Insights into the Effects of Mesenchymal Stem Cell-Derived Secretome in Parkinson’s Disease
Abstract
1. Introduction
1.1. Cellular and Molecular Mechanisms of PD
1.2. Searching for New Biomarkers and Therapeutic Approaches for PD
2. The Fate of MSC-Derived Secretome
2.1. Positive Effects of MSCs in PD
2.2. Positive Effects of MSC-Derived Conditioned Medium in PD
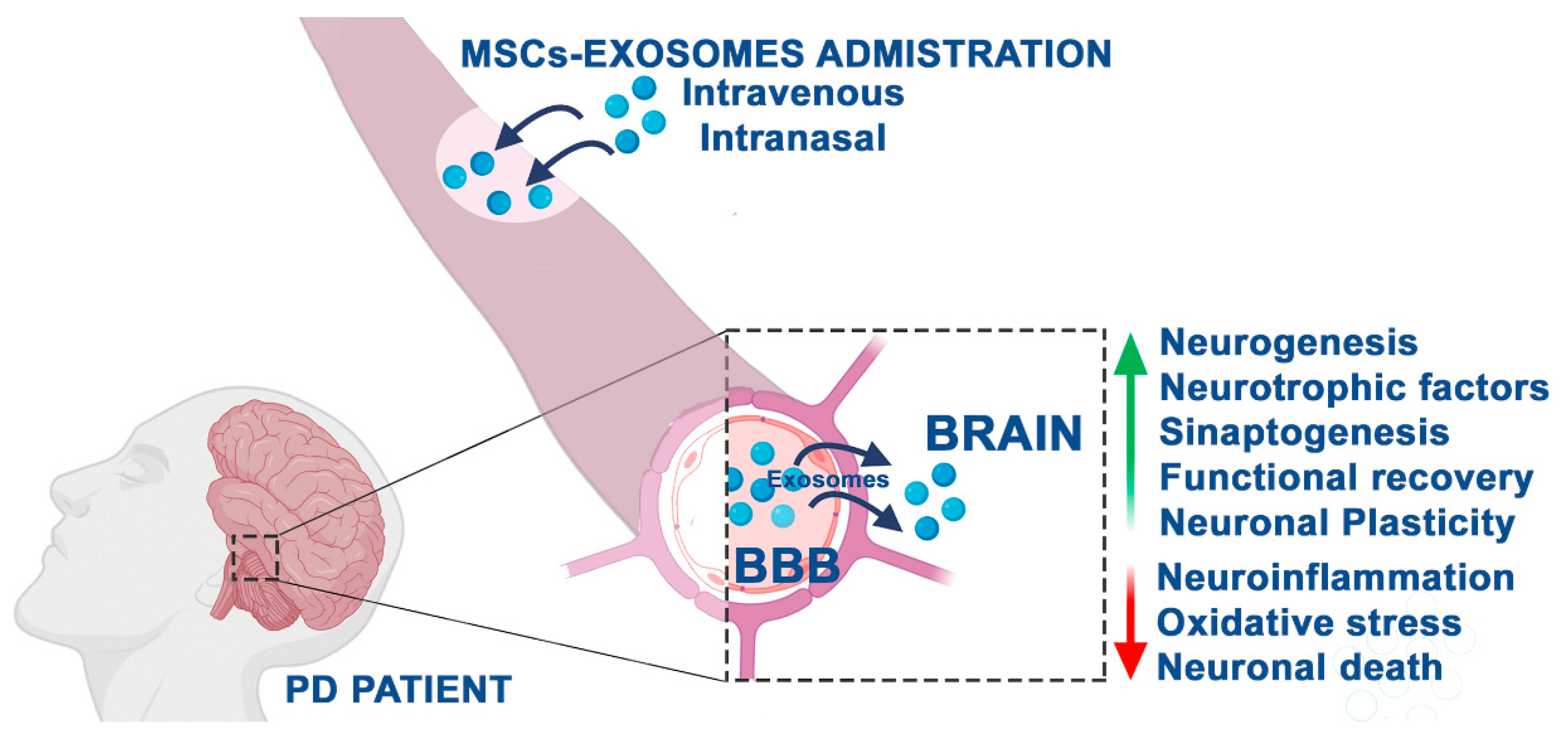
2.3. Positive Effects of MSC-Derived Exosomes in PD
3. MSC-Secretome: miRNA Relevance and Theranostic Applications in PD
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EVs | Extravesicles |
| MenSCs | Menstrual blood-derived mesenchymal stem cells |
| MPP+. | 1-methyl-4-phenylpyridinium |
| BMMSCs | Bone marrow mesenchymal stem cells |
| MSCs | Mesenchymal stem cells |
| hucMSCs | Human umbilical cord mesenchymal stem cells |
| PD | Parkinson’s disease |
| 6-OHDA | 6-hydroxydopamine |
| ASC | Adipose stem cells |
| CM | Conditioned media |
| SHED | Human exfoliated deciduous teeth |
| TH | Tyrosine hydroxylase |
References
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.-L.; Zhang, Y.; Li, X.; Fu, Q.-L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Wu, K.-C.; Harn, H.-J.; Lin, S.-Z.; