Artemisia annua, a Traditional Plant Brought to Light
Abstract
1. Introduction
2. Chemical Compounds from Artemisia annua and Their Biological Activities
2.1. Monoterpenes
2.2. Sesquiterpenes
3. Phenolic Compounds
4. Coumarins
5. Biological Activities of Artemisia annua
5.1. Antioxidant Activities
5.2. Antidiabetic Activities
5.3. Cytotoxic and Antitumor Effects
5.4. Immunomodulatory Effects
5.5. Antibacterial and Antifungal Activities
5.6. Antiviral Activities
5.7. Antiparasitic Activities
5.7.1. Antiplasmodial Activity
5.7.2. Anti-Helminthic Activities
5.7.3. Activity against Other Protozoa
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABTS | Acide 2,2′-azino-bis(3-éthylbenzothiazoline-6-sulphonique) |
| ACP | Acidic Phosphatases |
| ART | Artesunate |
| AD | Alzheimer’s Disease |
| ALDO | Aldolase |
| ALP | Alkaline Phosphatases |
| BAFF | B cell-activating factor |
| BKV | Polyomavirus BK |
| BVDV | Bovine Viral Diarrhea Virus |
| CAM | Chorioallantoic Membrane |
| CMV | CytoMegaloVirus |
| COX-2 | Cyclooxygenase 2 |
| CytC | Cytochrome C peroxidase |
| DAPI | Phenylindole dihydrochloride |
| DENV | Dengue Virus |
| DHA | Dihydroartemisinin |
| DPPH | 2,2-diphényl-1-picrylhydrazyle |
| DSS | Sulfate Sodium Salt |
| EBV | Epstein-Barr Virus |
| ENO | Enolase |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| G6PD | Glucose-6-Phosphate Dehydrogenase |
| GPI | Glucose-6-Phosphate Isomerase |
| GPx | Glutathione Peroxidase |
| GR | Glutathione Reductase |
| GRα | Glucocorticoid receptor α |
| GSH | Glutathione |
| GST | Glutathione S-Transferase |
| HBsAg | HBV surface antigen |
| HBV | Hepatitis B Virus |
| HCMV | Human Cytomegalovirus |
| HCV | Hepatitis C Virus |
| HHC-6A | Human Herpes Virus 6A |
| HIF-1α | Hypoxia-Inducible Factor-1α |
| HK | Hexokinase |
| HPV | Human Papillomavirus |
| HSV-1 | Herpes Simplex Virus 1 |
| IBD | Inflammatory Bowel Disease |
| IL-1β | Interleukin 1 beta |
| iNOS | Inducible Nitric Oxide Synthase |
| IP3 | Inositol trisphosphate |
| JCPyV | Human JC Polyomavirus |
| LDH | Lactate Dehydrogenase |
| LDL | Low Density Lipoprotein |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen Activated Protein Kinase |
| MCP-1 | Monocyte Chemoattractant Protein 1 |
| MDH | Malate Dehydrogenase |
| ME | Malic Enzyme |
| MMP-2 | Metalloproteinase-2 |
| MPI | Mannose-6-Phosphateisomerase |
| NF-κB | Nuclear Factor κB |
| Nrf-2 | Nuclear Factor (erythroid-derived 2)-like 2 |
| ORAC | Oxygen Radical Absorbance Capacity |
| PARP | Poly (ADP-ribose) Polymerase |
| PFK | Phosphofructokinase |
| PGAM | Phosphoglycerate Mutase |
| PGD | 6-Phosphogluconate Dehydrogenase |
| PGK | Phosphoglycerate Kinase |
| PI3K | Phosphoinositide 3-kinase |
| PK | Pyruvate Kinase |
| PLCγ | Phospholipase Cγ |
| RA | Rheumatoid Arthritis |
| ROS | Reactive Oxygen Species |
| SLE | Systemic Lupus Erythematosus |
| SM905 | 1-(12β-dihydroartemisinoxy)-2-hydroxy-3-tert-butylaminopropane maleate, new water-soluble derivative |
| SM934 | β-aminoarteether maleate, new water-soluble derivative |
| SOD | Superoxide Dismutase |
| TGF-1β | Transforming Growth Factor beta 1 |
| TNBC | Triple Negative Breast cancer |
| TNBS | Trinitrobenzene Sulfonic acid |
| TNF-α | Tumor Necrosis Factor alpha |
| VEGF | Vascular Endothelial Growth Factor |
References
- Bora, K.S.; Sharma, A. The Genus Artemisia: A Comprehensive Review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef]
- Sanz, M.; Vilatersana, R.; Hidalgo, O.; Garcia-Jacas, N.; Susanna, A.; Schneeweiss, G.M.; Vallès, J. Molecular Phylogeny and Evolution of Floral Characters of Artemisia and Allies (Anthemideae, Asteraceae): Evidence from NrDNA ETS and ITS Sequences. TAXON 2008, 57, 66–78. [Google Scholar] [CrossRef]
- Alesaeidi, S.; Miraj, S. A Systematic Review of Anti-Malarial Properties, Immunosuppressive Properties, Anti-Inflammatory Properties, and Anti-Cancer Properties of Artemisia Annua. Electron. Physician 2016, 8, 3150–3155. [Google Scholar] [CrossRef] [PubMed]
- Funk, V.A.; Bayer, R.J.; Keeley, S.; Chan, R.; Watson, L.; Gemeinholzer, B.; Schilling, E.; Panero, J.L.; Baldwin, B.G.; Garcia-Jacas, N.; et al. Everywhere but Antarctica: Using a Supertree to Understand the Diversity and Distribution of the Compositae. Biol. Skr. 2005, 55, 343–374. [Google Scholar]
- Vallès, J.; Garcia, S.; Hidalgo, O.; Martín, J.; Pellicer, J.; Sanz, M.; Garnatje, T. Biology, Genome Evolution, Biotechnological Issues and Research Including Applied Perspectives in Artemisia (Asteraceae). Adv. Bot. Res. 2011. [Google Scholar] [CrossRef]
- Jarvis, C.E. A List of Linnaean Generic Names and Their Types; Koeltz Sciebtific Books; International Association for Plant Taxonomy: Bratislava, Slovakia, 1993; Volume 127, p. 100. [Google Scholar]
- Willcox, M. Artemisia Species: From Traditional Medicines to Modern Antimalarials—and Back Again. J. Altern. Complement. Med. 2009, 15, 101–109. [Google Scholar] [CrossRef]
- Ko, Y.S.; Lee, W.S.; Panchanathan, R.; Joo, Y.N.; Choi, Y.H.; Kim, G.S.; Jung, J.-M.; Ryu, C.H.; Shin, S.C.; Kim, H.J. Polyphenols from Artemisia Annua L Inhibit Adhesion and EMT of Highly Metastatic Breast Cancer Cells MDA-MB-231. Phytother. Res. 2016, 30, 1180–1188. [Google Scholar] [CrossRef]
- L’Artemisia annua. La Maison de l’Artemisia-Cette Plante Peut Sauver des Millions de Vie. Available online: https://maison-artemisia.org/l-artemisia-du-cote-agronomique/artemisia-annua/ (accessed on 3 June 2020).
- Hsu, E. The History of Qing Hao in the Chinese Materia Medica. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 505–508. [Google Scholar] [CrossRef]
- Ćavar, S.; Maksimović, M.; Vidic, D.; Parić, A. Chemical Composition and Antioxidant and Antimicrobial Activity of Essential Oil of Artemisia Annua L. from Bosnia. Ind. Crop. Prod. 2012, 37, 479–485. [Google Scholar] [CrossRef]
- Mueller, M.S.; Karhagomba, I.B.; Hirt, H.M.; Wemakor, E. The Potential of Artemisia Annua L. as a Locally Produced Remedy for Malaria in the Tropics: Agricultural, Chemical and Clinical Aspects. J. Ethnopharmacol. 2000, 73, 487–493. [Google Scholar] [CrossRef]
- Gupta, P.C.; Dutta, B.; Pant, D.; Joshi, P.; Lohar, D.R. In Vitro Antibacterial Activity of Artemisia Annua Linn. Growing in India. Int. J. Green Pharm. 2009, 3. [Google Scholar] [CrossRef]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. Genus: A Review of Bioactive Essential Oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Cui, L.; Chang, X.; Guan, D. Biosynthesis and Characterization of Zinc Oxide Nanoparticles from Artemisia Annua and Investigate Their Effect on Proliferation, Osteogenic Differentiation and Mineralization in Human Osteoblast-like MG-63 Cells. J. Photochem. Photobiol. B 2020, 202, 111652. [Google Scholar] [CrossRef]
- Lubbe, A.; Seibert, I.; Klimkait, T.; van der Kooy, F. Ethnopharmacology in Overdrive: The Remarkable Anti-HIV Activity of Artemisia Annua. J. Ethnopharmacol. 2012, 141, 854–859. [Google Scholar] [CrossRef]
- Ho, W.E.; Peh, H.Y.; Chan, T.K.; Wong, W.S.F. Artemisinins: Pharmacological Actions beyond Anti-Malarial. Pharmacol. Ther. 2014, 142, 126–139. [Google Scholar] [CrossRef]
- Kim, M.H.; Seo, J.Y.; Liu, K.H.; Kim, J.-S. Protective Effect of Artemisia Annua L. Extract against Galactose-Induced Oxidative Stress in Mice. PLoS ONE 2014, 9, e101486. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Zhang, D.; Zhang, Y.; Wen, Y.; Li, L.; Zheng, L. Tumoricidal Effects of a Selenium (Se)-Polysaccharide from Ziyang Green Tea on Human Osteosarcoma U-2 OS Cells. Carbohydr. Polym. 2013, 98, 1186–1190. [Google Scholar] [CrossRef]
- Castilho, P.C.; Gouveia, S.C.; Rodrigues, A.I. Quantification of Artemisinin in Artemisia Annua Extracts by 1H-NMR. Phytochem. Anal. 2008, 19, 329–334. [Google Scholar] [CrossRef]
- Chaudhary, V.; Kapoor, R.; Bhatnagar, A.K. Effectiveness of Two Arbuscular Mycorrhizal Fungi on Concentrations of Essential Oil and Artemisinin in Three Accessions of Artemisia Annua L. Appl. Soil Ecol. 2008, 40, 174–181. [Google Scholar] [CrossRef]
- Slezakova, S.; Ruda-Kucerova, J. Anticancer Activity of Artemisinin and Its Derivatives. Anticancer Res. 2017, 37, 5995–6003. [Google Scholar] [CrossRef]
- Tse, E.G.; Korsik, M.; Todd, M.H. The Past, Present and Future of Anti-Malarial Medicines. Malar. J. 2019, 18, 93. [Google Scholar] [CrossRef]
- Brisibe, E.A.; Umoren, U.E.; Brisibe, F.; Magalhäes, P.M.; Ferreira, J.F.S.; Luthria, D.; Wu, X.; Prior, R.L. Nutritional Characterisation and Antioxidant Capacity of Different Tissues of Artemisia Annua L. Food Chem. 2009, 115, 1240–1246. [Google Scholar] [CrossRef]
- Van der Kooy, F.; Sullivan, S.E. The Complexity of Medicinal Plants: The Traditional Artemisia Annua Formulation, Current Status and Future Perspectives. J. Ethnopharmacol. 2013, 150. [Google Scholar] [CrossRef]
- Bhakuni, R.S.; Jain, D.C.; Sharma, R.P.; Kumar, S. Secondary Metabolites of Artemisia Annua and Their Biological Activity. Curr. Sci. 2001, 80, 35–48. [Google Scholar]
- Li, K.-M.; Dong, X.; Ma, Y.-N.; Wu, Z.-H.; Yan, Y.-M.; Cheng, Y.-X. Antifungal Coumarins and Lignans from Artemisia Annua. Fitoterapia 2019, 134, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Yw, Z.; Fy, N.; Yl, S.; Sy, W.; Wz, H.; Zz, W.; Wei, X. Chemical Constituents from Artemisia Annua. Zhongguo Zhong Yao Za Zhi 2014, 39, 4816–4821. [Google Scholar]
