A Novel Methoxybenzyl 5-Nitroacridone Derivative Effectively Triggers G1 Cell Cycle Arrest in Chronic Myelogenous Leukemia K562 Cells by Inhibiting CDK4/6-Mediated Phosphorylation of Rb
Abstract
:1. Introduction
2. Results
2.1. Multivariate Statistical Analysis and Changes in Metabolites
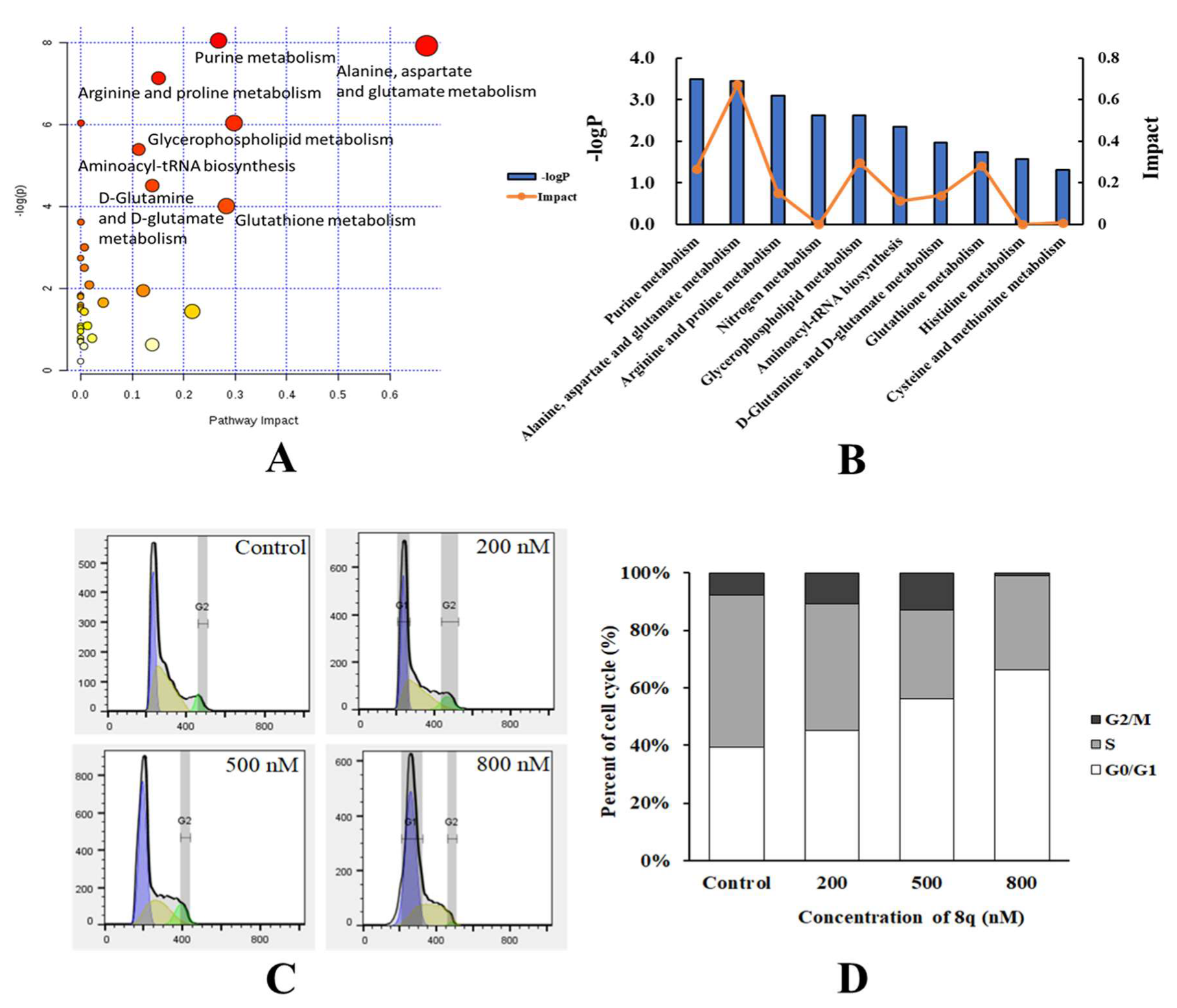
2.2. Metabolic Pathway Analysis
2.3. 8q Effectively Triggered G1 Cell Cycle Arrest in K562 Cells
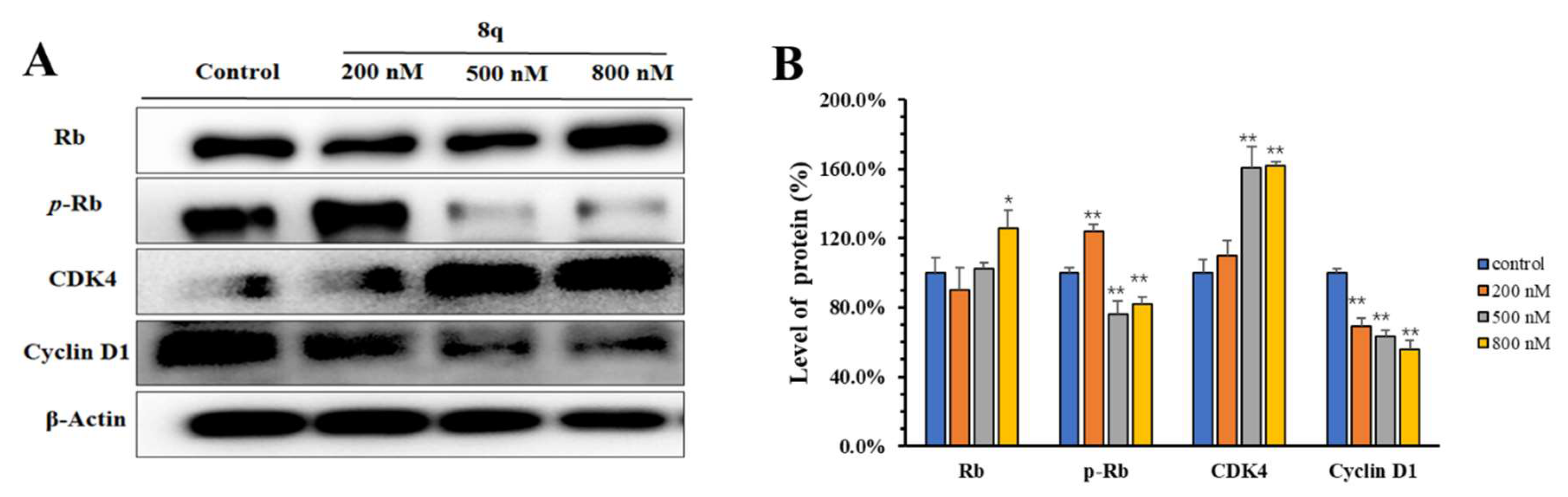
2.4. 8q Showed Concentration-Dependent Inhibition of the Phosphorylation of the CDK4/6 Substrate Rb
2.5. Homology Modeling of CDK4/6 Protein and Molecular Docking
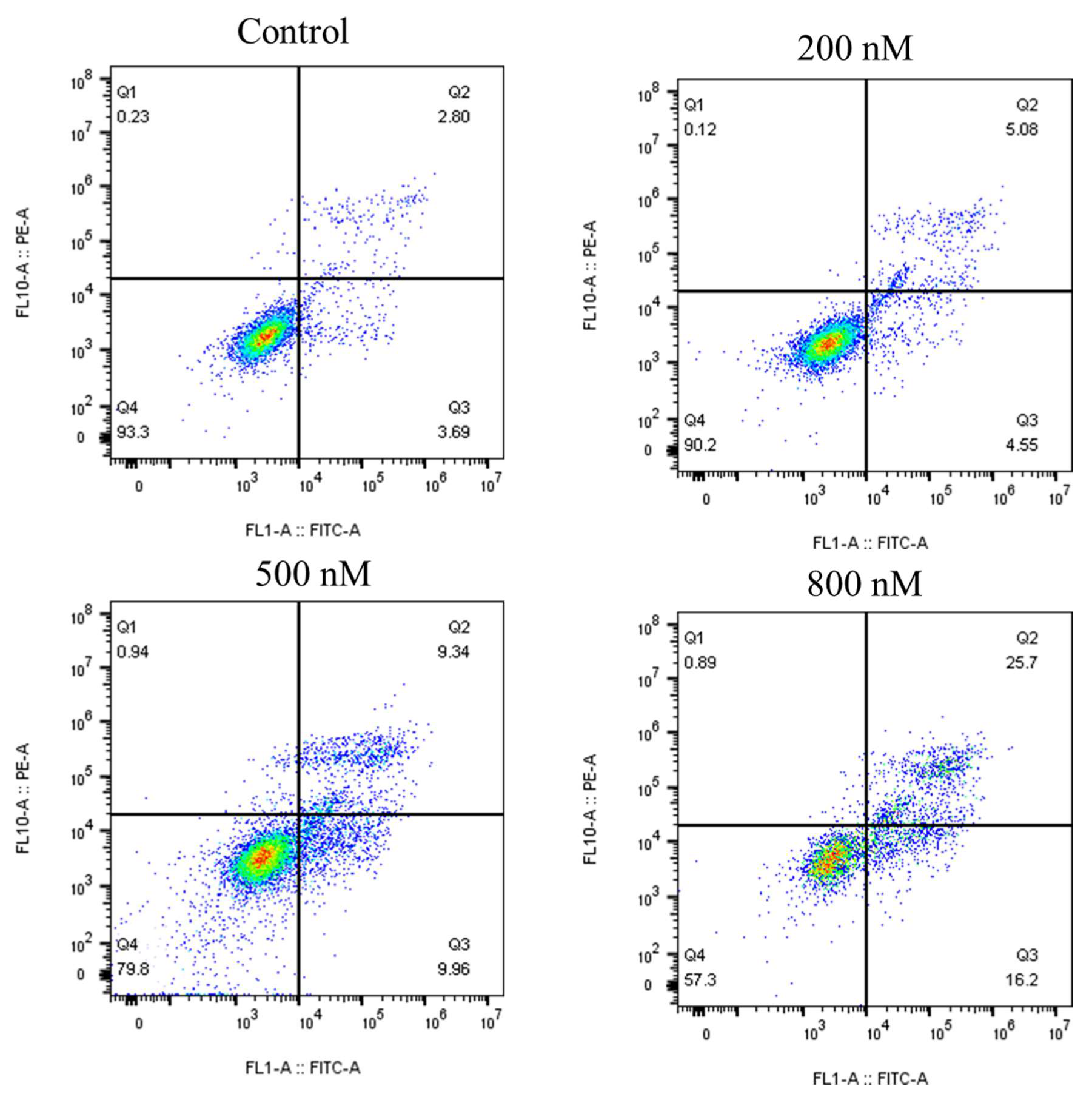
2.6. 8q Significantly Induced Apoptosis in K562 Cells
2.7. 8q Induced Apoptosis through the Caspase Pathway
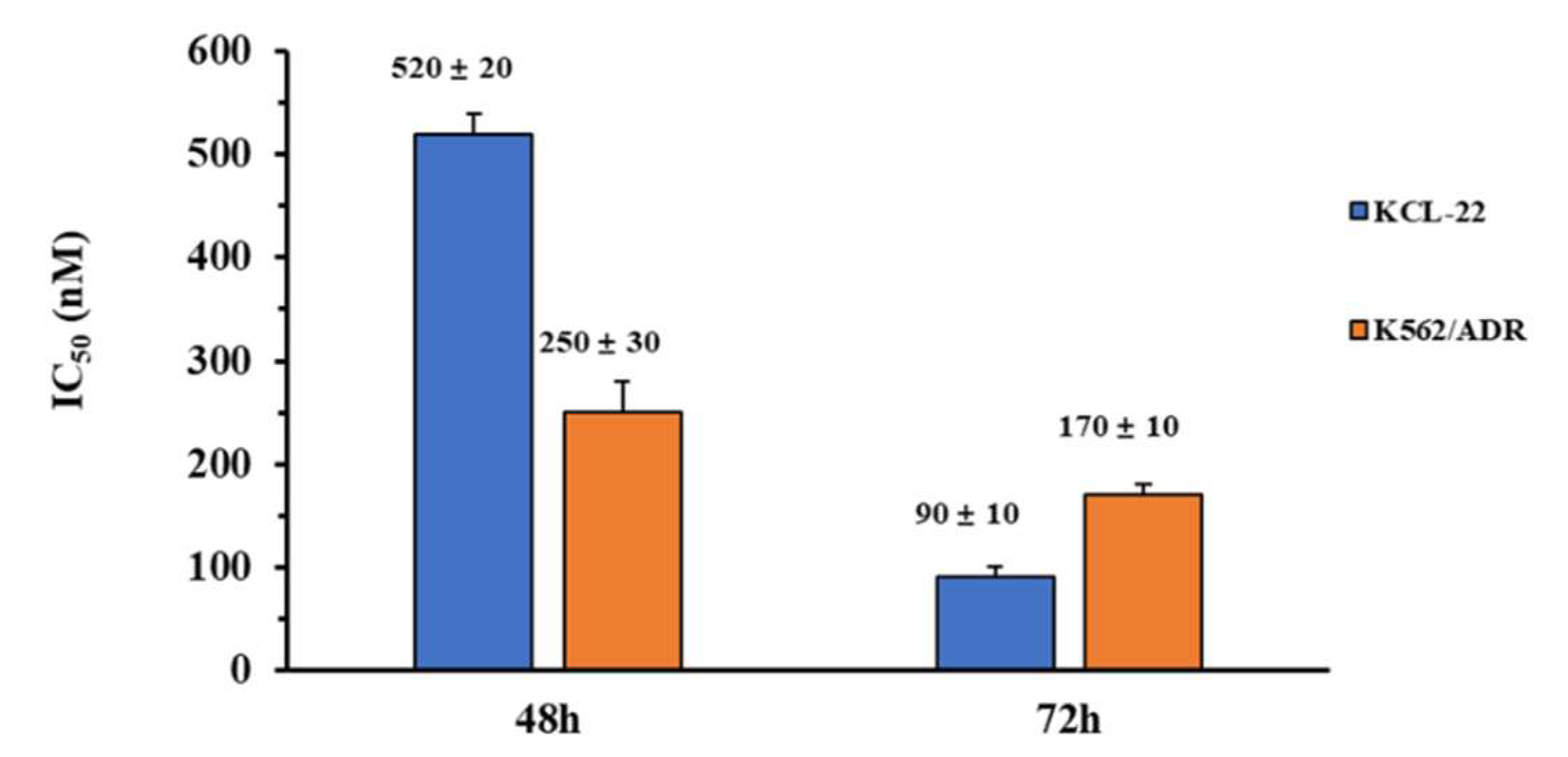
2.8. In Vitro Anti-Proliferation Activity of KCL-22 and K562/ADR Cells
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Apoptosis and Necrosis Detection Assay
4.3. Metabolomics Analysis Conditions
4.4. Flow Cytometric Analysis for Cell Cycle
4.5. Western Blotting
4.6. In Vitro Anti-Proliferative Assay
4.7. Homology Modeling
4.8. Molecular Docking
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miranda-Filho, A.; Piñeros, M.; Ferlay, J.; Soerjomataram, I.; Monnereau, A.; Bray, F. Epidemiological patterns of leukaemia in 184 countries: A population-based study. Lancet Haematol. 2018, 5, e14–e24. [Google Scholar] [CrossRef]
- Soverini, S.; De Benedittis, C.; Mancini, M.; Martinelli, G. Best Practices in Chronic Myeloid Leukemia Monitoring and Management. Oncologist 2016, 21, 626–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaehler, M.; Nagel, I.; Bruckmüller, H.; Boehm, R.; Ammerpohl, O.; Cascorbi, I. Abstract 5846: Drug resistance in chronic myeloid leukemia: Impact of methylation on gene expression in imatinib and nilotinib resistance. Cancer Res. 2018, 78, 5846. [Google Scholar]
- Zhang, B.; Wang, N.; Zhang, C.; Gao, C.; Zhang, W.; Chen, K.; Wu, W.; Chen, Y.Z.; Tan, C.; Liu, F.; et al. Novel multi-substituted benzyl acridone derivatives as survivin inhibitors for hepatocellular carcinoma treatment. Eur. J. Med. Chem. 2017, 129, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, K.; Wang, N.; Gao, C.; Sun, Q.; Li, L.; Chen, Y.Z.; Tan, C.; Liu, H.; Jiang, Y. Molecular design, synthesis and biological research of novel pyridyl acridones as potent DNA-binding and apoptosis-inducing agents. Eur. J. Med. Chem. 2015, 93, 214–226. [Google Scholar] [CrossRef]
