Advanced Strategies for the Regeneration of Lumbar Disc Annulus Fibrosus
Abstract
:1. Introduction
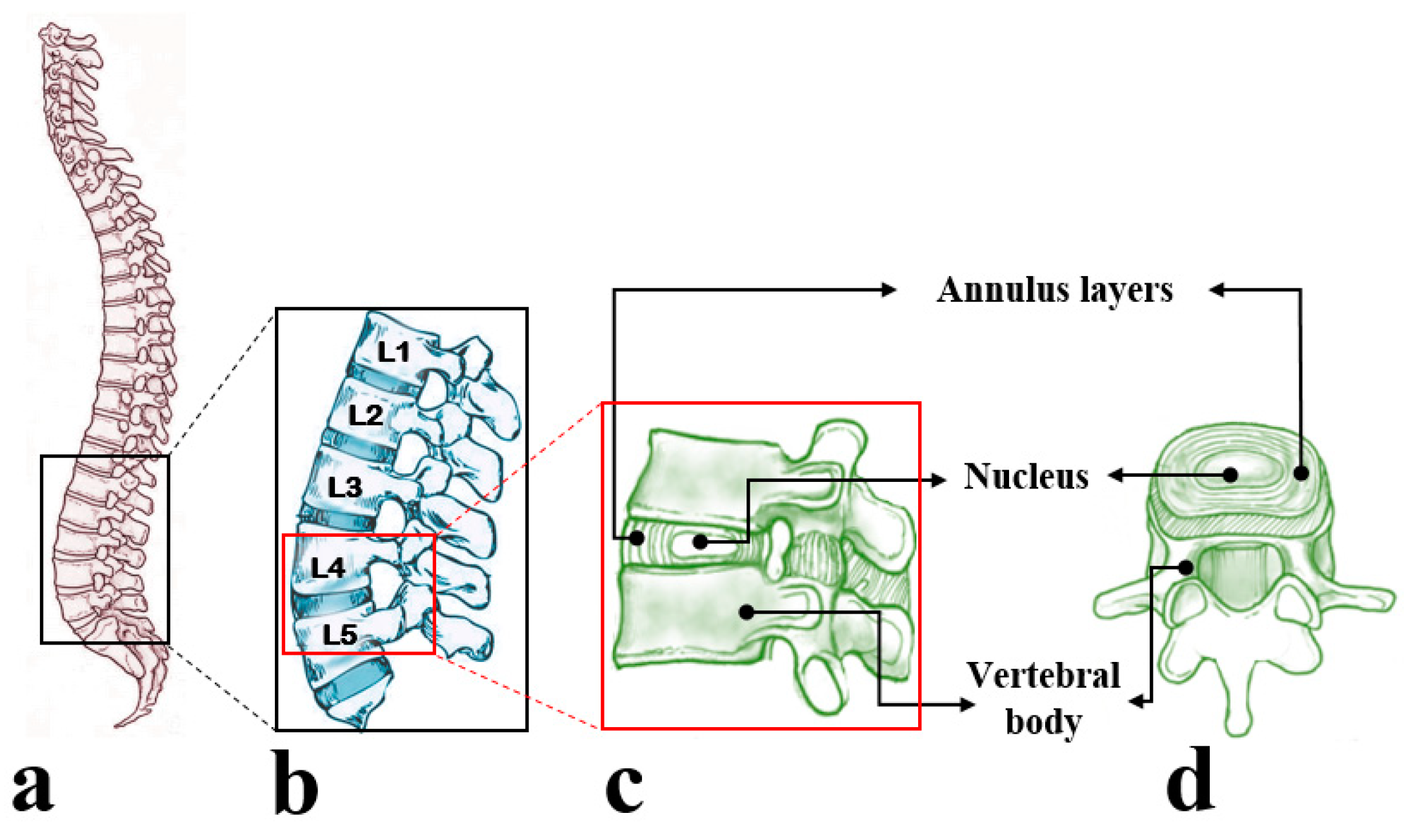
2. The Anatomy and Physiology of the Annulus Fibrosus
2.1. Structure and Composition of the Annulus Fibrosus
2.2. Annulus Fibrosus Cells
3. Repair Strategies for the Annulus Fibrosus
4. Regenerative Strategies
5. Future Outlook
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LBP | Low back pain |
| IVD | Intervertebral disc |
| AF | Annulus Fibrosus |
| NP | Nucleus Pulposus |
| ECM | Extracellular Matrix |
| ILM | Inter-lamellar matrix |
| GDF | Growth differentiation factors |
| YLD | Years lived with disability |
References
- Chan, S.C.W.; Ferguson, S.J.; Gantenbein-Ritter, B. The effects of dynamic loading on the intervertebral disc. Eur. Spine J. 2011, 20, 1796–1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urban, J.P.; Fairbank, J.C. Current perspectives on the role of biomechanical loading and genetics in development of disc degeneration and low back pain; a narrative review. J. Biomech. 2019, 109573. [Google Scholar] [CrossRef] [PubMed]
- Kupka, J.; Kohler, A.; El Bagdadi, K.; Bostelmann, R.; Brenneis, M.; Fleege, C.; Chan, D.; Zaucke, F.; Meurer, A.; Rickert, M. Adrenoceptor Expression during Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2020, 21, 2085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Fernández, C.; Francisco, V.; Pino, J.; Mera, A.; González-Gay, M.A.; Gómez, R.; Lago, F.; Gualillo, O. Molecular Relationships among Obesity, Inflammation and Intervertebral Disc Degeneration: Are Adipokines the Common Link? Int. J. Mol. Sci. 2019, 20, 2030. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Berg, S.; Alkass, K.; Druid, H.; Hart, D.A.; Svensson, C.I.; Kosek, E. NF-κB-Associated pain-related neuropeptide expression in patients with degenerative disc disease. Int. J. Mol. Sci. 2019, 20, 658. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.; Dong, W.; Liu, S.; Cheung, J.P.Y.; Kwan, K.Y.H.; Zeng, X.; Zhang, K.; Sun, Z.; Wang, X.; Cheung, K.M.C. The prevalence and years lived with disability caused by low back pain in China, 1990 to 2016: Findings from the global burden of disease study 2016. Pain 2019, 160, 237. [Google Scholar] [CrossRef]
- Luo, X.; Pietrobon, R.; Sun, S.X.; Liu, G.G.; Hey, L. Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine 2004, 29, 79–86. [Google Scholar] [CrossRef]
- Walker, B.; Muller, R.; Grant, W. Low back pain in Australian adults: The economic burden. Asia Pac. J. Public Health 2003, 15, 79–87. [Google Scholar] [CrossRef]
- Sabnis, A.B.; Diwan, A.D. The timing of surgery in lumbar disc prolapse: A systematic review. Indian J. Orthop. 2014, 48, 127. [Google Scholar]
- Wu, P.H.; Kim, H.S.; Jang, I.-T. Intervertebral Disc Diseases PART 2: A Review of the Current Diagnostic and Treatment Strategies for Intervertebral Disc Disease. Int. J. Mol. Sci. 2020, 21, 2135. [Google Scholar] [CrossRef] [Green Version]
- Kishen, T.J.; Diwan, A.D. Fusion versus disk replacement for degenerative conditions of the lumbar and cervical spine: Quid est testimonium? Orthop. Clin. 2010, 41, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Diwan, A.D.; Parvataneni, H.K.; Khan, S.N.; Sandhu, H.S.; Girardi, F.P.; Cammisa, F.P. Current concepts in intervertebral disk restoration. Orthop. Clin. 2000, 31, 453–464. [Google Scholar] [CrossRef]
- Vicars, R.; Hyde, P.; Brown, T.; Tipper, J.; Ingham, E.; Fisher, J.; Hall, R. The effect of anterior–posterior shear load on the wear of ProDisc-L TDR. Eur. Spine J. 2010, 19, 1356–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tipper, J.L.; Ingham, E.; Hailey, J.L.; Besong, A.A.; Fisher, J.; Wroblewski, B.M.; Stone, M.H. Quantitative analysis of polyethylene wear debris, wear rate and head damage in retrieved Charnley hip prostheses. J. Mater. Sci. Mater. Med. 2000, 11, 117–124. [Google Scholar] [CrossRef]
- Endo, M.; Tipper, J.L.; Barton, D.C.; Stone, M.H.; Ingham, E.; Fisher, J. Comparison of wear, wear debris and functional biological activity of moderately crosslinked and non-crosslinked polyethylenes in hip prostheses. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2002, 216, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Suñer, S.; Gowland, N.; Craven, R.; Joffe, R.; Emami, N.; Tipper, J.L. Ultrahigh molecular weight polyethylene/graphene oxide nanocomposites: Wear characterization and biological response to wear particles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 183–190. [Google Scholar]
- Long, R.G.; Torre, O.M.; Hom, W.W.; Assael, D.J.; Iatridis, J.C. Design requirements for annulus fibrosus repair: Review of forces, displacements, and material properties of the intervertebral disk and a summary of candidate hydrogels for repair. J. Biomech. Eng. 2016, 138. [Google Scholar] [CrossRef]
- Veres, S.P.; Robertson, P.A.; Broom, N.D. ISSLS Prize Winner: Microstructure and Mechanical Disruption of the Lumbar Disc Annulus Part II: How the Annulus Fails Under Hydrostatic Pressure. Spine 2008, 33, 2711–2720. [Google Scholar] [CrossRef] [Green Version]
- Ishiguro, H.; Kaito, T.; Yarimitsu, S.; Hashimoto, K.; Okada, R.; Kushioka, J.; Chijimatsu, R.; Takenaka, S.; Makino, T.; Sakai, Y. Intervertebral disc regeneration with an adipose mesenchymal stem cell-derived tissue-engineered construct in a rat nucleotomy model. Acta Biomater. 2019, 87, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Leon, E.A.; Bielajew, B.J.; Hu, J.C.; Athanasiou, K.A. Engineering self-assembled neomenisci through combination of matrix augmentation and directional remodeling. Acta Biomater. 2020. [Google Scholar] [CrossRef]
- Takeoka, Y.; Yurube, T.; Nishida, K. Gene therapy approach for intervertebral disc degeneration: An update. Neurospine 2020, 17, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krouwels, A.; Iljas, J.D.; Kragten, A.H.; Dhert, W.J.; Öner, F.C.; Tryfonidou, M.A.; Creemers, L.B. Bone Morphogenetic Proteins for Nucleus Pulposus Regeneration. Int. J. Mol. Sci. 2020, 21, 2720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tendulkar, G.; Ehnert, S.; Sreekumar, V.; Chen, T.; Kaps, H.-P.; Golombek, S.; Wendel, H.-P.; Nüssler, A.K.; Avci-Adali, M. Exogenous Delivery of Link N mRNA into Chondrocytes and MSCs—The Potential Role in Increasing Anabolic Response. Int. J. Mol. Sci. 2019, 20, 1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, E.Y.; Sloan, S.R., Jr.; Wipplinger, C.; Kirnaz, S.; Härtl, R.; Bonassar, L.J. Proteoglycan removal by chondroitinase ABC improves injectable collagen gel adhesion to annulus fibrosus. Acta Biomater. 2019, 97, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Chuah, Y.J.; Tan, J.R.; Wu, Y.; Lim, C.S.; Hee, H.T.; Kang, Y.; Wang, D.-A. Scaffold-Free Tissue Engineering with Aligned Bone Marrow Stromal Cell Sheets to Recapitulate the Microstructural and Biochemical Composition of Annulus Fibrosus. Acta Biomater. 2020, 107, 129–137. [Google Scholar] [CrossRef]
- Long, R.G.; Ferguson, S.J.; Benneker, L.M.; Sakai, D.; Li, Z.; Pandit, A.; Grijpma, D.W.; Eglin, D.; Zeiter, S.; Schmid, T. Morphological and biomechanical effects of annulus fibrosus injury and repair in an ovine cervical model. JOR Spine 2019, e1074. [Google Scholar] [CrossRef]
- Cassidy, J.; Hiltner, A.; Baer, E. Hierarchical structure of the intervertebral disc. Connect. Tissue Res. 1989, 23, 75–88. [Google Scholar] [CrossRef]
- Tavakoli, J.; Elliott, D.M.; Costi, J.J. Structure and mechanical function of the inter-lamellar matrix of the annulus fibrosus in the disc. J. Orthop. Res. 2016, 34, 1307–1315. [Google Scholar] [CrossRef]
- Tavakoli, J.; Costi, J.J. New findings confirm the viscoelastic behaviour of the inter-lamellar matrix of the disc annulus fibrosus in radial and circumferential directions of loading. Acta Biomater. 2018, 71, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, J.; Costi, J.J. Ultrastructural organization of elastic fibres in the partition boundaries of the annulus fibrosus within the intervertebral disc. Acta Biomater. 2018, 68, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, J.; Costi, J.J. Development of a rapid matrix digestion technique for ultrastructural analysis of elastic fibers in the intervertebral disc. J. Mech. Behav. Biomed. Mater. 2017, 71, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Costi, J.J. A method for visualization and isolation of elastic fibres in annulus fibrosus of the disc. Mater. Sci. Eng. C 2018, 93, 299–304. [Google Scholar] [CrossRef]
- Tavakoli, J.; Costi, J.J. New insights into the viscoelastic and failure mechanical properties of the elastic fiber network of the inter-lamellar matrix in the annulus fibrosus of the disc. Acta Biomater. 2018, 77, 292–300. [Google Scholar] [CrossRef]
- Tavakoli, J.; Elliott, D.; Costi, J. The ultra-structural organization of the elastic network in the intra-and inter-lamellar matrix of the intervertebral disc. Acta Biomater. 2017, 58, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Colombier, P.; Camus, A.; Lescaudron, L.; Clouet, J.; Guicheux, J. Intervertebral disc regeneration: A great challenge for tissue engineers. Trends Biotechnol. 2014, 32, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Andersson, G.B. Stem cell therapy for intervertebral disc regeneration: Obstacles and solutions. Nature Rev. Rheumatol. 2015, 11, 243–256. [Google Scholar] [CrossRef]
- Vonk, L.A.; Kroeze, R.J.; Doulabi, B.Z.; Hoogendoorn, R.J.; Huang, C.; Helder, M.N.; Everts, V.; Bank, R.A. Caprine articular, meniscus and intervertebral disc cartilage: An integral analysis of collagen network and chondrocytes. Matrix Biol. 2010, 29, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Bruehlmann, S.B.; Rattner, J.B.; Matyas, J.R.; Duncan, N.A. Regional variations in the cellular matrix of the annulus fibrosus of the intervertebral disc. J. Anat. 2002, 201, 159–171. [Google Scholar] [CrossRef]
- Hayes, A.J.; Smith, S.M.; Gibson, M.A.; Melrose, J. Comparative Immunolocalization of the Elastin Fiber–Associated Proteins Fibrillin-1, LTBP-2, and MAGP-1 With Components of the Collagenous and Proteoglycan Matrix of the Fetal Human Intervertebral Disc. Spine 2011, 36, E1365–E1372. [Google Scholar] [CrossRef]
- Bowles, R.D.; Setton, L.A. Biomaterials for intervertebral disc regeneration and repair. Biomaterials 2017, 129, 54–67. [Google Scholar] [CrossRef]
- Bailey, A.; Araghi, A.; Blumenthal, S.; Huffmon, G.V. Prospective, multicenter, randomized, controlled study of anular repair in lumbar discectomy: Two-year follow-up. Spine 2013, 38, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.H.; Balkovec, C.; Akens, M.K.; Chan, A.H.W.; Harrison, R.D.; Oakden, W.; Yee, A.J.M.; McGill, S.M. Closure of the annulus fibrosus of the intervertebral disc using a novel suture application device—in vivo porcine and ex vivo biomechanical evaluation. Spine J. 2016, 7, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.J.; Cheng, C.K.; Sun, J.S.; Liao, C.J.; Wang, Y.H.; Tsuang, Y.H. The effect of a new anular repair after discectomy in intervertebral disc degeneration: An experimental study using a porcine spine model. Spine 2011, 36, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Ahlgren, B.D.; Lui, W.; Herkowitz, H.N.; Panjabi, M.M.; Guiboux, J.P. Effect of anular repair on the healing strength of the intervertebral disc: A sheep model. Spine 2000, 25, 2165–2170. [Google Scholar] [CrossRef]
