The Biological and Clinical Role of the Long Non-Coding RNA LOC642852 in Ovarian Carcinoma
Abstract
:1. Introduction
2. Results
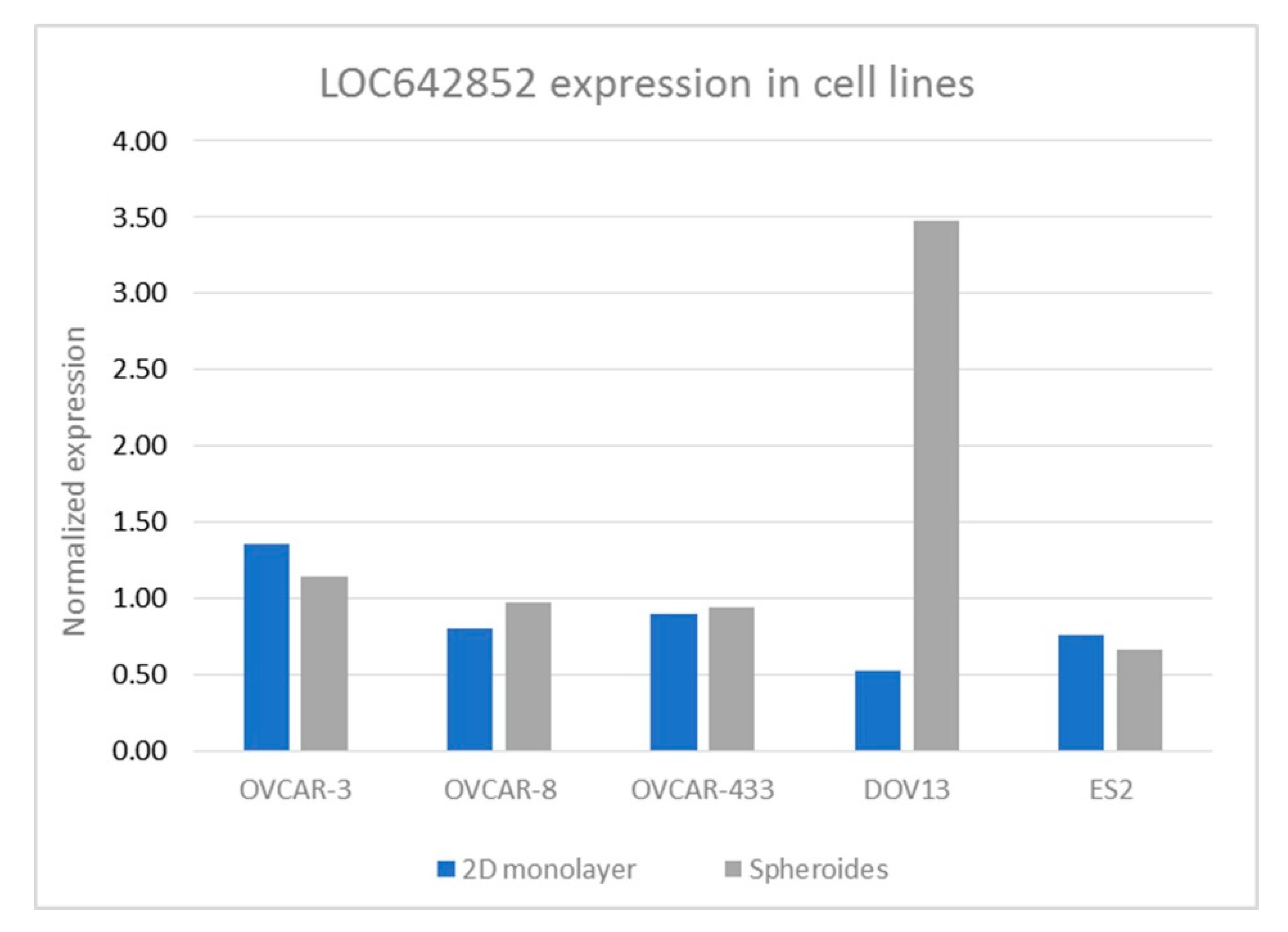
2.1. LOC642852 in OC Cell Lines
2.2. LOC642852 KO Affects Invasion and Migration, but Not MMP Activity, Proliferation or Spheroid Formation
2.3. LOC642852 and Cell Signaling
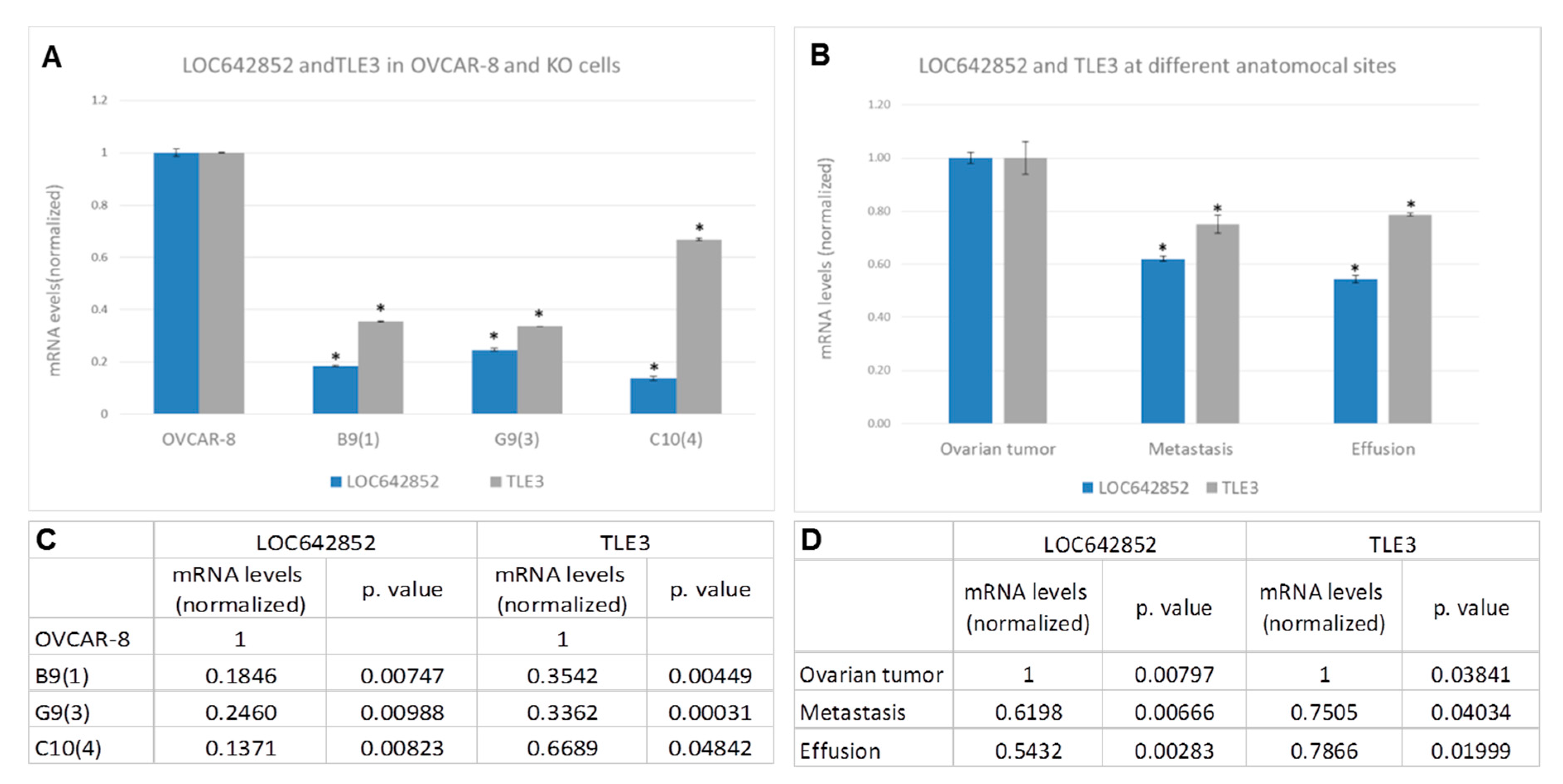
2.4. LOC642852 is Overexpressed in Solid Specimens Compared to HGSC Effusions
2.5. Identification of the Potential Biological Role of LOC642852 in OC
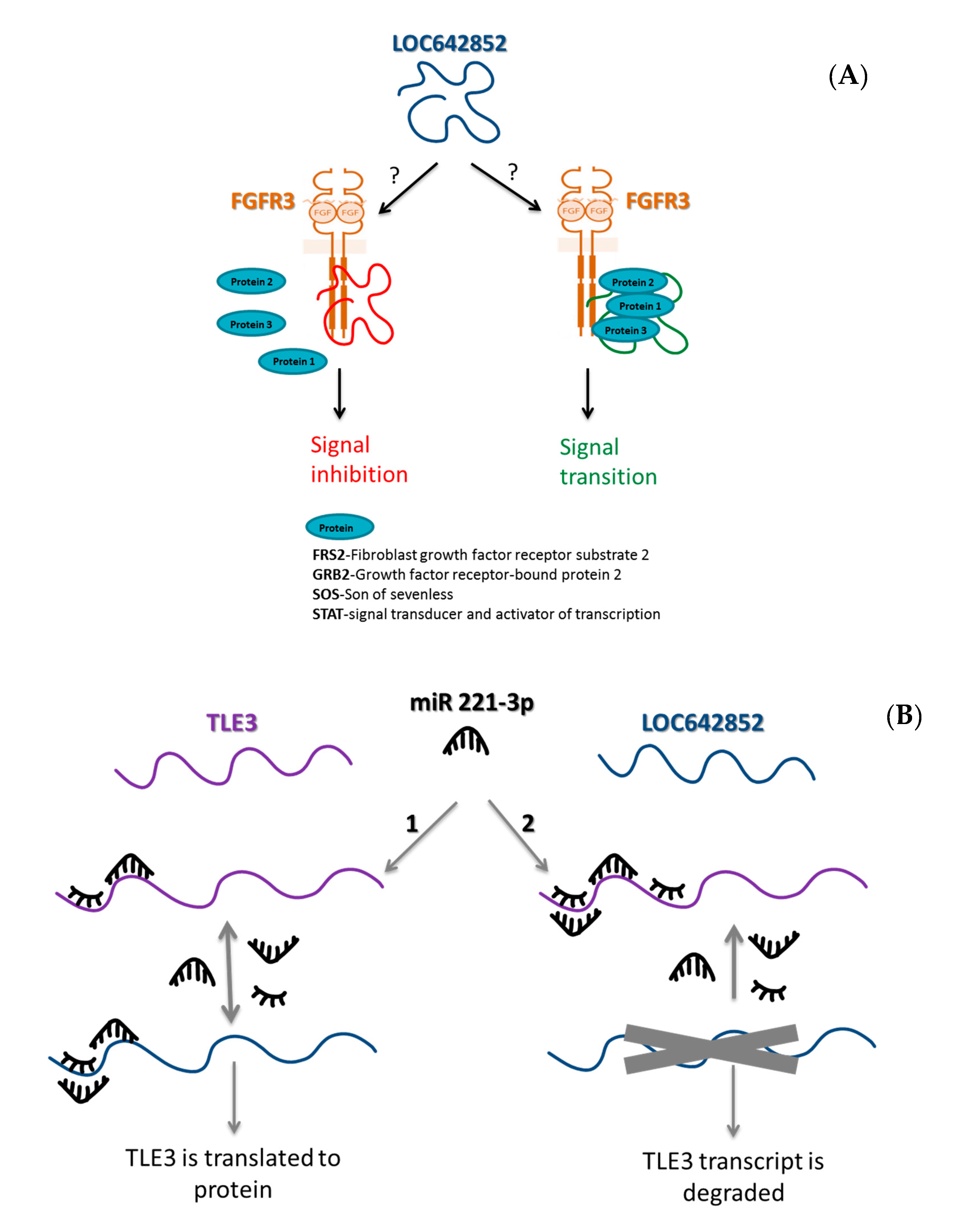
2.5.1. LOC642852/TLE3 ceRNA Network
2.5.2. LOC642852 and FGFR3
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Patients and Specimens
4.3. CRISPR/Cas9 KO
4.3.1. Guides
4.3.2. Transfection
4.4. Spheroid Formation
4.5. Scratch Assay
4.6. Cell Proliferation Assay
4.7. Matrigel Invasion Assay and Motility Assay
4.8. Matrix Metalloproteinase (MMP) Activity Assay (Zymography)
4.9. Western Blot
4.10. RNA Extraction and cDNA Generation
4.11. Quantitative Real-Time Reverse-Transcription Polymerase Chain Reaction (qRT-PCR) and RT-PCR
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fu, X.D. Non-coding RNA: A new frontier in regulatory biology. Natl. Sci Rev. 2014, 1, 190–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarroux, J.; Morillon, A.; Pinskaya, M. Long