- Cui, Z.; Li, X.; Li, L.; Zhang, B.; Gao, C.; Chen, Y.; Tan, C.; Liu, H.; Xie, W.; Yang, T.; et al. Design, synthesis and evaluation of acridine derivatives as multi-target Src and MEK kinase inhibitors for anti-tumor treatment. Bioorganic Med. Chem. 2016, 24, 261–269. [Google Scholar] [CrossRef]
- Arlin, Z.A. A Special Role for Amsacrine in the Treatment of Acute Leukemia. Cancer Investig. 1989, 7, 607–609. [Google Scholar] [CrossRef]
- Zhang, B.; Li, X.; Li, B.; Gao, C.; Jiang, Y. Acridine and its derivatives: A patent review (2009–2013). Expert Opin. Ther. Pat. 2014, 24, 647–664. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, B.; Jin, F.; Gao, D.; Liu, F.; Liu, H.; Jiang, Y. Combing metabolomics with bioanalysis methods to study the antitumor mechanism of the new acridone derivative 8q on CCRF-CEM cells: 8q induced mitochondrial-mediated apoptosis and targeted the PI3K/AKT/FOXO1 pathway. J. Pharm. Biomed. Anal. 2018, 160, 314–322. [Google Scholar] [CrossRef]
- Chan, C.Y.; Zhao, H.; Pugh, R.J.; Pedley, A.M.; French, J.; Jones, S.A.; Zhuang, X.; Jinnah, H.; Huang, T.J.; Benkovic, S.J. Purinosome formation as a function of the cell cycle. Proc. Natl. Acad. Sci. USA 2015, 112, 1368–1373. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Ren, W.; Huang, X.; Deng, J.; Li, T.; Yin, J. Potential Mechanisms Connecting Purine Metabolism and Cancer Therapy. Front. Immunol. 2018, 9, 1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.M.; Kim, J.Y.; Kim, S. Molecular network and functional implications of macromolecular tRNA synthetase complex. Biochem. Biophys. Res. Commun. 2003, 303, 985–993. [Google Scholar] [CrossRef]
- Gomez, M.A.R.; Ibba, M. Aminoacyl-tRNA Synthetases. RNA 2020, 69, 617–650. [Google Scholar] [CrossRef]
- Liang, F.; Kelly, S.; Suk, N.; Alexandre, A.; Carla, P.; Lennart, R.; Debra, T.H.; Dieter, S.L. Aminoacyl-tRNA synthesis by pre-translational amino acid modification. RNA Biol. 2004, 1, 15–19. [Google Scholar]
- Liu, W.; Jin, F.; Gao, D.; Song, L.; Ding, C.; Liu, H. Metabolomics analysis reveals aminoquinazolin derivative 9d-induced oxidative stress and cell cycle arrest in A549 cells. RSC Adv. 2017, 7, 13149–13158. [Google Scholar] [CrossRef] [Green Version]
- Zha, C.; Deng, W.; Fu, Y.; Tang, S.; Lan, X.; Ye, Y.; Su, Y.; Jiang, L.; Chen, Y.; Huang, Y.; et al. Design, synthesis and biological evaluation of tetrahydronaphthyridine derivatives as bioavailable CDK4/6 inhibitors for cancer therapy. Eur. J. Med. Chem. 2018, 148, 140–153. [Google Scholar] [CrossRef]
- Harper, J.W.; Adami, G.R.; Wei, N.; Keyomarsi, K.; Elledge, S.J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993, 75, 805–816. [Google Scholar] [CrossRef]
- Jansen, V.M.; Bhola, N.E.; Bauer, J.A.; Formisano, L.; Lee, K.M.; Hutchinson, K.E.; Witkiewicz, A.K.; Moore, P.D.; Estrada, M.V.; Sánchez, V. Kinome-wide RNA interference screen reveals a role for PDK1 in acquired resistance to CDK4/6 inhibition in ER-positive breast cancer. Cancer Res. 2017, 77, 2488–2499. [Google Scholar] [CrossRef] [Green Version]
- Goel, S.; DeCristo, M.J.; McAllister, S.S.; Zhao, J.J. CDK4/6 Inhibition in Cancer: Beyond Cell Cycle Arrest. Trends Cell Biol. 2018, 28, 911–925. [Google Scholar] [CrossRef]
- Tian, Y.-Z.; Liu, Y.-P.; Tian, S.-C.; Ge, S.-Y.; Wu, Y.-J.; Zhang, B.-L. Antitumor activity of ginsenoside Rd in gastric cancer via up-regulation of Caspase-3 and Caspase-9. Pharmazie 2020, 75, 147–150. [Google Scholar]
- Indran, I.R.; Tufo, G.; Pervaiz, S.; Brenner, C. Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Biochim. Biophys. Acta (BBA) Bioenerg. 2011, 1807, 735–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Médoc, M.; Dhilly, M.; Matesic, L.; Toutain, J.; Krauseheuer, A.M.; Delamare, J.; Fraser, B.H.; Touzani, O.; Barré, L.; Greguric, I. In vivo evaluation of radio fluorinated caspase-3/7 inhibitors as radiotracers for apoptosis imaging and comparison with [18F] ML-10 in a stroke model in the rat. Mol. Imaging Biol. 2016, 18, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Schulze-Gahmen, U. Toward Understanding the Structural Basis of Cyclin-Dependent Kinase 6 Specific Inhibition. J. Med. Chem. 2006, 49, 3826–3831. [Google Scholar] [CrossRef] [PubMed]
- Verdonk, M.L.; Cole, J.C.; Hartshorn, M.J.; Murray, C.W.; Taylor, R.D. Improved protein—Ligand docking using GOLD. Proteins Struct. Funct. Bioinform. 2003, 52, 609–623. [Google Scholar] [CrossRef]
- Tan, K.-L.; Bin Ali, A.; Du, Y.; Fu, H.; Jin, H.-X.; Chin, T.-M.; Khan, M.; Go, M.-L. Synthesis and Evaluation of Bisbenzylidenedioxotetrahydrothiopranones as Activators of Endoplasmic Reticulum (ER) Stress Signaling Pathways and Apoptotic Cell Death in Acute Promyelocytic Leukemic Cells. J. Med. Chem. 2014, 57, 5904–5918. [Google Scholar] [CrossRef]
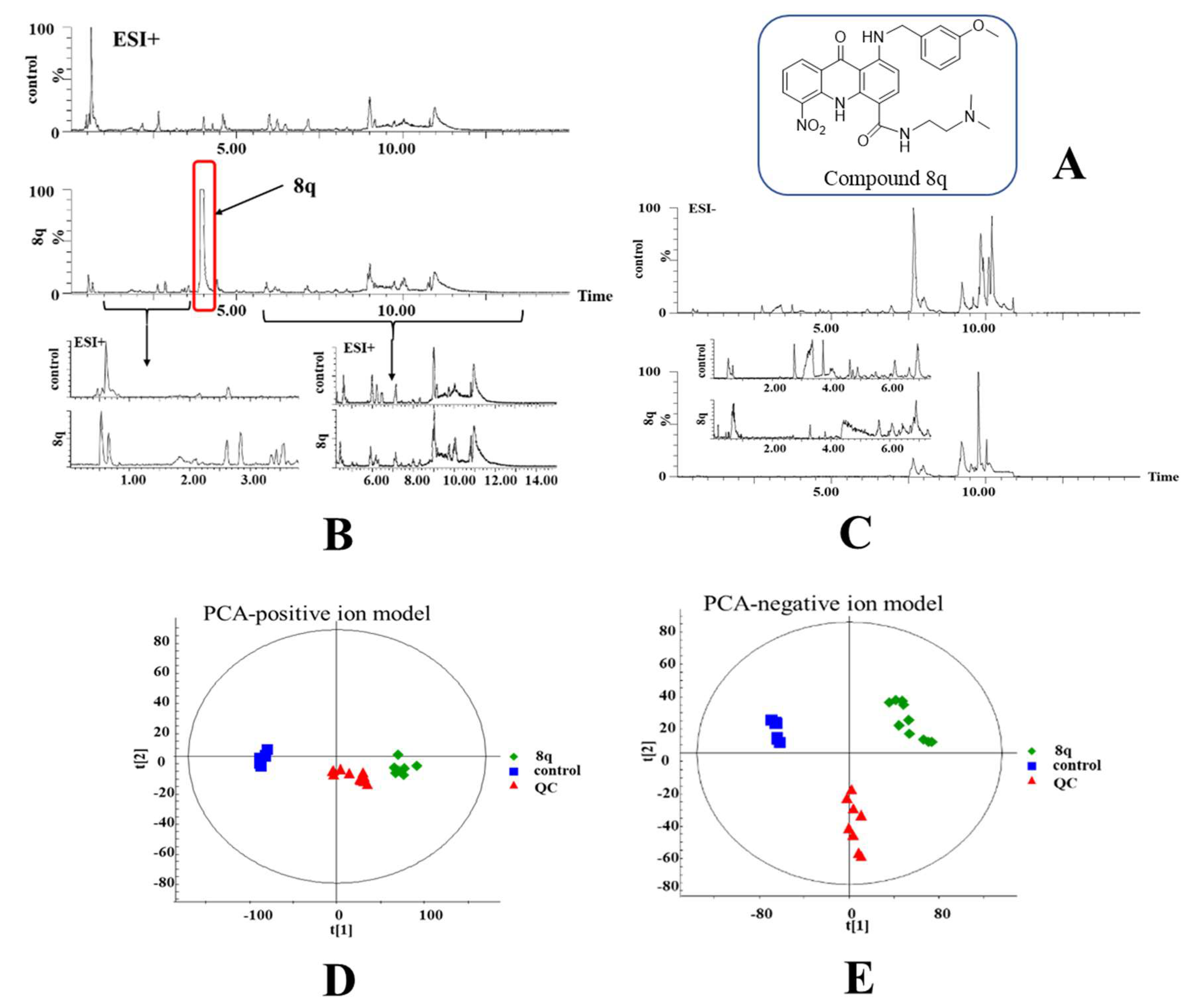