- El-Askary, H.; Handoussa, H.; Badria, F.; El-Khatib, A.H.; Alsayari, A.; Linscheid, M.W.; Abdel Motaal, A. Characterization of Hepatoprotective Metabolites from Artemisia Annua and Cleome Droserifolia Using HPLC/PDA/ESI/MS–MS. Rev. Bras. De Farmacogn. 2019, 29, 213–220. [Google Scholar] [CrossRef]
- Lang, S.J.; Schmiech, M.; Hafner, S.; Paetz, C.; Steinborn, C.; Huber, R.; Gaafary, M.E.; Werner, K.; Schmidt, C.Q.; Syrovets, T.; et al. Antitumor Activity of an Artemisia Annua Herbal Preparation and Identification of Active Ingredients. Phytomedicine 2019, 62, 152962. [Google Scholar] [CrossRef]
- Wan, X.L.; Niu, Y.; Zheng, X.C.; Huang, Q.; Su, W.P.; Zhang, J.F.; Zhang, L.L.; Wang, T. Antioxidant Capacities of Artemisia Annua L. Leaves and Enzymatically Treated Artemisia Annua L. in Vitro and in Broilers. Anim. Feed Sci. Technol. 2016, 221, 27–34. [Google Scholar] [CrossRef]
- Song, Y.; Desta, K.T.; Kim, G.-S.; Lee, S.J.; Lee, W.S.; Kim, Y.-H.; Jin, J.S.; Abd El-Aty, A.M.; Shin, H.-C.; Shim, J.-H.; et al. Polyphenolic Profile and Antioxidant Effects of Various Parts of Artemisia Annua L. Biomed. Chromatogr. 2016, 30, 588–595. [Google Scholar] [CrossRef]
- Li, Y.-J.; Guo, Y.; Yang, Q.; Weng, X.-G.; Yang, L.; Wang, Y.-J.; Chen, Y.; Zhang, D.; Li, Q.; Liu, X.-C.; et al. Flavonoids Casticin and Chrysosplenol D from Artemisia Annua L. Inhibit Inflammation in Vitro and in Vivo. Toxicol. Appl. Pharmacol. 2015, 286, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Casticin Alleviates Lipopolysaccharide-Induced Inflammatory Responses and Expression of Mucus and Extracellular Matrix in Human Airway Epithelial Cells through Nrf2/Keap1 and NF-ΚB Pathways. Phytother. Res. 2018, 32, 1346–1353. [Google Scholar] [CrossRef]
- Shin, N.-R.; Ryu, H.-W.; Ko, J.-W.; Park, S.-H.; Yuk, H.-J.; Kim, H.-J.; Kim, J.-C.; Jeong, S.-H.; Shin, I.-S. Artemisia Argyi Attenuates Airway Inflammation in Ovalbumin-Induced Asthmatic Animals. J. Ethnopharmacol. 2017, 209, 108–115. [Google Scholar] [CrossRef]
- Qiu, F.; Wu, S.; Lu, X.; Zhang, C.; Li, J.; Gong, M.; Wang, M. Quality Evaluation of the Artemisinin-Producing Plant Artemisia Annua L. Based on Simultaneous Quantification of Artemisinin and Six Synergistic Components and Hierarchical Cluster Analysis. Ind. Crop. Prod. 2018, 118, 131–141. [Google Scholar] [CrossRef]
- Zhang, X.-B.; Guo, L.-P.; Qiu, Z.-D.; Qu, X.-B.; Wang, H.; Jing, Z.-X.; Huang, L.-Q. [Analysis of spatial distribution of artemisinin in Artemisia annua in China]. Zhongguo Zhong Yao Za Zhi 2017, 42, 4277–4281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-B.; Zhao, Y.-P.; Huang, X.-W.; Qiu, Z.-D.; Guo, L.-P.; Qu, X.-B.; Huang, L.-Q. [Review on study of Dao-di herbs Artemisiae Annuae Herba]. Zhongguo Zhong Yao Za Zhi 2016, 41, 2015–2018. [Google Scholar] [CrossRef]
- Gouveia, S.C.; Castilho, P.C. Artemisia Annua L.: Essential Oil and Acetone Extract Composition and Antioxidant Capacity. Ind. Crop. Prod. 2013, 45, 170–181. [Google Scholar] [CrossRef]
- Marlet, C.; Lognay, G. Les monoterpènes: Sources et implications dans la qualité de l’air intérieur. Biotechnol. Agron. Soc. Environ. 2011, 15, 611–622. [Google Scholar]
- Janaćković, P.; Rajčević, N.; Gavrilović, M.; Novaković, J.; Giweli, A.; Stešević, D.; Marin, P.D. Essential Oil Composition of Five Artemisia (Compositae) Species in Regards to Chemophenetics. Biochem. Syst. Ecol. 2019, 87, 103960. [Google Scholar] [CrossRef]
- De Magalhães, P.M.; Pereira, B.; Sartoratto, A. Yields of antimalarial artemisia Annua L. species. Acta Hortic. 2004, 629, 421–424. [Google Scholar] [CrossRef]
- Durden, K.; Sellars, S.; Cowell, B.; Brown, J.J.; Pszczolkowski, M.A. Artemisia Annua Extracts, Artemisinin and 1,8-Cineole, Prevent Fruit Infestation by a Major, Cosmopolitan Pest of Apples. Pharm. Biol. 2011, 49, 563–568. [Google Scholar] [CrossRef][Green Version]
- Santos, F.A.; Rao, V.S.N. Antiinflammatory and Antinociceptive Effects of 1,8-Cineole a Terpenoid Oxide Present in Many Plant Essential Oils. Phytother. Res. 2000, 14, 240–244. [Google Scholar] [CrossRef]
- Vilela, G.R.; de Almeida, G.S.; D’Arce, M.A.B.R.; Moraes, M.H.D.; Brito, J.O.; da Silva, M.F.d.G.F.; Silva, S.C.; de Stefano Piedade, S.M.; Calori-Domingues, M.A.; da Gloria, E.M. Activity of Essential Oil and Its Major Compound, 1,8-Cineole, from Eucalyptus Globulus Labill., against the Storage Fungi Aspergillus Flavus Link and Aspergillus Parasiticus Speare. J. Stored Prod. Res. 2009, 45, 108–111. [Google Scholar] [CrossRef]
- Murata, S.; Shiragami, R.; Kosugi, C.; Tezuka, T.; Yamazaki, M.; Hirano, A.; Yoshimura, Y.; Suzuki, M.; Shuto, K.; Ohkohchi, N.; et al. Antitumor Effect of 1, 8-Cineole against Colon Cancer. Oncol. Rep. 2013, 30, 2647–2652. [Google Scholar] [CrossRef]
- De Figuêiredo, F.R.; Monteiro, Á.B.; de Menezes, I.R.; dos Sales, V.S.; do Nascimento, E.; de Souza Rodrigues, C.; Bitu Primo, A.J.; da Cruz, L.; do Amaro, É.N.; de Araújo Delmondes, G.; et al. Effects of the Hyptis Martiusii Benth. Leaf Essential Oil and 1,8-Cineole (Eucalyptol) on the Central Nervous System of Mice. Food Chem. Toxicol. 2019, 133, 110802. [Google Scholar] [CrossRef]
- Sampath, S.; Subramani, S.; Janardhanam, S.; Subramani, P.; Yuvaraj, A.; Chellan, R. Bioactive Compound 1,8-Cineole Selectively Induces G2/M Arrest in A431 Cells through the Upregulation of the P53 Signaling Pathway and Molecular Docking Studies. Phytomedicine 2018, 46, 57–68. [Google Scholar] [CrossRef]
- Rodenak-Kladniew, B.; Castro, A.; Stärkel, P.; Galle, M.; Crespo, R. 1,8-Cineole Promotes G0/G1 Cell Cycle Arrest and Oxidative Stress-Induced Senescence in HepG2 Cells and Sensitizes Cells to Anti-Senescence Drugs. Life Sci. 2020, 243, 117271. [Google Scholar] [CrossRef]
- Yang, H.; Woo, J.; Pae, A.N.; Um, M.Y.; Cho, N.-C.; Park, K.D.; Yoon, M.; Kim, J.; Lee, C.J.; Cho, S. α-Pinene, a Major Constituent of Pine Tree Oils, Enhances Non-Rapid Eye Movement Sleep in Mice through GABAA-Benzodiazepine Receptors. Mol. Pharm. 2016, 90, 530–539. [Google Scholar] [CrossRef]
- Albuquerque, M.R.J.R.; Costa, S.M.O.; Bandeira, P.N.; Santiago, G.M.P.; Andrade-Neto, M.; Silveira, E.R.; Pessoa, O.D.L. Nematicidal and Larvicidal Activities of the Essential Oils from Aerial Parts of Pectis Oligocephala and Pectis Apodocephala Baker. An. Da Acad. Bras. De Ciências 2007, 79, 209–213. [Google Scholar] [CrossRef]
- Rivas da Silva, A.C.; Lopes, P.M.; Barros de Azevedo, M.M.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological Activities of α-Pinene and β-Pinene Enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef]
- Kim, M.; Sowndhararajan, K.; Park, S.J.; Kim, S. Effect of Inhalation of Isomers, (+)-α-Pinene and (+)-β-Pinene on Human Electroencephalographic Activity According to Gender Difference. Eur. J. Integr. Med. 2018, 17, 33–39. [Google Scholar] [CrossRef]
- Benelli, G.; Govindarajan, M.; Rajeswary, M.; Vaseeharan, B.; Alyahya, S.A.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Maggi, F. Insecticidal Activity of Camphene, Zerumbone and α-Humulene from Cheilocostus Speciosus Rhizome Essential Oil against the Old-World Bollworm, Helicoverpa Armigera. Ecotoxicol. Environ. Saf. 2018, 148, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Okuniewski, M.; Paduszyński, K.; Domańska, U. Thermodynamic Study of Molecular Interactions in Eutectic Mixtures Containing Camphene. J. Phys. Chem. B 2016, 120, 12928–12936. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, D.; Hu, J.; Jia, Q.; Xu, W.; Su, D.; Song, H.; Xu, Z.; Cui, J.; Zhou, M.; et al. A Clinical and Mechanistic Study of Topical Borneol-induced Analgesia. Embo Mol. Med. 2017, 9, 802–815. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Ryu, A.-R.; Jin, S.; Jeon, Y.-M.; Lee, M.-Y. Chlorin E6-Mediated Photodynamic Therapy Suppresses P. Acnes-Induced Inflammatory Response via NFκB and MAPKs Signaling Pathway. PLoS ONE 2017, 12, e0170599. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, R.; Li, H.; Zhu, J.; Pan, Y.; Guo, Q. Analgesic and Anti-Inflammatory Effects and Mechanism of Action of Borneol on Photodynamic Therapy of Acne. Environ. Toxicol. Pharmacol. 2020, 75, 103329. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, Z.; Xiong, Y.; Huang, X.; Mei, Z.; Hong, Z. Anti-Inflammatory and Blood Stasis Activities of Essential Oil Extracted from Artemisia Argyi Leaf in Animals. J. Nat. Med. 2016, 70, 531–538. [Google Scholar] [CrossRef]
- Chen, X.; Lin, Z.; Liu, A.; Ye, J.; Luo, Y.; Luo, Y.; Mao, X.; Liu, P.; Pi, R. The Orally Combined Neuroprotective Effects of Sodium Ferulate and Borneol against Transient Global Ischaemia in C57 BL/6J Mice. J. Pharm. Pharmacol. 2010, 62, 915–923. [Google Scholar] [CrossRef]
- Ho, D.-D.; Lau, C.-P.; Ng, K.-H.; Kong, Y.-C.; Cheng, K.-F.; Chan, K.-P. Anti-Implantation Activity of S(−)- and R(+)-Camphor-Yuehchukene in Rats. Eur. J. Pharmacol. 1991, 205, 209–212. [Google Scholar] [CrossRef]
- Ng, P.C.; Ho, D.D.; Ng, K.H.; Kong, Y.C.; Cheng, K.F.; Stone, G. Mixed Estrogenic and Anti-Estrogenic Activities of Yuehchukene--a Bis-Indole Alkaloid. Eur. J. Pharmacol. 1994, 264, 1–12. [Google Scholar] [CrossRef]