Non Coding RNA Biology; Rao, M.R.S., Ed.; Advances in Experimental Medicine and Biology Springer Nature Singapore Pte Ltd.: Singapore, 2017; Volume 1008, pp. 1–46. [Google Scholar]
- Bhan, A.; Soleimani, M.; Mandal, S.S. Long noncoding RNA and cancer: A new paradigm. Cancer Res. 2017, 77, 3965–3981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.Y.; Lu, A.Q.; Chen, L.J. LncRNAs in ovarian cancer. Clin. Chim. Acta. 2019, 490, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Li, J.; Wei, B. Long non-coding RNAs in ovarian cancer. J. Exp. Clin. Cancer Res. 2018, 37, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, M.K.; Doxtater, K.; Keramatnia, F.; Zacheaus, C.; Yallapu, M.M.; Jaddi, M. Role of lncRNAs in ovarian cancer: Defining new biomarkers for therapeutic purposes. Drug Discov Today 2018, 23, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Vaksman, O.; Stavnes, H.T.; Kaern, J.; Trope, C.G.; Davidson, B.; Reich, R. miRNA profiling along tumour progression in ovarian carcinoma. J. Cell. Mol. Med. 2011, 15, 1593–1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippov-Levy, N.; Cohen-Schussheim, H.; Tropé, C.G.; Hetland Falkenthal, T.E.; Smith, Y.; Davidson, B.; Reich, R. Expression and clinical role of long non-coding RNA in high-grade serous carcinoma. Gynecol. Oncol. 