| Ion Model | Name | Ret. Time | m/z | ∆ppm | MS/MS | q Value | FC a |
|---|---|---|---|---|---|---|---|
| Positive ion mode | Spermine | 0.4484 | 203.2231 | 0 | 84.0; 112.1; 129.1 | 0.000 | 0.027 |
| Neurine | 0.5467 | 104.105 | 19 | 104.1 | 0.000 | 0.225 | |
| l-Proline | 0.5739 | 116.0691 | 12 | 70.0; 116.0 | 0.000 | 0.021 | |
| Adenosine 3′-monophosphate | 0.5884 | 348.0696 | 2 | 136.0; 348.0 | 0.000 | 0.034 | |
| Glutathione | 0.6219 | 308.09 | 3 | 58.9; 76.0; 84.0; 116.0; 130.0; 140.0; 144.0; 162.0; 179.0; 187.0; 215.0; 233.0; 245.0; 291.0; 308.0 | 0.000 | con b | |
| 3′-Keto-3′-deoxy-AMP | 0.6226 | 346.0473 | 21 | 136.0; 346.0 | 0.000 | 0.001 | |
| Piperidine | 0.6272 | 86.0936 | 32 | 69.0; 86.1 | 0.000 | 0.028 | |
| l-Leucine | 0.8145 | 132.1013 | 4 | 69.0; 86.0 | 0.000 | 0.026 | |
| Piperidine | 0.8172 | 86.094 | 28 | 69.0; 86.0 | 0.000 | 0.007 | |
| Xestoaminol C | 4.5328 | 230.2476 | 1 | 212.2; 230.2 | 0.001 | 0.815 | |
| C16 Sphinganine | 4.6369 | 274.2741 | 0 | 256.2; 274.2 | 0.003 | con | |
| PC(16:0/0:0) | 5.7318 | 496.3372 | 5 | 86.0; 184.0; 478.3; 496.3 | 0.000 | 0.097 | |
| PC(22:2/0:0) | 5.8967 | 576.4092 | 11 | 277.2; 559.3; 576.4 | 0.000 | 0.239 | |
| PC(16:0/0:0) | 5.9447 | 496.3376 | 4 | 104.1; 184.0; 258.1; 313.2; 419.2; 478.3; 496.3 | 0.000 | 0.487 | |
| PE(18:1/0:0) | 6.1807 | 480.3233 | 30 | 155.0; 339.2; 462.3; 480.3 | 0.000 | 0.668 | |
| PE(18:0/0:0) | 7.0864 | 482.3245 | 0 | 341.3; 462.2; 482.3 | 0.000 | 0.214 | |
| PC(0:0/18:0) | 7.1339 | 524.3699 | 2 | 104.1; 184.0; 341.3; 506.3; 524.3 | 0.000 | 0.578 | |
| PC(20:1/0:0) | 7.3169 | 550.3867 | 0 | 104.1; 184.0; 532.3; 553.3 | 0.000 | 0.197 | |
| PC(18:0/18:2) | 8.8141 | 786.6016 | 1 | 184.0; 605.5; 786.6 | 0.249 | 0.548 | |
| PC(18:1/18:1) | 9.2222 | 786.601 | 0 | 184.0; 522.3; 603.5; 786.6 | 0.024 | 0.297 | |
| PC(18:3/18:1) | 9.3872 | 782.571 | 2 | 184.0; 603.5; 782.5 | 0.000 | 0.375 | |
| PC(18:1/18:1) | 9.4376 | 786.6019 | 1 | 184.0; 339.2; 504.3; 522.3; 786.6 | 0.035 | 0.172 | |
| 13E-Docosenamide/13Z-Docosenamide | 9.9366 | 338.3416 | 0 | 303.3; 321.3; 338.3 | 0.025 | 1.613 | |
| PC(18:1/18:2) | 10.7816 | 784.5947 | 12 | 86.0; 184.0; 504.3; 784.5 | 0.000 | 0.381 | |
| PC(18:1/16:1) | 10.7869 | 758.5774 | 10 | 184.0; 504.3; 758.5 | 0.013 | 0.279 | |
| PC(18:1/18:1) | 10.7904 | 786.6056 | 6 | 184.0; 603.5; 786.6 | 0.035 | 0.440 | |
| PC(16:1/18:0) | 10.7926 | 760.5899 | 6 | 86.0; 184.0; 577.5; 760.5 | 0.231 | 0.617 | |