- Heuer, F.; Ulrich, S.; Claes, L.; Wilke, H.J. Biomechanical evaluation of conventional anulus fibrosus closure methods required for nucleus replacement. Laboratory investigation. J. Neurosurg. Spine 2008, 9, 307–313. [Google Scholar] [CrossRef]
- Kloth, D.; Fenton, D.; Andersson, G.; Block, J. Intradiscal electrothermal therapy (IDET) for the treatment of discogenic low back pain: Patient selection and indications for use. Pain Physician 2008, 11, 659–668. [Google Scholar]
- Maurer, P.; Block, J.E.; Squillante, D. Intradiscal electrothermal therapy (IDET) provides effective symptom relief in patients with discogenic low back pain. Clin. Spine Surg. 2008, 21, 55–62. [Google Scholar] [CrossRef]
- Fukui, S.; Nitta, K.; Iwashita, N.; Tomie, H.; Nosaka, S.; Rohof, O. Results of intradiscal pulsed radiofrequency for lumbar discogenic pain: Comparison with intradiscal electrothermal therapy. Korean J. Pain 2012, 25, 155–160. [Google Scholar] [CrossRef]
- Fukui, S.; Nitta, K.; Iwashita, N.; Tomie, H.; Nosaka, S.; Rohof, O. Intradiscal pulsed radiofrequency for chronic lumbar discogenic low back pain: A one year prospective outcome study using discoblock for diagnosis. Pain Physician 2013, 16, 435–442. [Google Scholar]
- Kim, H.S.; Wu, P.H.; Jang, I.-T. Lumbar degenerative disease part 1: Anatomy and pathophysiology of intervertebral discogenic pain and radiofrequency ablation of basivertebral and sinuvertebral nerve treatment for chronic discogenic back pain: A prospective case series and review of literature. Int. J. Mol. Sci. 2020, 21, 1483. [Google Scholar]
- Hahn, B.S.; Ji, G.Y.; Moon, B.; Shin, D.A.; Ha, Y.; Kim, K.N.; Yoon, D.H. Use of annular closure device (Barricaid®) for preventing lumbar disc Reherniation: One-year results of three cases. Korean J. Neurotrauma 2014, 10, 119–122. [Google Scholar] [CrossRef]
- Choy, W.J.; Phan, K.; Diwan, A.D.; Ong, C.S.; Mobbs, R.J. Annular closure device for disc herniation: Meta-analysis of clinical outcome and complications. BMC Musculoskelet. Disord. 2018, 19, 290. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.L.; Grahovac, G.; Vukas, D.; Vilendecic, M.; Ledic, D.; McGirt, M.J.; Carragee, E.J. Effect of an Annular Closure Device (Barricaid) on Same-Level Recurrent Disk Herniation and Disk Height Loss After Primary Lumbar Discectomy: Two-year Results of a Multicenter Prospective Cohort Study. Clin. Spine Surg. 2016, 29, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Trummer, M.; Eustacchio, S.; Barth, M.; Klassen, P.D.; Stein, S. Protecting facet joints post-lumbar discectomy: Barricaid annular closure device reduces risk of facet degeneration. Clin. Neurol. Neurosurg. 2013, 115, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; van Kooten, T.G.; Kranenburg, H.-J.C.; Meij, B.P.; Behl, M.; Lendlein, A.; Grijpma, D.W. An annulus fibrosus closure device based on a biodegradable shape-memory polymer network. Biomaterials 2013, 34, 8105–8113. [Google Scholar] [CrossRef] [PubMed]
- Stephen, R.S., Jr.; Marianne, L.; Ibrahim, H.; Roger, H.; Lawrence, J.B. Biologic Annulus Fibrosus Repair: A Review of Preclinical In Vivo Investigations. Tissue Eng. Part B Rev. 2018, 24, 179–190. [Google Scholar]
- Yin, W.; Pauza, K.; Olan, W.J.; Doerzbacher, J.F.; Thorne, K.J. Intradiscal injection of fibrin sealant for the treatment of symptomatic lumbar internal disc disruption: Results of a prospective multicenter pilot study with 24-month follow-Up. Pain Med. 2014, 15, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Buser, Z.; Liu, J.; Thorne, K.J.; Coughlin, D.; Lotz, J.C. Inflammatory response of intervertebral disc cells is reduced by fibrin sealant scaffold in vitro. J. Tissue Eng. Regen. Med. 2014, 8, 77–84. [Google Scholar] [CrossRef]
- Grunert, P.; Borde, B.H.; Towne, S.B.; Moriguchi, Y.; Hudson, K.D.; Bonassar, L.J.; Härtl, R. Riboflavin crosslinked high-density collagen gel for the repair of annular defects in intervertebral discs: An in vivo study. Acta Biomater. 2015, 26, 215–224. [Google Scholar] [CrossRef]
- Slusarewicz, P.; Zhu, K.; Kirking, B.; Toungate, J.; Hedman, T. Optimization of protein crosslinking formulations for the treatment of degenerative disc disease. Spine 2011, 36, E7. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.-C.; Kuo, Y.-W.; Chang, Y.-C.; Nikkhoo, M.; Wang, J.-L. Rheological and dynamic integrity of simulated degenerated disc and consequences after cross-linker augmentation. Spine 2013, 38, E1446–E1453. [Google Scholar] [CrossRef] [PubMed]
- Kirking, B.; Hedman, T.; Criscione, J. Changes in the interfacial shear resistance of disc annulus fibrosus from genipin crosslinking. J. Biomech. 2014, 47, 293–296. [Google Scholar] [CrossRef] [Green Version]
- Schek, R.; Michalek, A.; Iatridis, J. Genipin-crosslinked fibrin hydrogels as a potential adhesive to augment intervertebral disc annulus repair. Eur. Cells Mater. 2011, 21, 373. [Google Scholar] [CrossRef]
- Mizuno, H.; Roy, A.K.; Vacanti, C.A.; Kojima, K.; Ueda, M.; Bonassar, L.J. Tissue-Engineered Composites of Anulus Fibrosus and Nucleus Pulposus for Intervertebral Disc Replacement. Spine 2004, 29, 1290–1297. [Google Scholar] [CrossRef]
- Saad, L.; Spector, M. Effects of collagen type on the behavior of adult canine annulus fibrosus cells in collagen-glycosaminoglycan scaffolds. J. Biomed. Mater. Res. Part A 2004, 71, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Wilda, H.; Gough, J.E. In vitro studies of annulus fibrosus disc cell attachment, differentiation and matrix production on PDLLA/45S5 Bioglass composite films. Biomaterials 2006, 27, 5220–5229. [Google Scholar] [CrossRef]
- Shen, B.; Wei, A.; Bhargav, D.; Kishen, T.; Diwan, A.D. Hyaluronan: Its potential application in intervertebral disc regeneration. Orthop. Res. Rev. 2010. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, J. Tissue engineering of the intervertebral disc’s annulus fibrosus: A scaffold-based review study. Tissue Eng. Regenrattive Med. 2016. [Google Scholar] [CrossRef]
- Growney Kalaf, E.A.; Flores, R.; Bledsoe, J.G.; Sell, S.A. Characterization of slow-gelling alginate hydrogels for intervertebral disc tissue-engineering applications. Mater. Sci. Eng. C 2016, 63, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Bowles, R.D.; Williams, R.M.; Zipfel, W.R.; Bonassar, L.J. Self-assembly of aligned tissue-engineered annulus fibrosus and intervertebral disc composite via collagen gel contraction. Tissue Eng. Part A 2010, 16, 1339–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsdale, T.; Bard, J. Collagen Substrata for Studies on Cell Behavior. J. Cell Biol. 1972, 54, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Borde, B.; Grunert, P.; Hartl, R.; Bonassar, L.J. Injectable, high-density collagen gels for annulus fibrosus repair: An in vitro rat tail model. J. Biomed. Mater. Res. A 2014. [Google Scholar]
- Bowles, R.D.; Gebhard, H.H.; Hartl, R.; Bonassar, L.J. Tissue-engineered intervertebral discs produce new matrix, maintain disc height, and restore biomechanical function to the rodent spine. Proc. Natl. Acad. Sci. USA 2011, 108, 13106–13111. [Google Scholar] [CrossRef] [Green Version]
- Chik, T.K.; Ma, X.Y.; Choy, T.H.; Li, Y.Y.; Diao, H.J.; Teng, W.K.; Han, S.J.; Cheung, K.M.; Chan, B.P. Photochemically crosslinked collagen annulus plug: A potential solution solving the leakage problem of cell-based therapies for disc degeneration. Acta Biomater. 2013, 9, 8128–8139. [Google Scholar] [CrossRef] [PubMed]
- Pirvu, T.; Blanquer, S.B.G.; Benneker, L.M.; Grijpma, D.W.; Richards, R.G.; Alini, M.; Eglin, D.; Grad, S.; Li, Z. A combined biomaterial and cellular approach for annulus fibrosus rupture repair. Biomaterials 2015, 42, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Feng, G.; Shen, F.H.; Laurencin, C.T.; Li, X. Biphasic scaffold for annulus fibrosus tissue regeneration. Biomaterials 2008, 29, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, G.; Mozetic, P.; Rainer, A.; Centola, M.; Loppini, M.; Trombetta, M.; Denaro, V. Bioactive electrospun scaffold for annulus fibrosus repair and regeneration. Eur. Spine J. 2012, 21, 20–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillaume, O.; Daly, A.; Lennon, K.; Gansau, J.; Buckley, S.F.; Buckley, C.T. Shape-memory porous alginate scaffolds for regeneration of the annulus fibrosus: Effect of TGF-β3 supplementation and oxygen culture conditions. Acta Biomater 2014, 10, 1985–1995. [Google Scholar] [CrossRef]
- Xu, X.; Hu, J.; Lu, H. Histological observation of a gelatin sponge transplant loaded with bone marrow-derived mesenchymal stem cells combined with platelet-rich plasma in repairing an annulus defect. PLoS ONE 2017, 12, e0171500. [Google Scholar] [CrossRef] [Green Version]
- McGuire, R.; Borem, R.; Mercuri, J. The fabrication and characterization of a multi-laminate, angle-ply collagen patch for annulus fibrosus repair. J. Tissue Eng. Regen. Med. 2016, 11, 3488–3493. [Google Scholar] [CrossRef]
- Cunha, C.; Teixeira, G.Q.; Ribeiro-Machado, C.; Pereira, C.L.; Ferreira, J.R.; Molinos, M.; Santos, S.G.; Barbosa, M.A.; Goncalves, R.M. Modulation of the In Vivo Inflammatory Response by Pro-Versus Anti-Inflammatory Intervertebral Disc Treatments. Int. J. Mol. Sci. 2020, 21, 1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kameda, T.; Zvick, J.; Vuk, M.; Sadowska, A.; Tam, W.K.; Leung, V.Y.; Bölcskei, K.; Helyes, Z.; Applegate, L.A.; Hausmann, O.N. Expression and Activity of TRPA1 and TRPV1 in the Intervertebral Disc: Association with Inflammation and Matrix Remodeling. Int. J. Mol. Sci. 2019, 20, 1767. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.-R.; Bau, D.-T.; Chen, T.-S.; Chuang, I.; Tsai, C.-F.; Chang, P.-C.; Hsu, H.-C.; Lu, D.-Y. Pro-Inflammatory Stimuli Influence Expression of Intercellular Adhesion Molecule 1 in Human Anulus Fibrosus Cells through FAK/ERK/GSK3 and PKCδ Signaling Pathways. Int. J. Mol. Sci. 2019, 20, 77. [Google Scholar] [CrossRef] [Green Version]
- Stich, S.; Möller, A.; Cabraja, M.; Krüger, J.P.; Hondke, S.; Endres, M.; Ringe, J.; Sittinger, M. Chemokine CCL25 induces migration and extracellular matrix production of anulus fibrosus-derived cells. Int. J. Mol. Sci. 2018, 19, 2207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Luca, P.; De Girolamo, L.; Perucca Orfei, C.; Viganò, M.; Cecchinato, R.; Brayda-Bruno, M.; Colombini, A. Vitamin D’s effect on the proliferation and inflammation of human intervertebral disc cells in relation to the functional vitamin D receptor gene FokI polymorphism. Int. J. Mol. Sci. 2018, 19, 2002. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.A.; Khan, S.N.; Diwan, A.D. The molecular basis of intervertebral disk degeneration. Orthop. Clin. N. Am. 2003, 34, 209–219. [Google Scholar] [CrossRef]
