.; Escobar, P.F.; Belinson, J.; Mok, S.C.; Xu, Y. Hypoxia enhances lysophosphatidic acid responsiveness in ovarian cancer cells and lysophosphatidic acid induces ovarian tumor metastasis in vivo. Cancer Res. 2006, 66, 7983–7990. [Google Scholar] [CrossRef]
- Aki, Y.; Kondo, A.; Nakamura, H.; Togari, A. Lysophosphatidic acid-stimulated interleukin-6 and -8 synthesis through LPA1 receptors on human osteoblasts. Arch. Oral. Biol. 2008, 53, 207–213. [Google Scholar] [CrossRef]
- Chen, R.J.; Chen, S.U.; Chou, C.H.; Lin, M.C. Lysophosphatidic acid receptor 2/3-mediated IL-8-dependent angiogenesis in cervical cancer cells. Int. J. Cancer 2012, 131, 789–802. [Google Scholar] [CrossRef]
- Shimada, H.; Rajagopalan, L.E. Rho kinase-2 activation in human endothelial cells drives lysophosphatidic acid-mediated expression of cell adhesion molecules via NF-kappaB p65. J. Biol. Chem. 2010, 285, 12536–12542. [Google Scholar] [CrossRef] [PubMed]
- Brault, S.; Gobeil, F., Jr.; Fortier, A.; Honoré, J.C.; Joyal, J.S.; Sapieha, P.S.; Kooli, A.; Martin, E.; Hardy, P.; Ribeiro-da-Silva, A.; et al. Lysophosphatidic acid induces endothelial cell death by modulating the redox environment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1174–R1183. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Rashid, M.H.; Fujita, R.; Contos, J.J.; Chun, J.; Ueda, H. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat. Med. 2004, 10, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Strunnikova, N.V.; Maminishkis, A.; Barb, J.J.; Wang, F.; Zhi, C.; Sergeev, Y.; Chen, W.; Edwards, A.O.; Stambolian, D.; Abecasis, G.; et al. Transcriptome analysis and molecular signature of human retinal pigment epithelium. Hum. Mol. Genet. 2010, 19, 2468–2486. [Google Scholar] [CrossRef]
- Zhou, W.L.; Sugioka, M.; Yamashita, M. Lysophosphatidic acid-induced Ca(2+) mobilization in the neural retina of chick embryo. J. Neurobiol. 1999, 41, 495–504. [Google Scholar] [CrossRef]
- Fincher, J.; Whiteneck, C.; Birgbauer, E. G-protein-coupled receptor cell signaling pathways mediating embryonic chick retinal growth cone collapse induced by lysophosphatidic acid and sphingosine-1-phosphate. Dev. Neurosci. 2014, 36, 443–453. [Google Scholar] [CrossRef]
- Lidgerwood, G.E.; Morris, A.J.; Conquest, A.; Daniszewski, M.; Rooney, L.A.; Lim, S.Y.; Hernández, D.; Liang, H.H.; Allen, P.; Connell, P.P.; et al. Role of lysophosphatidic acid in the retinal pigment epithelium and photoreceptors. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2018, 1863, 750–761. [Google Scholar] [CrossRef]
- Yasuda, D.; Kobayashi, D.; Akahoshi, N.; Ohto-Nakanishi, T.; Yoshioka, K.; Takuwa, Y.; Mizuno, S.; Takahashi, S.; Ishii, S. Lysophosphatidic acid-induced YAP/TAZ activation promotes developmental angiogenesis by repressing Notch ligand Dll4. J. Clin. Investig. 2019, 129, 4332–4349. [Google Scholar] [CrossRef]
- Abu El-Asrar, A.M.; Nawaz, M.I.; Mohammad, G.; Siddiquei, M.M.; Alam, K.; Mousa, A.; Opdenakker, G. Expression of bioactive lysophospholipids and processing enzymes in the vitreous from patients with proliferative diabetic retinopathy. Lipids Health Dis. 2014, 13, 187. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abu El-Asrar, A.M.; Mohammad, G.; Nawaz, M.I.; Siddiquei, M.M.; Kangave, D.; Opdenakker, G. Expression of lysophosphatidic acid, autotaxin and acylglycerol kinase as biomarkers in diabetic retinopathy. Acta Diabetol. 