| PC(14:1/17:0) | 10.7938 | 718.5497 | 16 | 184.0; 577.5; 718.5 | 0.010 | 0.338 | |
| PC(20:3/18:0) | 10.7944 | 812.64 | 29 | 184.0; 504.3; 812.6 | 0.122 | 0.402 | |
| PC(P-16:0/15:1) | 10.7954 | 702.5472 | 5 | 184.0; 702.5 | 0.100 | 0.559 | |
| SM(d18:1/16:0) | 10.7964 | 703.5721 | 3 | 86.0; 184.0;7 03.5 | 0.023 | 0.181 | |
| PE(18:1/18:1) | 10.7979 | 744.5627 | 11 | 265.2; 603.5; 744.5 | 0.003 | 0.365 | |
| PE(18:1/0:0) | 10.8012 | 480.3095 | 2 | 339.2; 480.3 | 0.000 | 0.614 |
| Ion Model | Name | Ret. Time | m/z | ∆ppm | MS/MS | q Value | FC a |
|---|---|---|---|---|---|---|---|
| Negative ion mode | UDP-glucose/UDP-D-galactose | 0.5493 | 565.0425 | 9 | 78.9; 96.9; 241.0; 323.0; 385.0; 565.0 | 0.000 | 0.002 |
| Uridine diphosphate-N-acetylglucosamine | 0.5502 | 606.0683 | 9 | 78.9; 158.9; 272.9; 282.0; 384.9; 403.0; 606.0 | 0.000 | 0.002 | |
| ADP | 0.5611 | 426.0179 | 9 | 78.9; 134.0; 158.9; 272.9; 328.0; 408.0; 426.0 | 0.000 | 0.004 | |
| Inosine 5′-monophosphate (IMP) | 0.5714 | 347.0376 | 6 | 78.9; 96.9; 347.0 | 0.000 | 0.011 | |
| 5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranosyl 5′-monophosphate | 0.5799 | 337.0528 | 7 | 78.9; 96.9; 337.0 | 0.000 | 0.007 | |
| l-Aspartic Acid | 0.5844 | 132.0255 | 35 | 88.0; 114.0; 132.0 | 0.000 | 0.009 | |
| l-Glutamate | 0.5857 | 146.0426 | 22 | 102.0; 128.0; 146.0 | 0.000 | 0.049 | |
| d-Glycerol 1-phosphate | 0.5858 | 171.0083 | 11 | 78.9; 89.0; 171.0 | 0.000 | 0.012 | |
| Sulfuric acid | 0.591 | 96.9602 | 0 | 78.9; 96.9 | 0.000 | 0.235 | |
| Adenosine monophosphate | 0.6116 | 346.0515 | 12 | 78.9; 96.9; 211.0; 346.0 | 0.000 | 0.026 | |
| l-Glutamine | 0.6118 | 145.0599 | 13 | 102.0; 128.0; 146.9 | 0.000 | 0.003 | |
| l-Leucine | 0.7127 | 130.0842 | 24 | 130.0 | 0.000 | 0.077 | |
| Cumanin | 4.6125 | 265.1467 | 8 | 96.9; 265.1 | 0.007 | 0.143 | |
| Dehydroabietic acid | 5.6388 | 299.2017 | 0 | 299.2 | 0.000 | 2.076 | |
| PE(P-16:0/0:0) | 6.3388 | 436.2828 | 1 | 153.0; 196.0; 239.2; 436.2 | 0.002 | 1.535 | |
| Arginyl-Glutamine | 6.4253 | 301.1607 | 7 | 217.0; 286.1; 301.1 | 0.000 | 2.691 | |
| Δ2-trans-Hexadecenoic Acid | 6.4529 | 253.2172 | 0 | 84.5; 253.2 | 0.012 | 2.286 | |
| Arachidonic Acid (peroxide free) | 6.7046 | 303.2337 | 2 | 205.1; 259.2; 303.2 | 0.000 | 71.665 | |
| Arginyl-Gamma-glutamate | 6.8151 | 301.1606 | 7 | 301.1 | 0.000 | 2.560 | |
| PE(18:0/0:0) | 7.2382 | 480.3079 | 3 | 196.0; 283.2; 480.3 | 0.000 | 0.416 | |