- Chatterjie, N.; Alexander, G.J. Anticonvulsant Properties of Spirohydantoins Derived from Optical Isomers of Camphor. Neurochem. Res. 1986, 11, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Laude, E.A.; Morice, A.H.; Grattan, T.J. The Antitussive Effects of Menthol, Camphor and Cineole in Conscious Guinea-Pigs. Pulm. Pharmacol. 1994, 7, 179–184. [Google Scholar] [CrossRef]
- Tinwell, H.; Lefevre Paul, A.; Moffat Graeme, J.; Burns, A.; Odum, J.; Spurway, T.D.; Orphanides, G.; Ashby, J. Confirmation of Uterotrophic Activity of 3-(4-Methylbenzylidine)Camphor in the Immature Rat. Environ. Health Perspect. 2002, 110, 533–536. [Google Scholar] [CrossRef]
- Park, T.J.; Seo, H.K.; Kang, B.J.; Kim, K.T. Noncompetitive Inhibition by Camphor of Nicotinic Acetylcholine Receptors. Biochem. Pharmacol. 2001, 61, 787–793. [Google Scholar] [CrossRef]
- Schlumpf, M.; Cotton, B.; Conscience, M.; Haller, V.; Steinmann, B.; Lichtensteiger, W. In Vitro and in Vivo Estrogenicity of UV Screens. Environ. Health Perspect 2001, 109, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Holbech, H.; Nørum, U.; Korsgaard, B.; Poul, B. The Chemical UV-Filter 3-Benzylidene Camphor Causes an Oestrogenic Effect in an in Vivo Fish Assay. Pharmacol. Toxicol. 2002, 91, 204–208. [Google Scholar] [CrossRef]
- Mueller, S.O.; Kling, M.; Arifin Firzani, P.; Mecky, A.; Duranti, E.; Shields-Botella, J.; Delansorne, R.; Broschard, T.; Kramer, P.-J. Activation of Estrogen Receptor α and ERβ by 4-Methylbenzylidene-Camphor in Human and Rat Cells: Comparison with Phyto- and Xenoestrogens. Toxicol. Lett. 2003, 142, 89–101. [Google Scholar] [CrossRef]
- Arakaki, N.; Shimoji, Y.; Wakamura, S. Camphor: An Attractant for the Cupreous Polished Chafer, Protaetia Pryeri Pryeri (Janson) (Coleoptera: Scarabaeidae). Appl. Entomol. Zool. 2009, 44, 621–625. [Google Scholar] [CrossRef][Green Version]
- Zhao, M.; Du, J. Anti-Inflammatory and Protective Effects of D-Carvone on Lipopolysaccharide (LPS)-Induced Acute Lung Injury in Mice. J. King Saud Univ.-Sci. 2020, 32, 1592–1596. [Google Scholar] [CrossRef]
- Vinothkumar, R.; Sudha, M.; Viswanathan, P.; Kabalimoorthy, J.; Balasubramanian, T.; Nalini, N. Modulating Effect of D-Carvone on 1,2-Dimethylhydrazine-Induced Pre-Neoplastic Lesions, Oxidative Stress and Biotransforming Enzymes, in an Experimental Model of Rat Colon Carcinogenesis. Cell Prolif. 2013, 46, 705–720. [Google Scholar] [CrossRef]
- Moro, I.J.; Gondo, G.D.G.A.; Pierri, E.G.; Pietro, R.C.L.R.; Soares, C.P.; de Sousa, D.P.; Santos, A.G. Evaluation of Antimicrobial, Cytotoxic and Chemopreventive Activities of Carvone and Its Derivatives. Braz. J. Pharm. Sci. 2017, 53. [Google Scholar] [CrossRef]
- De Cássia da Silveira e Sá, R.; Andrade, L.N.; de Sousa, D.P. A Review on Anti-Inflammatory Activity of Monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef] [PubMed]
- Bier, M.C.J.; Medeiros, A.B.P.; De Kimpe, N.; Soccol, C.R. Evaluation of Antioxidant Activity of the Fermented Product from the Biotransformation of R-(+)-Limonene in Solid-State Fermentation of Orange Waste by Diaporthe Sp. Biotechnol. Res. Innov. 2019, 3, 168–176. [Google Scholar] [CrossRef]
- Bacanlı, M.; Başaran, A.A.; Başaran, N. The Antioxidant and Antigenotoxic Properties of Citrus Phenolics Limonene and Naringin. Food Chem. Toxicol. 2015, 81, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Chidambara Murthy, K.N.; Jayaprakasha, G.K.; Patil, B.S. D-Limonene Rich Volatile Oil from Blood Oranges Inhibits Angiogenesis, Metastasis and Cell Death in Human Colon Cancer Cells. Life Sci. 2012, 91, 429–439. [Google Scholar] [CrossRef]
- Quiroga, P.R.; Nepote, V.; Baumgartner, M.T. Contribution of Organic Acids to α-Terpinene Antioxidant Activity. Food Chem. 2019, 277, 267–272. [Google Scholar] [CrossRef]
- Bejeshk, M.A.; Samareh Fekri, M.; Najafipour, H.; Rostamzadeh, F.; Jafari, E.; Rajizadeh, M.A.; Masoumi-Ardakani, Y. Anti-Inflammatory and Anti-Remodeling Effects of Myrtenol in the Lungs of Asthmatic Rats: Histopathological and Biochemical Findings. Allergol. Et Immunopathol. 2019, 47, 185–193. [Google Scholar] [CrossRef]
- Sepici, A.; Gürbüz, I.; Çevik, C.; Yesilada, E. Hypoglycaemic Effects of Myrtle Oil in Normal and Alloxan-Diabetic Rabbits. J. Ethnopharmacol. 2004, 93, 311–318. [Google Scholar] [CrossRef]
- Aleksic, V.; Knezevic, P. Antimicrobial and Antioxidative Activity of Extracts and Essential Oils of Myrtus Communis L. Microbiol. Res. 2014, 169, 240–254. [Google Scholar] [CrossRef]
- Clark, A.M. Natural Products as a Resource for New Drugs. Pharm. Res. 1996, 13, 1133–1144. [Google Scholar] [CrossRef]
- Fu, C.; Yu, P.; Wang, M.; Qiu, F. Phytochemical Analysis and Geographic Assessment of Flavonoids, Coumarins and Sesquiterpenes in Artemisia Annua L. Based on HPLC-DAD Quantification and LC-ESI-QTOF-MS/MS Confirmation. Food Chem. 2020, 312, 126070. [Google Scholar] [CrossRef]
- Dandan, Z.; Jianjiang, Z. Two Cytotoxic Sesquiterpenes from Hairy Root Cultures of Artemisia Annua L. Induced Apoptosis of Highly Metastatic Lung Carcinoma Cell Line 95-D. J. Biosci. Bioeng. 2009, 108, S24–S25. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.-M.; Shan, F.; Wu, G.-S.; Ding, J.; Xiao, D.; Han, J.-X.; Atassi, G.; Leonce, S.; Caignard, D.-H.; et al. Synthesis and Cytotoxicity of Dihydroartemisinin Ethers Containing Cyanoarylmethyl Group. Bioorg. Med. Chem. 2003, 11, 977–984. [Google Scholar] [CrossRef]
- Nam, W.; Tak, J.; Ryu, J.-K.; Jung, M.; Yook, J.-I.; Kim, H.-J.; Cha, I.-H. Effects of Artemisinin and Its Derivatives on Growth Inhibition and Apoptosis of Oral Cancer Cells. Head Neck 2007, 29, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Zhai, D.-D.; Supaibulwatana, K.; Zhong, J.-J. Inhibition of Tumor Cell Proliferation and Induction of Apoptosis in Human Lung Carcinoma 95-D Cells by a New Sesquiterpene from Hairy Root Cultures of Artemisia Annua. Phytomedicine 2010, 17, 856–861. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, B.; Ashraf, U.; Zhang, H.; Cao, C.; Li, Q.; Chen, Z.; Imran, M.; Chen, H.; Cao, S.; et al. Artemisinin Inhibits the Replication of Flaviviruses by Promoting the Type I Interferon Production. Antivir. Res. 2020. [Google Scholar] [CrossRef]
- Wong, Y.K.; Xu, C.; Kalesh, K.A.; He, Y.; Lin, Q.; Wong, W.S.F.; Shen, H.-M.; Wang, J. Artemisinin as an Anticancer Drug: Recent Advances in Target Profiling and Mechanisms of Action. Med. Res. Rev. 2017, 37, 1492–1517. [Google Scholar] [CrossRef] [PubMed]
- Idowu, A.O.; Bhattacharyya, S.; Gradus, S.; Oyibo, W.; George, Z.; Black, C.; Igietseme, J.; Azenabor, A.A. Plasmodium Falciparum Treated with Artemisinin-Based Combined Therapy Exhibits Enhanced Mutation, Heightened Cortisol and TNF-α Induction. Int. J. Med. Sci. 2018, 15, 1449–1457. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; You, F.; Xue, J. Novel Use for Old Drugs: The Emerging Role of Artemisinin and Its Derivatives in Fibrosis. Pharmacol. Res. 2020. [Google Scholar] [CrossRef]
- Martino, E.; Tarantino, M.; Bergamini, M.; Castelluccio, V.; Coricello, A.; Falcicchio, M.; Lorusso, E.; Collina, S. Artemisinin and Its Derivatives; Ancient Tradition Inspiring the Latest Therapeutic Approaches against Malaria. Future Med. Chem. 2019, 11, 1443–1459. [Google Scholar] [CrossRef]
- Suberu, J.O.; Gorka, A.P.; Jacobs, L.; Roepe, P.D.; Sullivan, N.; Barker, G.C.; Lapkin, A.A. Anti-Plasmodial Polyvalent Interactions in Artemisia Annua L. Aqueous Extract--Possible Synergistic and Resistance Mechanisms. PLoS ONE 2013, 8, e80790. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Herrmann, F.; Tahrani, A.; Wink, M. Cytotoxic Activity of Secondary Metabolites Derived from Artemisia Annua L. towards Cancer Cells in Comparison to Its Designated Active Constituent Artemisinin. Phytomedicine 2011, 18, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.X.; Yang, L.; Li, Y.J.; Zhang, D.; Chen, Y.; Kostecká, P.; Kmoníèková, E.; Zídek, Z. Effects of Sesquiterpene, Flavonoid and Coumarin Types of Compounds from Artemisia Annua L. on Production of Mediators of Angiogenesis. Pharmacol. Rep. 2013, 65, 410–420. [Google Scholar] [CrossRef]
- Sharma, G.; Kapoor, H.; Chopra, M.; Kumar, K.; Agrawal, V. Strong Larvicidal Potential of Artemisia Annua Leaf Extract against Malaria (Anopheles Stephensi Liston) and Dengue (Aedes Aegypti L.) Vectors and Bioassay-Driven Isolation of the Marker Compounds. Parasitol. Res. 2014, 113, 197–209. [Google Scholar] [CrossRef]
- Flobinus, A.; Taudon, N.; Desbordes, M.; Labrosse, B.; Simon, F.; Mazeron, M.-C.; Schnepf, N. Stability and Antiviral Activity against Human Cytomegalovirus of Artemisinin Derivatives. J. Antimicrob. Chemother. 2014, 69, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, M.-H.; Lee, J.-H.; Jung, E.; Yoo, E.-S.; Park, D. Artemisinic Acid Is a Regulator of Adipocyte Differentiation and C/EBP δ Expression. J. Cell Biochem. 2012, 113, 2488–2499. [Google Scholar] [CrossRef]
- Efferth, T. From Ancient Herb to Modern Drug: Artemisia Annua and Artemisinin for Cancer Therapy. Semin. Cancer Biol. 2017, 46, 65–83. [Google Scholar] [CrossRef]