2013, 50, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Dacheva, I.; Ullmer, C.; Ceglowska, K.; Nogoceke, E.; Hartmann, G.; Müller, S.; Rejdak, R.; Nowomiejska, K.; Reich, M.; Nobl, M.; et al. LYSOPHOSPHATIDIC ACIDS AND AUTOTAXIN IN RETINAL VEIN OCCLUSION. Retina 2016, 36, 2311–2318. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Moaddel, R.; Cotch, M.F.; Jonasson, F.; Eiriksdottir, G.; Harris, T.B.; Launer, L.J.; Sun, K.; Klein, R.; Schaumberg, D.A.; et al. Serum lipids in adults with late age-related macular degeneration: A case-control study. Lipids Health Dis. 2019, 18, 7. [Google Scholar] [CrossRef]
- Van Echten-Deckert, G.; Alam, S. Sphingolipid metabolism - an ambiguous regulator of autophagy in the brain. Biol. Chem. 2018, 399, 837–850. [Google Scholar] [CrossRef]
- Bandet, C.L.; Tan-Chen, S.; Bourron, O.; Le Stunff, H.; Hajduch, E. Sphingolipid Metabolism: New Insight into Ceramide-Induced Lipotoxicity in Muscle Cells. Int. J. Mol. Sci. 2019, 20, 479. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Coant, N.; Sakamoto, W.; Mao, C.; Hannun, Y.A. Ceramidases, roles in sphingolipid metabolism and in health and disease. Adv. Biol. Regul. 2017, 63, 122–131. [Google Scholar] [CrossRef]
- Sanchez, T.; Hla, T. Structural and functional characteristics of S1P receptors. J. Cell Biochem. 2004, 92, 913–922. [Google Scholar] [CrossRef]
- Takabe, K.; Paugh, S.W.; Milstien, S.; Spiegel, S. “Inside-out” signaling of sphingosine-1-phosphate: Therapeutic targets. Pharmacol. Rev. 2008, 60, 181–195. [Google Scholar] [CrossRef]
- Spiegel, S.; Milstien, S. Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat. Rev. Mol. Cell. Biol. 2003, 4, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Siow, D.L.; Anderson, C.D.; Berdyshev, E.V.; Skobeleva, A.; Natarajan, V.; Pitson, S.M.; Wattenberg, B.W. Sphingosine kinase localization in the control of sphingolipid metabolism. Adv. Enzyme Regul. 2011, 51, 229–244. [Google Scholar] [CrossRef][Green Version]
- Strub, G.M.; Paillard, M.; Liang, J.; Gomez, L.; Allegood, J.C.; Hait, N.C.; Maceyka, M.; Price, M.M.; Chen, Q.; Simpson, D.C.; et al. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 2011, 25, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Bryan, A.M.; Del Poeta, M. Sphingosine-1-phosphate receptors and innate immunity. Cell Microbiol. 2018, 20, e12836. [Google Scholar] [CrossRef] [PubMed]
- Gräler, M.H.; Bernhardt, G.; Lipp, M. EDG6, a novel G-protein-coupled receptor related to receptors for bioactive lysophospholipids, is specifically expressed in lymphoid tissue. Genomics 1998, 53, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Im, D.S.; Heise, C.E.; Ancellin, N.; O’Dowd, B.F.; Shei, G.J.; Heavens, R.P.; Rigby, M.R.; Hla, T.; Mandala, S.; McAllister, G.; et al. Characterization of a novel sphingosine 1-phosphate receptor, Edg-8. J. Biol. Chem. 2000, 275, 14281–14286. [Google Scholar] [CrossRef]
- Zhao, J.; Tan, Y.; Wang, L.; Su, X.; Shi, Y. Serum sphingosine-1-phosphate levels and Sphingosine-1-Phosphate gene polymorphisms in acute respiratory distress syndrome: A multicenter prospective study. J. Transl. Med. 2020, 18, 156. [Google Scholar] [CrossRef]
- Poppe, A.; Moritz, E.; Geffken, M.; Schreiber, J.; Greiwe, G.; Amschler, K.; Wruck, M.L.; Schwedhelm, E.; Daum, G.; Kluge, S.; et al. Analyses of sphingosine-1-phosphate in the context of transfusion: How much is in stored blood products and in patient blood? Transfusion 2019, 59, 3071–3076. [Google Scholar] [CrossRef]