- de Oliveira, C.P.; Rodrigues, L.M.R.; Fregni, M.V.V.D.; Gotfryd, A.; Made, A.M.; da Silva Pinhal, M.A. Extracellular matrix remodeling in experimental intervertebral disc degeneration. Acta Ortop. Bras. 2013, 21, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuller, E.S.; Shu, C.; Smith, M.M.; Little, C.B.; Melrose, J. Hyaluronan oligosaccharides stimulate matrix metalloproteinase and anabolic gene expression in vitro by intervertebral disc cells and annular repair in vivo. J. Tissue Eng. Regen. Med. 2018, 12, e216–e226. [Google Scholar] [CrossRef]
- Cazzanelli, P.; Wuertz-Kozak, K. MicroRNAs in Intervertebral Disc Degeneration, Apoptosis, Inflammation, and Mechanobiology. Int. J. Mol. Sci. 2020, 21, 3601. [Google Scholar] [CrossRef]
- Masuda, K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur. Spine J. 2008, 17, 441. [Google Scholar] [CrossRef] [Green Version]
- Wei, A.; Brisby, H.; Chung, S.A.; Diwan, A.D. Bone morphogenetic protein-7 protects human intervertebral disc cells in vitro from apoptosis. Spine J. 2008, 8, 466–474. [Google Scholar] [CrossRef]
- Wei, A.; Williams, L.A.; Bhargav, D.; Shen, B.; Kishen, T.; Duffy, N.; Diwan, A.D. BMP13 Prevents the Effects of Annular Injury in an Ovine Model. Int. J. Biol. Sci. 2009, 5, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.Y.; Wei, A.-Q.; Shen, B.; Williams, L.; Diwan, A.D. Cartilage derived morphogenetic protein-2 induces cell migration and its chondrogenic potential in C28/I2 cells. Int. J. Spine Surg. 2015, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodgkinson, T.; Shen, B.; Diwan, A.; Hoyland, J.A.; Richardson, S.M. Therapeutic potential of growth differentiation factors in the treatment of degenerative disc diseases. JOR Spine 2019, 2, e1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendtsen, M.; Bunger, C.; Colombier, P.; Le Visage, C.; Roberts, S.; Sakai, D.; Urban, J.P. Biological challenges for regeneration of the degenerated disc using cellular therapies. Acta Orthop. 2016, 87, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Gruber, H.E.; Riley, F.E.; Hoelscher, G.L.; Ingram, J.A.; Bullock, L.; Hanley, E.N. Human annulus progenitor cells: Analyses of this viable endogenous cell population. J. Orthop. Res. 2016, 34, 1351–1360. [Google Scholar] [CrossRef]
- Stich, S.; Jagielski, M.; Fleischmann, A.; Meier, C.; Bussmann, P.; Kohl, B.; Schmidt, J.; Krüger, J.-P.; Endres, M.; Cabraja, M. Degeneration of Lumbar Intervertebral Discs: Characterization of Anulus Fibrosus Tissue and Cells of Different Degeneration Grades. Int. J. Mol. Sci. 2020, 21, 2165. [Google Scholar] [CrossRef] [Green Version]
- Williams, L.A.; Bhargav, D.; Diwan, A.D. Unveiling the bmp13 enigma: Redundant morphogen or crucial regulator? Int. J. Biolog. Sci. 2008, 4, 318. [Google Scholar] [CrossRef]
- Razban, V.; Lotfi, A.S.; Soleimani, M.; Ahmadi, H.; Massumi, M.; Khajeh, S.; Ghaedi, M.; Arjmand, S.; Najavand, S.; Khoshdel, A. HIF-1α overexpression induces angiogenesis in mesenchymal stem cells. BioRes. Open Access 2012, 1, 174–183. [Google Scholar] [CrossRef]
- Attar, M.; Arefian, E.; Nabiuni, M.; Adegani, F.J.; Bakhtiari, S.H.A.; Karimi, Z.; Barzegar, M.; Soleimani, M. MicroRNA 17–92 expressed by a transposone-based vector changes expression level of cell-cycle-related genes. Cell Biol. Int. 2012, 36, 1005–1012. [Google Scholar] [CrossRef]
- Williams, L.A.; Wei, A.; Bhargav, D.; Diwan, A.D. Cartilage derived morphogenetic protein 2 – A potential therapy for intervertebral disc regeneration? Biologicals 2014, 42, 65–73. [Google Scholar] [CrossRef]
- Miyazaki, S.; Diwan, A.D.; Kato, K.; Cheng, K.; Bae, W.C.; Sun, Y.; Yamada, J.; Muehleman, C.; Lenz, M.E.; Inoue, N.; et al. ISSLS PRIZE IN BASIC SCIENCE 2018: Growth differentiation factor-6 attenuated pro-inflammatory molecular changes in the rabbit anular-puncture model and degenerated disc-induced pain generation in the rat xenograft radiculopathy model. Eur. Spine J. 2018, 27, 739–751. [Google Scholar] [CrossRef]
- Tendulkar, G.; Chen, T.; Ehnert, S.; Kaps, H.P.; Nüssler, A.K. Intervertebral disc nucleus repair: Hype or hope? Int. J. Mol. Sci. 2019, 20, 3622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soleimani, M.; Nadri, S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nature Protoc. 2009, 4, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Brisby, H.; Tao, H.; Ma, D.D.; Diwan, A.D. Cell therapy for disc degeneration—potentials and pitfalls. Orthop. Clin. N. Am. 2004, 35, 85–93. [Google Scholar] [CrossRef]
- Shafiee, A.; Seyedjafari, E.; Soleimani, M.; Ahmadbeigi, N.; Dinarvand, P.; Ghaemi, N. A comparison between osteogenic differentiation of human unrestricted somatic stem cells and mesenchymal stem cells from bone marrow and adipose tissue. Biotechnol. Lett. 2011, 33, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Nadri, S.; Soleimani, M. Comparative analysis of mesenchymal stromal cells from murine bone marrow and amniotic fluid. Cytotherapy 2007, 9, 729–737. [Google Scholar] [CrossRef]
- Thorpe, A.A.; Bach, F.C.; Tryfonidou, M.A.; Le Maitre, C.L.; Mwale, F.; Diwan, A.D.; Ito, K. Leaping the hurdles in developing regenerative treatments for the intervertebral disc from preclinical to clinical. JOR Spine 2018, 1, e1027. [Google Scholar] [CrossRef]
- Smith, L.J.; Silverman, L.; Sakai, D.; Le Maitre, C.L.; Mauck, R.L.; Malhotra, N.R.; Lotz, J.C.; Buckley, C.T. Advancing cell therapies for intervertebral disc regeneration from the lab to the clinic: Recommendations of the ORS spine section. JOR Spine 2018, 1, e1036. [Google Scholar] [CrossRef]
- Sakai, D.; Schol, J. Cell therapy for intervertebral disc repair: Clinical perspective. J. Orthop. Translat. 2017, 9, 8–18. [Google Scholar] [CrossRef]