- Weathers, P.J.; Arsenault, P.R.; Covello, P.S.; McMickle, A.; Teoh, K.H.; Reed, D.W. Artemisinin Production in Artemisia Annua: Studies in Planta and Results of a Novel Delivery Method for Treating Malaria and Other Neglected Diseases. Phytochem. Rev. 2011, 10, 173–183. [Google Scholar] [CrossRef]
- Ferreira, J.F.S.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia Annua L. as Antioxidants and Their Potential Synergism with Artemisinin against Malaria and Cancer. Molecules 2010, 15, 3135–3170. [Google Scholar] [CrossRef]
- Han, J.; Ye, M.; Qiao, X.; Xu, M.; Wang, B.; Guo, D.-A. Characterization of Phenolic Compounds in the Chinese Herbal Drug Artemisia Annua by Liquid Chromatography Coupled to Electrospray Ionization Mass Spectrometry. J. Pharm. Biomed. Anal. 2008, 47, 516–525. [Google Scholar] [CrossRef]
- Lai, J.-P.; Lim, Y.H.; Su, J.; Shen, H.-M.; Ong, C.N. Identification and Characterization of Major Flavonoids and Caffeoylquinic Acids in Three Compositae Plants by LC/DAD-APCI/MS. J. Chromatogr. B 2007, 848, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.S.; Cavaco, T.; Brodelius, M. Phenolic Composition and Antioxidant Capacity of Six Artemisia Species. Ind. Crop. Prod. 2011, 33, 382–388. [Google Scholar] [CrossRef]
- Tsevegsuren, N.; Edrada, R.; Lin, W.; Ebel, R.; Torre, C.; Ortlepp, S.; Wray, V.; Proksch, P. Biologically Active Natural Products from Mongolian Medicinal Plants Scorzonera Divaricata and Scorzonera Pseudodivaricata. J. Nat. Prod. 2007, 70, 962–967. [Google Scholar] [CrossRef]
- Zidorn, C.; Petersen, B.O.; Udovičić, V.; Larsen, T.O.; Duus, J.Ø.; Rollinger, J.M.; Ongania, K.-H.; Ellmerer, E.P.; Stuppner, H. Podospermic Acid, 1,3,5-Tri-O-(7,8-Dihydrocaffeoyl)Quinic Acid from Podospermum Laciniatum (Asteraceae). Tetrahedron Lett. 2005, 46, 1291–1294. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Liu, X.; Wu, H.-R.; He, X.-F.; Bi, Y.-R.; Zhu, Y.; Liu, Z.-L. Radical Scavenging Activity and Cytotoxicity of Active Quinic Acid Derivatives from Scorzonera Divaricata Roots. Food Chem. 2013, 138, 2057–2063. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, S.E.; Prinsloo, G.; Heyman, H.M.; Oosthuizen, C.B.; Klimkait, T.; Meyer, J.J.M. Anti-HIV-1 Activity of Quinic Acid Isolated from Helichrysum Mimetes Using NMR-Based Metabolomics and Computational Analysis. S. Afr. J. Bot. 2019, 126, 328–339. [Google Scholar] [CrossRef]
- Zhang, J.-L.; Yan, R.-J.; Yu, N.; Zhang, X.; Chen, D.-J.; Wu, T.; Xin, J.-G. A New Caffeic Acid Tetramer from the Dracocephalum Moldavica L. Nat. Prod. Res. 2018, 32, 370–373. [Google Scholar] [CrossRef]
- Choi, H.G.; Tran, P.T.; Lee, J.-H.; Min, B.S.; Kim, J.A. Anti-Inflammatory Activity of Caffeic Acid Derivatives Isolated from the Roots of Salvia Miltiorrhiza Bunge. Arch. Pharm. Res. 2018, 41, 64–70. [Google Scholar] [CrossRef]
- Langland, J.; Jacobs, B.; Wagner, C.E.; Ruiz, G.; Cahill, T.M. Antiviral Activity of Metal Chelates of Caffeic Acid and Similar Compounds towards Herpes Simplex, VSV-Ebola Pseudotyped and Vaccinia Viruses. Antivir. Res. 2018, 160, 143–150. [Google Scholar] [CrossRef]
- Mishra, M.; Panta, R.; Miyares, M. Influence of Coffee and Its Components on Breast Cancer: A Review. Asian Pac. J. Trop. Dis. 2016, 6, 827–831. [Google Scholar] [CrossRef]
- Habtemariam, S. Protective Effects of Caffeic Acid and the Alzheimer’s Brain. Available online: http://www.eurekaselect.com/147781/article (accessed on 5 May 2020).
- Adisakwattana, S. Cinnamic Acid and Its Derivatives: Mechanisms for Prevention and Management of Diabetes and Its Complications. Nutrients 2017, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Murillo, A.G.; Fernandez, M.L. The Relevance of Dietary Polyphenols in Cardiovascular Protection. Curr. Pharm. Des. 2017, 23, 2444–2452. [Google Scholar] [CrossRef]
- Silva, T.; Oliveira, C.; Borges, F. Caffeic Acid Derivatives, Analogs and Applications: A Patent Review (2009–2013). Expert Opin. Ther. Pat. 2014, 24, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zhang, H.; Cai, X.; Fang, W.; Chai, D.; Wen, Y.; Chen, H.; Chu, F.; Zhang, Y. Luteolin Promotes Cell Apoptosis by Inducing Autophagy in Hepatocellular Carcinoma. Cell. Physiol. Biochem. 2017, 43, 1803–1812. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Tian, X.-H.; Yi, Y.-S.; Jiang, W.-S.; Zhou, Y.-J.; Cheng, W.-J. Luteolin-Induced Protection of H2O2-Induced Apoptosis in PC12 Cells and the Associated Pathway. Mol. Med. Rep. 2015, 12, 7699–7704. [Google Scholar] [CrossRef]
- Nunes, C.; Almeida, L.; Barbosa, R.M.; Laranjinha, J. Luteolin Suppresses the JAK/STAT Pathway in a Cellular Model of Intestinal Inflammation. Food Funct. 2017, 8, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.-H.; Yao, X.-L.; Zhang, Y.; Zhang, S.-F.; Hu, J.-C. Luteolin Could Improve Cognitive Dysfunction by Inhibiting Neuroinflammation. Neurochem. Res. 2018, 43, 806–820. [Google Scholar] [CrossRef]
- Wei, B.; Lin, Q.; Ji, Y.-G.; Zhao, Y.-C.; Ding, L.-N.; Zhou, W.-J.; Zhang, L.-H.; Gao, C.-Y.; Zhao, W. Luteolin Ameliorates Rat Myocardial Ischaemia-Reperfusion Injury through Activation of Peroxiredoxin II. Br. J. Pharmacol. 2018, 175, 3315–3332. [Google Scholar] [CrossRef]
- Jang, C.H.; Moon, N.; Oh, J.; Kim, J.-S. Luteolin Shifts Oxaliplatin-Induced Cell Cycle Arrest at G0/G1 to Apoptosis in HCT116 Human Colorectal Carcinoma Cells. Nutrients 2019, 11, 770. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and Anti-Inflammatory Activities of Quercetin and Its Derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Duarte, J.; Pérez-Vizcaíno, F.; Zarzuelo, A.; Jiménez, J.; Tamargo, J. Vasodilator Effects of Quercetin in Isolated Rat Vascular Smooth Muscle. Eur. J. Pharmacol. 1993, 239. [Google Scholar] [CrossRef]
- Luna-Vázquez, F.J.; Ibarra-Alvarado, C.; Rojas-Molina, A.; Rojas-Molina, I.; Zavala-Sánchez, M.Á. Vasodilator Compounds Derived from Plants and Their Mechanisms of Action. Molecules 2013, 18, 5814–5857. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Mittal, S.; Sak, K.; Singhal, P.; Tuli, H.S. Molecular Mechanisms of Action of Quercetin in Cancer: Recent Advances. Tumor Biol. 2016, 37, 12927–12939. [Google Scholar] [CrossRef]
- Ezzati, M.; Yousefi, B.; Velaei, K.; Safa, A. A Review on Anti-Cancer Properties of Quercetin in Breast Cancer. Life Sci. 2020, 248, 117463. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singh, V.; Shin, H.-S. Therapeutic Potential of Quercetin as a Cardiovascular Agent. Eur. J. Med. Chem. 2018, 155, 889–904. [Google Scholar] [CrossRef]
- Ferreira, C.G.T.; Campos, M.G.; Felix, D.M.; Santos, M.R.; de Carvalho, O.V.; Diaz, M.A.N.; Fietto, J.L.R.; Bressan, G.C.; Silva-Júnior, A.; de Almeida, M.R. Evaluation of the Antiviral Activities of Bacharis Dracunculifolia and Quercetin on Equid Herpesvirus 1 in a Murine Model. Res. Vet. Sci. 2018, 120, 70–77. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Suganya, S.N.; Sumathi, T. Effect of Rutin against a Mitochondrial Toxin, 3-Nitropropionicacid Induced Biochemical, Behavioral and Histological Alterations-a Pilot Study on Huntington’s Disease Model in Rats. Metab. Brain Dis. 2017, 32, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhabi, N.A.; Arasu, M.V.; Park, C.H.; Park, S.U. An Up-to-Date Review of Rutin and Its Biological and Pharmacological Activities. Excli. J. 2015, 14, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An Important Scaffold for Medicinal Chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Shukla, S.; Gupta, S. Plant Flavonoid Apigenin Inactivates Akt to Trigger Apoptosis in Human Prostate Cancer: An in Vitro and in Vivo Study. Carcinogenesis 2008, 29, 2210–2217. [Google Scholar] [CrossRef]
- Bao, Y.-Y.; Zhou, S.-H.; Fan, J.; Wang, Q.-Y. Anticancer Mechanism of Apigenin and the Implications of GLUT-1 Expression in Head and Neck Cancers. Future Oncol. 2013, 9, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Mandal, M. Oxidative Stress Triggered by Naturally Occurring Flavone Apigenin Results in Senescence and Chemotherapeutic Effect in Human Colorectal Cancer Cells. Redox Biol. 2015, 5, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Bian, M.; Zhang, Y.; Du, X.; Xu, J.; Cui, J.; Gu, J.; Zhu, W.; Zhang, T.; Chen, Y. Apigenin-7-Diglucuronide Protects Retinas against Bright Light-Induced Photoreceptor Degeneration through the Inhibition of Retinal Oxidative Stress and Inflammation. Brain Res. 2017, 1663, 141–150. [Google Scholar] [CrossRef]
- Shukla, S.; Gupta, S. Apigenin: A Promising Molecule for Cancer Prevention. Pharm Res. 2010, 27, 962–978. [Google Scholar] [CrossRef]
- Jamali-Raeufy, N.; Baluchnejadmojarad, T.; Roghani, M.; Keimasi, S.; Goudarzi, M. Isorhamnetin Exerts Neuroprotective Effects in STZ-Induced Diabetic Rats via Attenuation of Oxidative Stress, Inflammation and Apoptosis. J. Chem. Neuroanat. 2019, 102, 101709. [Google Scholar] [CrossRef]
- Yang, J.H.; Kim, S.C.; Shin, B.Y.; Jin, S.H.; Jo, M.J.; Jegal, K.H.; Kim, Y.W.; Lee, J.R.; Ku, S.K.; Cho, I.J.; et al. O-Methylated Flavonol Isorhamnetin Prevents Acute Inflammation through Blocking of NF-ΚB Activation. Food Chem. Toxicol. 2013, 59, 362–372. [Google Scholar] [CrossRef]