- Hänel, P.; Andréani, P.; Gräler, M.H. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007, 21, 1202–1209. [Google Scholar] [CrossRef]
- Mizugishi, K.; Yamashita, T.; Olivera, A.; Miller, G.F.; Spiegel, S.; Proia, R.L. Essential role for sphingosine kinases in neural and vascular development. Mol. Cell Biol. 2005, 25, 11113–11121. [Google Scholar] [CrossRef]
- Liu, Y.; Wada, R.; Yamashita, T.; Mi, Y.; Deng, C.X.; Hobson, J.P.; Rosenfeldt, H.M.; Nava, V.E.; Chae, S.S.; Lee, M.J.; et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Investig. 2000, 106, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Mi, Y.; Liu, Y.; Sasaki, T.; Allende, M.L.; Wu, Y.P.; Yamashita, T.; Proia, R.L. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J. Biol. Chem. 2004, 279, 29367–29373. [Google Scholar] [CrossRef] [PubMed]
- Chumanevich, A.; Wedman, P.; Oskeritzian, C.A. Sphingosine-1-Phosphate/Sphingosine-1-Phosphate Receptor 2 Axis Can Promote Mouse and Human Primary Mast Cell Angiogenic Potential through Upregulation of Vascular Endothelial Growth Factor-A and Matrix Metalloproteinase-2. Mediat. Inflamm. 2016, 2016, 1503206. [Google Scholar] [CrossRef] [PubMed]
- Arjamaa, O.; Aaltonen, V.; Piippo, N.; Csont, T.; Petrovski, G.; Kaarniranta, K.; Kauppinen, A. Hypoxia and inflammation in the release of VEGF and interleukins from human retinal pigment epithelial cells. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 1757–1762. [Google Scholar] [CrossRef]
- Kalhori, V.; Kemppainen, K.; Asghar, M.Y.; Bergelin, N.; Jaakkola, P.; Törnquist, K. Sphingosine-1-Phosphate as a Regulator of Hypoxia-Induced Factor-1α in Thyroid Follicular Carcinoma Cells. PLoS ONE 2013, 8, e66189. [Google Scholar] [CrossRef]
- Haase, V.H. Hypoxia-inducible factors in the kidney. Am. J. Physiol. Ren. Physiol. 2006, 291, F271–F281. [Google Scholar] [CrossRef]
- Anelli, V.; Gault, C.R.; Cheng, A.B.; Obeid, L.M. Sphingosine kinase 1 is up-regulated during hypoxia in U87MG glioma cells. Role of hypoxia-inducible factors 1 and 2. J. Biol. Chem. 2008, 283, 3365–3375. [Google Scholar] [CrossRef]
- Qiao, Y.; Hu, R.; Wang, Q.; Qi, J.; Yang, Y.; Kijlstra, A.; Yang, P. Sphingosine 1-phosphate elicits proinflammatory responses in ARPE-19 cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8200–8207. [Google Scholar] [CrossRef]
- O’Sullivan, M.J.; Hirota, N.; Martin, J.G. Sphingosine 1-phosphate (S1P) induced interleukin-8 (IL-8) release is mediated by S1P receptor 2 and nuclear factor κB in BEAS-2B cells. PLoS ONE 2014, 9, e95566. [Google Scholar] [CrossRef]
- Lee, M.J.; Thangada, S.; Claffey, K.P.; Ancellin, N.; Liu, C.H.; Kluk, M.; Volpi, M.; Sha’afi, R.I.; Hla, T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 1999, 99, 301–312. [Google Scholar] [CrossRef]
- Sanchez, T.; Skoura, A.; Wu, M.T.; Casserly, B.; Harrington, E.O.; Hla, T. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, T.; Thangada, S.; Wu, M.T.; Kontos, C.D.; Wu, D.; Wu, H.; Hla, T. PTEN as an effector in the signaling of antimigratory G protein-coupled receptor. Proc. Natl. Acad. Sci. USA 2005, 102, 4312–4317. [Google Scholar] [CrossRef] [PubMed]
- Terao, R.; Honjo, M.; Ueta, T.; Obinata, H.; Izumi, T.; Kurano, M.; Yatomi, Y.; Koso, H.; Watanabe, S.; Aihara, M. Light Stress-Induced Increase of Sphingosine 1-Phosphate in Photoreceptors and Its Relevance to Retinal Degeneration. Int. J. Mol. Sci. 2019, 20, 3670. [Google Scholar] [CrossRef]