| Petroselinic acid/Oleic Acid | 7.9889 | 281.2483 | 1 | 281.2 | 0.000 | 3.103 | |
| 11Z,14Z-Eicosadienoic Acid | 8.4771 | 307.2647 | 1 | 307.2 | 0.000 | 7.295 | |
| Stearic acid | 9.222 | 283.2654 | 4 | 265.3; 283.2 | 0.000 | 2.336 | |
| PI(20:5/18:1) | 9.3598 | 881.5158 | 3 | 241.0; 281.2; 881.5 | 0.000 | 2.836 | |
| cis-gondoic acid/ 2E-Eicosenoic acid | 9.3917 | 309.2805 | 1 | 309.2 | 0.000 | 5.116 | |
| PS(18:0/19:1) | 9.4136 | 802.5604 | 0 | 283.2; 419.2; 701.5; 802.5 | 0.005 | 0.751 | |
| PS(18:0/18:1) | 9.4246 | 788.5407 | 5 | 152.9; 283.2; 419.2; 701.5; 788.5 | 0.001 | 0.545 | |
| PG(18:1/22:6) | 9.4363 | 819.525 | 8 | 281.2; 327.2; 419.2; 819.5 | 0.000 | 0.516 | |
| PI(20:4/16:0) | 9.5244 | 857.5121 | 7 | 241.0; 303.2; 391.2; 553.2; 857.5 | 0.000 | 3.782 | |
| PI(20:4/18:1) | 9.5572 | 883.5157 | 20 | 152.9; 222.9; 241.0; 303.2; 417.2; 579.2; 883.5 | 0.001 | 1.449 | |
| PG(18:3/18:1) | 9.5579 | 769.5007 | 2 | 152.9; 277.2; 281.2; 769.5 | 0.000 | 11.712 | |
| PI(16:1/18:1) | 9.5732 | 833.5135 | 6 | 152.9; 241.0; 253.2; 281.2; 389.2; 417.2; 579.2; 833.5 | 0.000 | 0.752 | |
| PG(20:3/18:1) | 9.6053 | 797.53 | 4 | 152.9; 281.2; 305.2; 765.6; 797.5 | 0.000 | 0.330 | |
| PS(18:0/18:1) | 9.6188 | 788.5404 | 5 | 152.9; 281.2; 283.2; 417.2; 419.2; 701.5; 788.5 | 0.000 | 0.086 | |
| PG(18:1/20:4) | 9.6202 | 795.5092 | 11 | 281.2; 303.2; 417.2; 795.5 | 0.000 | 16.215 | |
| PI(18:1/18:2) | 9.6278 | 859.5297 | 5 | 152.9; 241.0; 279/2; 281.2; 415.2; 417.2; 577.2; 579.2;859.5 | 0.007 | 1.146 | |
| 7,7-dimethyl-5,8-Eicosadienoic Acid | 9.6238 | 335.2971 | 4 | 335.2 | 0.000 | 5.874 | |
| PG(16:1/18:1) | 9.6489 | 745.4967 | 7 | 152.9; 253.2; 281.2; 389.2; 491.2; 673.5; 745.4 | 0.000 | 17.477 | |
| PI(20:2/20:4) | 9.659 | 909.5474 | 2 | 241.0; 303.2; 307.2; 439.2; 443.2; 909.5 | 0.000 | 1.953 | |
| Nervonic acid | 9.6611 | 365.3426 | 0 | 365.3 | 0.000 | 3.921 | |
| PG(18:2/18:1) | 9.6761 | 771.5117 | 8 | 152.9; 279.2; 281.2; 771.5 | 0.000 | 4.204 | |
| Docosanoic acid | 9.7185 | 339.3293 | 7 | 339.3 | 0.000 | 3.146 | |
| PG(18:1/17:1) | 9.7688 | 759.5153 | 3 | 152.9; 267.2; 281.2; 759.5 | 0.000 | 8.753 | |
| PI(20:4/18:0) | 9.7744 | 885.546 | 4 | 223.0; 241.0; 283.2; 303.2; 419.2; 581.3; 885.5 | 0.000 | 2.928 | |
| PI(18:0/22:5) | 9.7767 | 911.5626 | 3 | 152.9; 241.0; 329.2; 419.2; 581.3; 607.3; 911.5 | 0.000 | 1.520 | |
| PG(18:1/15:0) | 9.7803 | 733.4986 | 5 | 281.2; 733.4 | 0.000 | 3.943 | |
| PI(O-16:0/18:1) | 9.8087 | 821.5381 | 20 | 255.2; 281.2; 437.2; 745.5; 821.5 | 0.018 | 1.521 | |
| PA(17:0/16:1) | 9.8117 | 659.4584 | 11 | 253.2; 659.4 | 0.000 | 6.842 | |
| PI(18:1/18:1) | 9.8177 | 861.5442 | 6 | 78.9; 223.0; 241.0; 281.2; 417.2; 579.2; 792.5; 861.5 | 0.007 | 0.677 | |
| PG(18:1/18:1) | 9.833 | 773.5242 | 12 | 152.9; 241.2; 417.2; 509.2; 773.5 | 0.031 | 1.315 | |
| PG(20:2/18:1) | 9.84 | 799.5471 | 2 | 152.9; 281.2; 307.2; 799.5 | 0.000 | 0.177 | |
| PG(20:4/18:0) | 9.8432 | 797.5334 | 0 | 152.9; 260.2; 283.2; 303.2; 419.2; 511.3; 797.5 | 0.000 | 51.407 | |
| 5,9-hexacosadienoic acid | 9.8475 | 391.3566 | 3 | 391.3 | 0.001 | 2.517 | |
| PI(18:1/20:2) | 9.8834 | 887.5579 | 8 | 152.9; 223.0; 241.0; 307.2; 417.2; 443.2; 579.2; 887.5 | 0.000 | 0.308 | |
| PG(16:0/18:1) | 9.8925 | 747.5105 | 10 | 255.2; 281.2; 465.2; 747.5 | 0.000 | 0.437 | |
| PI(18:1/17:0) | 9.9228 | 849.5507 | 0 | 241.0; 281.2; 419.2; 567.3; 849.5 | 0.001 | 1.593 | |
| PI(22:4/18:0) | 9.9428 | 913.5793 | 2 | 152.9; 283.2; 331.2; 419.2; 443.2; 581.3; 605.3; 913.5 | 0.013 | 1.158 | |
| PG(18:1/20:1) | 9.9942 | 801.5621 | 3 | 152.9; 281.2; 309.2; 728.5; 801.5 | 0.000 | 0.162 | |
| PG(22:2/18:1) | 9.9951 | 827.5801 | 0 | 281.2; 335.2; 419.2;827.5 | 0.000 | 0.057 | |
| PI(18:0/18:1) | 10.0446 | 863.5568 | 10 | 152.9; 241.0; 281.2; 283.2; 417.2; 419.2; 581.3; 863.5 | 0.027 | 0.733 | |
| Lignoceric acid | 10.0555 | 367.3588 | 1 | 367.3 | 0.000 | 7.465 | |
| PA(18:1/17:0) | 10.0864 | 687.4864 | 15 | 152.9; 281.2; 423.2; 687.4 | 0.000 | 10.392 | |
| PA(19:1/18:1) | 10.0979 | 713.4849 | 38 | 152.9; 253.2; 281.2; 417.2; 713.4 | 0.000 | 4.158 | |
| PI(22:2/18:1) | 10.1148 | 915.599 | 2 | 152.9; 241.0; 417.2; 579.2; 915.5 | 0.000 | 0.475 | |
| PI(20:2/18:0) | 10.1387 | 889.5771 | 4 | 223.0; 241.0; 283.2; 307.2; 419.2; 443.2; 581.3; 599.3 | 0.000 | 0.380 | |
| PG(18:0/18:1) | 10.1847 | 775.5441 | 6 | 152.9; 281.2; 283.2; 419.2; 493.2; 511.3; 775.5 | 0.001 | 0.789 | |
| PE(P-18:1/18:3) | 10.2445 | 722.5086 | 6 | 152.9; 281.2; 413.1; 417.2; 722.5 | 0.002 | 0.404 | |
| PI(22:2/20:1) | 10.4522 | 943.6288 | 0 | 241.0; 445.2; 607.3 | 0.000 | 0.303 | |
| PS(18:0/18:1) | 10.5039 | 788.5415 | 4 | 152.9; 281.2; 283.2; 417.2; 419.2; 701.5; 788.5 | 0.020 | 0.212 | |
| PI(18:0/20:1) | 10.5194 | 891.5939 | 3 | 153.0; 223.0; 241.0; 283.2; 309.2; 419.2; 581.3; 891.5 | 0.003 | 0.020 | |
| PI(22:2/18:0) | 10.5319 | 917.6085 | 4 | 153.0; 223.0; 241.0; 335.2; 419.2; 581.3; 917.6 | 0.000 | 0.613 | |