- Wu, Q.; Kroon, P.A.; Shao, H.; Needs, P.W.; Yang, X. Differential Effects of Quercetin and Two of Its Derivatives, Isorhamnetin and Isorhamnetin-3-Glucuronide, in Inhibiting the Proliferation of Human Breast-Cancer MCF-7 Cells. J. Agric. Food Chem. 2018, 66, 7181–7189. [Google Scholar] [CrossRef]
- Yang, J.H.; Shin, B.Y.; Han, J.Y.; Kim, M.G.; Wi, J.E.; Kim, Y.W.; Cho, I.J.; Kim, S.C.; Shin, S.M.; Ki, S.H. Isorhamnetin Protects against Oxidative Stress by Activating Nrf2 and Inducing the Expression of Its Target Genes. Toxicol. Appl. Pharmacol. 2014, 274, 293–301. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, S.; Lee, H.S.; Kim, B.-K.; Ohuchi, K.; Shin, K.H. Inhibitory Effects of Isorhamnetin-3-O-Beta-D-Glucoside from Salicornia Herbacea on Rat Lens Aldose Reductase and Sorbitol Accumulation in Streptozotocin-Induced Diabetic Rat Tissues. Biol. Pharm. Bull. 2005, 28, 916–918. [Google Scholar] [CrossRef] [PubMed]
- Jamali-Raoufi, N.; Keimasi, S.; Baluchnejadmojarad, T. Isorhamnetin Mitigates Learning and Memory Disturbances in Streptozotocin-Induced Diabetic Rats. J. Basic Clin. Pathophysiol. 2018, 6, 37–42. [Google Scholar] [CrossRef]
- Yang, E.-J.; Kim, G.-S.; Jun, M.; Song, K.-S. Kaempferol Attenuates the Glutamate-Induced Oxidative Stress in Mouse-Derived Hippocampal Neuronal HT22 Cells. Food Funct. 2014, 5, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Rengarajan, T.; Nandakumar, N.; Palaniswami, R.; Nishigaki, Y.; Nishigaki, I. Kaempferol, a Potential Cytostatic and Cure for Inflammatory Disorders. Eur. J. Med. Chem. 2014, 86, 103–112. [Google Scholar] [CrossRef]
- Dang, Q.; Song, W.; Xu, D.; Ma, Y.; Li, F.; Zeng, J.; Zhu, G.; Wang, X.; Chang, L.S.; He, D.; et al. Kaempferol Suppresses Bladder Cancer Tumor Growth by Inhibiting Cell Proliferation and Inducing Apoptosis. Mol. Carcinog. 2015, 54, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, A.; Motegi, S.; Fujiwara, C.; Yamazaki, S.; Inoue, Y.; Uchiyama, A.; Akai, R.; Iwawaki, T.; Ishikawa, O. Inhibitory Effect of Kaempferol on Skin Fibrosis in Systemic Sclerosis by the Suppression of Oxidative Stress. J. Dermatol. Sci. 2019, 96, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Sadasivam, K.; Kumaresan, R. Antioxidant Behavior of Mearnsetin and Myricetin Flavonoid Compounds—A DFT Study. Spectrochim. Acta Part A 2011, 79, 282–293. [Google Scholar] [CrossRef]
- De Souza, P.; Gasparotto, A.; Crestani, S.; Stefanello, M.É.A.; Marques, M.C.A.; da Silva-Santos, J.E.; Kassuya, C.A.L. Hypotensive Mechanism of the Extracts and Artemetin Isolated from Achillea Millefolium L. (Asteraceae) in Rats. Phytomedicine 2011, 18, 819–825. [Google Scholar] [CrossRef]
- Lee, D.; Kim, C.-E.; Park, S.-Y.; Kim, K.O.; Hiep, N.T.; Lee, D.; Jang, H.-J.; Lee, J.W.; Kang, K.S. Protective Effect of Artemisia Argyi and Its Flavonoid Constituents against Contrast-Induced Cytotoxicity by Iodixanol in LLC-PK1 Cells. Int. J. Mol. Sci. 2018, 19, 1387. [Google Scholar] [CrossRef]
- Hu, J.; Ma, W.; Li, N.; Wang, K.-J.; Hu, J.; Ma, W.; Li, N.; Wang, K.-J. Antioxidant and Anti-Inflammatory Flavonoids from the Flowers of Chuju, a Medical Cultivar of Chrysanthemum Morifolim Ramat. J. Mex. Chem. Soc. 2017, 61, 282–289. [Google Scholar] [CrossRef]
- Li, W.-X.; Cui, C.-B.; Cai, B.; Wang, H.-Y.; Yao, X.-S. Flavonoids from Vitex Trifolia L. Inhibit Cell Cycle Progression at G2/M Phase and Induce Apoptosis in Mammalian Cancer Cells. J. Asian Nat. Prod. Res. 2005, 7, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Langa, E.; Pardo, J.I.; Giménez-Rota, C.; González-Coloma, A.; Hernáiz, M.J.; Mainar, A.M. Supercritical Anti-Solvent Fractionation of Artemisia Absinthium L. Conventional Extracts: Tracking Artemetin and Casticin. J. Supercrit. Fluids 2019, 151, 15–23. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Wong, S.K.; Chan, H.T. Casticin from Vitex Species: A Short Review on Its Anticancer and Anti-Inflammatory Properties. J. Integr. Med. 2018, 16, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jung, K.-H.; Lee, H.; Park, S.; Choi, W.; Bae, H. Casticin, an Active Compound Isolated from Vitex Fructus, Ameliorates the Cigarette Smoke-Induced Acute Lung Inflammatory Response in a Murine Model. Int. Immunopharmacol. 2015, 28, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Liou, C.-J.; Len, W.-B.; Wu, S.-J.; Lin, C.-F.; Wu, X.-L.; Huang, W.-C. Casticin Inhibits COX-2 and INOS Expression via Suppression of NF-ΚB and MAPK Signaling in Lipopolysaccharide-Stimulated Mouse Macrophages. J. Ethnopharmacol. 2014, 158, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.-C.; Wang, Y.; Liu, Y.-P.; Zhang, R.-Q.; Li, X.; Su, W.-H.; Long, F.; Luo, X.-D.; Peng, T. Inhibition of Enterovirus 71 Replication by Chrysosplenetin and Penduletin. Eur. J. Pharm. Sci. 2011, 44, 392–398. [Google Scholar] [CrossRef]
- Messaili, S.; Colas, C.; Fougère, L.; Destandau, E. Combination of Molecular Network and Centrifugal Partition Chromatography Fractionation for Targeting and Identifying Artemisia Annua L. Antioxidant Compounds. J. Chromatogr. A 2020, 1615, 460785. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Gong, F.-Y.; Wu, X.-X.; Sun, Y.; Li, Y.-H.; Chen, T.; Xu, Q. Anti-Inflammatory and Immunosuppressive Effect of Flavones Isolated from Artemisia Vestita. J. Ethnopharmacol. 2008, 120. [Google Scholar] [CrossRef]
- Sheng, X.; Sun, Y.; Yin, Y.; Chen, T.; Xu, Q. Cirsilineol Inhibits Proliferation of Cancer Cells by Inducing Apoptosis via Mitochondrial Pathway. J. Pharm. Pharmacol. 2008, 60, 1523–1529. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, X.-X.; Yin, Y.; Gong, F.-Y.; Shen, Y.; Cai, T.-T.; Zhou, X.-B.; Wu, X.-F.; Xu, Q. Novel Immunomodulatory Properties of Cirsilineol through Selective Inhibition of IFN-γ Signaling in a Murine Model of Inflammatory Bowel Disease. Biochem. Pharmacol. 2010, 79, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, Y.; Stampoulis, P.; Banskota, A.H.; Awale, S.; Tran, K.Q.; Saiki, I.; Kadota, S. Constituents of the Vietnamese Medicinal Plant Orthosiphon Stamineus. Chem. Pharm. Bull. 2000, 48, 1711–1719. [Google Scholar] [CrossRef]
- Nagao, T.; Abe, F.; Kinjo, J.; Okabe, H. Antiproliferative Constituents in Plants 10. Flavones from the Leaves of Lantana Montevidensis Briq. and Consideration of Structure-Activity Relationship. Biol. Pharm. Bull. 2002, 25, 875–879. [Google Scholar] [CrossRef]
- Androutsopoulos, V.; Arroo, R.R.J.; Hall, J.F.; Surichan, S.; Potter, G.A. Antiproliferative and Cytostatic Effects of the Natural Product Eupatorin on MDA-MB-468 Human Breast Cancer Cells Due to CYP1-Mediated Metabolism. Breast Cancer Res. 2008, 10, R39. [Google Scholar] [CrossRef]
- Chen, Z.; Liao, L.; Zhang, Z.; Wu, L.; Wang, Z. Comparison of Active Constituents, Acute Toxicity, Anti-Nociceptive and Anti-Inflammatory Activities of Porana Sinensis Hemsl., Erycibe Obtusifolia Benth. and Erycibe Schmidtii Craib. J. Ethnopharmacol. 2013, 150, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Jang, S.I.; Kim, Y.-J.; Chung, H.-T.; Yun, Y.-G.; Kang, T.-H.; Jeong, O.-S.; Kim, Y.-C. Scopoletin Suppresses Pro-Inflammatory Cytokines and PGE2 from LPS-Stimulated Cell Line, RAW 264.7 Cells. Fitoterapia 2004, 75, 261–266. [Google Scholar] [CrossRef]
- Pan, R.; Dai, Y.; Gao, X.; Xia, Y. Scopolin Isolated from Erycibe Obtusifolia Benth Stems Suppresses Adjuvant-Induced Rat Arthritis by Inhibiting Inflammation and Angiogenesis. Int. Immunopharmacol. 2009, 9, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.-Y.; Chen, C.-H.; Hsu, C.-C.; Chen, C.-C.; Tsai, Y.-C. Antioxidant Properties of Scopoletin Isolated from Sinomonium Acutum. Phytother. Res. 2003, 17, 823–825. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.-D.; Lee, B.-H.; Jeong, H.-J.; An, H.-J.; Park, S.-J.; Kim, H.-R.; Ko, S.-G.; Um, J.-Y.; Hong, S.-H.; Kim, H.-M. Use of Scopoletin to Inhibit the Production of Inflammatory Cytokines through Inhibition of the IκB/NF-ΚB Signal Cascade in the Human Mast Cell Line HMC-1. Eur. J. Pharmacol. 2007, 555, 218–225. [Google Scholar] [CrossRef]
- Thabet, A.A.; Youssef, F.S.; Korinek, M.; Chang, F.-R.; Wu, Y.-C.; Chen, B.-H.; El-Shazly, M.; Singab, A.N.B.; Hwang, T.-L. Study of the Anti-Allergic and Anti-Inflammatory Activity of Brachychiton Rupestris and Brachychiton Discolor Leaves (Malvaceae) Using in Vitro Models. BMC Complement. Altern. Med. 2018, 18, 299. [Google Scholar] [CrossRef]
- Iqbal, S.; Younas, U.; Chan, K.W.; Zia-Ul-Haq, M.; Ismail, M. Chemical Composition of Artemisia Annua L. Leaves and Antioxidant Potential of Extracts as a Function of Extraction Solvents. Molecules 2012, 17, 6020–6032. [Google Scholar] [CrossRef]
- Winkelman, M. Ethnobotanical Treatments of Diabetes in Baja California Norte. Med. Anthr. 1989, 11, 255–268. [Google Scholar] [CrossRef]
- Helal, E.G.E.; Abou-Aouf, N.; Khattab, A.L.M.; Zoair, M.A. Anti-Diabetic Effect of Artemisia Annua (Kaysom) in Alloxan-Induced Diabetic Rats. EJHM 2014, 57, 422–430. [Google Scholar] [CrossRef][Green Version]
- Woerdenbag, H.J.; Pras, N.; Bos, R.; Visser, J.F.; Hendriks, H.; Malingré, T.M. Analysis of Artemisinin and Related Sesquiterpenoids from Artemisia Annua L. by Combined Gas Chromatography/Mass Spectrometry. Phytochem. Anal. 1991, 2, 215–219. [Google Scholar] [CrossRef]