- Gaengel, K.; Niaudet, C.; Hagikura, K.; Laviña, B.; Muhl, L.; Hofmann, J.J.; Ebarasi, L.; Nyström, S.; Rymo, S.; Chen, L.L.; et al. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev. Cell 2012, 23, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, K.; Engelbrecht, E.; Niaudet, C.; Jung, B.; Gaengel, K.; Holton, K.; Swendeman, S.; Liu, C.H.; Levesque, M.V.; Kuo, A.; et al. Sphingosine 1-Phosphate Receptor Signaling Establishes AP-1 Gradients to Allow for Retinal Endothelial Cell Specialization. Dev. Cell 2020, 52, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Eresch, J.; Stumpf, M.; Koch, A.; Vutukuri, R.; Ferreirós, N.; Schreiber, Y.; Schröder, K.; Devraj, K.; Popp, R.; Huwiler, A.; et al. Sphingosine Kinase 2 Modulates Retinal Neovascularization in the Mouse Model of Oxygen-Induced Retinopathy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Skoura, A.; Sanchez, T.; Claffey, K.; Mandala, S.M.; Proia, R.L.; Hla, T. Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J. Clin. Investig. 2007, 117, 2506–2516. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Shen, J.; Dong, A.; Rashid, A.; Stoller, G.; Campochiaro, P.A. Blockade of sphingosine-1-phosphate reduces macrophage influx and retinal and choroidal neovascularization. J. Cell Physiol. 2009, 218, 192–198. [Google Scholar] [CrossRef]
- O’Brien, N.; Jones, S.T.; Williams, D.G.; Cunningham, H.B.; Moreno, K.; Visentin, B.; Gentile, A.; Vekich, J.; Shestowsky, W.; Hiraiwa, M.; et al. Production and characterization of monoclonal anti-sphingosine-1-phosphate antibodies. J. Lipid Res. 2009, 50, 2245–2257. [Google Scholar] [CrossRef]
- Terao, R.; Honjo, M.; Aihara, M. Apolipoprotein M Inhibits Angiogenic and Inflammatory Response by Sphingosine 1-Phosphate on Retinal Pigment Epithelium Cells. Int. J. Mol. Sci. 2017, 19, 112. [Google Scholar] [CrossRef]
- Terao, R.; Honjo, M.; Totsuka, K.; Miwa, Y.; Kurihara, T.; Aihara, M. The role of sphingosine 1-phosphate receptors on retinal pigment epithelial cells barrier function and angiogenic effects. Prostaglandins Other Lipid Mediat. 2019, 145, 106365. [Google Scholar] [CrossRef] [PubMed]
- Efficacy and Safety Study of iSONEP with & without Lucentis/Avastin/Eylea to Treat Wet AMD; Lpath, Inc.: San Diego, CA, USA, 2011; Available online: https://ClinicalTrials.gov/show/NCT01414153.
- Yonesu, K.; Kawase, Y.; Inoue, T.; Takagi, N.; Tsuchida, J.; Takuwa, Y.; Kumakura, S.; Nara, F. Involvement of sphingosine-1-phosphate and S1P1 in angiogenesis: Analyses using a new S1P1 antagonist of non-sphingosine-1-phosphate analog. Biochem. Pharmacol. 2009, 77, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- LaMontagne, K.; Littlewood-Evans, A.; Schnell, C.; O’Reilly, T.; Wyder, L.; Sanchez, T.; Probst, B.; Butler, J.; Wood, A.; Liau, G.; et al. Antagonism of sphingosine-1-phosphate receptors by FTY720 inhibits angiogenesis and tumor vascularization. Cancer Res. 2006, 66, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ahmed, S.; Stanley, D.; An, C. Eicosanoid-mediated immunity in insects. Dev. Comp. Immunol. 2018, 83, 130–143. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fatty Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]
- Wang, W.; Yang, H.; Johnson, D.; Gensler, C.; Decker, E.; Zhang, G. Chemistry and biology of ω-3 PUFA peroxidation-derived compounds. Prostaglandins Other Lipid Mediat. 2017, 132, 84–91. [Google Scholar] [CrossRef]
- Hardy, P.; Beauchamp, M.; Sennlaub, F.; Gobeil, F., Jr.; Tremblay, L.; Mwaikambo, B.; Lachapelle, P.; Chemtob, S. New insights into the retinal circulation: Inflammatory lipid mediators in ischemic retinopathy. Prostaglandins Leukot. Essent. Fatty Acids 2005, 72, 301–325. [Google Scholar] [CrossRef]