- Werner, B.C.; Li, X.; Shen, F.H. Stem cells in preclinical spine studies. Spine J. 2014, 14, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Costi, J.J.; Hearn, T.C.; Fazzalari, N.L. The effect of hydration on the stiffness of intervertebral discs in an ovine model. Clin. Biomech. 2002, 17, 446–455. [Google Scholar] [CrossRef]
- Wuertz, K.; Godburn, K.; Iatridis, J.C. MSC response to pH levels found in degenerating intervertebral discs. Biochem. Biophys. Res. Commun. 2009, 379, 824–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bibby, S.R.; Jones, D.A.; Ripley, R.M.; Urban, J.P. Metabolism of the intervertebral disc: Effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine 2005, 30, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Hampton, D.; Laros, G.; McCarron, R.; Franks, D. Healing potential of the anulus fibrosus. Spine 1989, 14, 398–401. [Google Scholar] [CrossRef]
- Key, J.A.; Ford, L.T. Experimental intervertebral-disc lesions. J. Bone Jt. Surg. 1948, 30, 621–630. [Google Scholar] [CrossRef]
- Smith, J.; Walmsley, R. Experimental incision of the intervertebral disc. J. Bone Jt. Surg. 1951, 33, 612–625. [Google Scholar] [CrossRef]
- Benneker, L.M.; Andersson, G.; Iatridis, J.C.; Sakai, D.; Hartl, R.; Ito, K.; Grad, S. Cell therapy for intervertebral disc repair: Advancing cell therapy from bench to clinics. Eur. Cell Mater. 2014, 27, 5–11. [Google Scholar] [CrossRef]
- Tan, H.; Marra, K.G. Injectable, biodegradable hydrogels for tissue engineering applications. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Nicodemus, G.D.; Bryant, S.J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng. Part B Rev. 2008, 14, 149–165. [Google Scholar] [CrossRef]
- Iwasa, J.; Ochi, M.; Uchio, Y.; Katsube, K.; Adachi, N.; Kawasaki, K. Effects of cell density on proliferation and matrix synthesis of chondrocytes embedded in atelocollagen gel. Artif. Organs 2003, 27, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Mochida, J.; Iwashina, T.; Hiyama, A.; Omi, H.; Imai, M.; Nakai, T.; Ando, K.; Hotta, T. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials 2006, 27, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Mochida, J.; Yamamoto, Y.; Nomura, T.; Okuma, M.; Nishimura, K.; Nakai, T.; Ando, K.; Hotta, T. Transplantation of mesenchymal stem cells embedded in Atelocollagen® gel to the intervertebral disc: A potential therapeutic model for disc degeneration. Biomaterials 2003, 24, 3531–3541. [Google Scholar] [CrossRef]
- Alini, M.; Li, W.; Markovic, P.; Aebi, M.; Spiro, R.C.; Roughley, P.J. The potential and limitations of a cell-seeded collagen/hyaluronan scaffold to engineer an intervertebral disc-like matrix. Spine 2003, 28, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, G.; Sowa, G.; Hubert, M.; Gilbertson, L.G.; Denaro, V.; Kang, J.D. Mesenchymal stem cells injection in degenerated intervertebral disc: Cell leakage may induce osteophyte formation. J. Tissue Eng. Regen. Med. 2012, 6, 348–355. [Google Scholar] [CrossRef]
- Wan, Y.; Feng, G.; Shen, F.H.; Balian, G.; Laurencin, C.T.; Li, X. Novel Biodegradable Poly(1,8-octanediol malate) for Annulus Fibrosus Regeneration. Macromol. Biosci. 2007, 7, 1217–1224. [Google Scholar] [CrossRef]
- Schneider, T.O.; Mueller, S.M.; Shortkroff, S.; Spector, M. Expression of α-smooth muscle actin in canine intervertebal disc cells in situ and in collagen-glycosaminoglycan matrices in vitro. J Orthop. Res. 1999, 17, 192–199. [Google Scholar] [CrossRef]
- Nerurkar, N.L.; Elliott, D.M.; Mauck, R.L. Mechanics of oriented electrospun nanofibrous scaffolds for annulus fibrosus tissue engineering. J. Orthop. Res. 2007, 25, 1018–1028. [Google Scholar] [CrossRef]
- Silva-Correia, J.; Correia, S.I.; Oliveira, J.M.; Reis, R.L. Tissue engineering strategies applied in the regeneration of the human intervertebral disk. Biotechnol. Adv. 2013, 31, 1514–1531. [Google Scholar] [CrossRef]
- Peroglio, M.; Grad, S.; Mortisen, D.; Sprecher, C.M.; Illien-Jünger, S.; Alini, M.; Eglin, D. Injectable thermoreversible hyaluronan-based hydrogels for nucleus pulposus cell encapsulation. Eur. Spine J. 2012, 21, 839–849. [Google Scholar] [CrossRef]
- Nesti, L.J.; Li, W.-J.; Shanti, R.M.; Jiang, Y.J.; Jackson, W.; Freedman, B.A.; Kuklo, T.R.; Giuliani, J.R.; Tuan, R.S. Intervertebral Disc Tissue Engineering Using a Novel Hyaluronic Acid–Nanofibrous Scaffold (HANFS) Amalgam. Tissue Eng. Part A 2008, 14, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Dahia, C.L.; Iatridis, J.C.; Risbud, M.V. New horizons in spine research: Disc biology, tissue engineering, biomechanics, translational, and clinical research. JOR Spine 2018, 1. [Google Scholar] [CrossRef]
- Buckley, C.T.; Hoyland, J.A.; Fujii, K.; Pandit, A.; Iatridis, J.C.; Grad, S. Critical aspects and challenges for intervertebral disc repair and regeneration—Harnessing advances in tissue engineering. JOR Spine 2018, 1, e1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, J.T.; Gullbrand, S.E.; Fields, A.J.; Purmessur, D.; Diwan, A.D.; Oxland, T.R.; Chiba, K.; Guilak, F.; Hoyland, J.A.; Iatridis, J.C. Publication trends in spine research from 2007 to 2016: Comparison of the Orthopaedic Research Society Spine Section and the International Society for the Study of the Lumbar Spine. JOR Spine 2018, 1, e1006. [Google Scholar] [CrossRef] [PubMed]
- Ashish, A.; Diwan, U.C. A Multi-centre, Randomised, Single-Blinded, Twelve-Month Follow-Up Trial to Evaluate the Safety and Effectiveness of the Kunovus Disc Device for the Preservation of Lumbar Disc Form and Function in Patients Undergoing Microdiscectomy for Sciatica due to Lumbar Disc Herniation. Available online: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=375184&showOriginal=true&isReview=true (accessed on 1 March 2020).