2018, 148, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Hu, Y.C.; Wang, S.; Lang, J.H. Altered long noncoding RNAs and survival outcomes in ovarian cancer: A systematic review and meta-analysis (PRISMA Compliant). Medicine (Baltimore) 2018, 97, e11481. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.K.; Ranganath, K.; Hammerman, P.S.; Vaklavas, C.; Mohindra, N.; Kalyan, A.; Matsangou, M.; Costa, R.; Carneiro, B.; Villaflor, V.M.; et al. Inhibition of the fibroblast growth factor receptor (FGFR) pathway: The current landscape and barriers to clinical application. Oncotarget 2017, 8, 16052–16074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.W.; Zeng, Y.Y.; Wei, W.T.; Cui, Y.M.; Sun, H.Y.; Cai, Y.L.; Nian, X.X.; Hu, Y.T.; Quan, Y.P.; Jiang, S.L. TLE3 represses colorectal cancer proliferation by inhibiting MAPK and AKT signaling pathways. J. Exp. Clin. Cancer Res. 2016, 35, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samimi, G.; Ring, B.Z.; Ross, D.T.; Seitz, R.S.; Sutherland, R.L.; O’Brien, P.M.; Hacker, N.F.; Huh, W.K. TLE3 expression is associated with sensitivity to taxane treatment in ovarian carcinoma. Cancer Epidemio.l Biomarkers Prev. 2012, 21, 273–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Wang, Y.; Sun, J.; Ma, H.; Guo, C. LINC00205 promotes proliferation, migration and invasion of HCC cells by targeting miR-122-5p. Pathol. Res. Pract. 2019, 215, 152515. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Li, Q.; Zhi, L.J.; Li, J.M.; Wang, Z.Y. LINC00205 modulates the expression of EPHX1 through the inhibition of miR-184 in hepatocellular carcinoma as a ceRNA. J. Cell. Physiol. 2020, 235, 3013–3021. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhang, Y.; Zhang, Q.; Chen, W.; Zhao, H.; Liang, J. A comprehensive genome-wide analysis of long noncoding RNA expression profile in hepatocellular carcinoma. Cancer Med. 2017, 6, 2932–2941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, S.; Wang, B.; Su, Y.; Li, S.; Liu, G.; Zhang, X. An eight-long noncoding RNA expression signature for colorectal cancer patients’ prognosis. J. Cell. Biochem. 2019, 120, 5636–5643. [Google Scholar] [CrossRef] [PubMed]
- Giulietti, M.; Righetti, A.; Principato, G.; Piva, F. LncRNA co-expression network analysis reveals novel biomarkers for pancreatic cancer. Carcinogenesis 2018, 39, 1016–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brassart, B.; Randoux, A.; Hornebeck, W.; Emonard, H. Regulation of matrix metalloproteinase-2 (gelatinase A, MMP-2), membrane-type matrix metalloproteinase-1 (MT1-MMP) and tissue inhibitor of