- Wang, H.; Ma, C.; Ma, L.; Du, Z.; Wang, H.; Ye, H.; Li, G.; Liu, B.; Xu, G. Secondary Metabolic Profiling and Artemisinin Biosynthesis of Two Genotypes of Artemisia Annua. Planta Med. 2009, 75, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Taleghani, A.; Emami, S.A.; Tayarani-Najaran, Z. Artemisia: A Promising Plant for the Treatment of Cancer. Bioorg. Med. Chem. 2020, 28, 115180. [Google Scholar] [CrossRef]
- Huo, J.; Lu, Y.; Xia, L.; Chen, D. Structural Characterization and Anticomplement Activities of Three Acidic Homogeneous Polysaccharides from Artemisia Annua. J. Ethnopharmacol. 2020, 247, 112281. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Olbrich, A.; Bauer, R. MRNA Expression Profiles for the Response of Human Tumor Cell Lines to the Antimalarial Drugs Artesunate, Arteether, and Artemether. Biochem. Pharmacol. 2002, 64, 617–623. [Google Scholar] [CrossRef]
- Efferth, T.; Sauerbrey, A.; Olbrich, A.; Gebhart, E.; Rauch, P.; Weber, H.O.; Hengstler, J.G.; Halatsch, M.-E.; Volm, M.; Tew, K.D.; et al. Molecular Modes of Action of Artesunate in Tumor Cell Lines. Mol. Pharm. 2003, 64, 382–394. [Google Scholar] [CrossRef]
- Efferth, T.; Oesch, F. Oxidative Stress Response of Tumor Cells: Microarray-Based Comparison between Artemisinins and Anthracyclines. Biochem. Pharmacol. 2004, 68, 3–10. [Google Scholar] [CrossRef]
- Efferth, T.; Volm, M. Glutathione-Related Enzymes Contribute to Resistance of Tumor Cells and Low Toxicity in Normal Organs to Artesunate. Vivo 2005, 19, 225–232. [Google Scholar]
- Efferth, T.; Giaisi, M.; Merling, A.; Krammer, P.H.; Li-Weber, M. Artesunate Induces ROS-Mediated Apoptosis in Doxorubicin-Resistant T Leukemia Cells. PLoS ONE 2007, 2, e693. [Google Scholar] [CrossRef] [PubMed]
- Sieber, S.; Gdynia, G.; Roth, W.; Bonavida, B.; Efferth, T. Combination Treatment of Malignant B Cells Using the Anti-CD20 Antibody Rituximab and the Anti-Malarial Artesunate. Int. J. Oncol. 2009, 35, 149–158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ooko, E.; Saeed, M.E.M.; Kadioglu, O.; Sarvi, S.; Colak, M.; Elmasaoudi, K.; Janah, R.; Greten, H.J.; Efferth, T. Artemisinin Derivatives Induce Iron-Dependent Cell Death (Ferroptosis) in Tumor Cells. Phytomedicine 2015, 22, 1045–1054. [Google Scholar] [CrossRef]
- Lin, R.; Zhang, Z.; Chen, L.; Zhou, Y.; Zou, P.; Feng, C.; Wang, L.; Liang, G. Dihydroartemisinin (DHA) Induces Ferroptosis and Causes Cell Cycle Arrest in Head and Neck Carcinoma Cells. Cancer Lett. 2016, 381, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Wojtkowiak-Giera, A.; Derda, M.; Kosik-Bogacka, D.; Kolasa-Wołosiuk, A.; Wandurska-Nowak, E.; Jagodziński, P.P.; Hadaś, E. The Modulatory Effect of Artemisia Annua L. on Toll-like Receptor Expression in Acanthamoeba Infected Mouse Lungs. Exp. Parasitol. 2019, 199, 24–29. [Google Scholar] [CrossRef]
- Yao, W.; Wang, F.; Wang, H. Immunomodulation of Artemisinin and Its Derivatives. Sci. Bull. 2016, 61, 1399–1406. [Google Scholar] [CrossRef]
- Wojtkowiak-Giera, A.; Derda, M.; Kosik-Bogacka, D.; Kolasa-Wołosiuk, A.; Solarczyk, P.; Cholewiński, M.; Wandurska-Nowak, E.; Jagodziński, P.P.; Hadaś, E. Influence of Artemisia Annua L. on Toll-like Receptor Expression in Brain of Mice Infected with Acanthamoeba Sp. Exp. Parasitol. 2018, 185, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The Roles of TLRs, RLRs and NLRs in Pathogen Recognition. Int. Immunol. 2009, 21, 317–337. [Google Scholar] [CrossRef]
- Cario, E.; Brown, D.; McKee, M.; Lynch-Devaney, K.; Gerken, G.; Podolsky, D.K. Commensal-Associated Molecular Patterns Induce Selective Toll-Like Receptor-Trafficking from Apical Membrane to Cytoplasmic Compartments in Polarized Intestinal Epithelium. Am. J. Pathol. 2002, 160, 165–173. [Google Scholar] [CrossRef]
- Li, B.; Li, J.; Pan, X.; Ding, G.; Cao, H.; Jiang, W.; Zheng, J.; Zhou, H. Artesunate Protects Sepsis Model Mice Challenged with Staphylococcus Aureus by Decreasing TNF-α Release via Inhibition TLR2 and Nod2 MRNA Expressions and Transcription Factor NF-ΚB Activation. Int. Immunopharmacol. 2010, 10, 344–350. [Google Scholar] [CrossRef]
- Huang, X.; Xie, Z.; Liu, F.; Han, C.; Zhang, D.; Wang, D.; Bao, X.; Sun, J.; Wen, C.; Fan, Y. Dihydroartemisinin Inhibits Activation of the Toll-like Receptor 4 Signaling Pathway and Production of Type I Interferon in Spleen Cells from Lupus-Prone MRL/Lpr Mice. Int. Immunopharmacol. 2014, 22, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Saadat, F.; Paola, R.D.; Mirshafiey, A. Artemether: A New Therapeutic Strategy in Experimental Rheumatoid Arthritis. Immunopharmacol. Immunotoxicol. 2005, 27, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Mirshafiey, A.; Saadat, F.; Attar, M.; Paola, R.D.; Sedaghat, R.; Cuzzocrea, S. Design of a New Line in Treatment of Experimental Rheumatoid Arthritis by Artesunate. Immunopharmacol. Immunotoxicol. 2006, 28, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-X.; Tang, W.; Zhou, R.; Wan, J.; Shi, L.-P.; Zhang, Y.; Yang, Y.-F.; Li, Y.; Zuo, J.-P. The New Water-Soluble Artemisinin Derivative SM905 Ameliorates Collagen-Induced Arthritis by Suppression of Inflammatory and Th17 Responses. Br. J. Pharmacol. 2008, 153, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, S.; Wang, Y.; Zhou, C.; Chen, G.; Shen, W.; Li, C.; Lin, W.; Lin, S.; Huang, H.; et al. Inhibitory Effect of the Antimalarial Agent Artesunate on Collagen-Induced Arthritis in Rats through Nuclear Factor Kappa B and Mitogen-Activated Protein Kinase Signaling Pathway. Transl. Res. 2013, 161, 89–98. [Google Scholar] [CrossRef]
- Xu, H.; He, Y.; Yang, X.; Liang, L.; Zhan, Z.; Ye, Y.; Yang, X.; Lian, F.; Sun, L. Anti-Malarial Agent Artesunate Inhibits TNF-α-Induced Production of Proinflammatory Cytokines via Inhibition of NF-ΚB and PI3 Kinase/Akt Signal Pathway in Human Rheumatoid Arthritis Fibroblast-like Synoviocytes. Rheumatol. Oxf. 2007, 46, 920–926. [Google Scholar] [CrossRef]
- He, Y.; Fan, J.; Lin, H.; Yang, X.; Ye, Y.; Liang, L.; Zhan, Z.; Dong, X.; Sun, L.; Xu, H. The Anti-Malaria Agent Artesunate Inhibits Expression of Vascular Endothelial Growth Factor and Hypoxia-Inducible Factor-1α in Human Rheumatoid Arthritis Fibroblast-like Synoviocyte. Rheumatol. Int. 2011, 31, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Jin, O.; Zhang, H.; Gu, Z.; Zhao, S.; Xu, T.; Zhou, K.; Jiang, B.; Wang, J.; Zeng, X.; Sun, L. A Pilot Study of the Therapeutic Efficacy and Mechanism of Artesunate in the MRL/Lpr Murine Model of Systemic Lupus Erythematosus. Cell. Mol. Immunol. 2009, 6, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.-F.; He, S.-J.; Li, X.; Wan, C.-P.; Yang, Y.; Zhang, X.-H.; He, P.-L.; Zhou, Y.; Zhu, F.-H.; Yang, Y.-F.; et al. SM934 Treated Lupus-Prone NZB×NZW F1 Mice by Enhancing Macrophage Interleukin-10 Production and Suppressing Pathogenic T Cell Development. PLoS ONE 2012, 7, e32424. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.-F.; He, S.-J.; Li, X.; Yang, Y.; He, P.-L.; Zhou, Y.; Zhu, F.-H.; Yang, Y.-F.; Li, Y.; Tang, W.; et al. Oral Administration of Artemisinin Analog SM934 Ameliorates Lupus Syndromes in MRL/Lpr Mice by Inhibiting Th1 and Th17 Cell Responses. Arthritis Rheum. 2011, 63, 2445–2455. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Dong, Y.; Tu, Y.; Lin, Z. Dihydroarteannuin Ameliorates Lupus Symptom of BXSB Mice by Inhibiting Production of TNF-Alpha and Blocking the Signaling Pathway NF-Kappa B Translocation. Int. Immunopharmacol. 2006, 6, 1243–1250. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, W.; Shi, X.; An, P.; Sun, W.; Wang, Z. Therapeutic Effect of Artemisinin on Lupus Nephritis Mice and Its Mechanisms. Acta Biochim. Biophys. Sin. 2010, 42, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, W.; Shi, X.; An, P.; Sun, W.; Qiao, C.; Wang, Z. Effect of Artemisinin Combined with Glucocorticoid on the Expressions of Glucocorticoid Receptor α MRNA, Glucocorticoid Receptor β MRNA and P300/CBP Protein in Lupus Nephritis Mice. Chin. J. Integr. Med. 2011, 17, 277–282. [Google Scholar] [CrossRef]
- Yang, Z.; Ding, J.; Yang, C.; Gao, Y.; Li, X.; Chen, X.; Peng, Y.; Fang, J.; Xiao, S. Immunomodulatory and Anti-Inflammatory Properties of Artesunate in Experimental Colitis. Curr. Med. Chem. 2012, 19, 4541–4551. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, H.; Wei, N.; Mei, X.; Zhang, S.; Liu, D.; Gao, Y.; Bai, S.; Liu, X.; Zhou, Y. Anti-Inflammatory and Immunomodulatory Mechanisms of Artemisinin on Contact Hypersensitivity. Int. Immunopharmacol. 2012, 12, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Ng, D.S.W.; Chan, T.K.; Guan, S.P.; Ho, W.E.; Koh, A.H.M.; Bian, J.S.; Lau, H.Y.A.; Wong, W.S.F. Anti-Allergic Action of Anti-Malarial Drug Artesunate in Experimental Mast Cell-Mediated Anaphylactic Models. Allergy 2013, 68, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Ho, W.E.; Goh, F.Y.; Guan, S.P.; Kong, L.R.; Lai, W.-Q.; Leung, B.P.; Wong, W.S.F. Anti-Malarial Drug Artesunate Attenuates Experimental Allergic Asthma via Inhibition of the Phosphoinositide 3-Kinase/Akt Pathway. PLoS ONE 2011, 6, e20932. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.E.; Cheng, C.; Peh, H.Y.; Xu, F.; Tannenbaum, S.R.; Ong, C.N.; Wong, W.S.F. Anti-Malarial Drug Artesunate Ameliorates Oxidative Lung Damage in Experimental Allergic Asthma. Free Radic. Biol. Med. 2012, 53, 498–507. [Google Scholar] [CrossRef]