- Fearing, B.V.; Hernandez, P.A.; Setton, L.A.; Chahine, N.O. Mechanotransduction and cell biomechanics of the intervertebral disc. JOR Spine 2018, 1, e1026. [Google Scholar] [CrossRef]
- Loibl, M.; Wuertz-Kozak, K.; Vadala, G.; Lang, S.; Fairbank, J.; Urban, J.P. Controversies in regenerative medicine: Should intervertebral disc degeneration be treated with mesenchymal stem cells? JOR Spine 2019, 2, e1043. [Google Scholar] [CrossRef]
- D’Este, M.; Eglin, D.; Alini, M. Lessons to be learned and future directions for intervertebral disc biomaterials. Acta Biomater. 2018, 78, 13–22. [Google Scholar] [CrossRef]
- Dehbari, N.; Tavakoli, J.; Khatrao, S.S.; Tang, Y. In situ polymerized hyperbranched polymer reinforced poly (acrylic acid) hydrogels. Mater. Chem. Front. 2017, 1, 1995–2004. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, J.; Amin, D.B.; Freeman, B.J.C.; Costi, J.J. The Biomechanics of the Inter-Lamellar Matrix and the Lamellae During Progression to Lumbar Disc Herniation: Which is the Weakest Structure? Ann. Biomed. Eng. 2018, 46, 1280–1291. [Google Scholar] [CrossRef]
- Martin, J.T.; Milby, A.H.; Chiaro, J.A.; Kim, D.H.; Hebela, N.M.; Smith, L.J.; Elliott, D.M.; Mauck, R.L. Translation of an engineered nanofibrous disc-like angle-ply structure for intervertebral disc replacement in a small animal model. Acta Biomater. 2014, 10, 2473–2481. [Google Scholar] [CrossRef] [Green Version]
- Nerurkar, N.L.; Baker, B.M.; Sen, S.; Wible, E.E.; Elliott, D.M.; Mauck, R.L. Nanofibrous biologic laminates replicate the form and function of the annulus fibrosus. Nature Mater. 2009, 8, 986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nerurkar, N.L.; Sen, S.; Huang, A.H.; Elliott, D.M.; Mauck, R.L. Engineered disc-like angle-ply structures for intervertebral disc replacement. Spine 2010, 35, 867. [Google Scholar] [CrossRef] [Green Version]
- Jammalamadaka, U.; Tappa, K. Recent advances in biomaterials for 3D printing and tissue engineering. J. Funct. Biomater. 2018, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.S.; Kwon, Y.W.; Kong, J.-S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.-B.; Lee, H.; Kim, J.H.; Cho, D.-W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 2018, 168, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, Y.; Qin, X.; Wa, Q. 3D printing of concentrated alginate/gelatin scaffolds with homogeneous nano apatite coating for bone tissue engineering. Mate. Des. 2018, 146, 12–19. [Google Scholar] [CrossRef]
- Costa, J.B.; Silva-Correia, J.; Ribeiro, V.P.; da Silva Morais, A.; Oliveira, J.M.; Reis, R.L. Engineering patient-specific bioprinted constructs for treatment of degenerated intervertebral disc. Mater. Today Commun. 2019, 19, 506–512. [Google Scholar] [CrossRef]
- Oner, T.; Cengiz, I.; Pitikakis, M.; Cesario, L.; Parascandolo, P.; Vosilla, L.; Viano, G.; Oliveira, J.; Reis, R.; Silva-Correia, J. 3D segmentation of intervertebral discs: From concept to the fabrication of patient-specific scaffolds. J. 3D Print. Med. 2017, 1, 91–101. [Google Scholar] [CrossRef]
- Doyle, A.D.; Yamada, K.M. Mechanosensing via cell-matrix adhesions in 3D microenvironments. Exp. Cell Res. 2016, 343, 60–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jokinen, J.; Dadu, E.; Nykvist, P.; Kapyla, J.; White, D.J.; Ivaska, J.; Vehvilainen, P.; Reunanen, H.; Larjava, H.; Hakkinen, L.; et al. Integrin-mediated cell adhesion to type I collagen fibrils. J. Biol. Chem. 2004, 279, 31956–31963. [Google Scholar] [CrossRef] [Green Version]
- Kesti, M.; Müller, M.; Becher, J.; Schnabelrauch, M.; D’Este, M.; Eglin, D.; Zenobi-Wong, M. A versatile bioink for three-dimensional printing of cellular scaffolds based on thermally and photo-triggered tandem gelation. Acta Biomater. 2015, 11, 162–172. [Google Scholar] [CrossRef] [Green Version]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. Part A 2013, 101, 1255–1264. [Google Scholar] [CrossRef] [Green Version]