metalloproteinases-2 (TIMP-2) expression by elastin-derived peptides in human HT-1080 fibrosarcoma cell line. Clin. Exp. Metastasis 1998, 16, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Carcangiu, M.L.; Herrington, C.S.; Young, R.H. (Eds.) WHO Classification of Tumors of Female Reproductive Organs, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2014; Volume 6, pp. 11–83. [Google Scholar]
- Davidson, B. Ovarian carcinoma. In Serous Effusions—Etiology, Diagnosis, Prognosis and Therapy, 2nd ed.; Davidson, B., Firat, P., Michael, C.W., Eds.; Springer: London, UK, 2018; pp. 167–204. [Google Scholar]


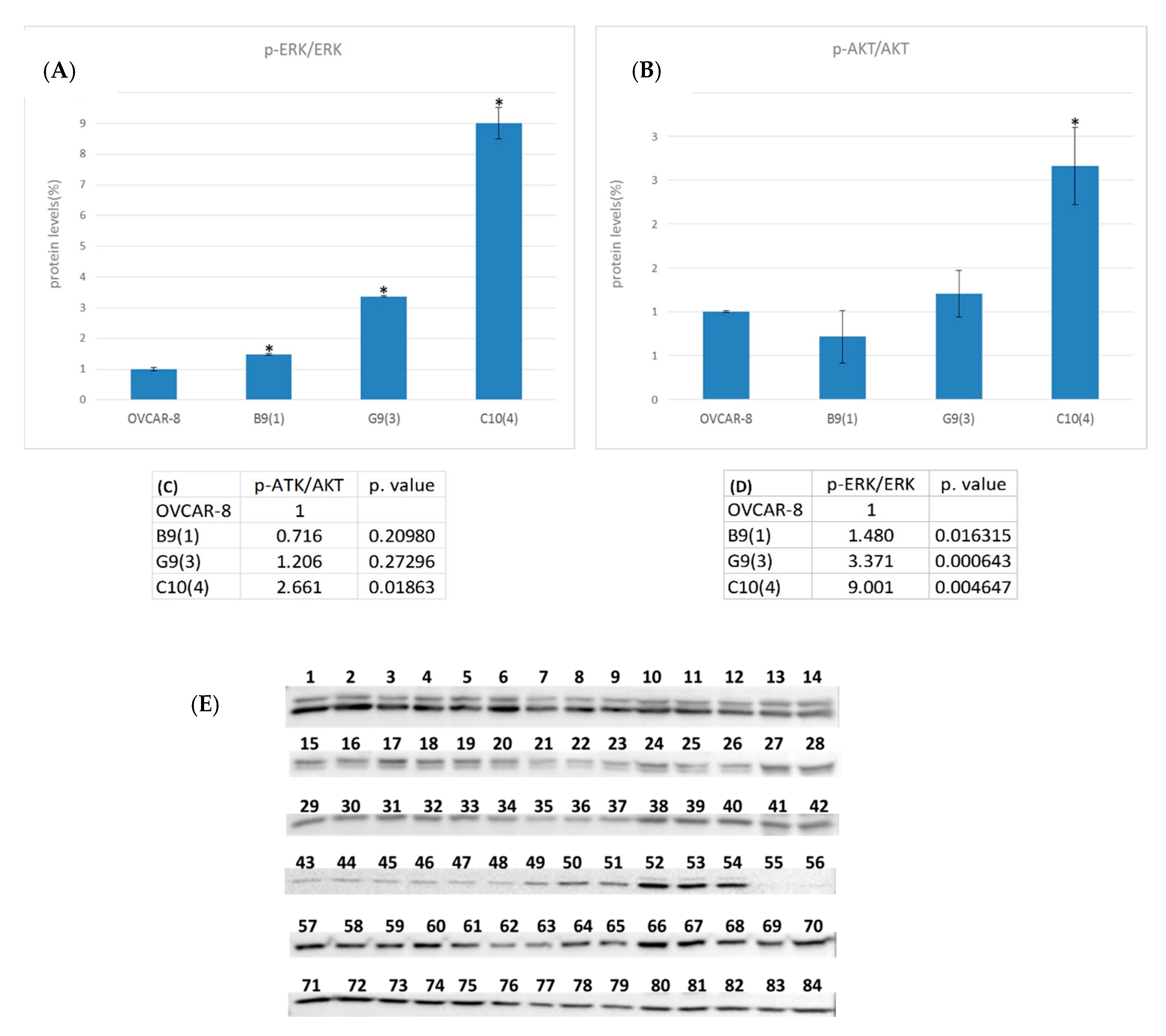

| Protein | OVCAR-8 | B9(1) | G9(3) | C10(4) | HeLa |
|---|---|---|---|---|---|
| ERK | 1–3 | 4–6 | 7–9 | 10–12 | 13–14 |
| AKT | 15–17 | 18–20 | 21–23 | 24–26 | 27–28 |
| GAPDH | 29–31 | 32–34 | 35–37 | 38–40 | 41–42 |
| p-ERK | 43–45 | 46–48 | 49–51 | 52–54 | 55–56 |
| p-AKT | 57–59 | 60–62 | 63–65 | 66–68 | 69–70 |
| GAPDH | 71–73 | 74–76 | 77–79 | 80–82 | 83–84 |
| Parameter | Distribution | |
|---|---|---|
| Age (mean) | 38–81 years (62) | |
| FIGO stage | ||
| III | 52 | |
| IV | 33 | |
| Residual disease | ||
| Primary debulking surgery (n = 56) | 0 cm | 8 |
| ≤1 cm | 20 | |
| >1 cm | 25 | |
| NA | 3 | |
| Interval debulking surgery (n = 25) | 0 cm | 10 |