| Arachidic Acid/Phytanic Acid | 10.5858 | 311.2971 | 4 | 311.2 | 0.000 | 2.173 | |
| 13Z-Docosenoic Acid | 10.6089 | 337.3124 | 3 | 337.3 | 0.000 | 2.192 | |
| Oleic Acid | 10.8021 | 281.2501 | 5 | 281.2 | 0.001 | 1.746 | |
| PA(20:2/18:1) | 10.8195 | 725.4859 | 36 | 152.9; 281.2; 417.2; 462.3 | 0.011 | 0.607 | |
| PE(18:1/18:1) | 10.8206 | 742.5396 | 0 | 196.0; 281.2; 460.2; 478.2; 742.5 | 0.003 | 0.334 | |
| PI(20:2/18:0) | 10.8474 | 889.5809 | 2 | 241.0; 283.2; 307.2; 889.5 | 0.008 | 0.570 | |
| PS(22:1/18:1 | 10.8657 | 842.5978 | 7 | 281.2; 755.5; 842.5 | 0.000 | 0.147 | |
| PS(18:0/19:1 | 10.8682 | 802.5587 | 2 | 152.9; 281.2; 419.2; 710.5; 715.2; 802.5 | 0.000 | 0.420 | |
| PS(18:0/18:1) | 10.8685 | 788.5394 | 6 | 152.9; 283.2; 419.2; 701.5; 788.5 | 0.000 | 0.365 | |
| PS(18:1/18:1) | 10.8723 | 786.5269 | 2 | 152.9; 281.2; 417.2; 699.4; 701.5; 786.5 | 0.000 | 0.240 | |
| PS(18:0/22:6) | 10.8727 | 834.5257 | 3 | 283.2; 419.2; 463.2; 747.4; 834.5 | 0.000 | 0.350 | |
| PS(18:1/16:0) | 10.8735 | 760.5147 | 1 | 152.9; 255.2; 281.2; 391.2; 673.5 | 0.000 | 0.109 | |
| PI(20:4/18:0) | 10.8741 | 885.55 | 0 | 241.0; 419.2; 581.3; 885.5 | 0.000 | 2.550 | |
| PG(18:0/17:1) | 10.8803 | 761.5346 | 1 | 152.9; 283.2; 391.2; 419.2; 687.5; 761.5 | 0.001 | 0.086 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Zhang, T.; Zhang, T.-Y.; Wang, N.; He, S.; Wu, B.; Jin, H.-X. A Novel Methoxybenzyl 5-Nitroacridone Derivative Effectively Triggers G1 Cell Cycle Arrest in Chronic Myelogenous Leukemia K562 Cells by Inhibiting CDK4/6-Mediated Phosphorylation of Rb. Int. J. Mol. Sci. 2020, 21, 5077. https://doi.org/10.3390/ijms21145077
Zhang B, Zhang T, Zhang T-Y, Wang N, He S, Wu B, Jin H-X. A Novel Methoxybenzyl 5-Nitroacridone Derivative Effectively Triggers G1 Cell Cycle Arrest in Chronic Myelogenous Leukemia K562 Cells by Inhibiting CDK4/6-Mediated Phosphorylation of Rb. International Journal of Molecular Sciences. 2020; 21(14):5077. https://doi.org/10.3390/ijms21145077
Chicago/Turabian StyleZhang, Bin, Ting Zhang, Tian-Yi Zhang, Ning Wang, Shan He, Bin Wu, and Hai-Xiao Jin. 2020. "A Novel Methoxybenzyl 5-Nitroacridone Derivative Effectively Triggers G1 Cell Cycle Arrest in Chronic Myelogenous Leukemia K562 Cells by Inhibiting CDK4/6-Mediated Phosphorylation of Rb" International Journal of Molecular Sciences 21, no. 14: 5077. https://doi.org/10.3390/ijms21145077