- Yanni, S.E.; Clark, M.L.; Yang, R.; Bingaman, D.P.; Penn, J.S. The effects of nepafenac and amfenac on retinal angiogenesis. Brain Res. Bull. 2010, 81, 310–319. [Google Scholar] [CrossRef][Green Version]
- Woodward, D.F.; Wang, J.W.; Ni, M.; Bauer, A.; Martos, J.L.; Carling, R.W.; Poloso, N.J. In vivo studies validating multitargeting of prostanoid receptors for achieving superior anti-inflammatory effects. FASEB J. 2017, 31, 368–375. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, X.; Jeschke, U.; von Schönfeldt, V. COX-2-PGE(2)-EPs in gynecological cancers. Arch. Gynecol. Obstet. 2020, 301, 1365–1375. [Google Scholar] [CrossRef]
- Nakao, A.; Allen, M.L.; Sonnenburg, W.K.; Smith, W.L. Regulation of cAMP metabolism by PGE2 in cortical and medullary thick ascending limb of Henle’s loop. Am. J. Physiol. 1989, 256, C652–C657. [Google Scholar] [CrossRef] [PubMed]
- Namba, T.; Sugimoto, Y.; Negishi, M.; Irie, A.; Ushikubi, F.; Kakizuka, A.; Ito, S.; Ichikawa, A.; Narumiya, S. Alternative splicing of C-terminal tail of prostaglandin E receptor subtype EP3 determines G-protein specificity. Nature 1993, 365, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Bos, C.L.; Richel, D.J.; Ritsema, T.; Peppelenbosch, M.P.; Versteeg, H.H. Prostanoids and prostanoid receptors in signal transduction. Int. J. Biochem. Cell Biol. 2004, 36, 1187–1205. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K. Regulation by prostaglandin E2 and histamine of angiogenesis in inflammatory granulation tissue. Yakugaku Zasshi 2003, 123, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.; Nandi, P.; Omar, A.; Ugwuagbo, K.C.; Lala, P.K. EP4 as a Therapeutic Target for Aggressive Human Breast Cancer. Int. J. Mol. Sci. 2018, 19, 1019. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Croix, B.S. Improving VEGF-targeted therapies through inhibition of COX-2/PGE2 signaling. Mol. Cell Oncol. 2014, 1, e969154. [Google Scholar] [CrossRef]
- Dufour, M.; Faes, S.; Dormond-Meuwly, A.; Demartines, N.; Dormond, O. PGE2-induced colon cancer growth is mediated by mTORC1. Biochem. Biophys. Res. Commun. 2014, 451, 587–591. [Google Scholar] [CrossRef]
- Tang, J.; Shen, Y.; Chen, G.; Wan, Q.; Wang, K.; Zhang, J.; Qin, J.; Liu, G.; Zuo, S.; Tao, B.; et al. Activation of E-prostanoid 3 receptor in macrophages facilitates cardiac healing after myocardial infarction. Nat. Commun. 2017, 8, 14656. [Google Scholar] [CrossRef]
- Taniguchi, T.; Fujino, H.; Israel, D.D.; Regan, J.W.; Murayama, T. Human EP3(I) prostanoid receptor induces VEGF and VEGF receptor-1 mRNA expression. Biochem. Biophys. Res. Commun. 2008, 377, 1173–1178. [Google Scholar] [CrossRef]
- Woodward, D.F.; Wang, J.W.; Ni, M.; Bauer, A.J.; Poloso, N.J. In Vivo Choroidal Neovascularization and Macrophage Studies Provide Further Evidence for a Broad Role of Prostacyclin in Angiogenesis. J. Ocul. Pharmacol. Ther. 2019, 35, 98–105. [Google Scholar] [CrossRef]
- Takeuchi, K.; Amagase, K. Roles of Cyclooxygenase, Prostaglandin E2 and EP Receptors in Mucosal Protection and Ulcer Healing in the Gastrointestinal Tract. Curr. Pharm. Des. 2018, 24, 2002–2011. [Google Scholar] [CrossRef] [PubMed]
- Duncan, W.C.; Nio-Kobayashi, J. Targeting angiogenesis in the pathological ovary. Reprod. Fertil. Dev. 2013, 25, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Renz, H.; Gong, J.H.; Schmidt, A.; Nain, M.; Gemsa, D. Release of tumor necrosis factor-alpha from macrophages. Enhancement and suppression are dose-dependently regulated by prostaglandin E2 and cyclic nucleotides. J. Immunol. 1988, 141, 2388–2393. [Google Scholar] [PubMed]
- Obermajer, N.; Muthuswamy, R.; Odunsi, K.; Edwards, R.P.; Kalinski, P. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res. 2011, 71, 7463–7470. [Google Scholar] [CrossRef]
- Yanni, S.E.; McCollum, G.W.; Penn, J.S. Genetic deletion of COX-2 diminishes VEGF production in mouse retinal Müller cells. Exp. Eye Res. 2010, 91, 34–41. [Google Scholar] [CrossRef]
- Chen, D.; Tang, J.; Wan, Q.; Zhang, J.; Wang, K.; Shen, Y.; Yu, Y. E-Prostanoid 3 Receptor Mediates Sprouting Angiogenesis Through Suppression of the Protein Kinase A/β-Catenin/Notch Pathway. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 856–866. [Google Scholar] [CrossRef]
- Yanni, S.E.; Barnett, J.M.; Clark, M.L.; Penn, J.S. The role of PGE2 receptor EP4 in pathologic ocular angiogenesis. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5479–5486. [Google Scholar] [CrossRef]
- Liclican, E.L.; Nguyen, V.; Sullivan, A.B.; Gronert, K. Selective activation of the prostaglandin E2 circuit in chronic injury-induced pathologic angiogenesis. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6311–6320. [Google Scholar] [CrossRef]
- Wilkinson-Berka, J.L.; Alousis, N.S.; Kelly, D.J.; Gilbert, R.E. COX-2 inhibition and retinal angiogenesis in a mouse model of retinopathy of prematurity. Investig. Ophthalmol. Vis. Sci. 2003, 44, 974–979. [Google Scholar] [CrossRef]
- Takahashi, H.; Yanagi, Y.; Tamaki, Y.; Uchida, S.; Muranaka, K. COX-2-selective inhibitor, etodolac, suppresses choroidal neovascularization in a mice model. Biochem. Biophys. Res. Commun. 2004, 325, 461–466. [Google Scholar] [CrossRef]
- Yamada, M.; Kawai, M.; Kawai, Y.; Mashima, Y. The effect of selective cyclooxygenase-2 inhibitor on corneal angiogenesis in the rat. Curr. Eye Res. 1999, 19, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Ottes Vasconcelos, R.; Nascimento Gomes, R.; Calviello, G. Protective Effects of ω-3 PUFA in Anthracycline-Induced Cardiotoxicity: A Critical Review. Int. J. Mol. Sci. 2017, 18, 2689. [Google Scholar] [CrossRef] [PubMed]
- SanGiovanni, J.P.; Chew, E.Y. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog. Retin. Eye Res. 2005, 24, 87–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Esselman, W.J.; Jump, D.B.; Busik, J.V. Anti-inflammatory effect of docosahexaenoic acid on cytokine-induced adhesion molecule expression in human retinal vascular endothelial cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4342–4347. [Google Scholar] [CrossRef]
- Stahl, A.; Sapieha, P.; Connor, K.M.; Sangiovanni, J.P.; Chen, J.; Aderman, C.M.; Willett, K.L.; Krah, N.M.; Dennison, R.J.; Seaward, M.R.; et al. Short communication: PPAR gamma mediates a direct antiangiogenic effect of omega 3-PUFAs in proliferative retinopathy. Circ. Res. 2010, 107, 495–500. [Google Scholar] [CrossRef]
- Ma, Q.; Shen, J.H.; Shen, S.R.; Das, U.N. Bioactive lipids in pathological retinopathy. Crit. Rev. Food Sci. Nutr. 2014, 54, 1–16. [Google Scholar] [CrossRef]
- Suzumura, A.; Kaneko, H.; Funahashi, Y.; Takayama, K.; Nagaya, M.; Ito, S.; Okuno, T.; Hirakata, T.; Nonobe, N.; Kataoka, K.; et al. n-3 Fatty Acid and Its Metabolite 18-HEPE Ameliorate Retinal Neuronal Cell Dysfunction by Enhancing Müller BDNF in Diabetic Retinopathy. Diabetes 2020, 69, 724–735. [Google Scholar] [CrossRef]
- Connor, K.M.; SanGiovanni, J.P.; Lofqvist, C.; Aderman, C.M.; Chen, J.; Higuchi, A.; Hong, S.; Pravda, E.A.; Majchrzak, S.; Carper, D.; et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007, 13, 868–873. [Google Scholar] [CrossRef]
- Murata, T.; He, S.; Hangai, M.; Ishibashi, T.; Xi, X.P.; Kim, S.; Hsueh, W.A.; Ryan, S.J.; Law, R.E.; Hinton, D.R. Peroxisome proliferator-activated receptor-gamma ligands inhibit choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2309–2317. [Google Scholar]
- SanGiovanni, J.P.; Chen, J.; Sapieha, P.; Aderman, C.M.; Stahl, A.; Clemons, T.E.; Chew, E.Y.; Smith, L.E. DNA sequence variants in PPARGC1A, a gene encoding a coactivator of the ω-3 LCPUFA sensing PPAR-RXR transcription complex, are associated with NV AMD and AMD-associated loci in genes of complement and VEGF signaling pathways. PLoS ONE 2013, 8, e53155. [Google Scholar] [CrossRef] [PubMed]
- Sangiovanni, J.P.; Agrón, E.; Meleth, A.D.; Reed, G.F.; Sperduto, R.D.; Clemons, T.E.; Chew, E.Y. {omega}-3 Long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: AREDS report 30, a prospective cohort study from the Age-Related Eye Disease Study. Am. J. Clin. Nutr. 2009, 90, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Augood, C.; Chakravarthy, U.; Young, I.; Vioque, J.; de Jong, P.T.; Bentham, G.; Rahu, M.; Seland, J.; Soubrane, G.; Tomazzoli, L.; et al. Oily fish consumption, dietary docosahexaenoic acid and eicosapentaenoic acid intakes, and associations with neovascular age-related macular degeneration. Am. J. Clin. Nutr. 2008, 88, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, J.; Sun, Y.; Fu, Z.; Liu, C.H.; Evans, L.; Tian, K.; Saba, N.; Fredrick, T.; Morss, P.; et al. Optimization of an Image-Guided Laser-Induced Choroidal Neovascularization Model in Mice. PLoS ONE 2015, 10, e0132643. [Google Scholar] [CrossRef] [PubMed]
- Rezende, F.A.; Lapalme, E.; Qian, C.X.; Smith, L.E.; SanGiovanni, J.P.; Sapieha, P. Omega-3 supplementation combined with anti-vascular endothelial growth factor lowers vitreal levels of vascular endothelial growth factor in wet age-related macular degeneration. Am. J. Ophthalmol. 2014, 158, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Rajasagi, N.K.; Reddy, P.B.; Suryawanshi, A.; Mulik, S.; Gjorstrup, P.; Rouse, B.T. Controlling herpes simplex virus-induced ocular inflammatory lesions with the lipid-derived mediator resolvin E1. J. Immunol. 2011, 186, 1735–1746. [Google Scholar] [CrossRef]
- Jin, Y.; Arita, M.; Zhang, Q.; Saban, D.R.; Chauhan, S.K.; Chiang, N.; Serhan, C.N.; Dana, R. Anti-angiogenesis effect of the novel anti-inflammatory and pro-resolving lipid mediators. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4743–4752. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terao, R.; Kaneko, H. Lipid Signaling in Ocular Neovascularization. Int. J. Mol. Sci. 2020, 21, 4758. https://doi.org/10.3390/ijms21134758
Terao R, Kaneko H. Lipid Signaling in Ocular Neovascularization. International Journal of Molecular Sciences. 2020; 21(13):4758. https://doi.org/10.3390/ijms21134758
Chicago/Turabian StyleTerao, Ryo, and Hiroki Kaneko. 2020. "Lipid Signaling in Ocular Neovascularization" International Journal of Molecular Sciences 21, no. 13: 4758. https://doi.org/10.3390/ijms21134758
APA StyleTerao, R., & Kaneko, H. (2020). Lipid Signaling in Ocular Neovascularization. International Journal of Molecular Sciences, 21(13), 4758. https://doi.org/10.3390/ijms21134758