- Chimene, D.; Lennox, K.K.; Kaunas, R.R.; Gaharwar, A.K. Advanced bioinks for 3D printing: A materials science perspective. Ann. Biomed. Eng. 2016, 44, 2090–2102. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Devarasetty, M.; Kang, H.-W.; Mead, I.; Bishop, C.; Shupe, T.; Lee, S.J.; Jackson, J.; Yoo, J.; Soker, S. A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs. Acta Biomater. 2015, 25, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Do, A.V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D printing of scaffolds for tissue regeneration applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokkinis, D.; Bouville, F.; Studart, A.R. 3D Printing of Materials with Tunable Failure via Bioinspired Mechanical Gradients. Adv. Mater. 2018, 30, 1705808. [Google Scholar] [CrossRef] [PubMed]




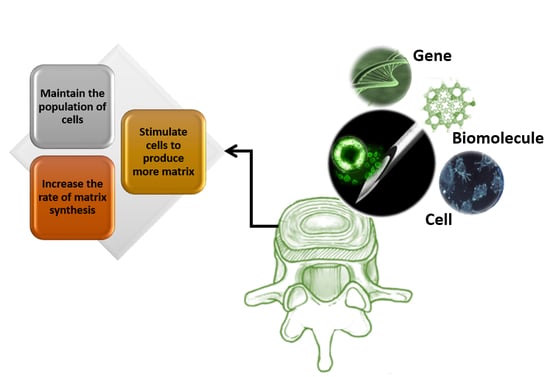
| Repair Strategies | ||
|---|---|---|
| Strategy | Current Status | Feasibility |
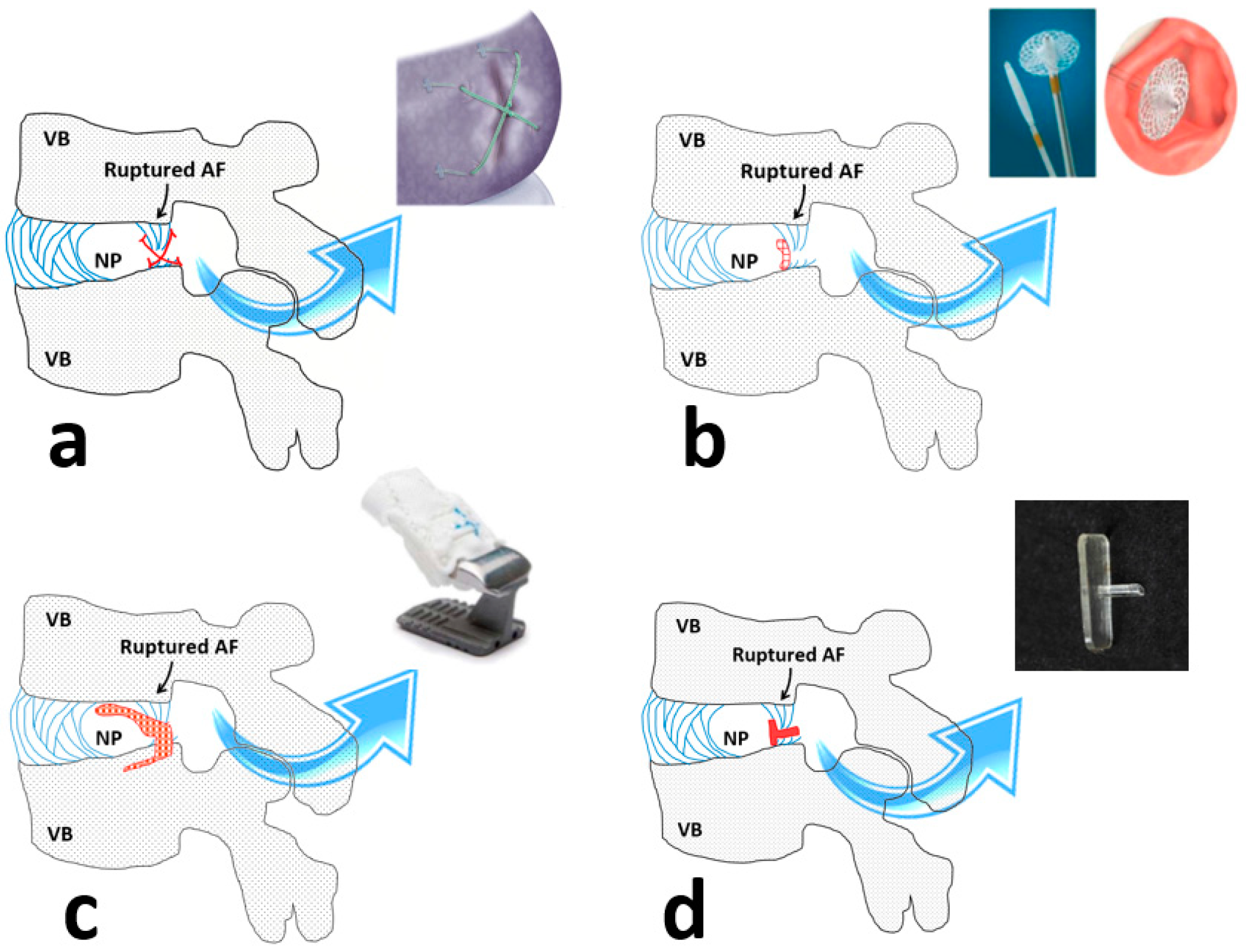
| Early AF closure device (Xclose®, Inclose®) | Not available anymore | Not effective to stop re-herniation and improve the rate or strength of the AF healing. |
| Suturing techniques | Rarely used | Are not reliable to sustain complex cyclic loading and not efficient yet. |
| Thermal therapies (pulse radiofrequency and intra-discal electro-thermal therapies) | In use | Are likely to be effective to seal the structural defects in the mildly ruptured AF. Sever AF defects are unlikely to be treated and their contribution to IVD degeneration in the long term is not known. |
| Advanced AF closure devices (Barricaid®) | Clinical trial | Being effective to reduce the rate of re-herniation and alleviate pain. The key limitation is being different from the native tissue in terms of structure and property, hence it may accelerate the degeneration process. |
| Injectable bio-adhesives | Laboratory trial | The appropriate properties (i.e., adhesion, mechanical strength) and long-term capacity to resist high stresses during daily activities have remained a major concern. |
| Polymeric implants | Laboratory trial | Not sufficient data available to evaluate their feasibility and no further clinical practice was reported. |
| Regenerative Strategies | ||
|---|---|---|
| Strategy | Current Status | Feasibility |
| Gene therapies | Laboratory trial | Being effective in accelerating the regeneration process, stimulation of ECM remodeling, and prevention of progression to AF injury. The frequent observation of AF cell apoptosis in degenerated IVDs is a barrier. |
| Biomolecule therapies (growth factors) | Laboratory trial | Being effective in cell differentiation, enhancing the healing process, and upregulating of healthy cell marker genes. The short lifetime of biomolecules is a limitation that restrains the efficacy of biomolecules to regenerate AF. |
| Cell therapies | preclinical studies | Being successful in ECM remodeling via direct transplantation of proliferated mesenchymal stem cells into the degenerated AF. Unlikely to regenerate highly degenerated IVDs due to the lack of (1) structural integrity to physically maintain the cells after injection, and (2) an appropriate biological environment important to prevent cell apoptosis. |
| Injectable cell delivery gels | Laboratory trial | Being effective to promote cell-based strategies via increasing cell retention time at the injection site. Injectable polymers have not been able to entirely address the current problems due to their low mechanical properties. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavakoli, J.; Diwan, A.D.; Tipper, J.L. Advanced Strategies for the Regeneration of Lumbar Disc Annulus Fibrosus. Int. J. Mol. Sci. 2020, 21, 4889. https://doi.org/10.3390/ijms21144889
Tavakoli J, Diwan AD, Tipper JL. Advanced Strategies for the Regeneration of Lumbar Disc Annulus Fibrosus. International Journal of Molecular Sciences. 2020; 21(14):4889. https://doi.org/10.3390/ijms21144889
Chicago/Turabian StyleTavakoli, Javad, Ashish D. Diwan, and Joanne L. Tipper. 2020. "Advanced Strategies for the Regeneration of Lumbar Disc Annulus Fibrosus" International Journal of Molecular Sciences 21, no. 14: 4889. https://doi.org/10.3390/ijms21144889
APA StyleTavakoli, J., Diwan, A. D., & Tipper, J. L. (2020). Advanced Strategies for the Regeneration of Lumbar Disc Annulus Fibrosus. International Journal of Molecular Sciences, 21(14), 4889. https://doi.org/10.3390/ijms21144889