- Dube, S.K.; Panda, P.S.; Agrawal, G.R.; Singh, D.K. Anaphylaxis to Artesunate? Indian J. Crit. Care Med. 2012, 16, 55–57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shi, J.-Q.; Zhang, C.-C.; Sun, X.-L.; Cheng, X.-X.; Wang, J.-B.; Zhang, Y.-D.; Xu, J.; Zou, H.-Q. Antimalarial Drug Artemisinin Extenuates Amyloidogenesis and Neuroinflammation in APPswe/PS1dE9 Transgenic Mice via Inhibition of Nuclear Factor-ΚB and NLRP3 Inflammasome Activation. Cns. Neurosci. Ther. 2013, 19, 262–268. [Google Scholar] [CrossRef]
- Wang, S.-J.; Sun, B.; Cheng, Z.-X.; Zhou, H.-X.; Gao, Y.; Kong, R.; Chen, H.; Jiang, H.-C.; Pan, S.-H.; Xue, D.-B.; et al. Dihydroartemisinin Inhibits Angiogenesis in Pancreatic Cancer by Targeting the NF-ΚB Pathway. Cancer Chemother. Pharm. 2011, 68, 1421–1430. [Google Scholar] [CrossRef]
- Bilia, A.R.; Santomauro, F.; Sacco, C.; Bergonzi, M.C.; Donato, R. Essential Oil of Artemisia Annua L.: An Extraordinary Component with Numerous Antimicrobial Properties. Evid. Based Complement. Altern. Med. 2014, 2014, 159819. [Google Scholar] [CrossRef]
- Stojanović, N.M.; Randjelović, P.J.; Mladenović, M.Z.; Ilić, I.R.; Petrović, V.; Stojiljković, N.; Ilić, S.; Radulović, N.S. Toxic Essential Oils, Part VI: Acute Oral Toxicity of Lemon Balm (Melissa Officinalis L.) Essential Oil in BALB/c Mice. Food Chem. Toxicol. 2019, 133, 110794. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, H.; Zheng, X.; Zhu, J.; Liu, L. Composition and Antimicrobial Activity of Essential Oil from the Aerial Part of Artemisia Annua. JMPR 2011, 5, 3629–3633. [Google Scholar]
- Juteau, F.; Masotti, V.; Bessière, J.M.; Dherbomez, M.; Viano, J. Antibacterial and Antioxidant Activities of Artemisia Annua Essential Oil. Fitoterapia 2002, 73, 532–535. [Google Scholar] [CrossRef]
- Verdian-rizi, M.R. Chemical Composition and Antimicrobial Activity of the Essential Oil of Artemisia Annua L. from Iran. Pharmacogn. Res. 2009, 1, 21. [Google Scholar]
- Massiha, A.; Khoshkholgh-Pahlaviani, M.M.; Issazadeh, K.; Bidarigh, S.; Zarrabi, S. Antibacterial Activity of Essential Oils and Plant Extracts of Artemisia (Artemisia Annua L.) in Vitro. Zahedan J. Res. Med. Sci. 2013, 15, 14–18. [Google Scholar]
- Viuda-Martos, M.; El Gendy, A.E.-N.G.S.; Sendra, E.; Fernández-López, J.; Abd El Razik, K.A.; Omer, E.A.; Pérez-Alvarez, J.A. Chemical Composition and Antioxidant and Anti-Listeria Activities of Essential Oils Obtained from Some Egyptian Plants. J. Agric. Food Chem. 2010, 58, 9063–9070. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.C.T.; Leme, E.E.; Delarmelina, C.; Soares, A.A.; Figueira, G.M.; Sartoratto, A. Activity of Essential Oils from Brazilian Medicinal Plants on Escherichia Coli. J. Ethnopharmacol. 2007, 111, 197–201. [Google Scholar] [CrossRef]
- Soylu, E.M.; Yiğitbaş, H.; Tok, F.M.; Soylu, S.; Kurt, Ş.; Baysal, Ö.; Kaya, A.D. Chemical Composition and Antifungal Activity of the Essential Oil of Artemisia Annua L. against Foliar and Soil-Borne Fungal Pathogens/Die Chemische Zusammensetzung Und Antimikrobielle Aktivität Das Ätherischen Öls von Artemisia Annua L. Gegen Blatt- Und Bodenbürtige Pilzliche Krankheitserreger. Z. Pflanzenkrankh. Pflanzenschutz/J. Plant Dis. Prot. 2005, 112, 229–239. [Google Scholar]
- Efferth, T. Beyond Malaria: The Inhibition of Viruses by Artemisinin-Type Compounds. Biotechnol. Adv. 2018, 36, 1730–1737. [Google Scholar] [CrossRef]
- Chang, Y.-S.; Woo, E.-R. Korean Medicinal Plants Inhibiting to Human Immunodeficiency Virus Type 1 (HIV-1) Fusion. Phytother. Res. 2003, 17, 426–429. [Google Scholar] [CrossRef]
- Oguariri, R.M.; Adelsberger, J.W.; Baseler, M.W.; Imamichi, T. Evaluation of the Effect of Pyrimethamine, an Anti-Malarial Drug, on HIV-1 Replication. Virus Res. 2010, 153, 269–276. [Google Scholar] [CrossRef]
- Jana, S.; Iram, S.; Thomas, J.; Hayat, M.Q.; Pannecouque, C.; Dehaen, W. Application of the Triazolization Reaction to Afford Dihydroartemisinin Derivatives with Anti-HIV Activity. Molecules 2017, 22, 303. [Google Scholar] [CrossRef]
- Efferth, T.; Marschall, M.; Wang, X.; Huong, S.-M.; Hauber, I.; Olbrich, A.; Kronschnabl, M.; Stamminger, T.; Huang, E.-S. Antiviral Activity of Artesunate towards Wild-Type, Recombinant, and Ganciclovir-Resistant Human Cytomegaloviruses. J. Mol. Med. 2002, 80, 233–242. [Google Scholar] [CrossRef]
- Efferth, T.; Romero, M.R.; Wolf, D.G.; Stamminger, T.; Marin, J.J.G.; Marschall, M. The Antiviral Activities of Artemisinin and Artesunate. Clin. Infect. Dis. 2008, 47, 804–811. [Google Scholar] [CrossRef]
- Milbradt, J.; Auerochs, S.; Korn, K.; Marschall, M. Sensitivity of Human Herpesvirus 6 and Other Human Herpesviruses to the Broad-Spectrum Antiinfective Drug Artesunate. J. Clin. Virol. 2009, 46, 24–28. [Google Scholar] [CrossRef]
- Arav-Boger, R.; He, R.; Chiou, C.-J.; Liu, J.; Woodard, L.; Rosenthal, A.; Jones-Brando, L.; Forman, M.; Posner, G. Artemisinin-Derived Dimers Have Greatly Improved Anti-Cytomegalovirus Activity Compared to Artemisinin Monomers. PLoS ONE 2010, 5, e10370. [Google Scholar] [CrossRef]
- He, R.; Mott, B.T.; Rosenthal, A.S.; Genna, D.T.; Posner, G.H.; Arav-Boger, R. An Artemisinin-Derived Dimer Has Highly Potent Anti-Cytomegalovirus (CMV) and Anti-Cancer Activities. PLoS ONE 2011, 6, e24334. [Google Scholar] [CrossRef]
- Reiter, C.; Fröhlich, T.; Zeino, M.; Marschall, M.; Bahsi, H.; Leidenberger, M.; Friedrich, O.; Kappes, B.; Hampel, F.; Efferth, T.; et al. New Efficient Artemisinin Derived Agents against Human Leukemia Cells, Human Cytomegalovirus and Plasmodium Falciparum: 2nd Generation 1,2,4-Trioxane-Ferrocene Hybrids. Eur. J. Med. Chem. 2015, 97, 164–172. [Google Scholar] [CrossRef]
- Hutterer, C.; Niemann, I.; Milbradt, J.; Fröhlich, T.; Reiter, C.; Kadioglu, O.; Bahsi, H.; Zeitträger, I.; Wagner, S.; Einsiedel, J.; et al. The Broad-Spectrum Antiinfective Drug Artesunate Interferes with the Canonical Nuclear Factor Kappa B (NF-ΚB) Pathway by Targeting RelA/P65. Antivir. Res. 2015, 124, 101–109. [Google Scholar] [CrossRef]
- Shapira, M.Y.; Resnick, I.B.; Chou, S.; Neumann, A.U.; Lurain, N.S.; Stamminger, T.; Caplan, O.; Saleh, N.; Efferth, T.; Marschall, M.; et al. Artesunate as a Potent Antiviral Agent in a Patient with Late Drug-Resistant Cytomegalovirus Infection after Hematopoietic Stem Cell Transplantation. Clin. Infect. Dis. 2008, 46, 1455–1457. [Google Scholar] [CrossRef] [PubMed]
- Naesens, L.; Bonnafous, P.; Agut, H.; De Clercq, E. Antiviral Activity of Diverse Classes of Broad-Acting Agents and Natural Compounds in HHV-6-Infected Lymphoblasts. J. Clin. Virol. 2006, 37, S69–S75. [Google Scholar] [CrossRef]
- Hakacova, N.; Klingel, K.; Kandolf, R.; Engdahl, E.; Fogdell-Hahn, A.; Higgins, T. First Therapeutic Use of Artesunate in Treatment of Human Herpesvirus 6B Myocarditis in a Child. J. Clin. Virol. 2013, 57, 157–160. [Google Scholar] [CrossRef]
- Qi, F.H.; Wang, Z.X.; Cai, P.P.; Zhao, L.; Gao, J.J.; Kokudo, N.; Li, A.Y.; Han, J.Q.; Tang, W. Traditional Chinese Medicine and Related Active Compounds: A Review of Their Role on Hepatitis B Virus Infection. Drug Discov. 2013, 7, 212–224. [Google Scholar] [CrossRef]
- Romero, M.R.; Efferth, T.; Serrano, M.A.; Castaño, B.; Macias, R.I.R.; Briz, O.; Marin, J.J.G. Effect of Artemisinin/Artesunate as Inhibitors of Hepatitis B Virus Production in an “in Vitro” Replicative System. Antivir. Res. 2005, 68, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Batty, K.T.; Davis, T.M.E.; Thu, L.T.A.; Quang Binh, T.; Kim Anh, T.; Ilett, K.F. Selective High-Performance Liquid Chromatographic Determination of Artesunate and α- and β-Dihydroartemisinin in Patients with Falciparum Malaria. J. Chromatogr. B 1996, 677, 345–350. [Google Scholar] [CrossRef]
- Sharma, B.N.; Marschall, M.; Henriksen, S.; Rinaldo, C.H. Antiviral Effects of Artesunate on Polyomavirus BK Replication in Primary Human Kidney Cells. Antimicrob. Agents Chemother. 2013. [Google Scholar] [CrossRef]
- Sharma, B.N.; Marschall, M.; Rinaldo, C.H. Antiviral Effects of Artesunate on JC Polyomavirus Replication in COS-7 Cells. Antimicrob. Agents Chemother. 2014, 58, 6724–6734. [Google Scholar] [CrossRef]
- Disbrow, G.L.; Baege, A.C.; Kierpiec, K.A.; Yuan, H.; Centeno, J.A.; Thibodeaux, C.A.; Hartmann, D.; Schlegel, R. Dihydroartemisinin Is Cytotoxic to Papillomavirus-Expressing Epithelial Cells In Vitro and In Vivo. Cancer Res. 2005, 65, 10854–10861. [Google Scholar] [CrossRef]
- Mondal, A.; Chatterji, U. Artemisinin Represses Telomerase Subunits and Induces Apoptosis in HPV-39 Infected Human Cervical Cancer Cells. J. Cell. Biochem. 2015, 116, 1968–1981. [Google Scholar] [CrossRef] [PubMed]
- Paeshuyse, J.; Coelmont, L.; Vliegen, I.; hemel, J.V.; Vandenkerckhove, J.; Peys, E.; Sas, B.; Clercq, E.D.; Neyts, J. Hemin Potentiates the Anti-Hepatitis C Virus Activity of the Antimalarial Drug Artemisinin. Biochem. Biophys. Res. Commun. 2006, 348, 139–144. [Google Scholar] [CrossRef]