| ≤1 cm | 6 | |
| >1 cm | 5 | |
| NA | 4 | |
| Only chemotherapy; no surgery | 4 | |
| CA 125 at diagnosis (range; median) | 11–43,800 (760) a | |
| Chemoresponse after primary treatment | ||
| CR | 40 | |
| PR | 25 | |
| SD | 5 | |
| PD | 7 | |
| NA b | 8 | |
| Name | Forward | Reverse |
|---|---|---|
| LOC642852 | GCTTTGTTTCAGGGTTTTGG | AGGTGTGTCCCCAGCTTCT |
| EGFR3 | CCACTCCCTCCATCTCCTG | GGATGCTGCCAAACTTGTTC |
| TLE3 | AGTGTGGAAAAGGCAACCAG | GACACAGAGAGGGCAAGAGG |
| RPLP0 | CCAACTACTTCCTTAAGATCATCCAACTA | ACATGCGGATCTGCTGCA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippov-Levy, N.; Reich, R.; Davidson, B. The Biological and Clinical Role of the Long Non-Coding RNA LOC642852 in Ovarian Carcinoma. Int. J. Mol. Sci. 2020, 21, 5237. https://doi.org/10.3390/ijms21155237
Filippov-Levy N, Reich R, Davidson B. The Biological and Clinical Role of the Long Non-Coding RNA LOC642852 in Ovarian Carcinoma. International Journal of Molecular Sciences. 2020; 21(15):5237. https://doi.org/10.3390/ijms21155237
Chicago/Turabian StyleFilippov-Levy, Natalie, Reuven Reich, and Ben Davidson. 2020. "The Biological and Clinical Role of the Long Non-Coding RNA LOC642852 in Ovarian Carcinoma" International Journal of Molecular Sciences 21, no. 15: 5237. https://doi.org/10.3390/ijms21155237