- Obeid, S.; Alen, J.; Nguyen, V.H.; Pham, V.C.; Meuleman, P.; Pannecouque, C.; Le, T.N.; Neyts, J.; Dehaen, W.; Paeshuyse, J. Artemisinin Analogues as Potent Inhibitors of In Vitro Hepatitis C Virus Replication. PLoS ONE 2013, 8, e81783. [Google Scholar] [CrossRef]
- Dai, R.; Xiao, X.; Peng, F.; Li, M.; Gong, G. Artesunate, an Anti-Malarial Drug, Has a Potential to Inhibit HCV Replication. Virus Genes 2016, 52, 22–28. [Google Scholar] [CrossRef]
- Romero, M.R.; Serrano, M.A.; Vallejo, M.; Efferth, T.; Alvarez, M.; Marin, J.J.G. Antiviral Effect of Artemisinin from Artemisia Annua against a Model Member of the Flaviviridae Family, the Bovine Viral Diarrhoea Virus (BVDV). Planta Med. 2006, 72, 1169–1174. [Google Scholar] [CrossRef]
- Blazquez, A.G.; Fernandez-Dolon, M.; Sanchez-Vicente, L.; Maestre, A.D.; Miguel, A.B.G.; Alvarez, M.l.; Serrano, M.A.; Jansen, H.; Efferth, T.; Marin, J.J.G.; et al. Novel Artemisinin Derivatives with Potential Usefulness against Liver/Colon Cancer and Viral Hepatitis. Bioorg. Med. Chem. 2013, 21, 4432–4441. [Google Scholar] [CrossRef] [PubMed]
- Thaha, M.; Pranawa, N.; Yogiantoro, M.; Tanimoto, M.; Tomino, Y. Acute Renal Failure in a Patient with Severe Malaria and Dengue Shock Syndrome. Clin. Nephrol. 2008, 70, 427–430. [Google Scholar] [CrossRef]
- Munyangi, J.; Cornet-Vernet, L.; Idumbo, M.; Lu, C.; Lutgen, P.; Perronne, C.; Ngombe, N.; Bianga, J.; Mupenda, B.; Lalukala, P.; et al. Artemisia Annua and Artemisia Afra Tea Infusions vs. Artesunate-Amodiaquine (ASAQ) in Treating Plasmodium Falciparum Malaria in a Large Scale, Double Blind, Randomized Clinical Trial. Phytomedicine 2019, 57, 49–56. [Google Scholar] [CrossRef]
- Hsu, E. Reflections on the ‘Discovery’ of the Antimalarial Qinghao. Br. J. Clin. Pharm. 2006, 61, 666–670. [Google Scholar] [CrossRef]
- White, N.J. Qinghaosu (Artemisinin): The Price of Success. Science 2008, 320, 330–334. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the Treatment of Malaria; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- LoVerde, P.T. Digenetic Trematodes. In Schistosomiasis; Toledo, R., Fried, B., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 45–70. [Google Scholar] [CrossRef]
- Steinmann, P.; Keiser, J.; Bos, R.; Tanner, M.; Utzinger, J. Schistosomiasis and Water Resources Development: Systematic Review, Meta-Analysis, and Estimates of People at Risk. Lancet Infect. Dis. 2006, 6, 411–425. [Google Scholar] [CrossRef]
- Saeed, M.E.M.; Krishna, S.; Greten, H.J.; Kremsner, P.G.; Efferth, T. Antischistosomal Activity of Artemisinin Derivatives in Vivo and in Patients. Pharmacol. Res. 2016, 110, 216–226. [Google Scholar] [CrossRef]
- Shalaby, H.A.; Abdel-Shafy, S.; Abdel-Rahman, K.A.; Derbala, A.A. Comparative in Vitro Effect of Artemether and Albendazole on Adult Toxocara Canis. Parasitol. Res. 2009, 105, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Golenser, J.; Waknine, J.H.; Krugliak, M.; Hunt, N.H.; Ge, G. Current Perspectives on the Mechanism of Action of Artemisinins. Int. J. Parasitol. 2006, 36, 1427–1441. [Google Scholar] [CrossRef]
- Abou Rayia, D.M.; Saad, A.E.; Ashour, D.S.; Oreiby, R.M. Implication of Artemisinin Nematocidal Activity on Experimental Trichinellosis: In Vitro and in Vivo Studies. Parasitol. Int. 2017, 66, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-J.; Jo, J.-O.; Cho, M.-K.; Yu, H.-S.; Ock, M.S.; Cha, H.-J. Trichinella Spiralis Infection Induces Angiogenic Factor Thymosin Β4 Expression. Vet. Parasitol. 2011, 181, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Echeverrigaray, S.; Zacaria, J.; Beltrão, R. Nematicidal Activity of Monoterpenoids Against the Root-Knot Nematode Meloidogyne Incognita. Phytopathology 2010, 100, 199–203. [Google Scholar] [CrossRef]
- Lam, N.S.; Long, X.; Su, X.-Z.; Lu, F. Artemisinin and Its Derivatives in Treating Helminthic Infections beyond Schistosomiasis. Pharmacol. Res. 2018, 133, 77–100. [Google Scholar] [CrossRef]
- Loo, C.S.N.; Lam, N.S.K.; Yu, D.; Su, X.-Z.; Lu, F. Artemisinin and Its Derivatives in Treating Protozoan Infections beyond Malaria. Pharmacol. Res. 2017, 117, 192–217. [Google Scholar] [CrossRef]
- Derda, M.; Hadaś, E.; Cholewiński, M.; Skrzypczak, Ł.; Grzondziel, A.; Wojtkowiak-Giera, A. Artemisia Annua L. as a Plant with Potential Use in the Treatment of Acanthamoebiasis. Parasitol. Res. 2016, 115, 1635–1639. [Google Scholar] [CrossRef]

| Molecule | Structure | Activities | Ref. |
|---|---|---|---|
| 1,8-cineole |  | Insecticidal, expectorant, anti-inflammatory, antibacterial, antifungal, antitumor | [43,44,45,46,47,48,49] |
| α-and-β-pinene |  | Antimicrobial, anti-hypertensive, antinociceptive, anti-inflammatory, flavor and fragrance purpose, food additive | [50,51,52,53] |
| Camphene |  | Insecticidal, antitumor, anti-inflammatory, antifungal, antigastriculcer | [54,55] |
| Borneol |  | Analgesia, anti-inflammatory, anesthesia, neuroprotective | [56,57,58,59,60] |
| Camphor |  | Anti-implantation, antiestrogenic, anticonvulsant, antitussive, uterotrophic, nicotinic receptor blocking, estrogenic, attractant, fragrance purpose, food additive | [61,62,63,64,65,66,67,68,69,70] |
| Carvone |  | Anti-inflammatory, anti-hyperlipidemic, anti-microbial, anti-carcinogenic, chemopreventive, anti-hypertensive, immunomodulator | [71,72,73,74] |
| Limonene |  | Antioxidant, antigenotoxic inhibition of angiogenesis, antitumor | [75,76,77] |
| α-terpinene |  | Antioxidant | [78] |
| Myrtenol |  | Analgesic, anti-inflammatory, antioxidant, mutagenic, antiaging, neuroprotective, anti-diabetic, antitumor, protects against LDL (Low Density Lipoprotein) oxidation and lung diseases. | [79,80,81,82] |
| Molecule | Structure | Activities | Ref. |
|---|---|---|---|
| Artemisinin |  | Antiviral, antitumor, antimalarial, antiparasitic, anti-inflammatory, antifibrotic | [88,89,90,91,92] |
| Arteannuin B |  | Antiviral, antitumor, anti-inflammatory, larvicidal | [93,94,95,96,97] |
| Artemisinic acid | 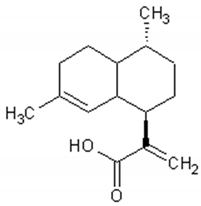 | Regulator of adipocyte differentiation | [98] |
| Molecule | Structure | Activities | Ref. |
|---|---|---|---|
| Quinic acid |  | Antioxidant, lipolytic, antiobesity, inhibitor of hepatic glucose-6-phosphate translocase, antiviral | [105,106,107,108] |
| Caffeic acid |  | Antiviral, antimicrobial, antiinflammatory, antitumor, antiAlzheimer, anti-diabetic, cardiovascular protector | [109,110,111,112,113,114,115,116] |
| Luteolin |  | Antitumor, antioxidant, antiinflammatory, nervous system protector, cardiovascular protector | [117,118,119,120,121,122] |
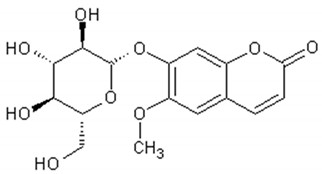
| Quercetin |  | Antioxidant, vasodilator effect, antiinflammatory antitumor, cardiovascular protector, neurodegenerative diseases protector, antiviral | [123,124,125,126,127,128,129,130] |
| Rutin |  | Antioxidant, cytoprotective, anti-inflammatory, immunomodulator, neuroprotective, neurodegenerative diseases protector, antitumor, antidiabetic, hypotensor, hyperlipidemia protector, antiviral | [131,132,133,134] |
| Apigenin |  | Antiinflammatory, antimicrobial, antitumor, antioxidant | [135,136,137,138,139] |
| Isorhamnetin |  | Antiinflammatory, antitumor, antioxidant, neuroprotective, antidiabetic | [140,141,142,143,144,145] |
| Kaempferol |  | Antiinflammatory, antioxidant, neuroprotective, antitumor | [146,147,148,149] |
| Mearnsetin |  | Antioxidant | [150] |
| Artemetin |  | Hypotensor, antitumor, antioxidant, antiinflammatory | [151,152,153,154,155] |
| Casticin |  | Antitumor, antiinflammatory, antioxidant, antiaging | [155,156,157,158] |
| Chrysosplenetin |  | Antiviral | [159] |
| Chrysoprenol D |  | Antiinflammatory, antitumor, antioxidant | [33,95,160] |
| Cirsilineol |  | Immunosuppressive, antitumor | [161,162,163] |
| Eupatorine | 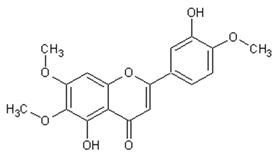 | Antitumor | [164,165,166] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Septembre-Malaterre, A.; Lalarizo Rakoto, M.; Marodon, C.; Bedoui, Y.; Nakab, J.; Simon, E.; Hoarau, L.; Savriama, S.; Strasberg, D.; Guiraud, P.; et al. Artemisia annua, a Traditional Plant Brought to Light. Int. J. Mol. Sci. 2020, 21, 4986. https://doi.org/10.3390/ijms21144986
Septembre-Malaterre A, Lalarizo Rakoto M, Marodon C, Bedoui Y, Nakab J, Simon E, Hoarau L, Savriama S, Strasberg D, Guiraud P, et al. Artemisia annua, a Traditional Plant Brought to Light. International Journal of Molecular Sciences. 2020; 21(14):4986. https://doi.org/10.3390/ijms21144986
Chicago/Turabian StyleSeptembre-Malaterre, Axelle, Mahary Lalarizo Rakoto, Claude Marodon, Yosra Bedoui, Jessica Nakab, Elisabeth Simon, Ludovic Hoarau, Stephane Savriama, Dominique Strasberg, Pascale Guiraud, and et al. 2020. "Artemisia annua, a Traditional Plant Brought to Light" International Journal of Molecular Sciences 21, no. 14: 4986. https://doi.org/10.3390/ijms21144986
APA StyleSeptembre-Malaterre, A., Lalarizo Rakoto, M., Marodon, C., Bedoui, Y., Nakab, J., Simon, E., Hoarau, L., Savriama, S., Strasberg, D., Guiraud, P., Selambarom, J., & Gasque, P. (2020). Artemisia annua, a Traditional Plant Brought to Light. International Journal of Molecular Sciences, 21(14), 4986. https://doi.org/10.3390/ijms21144986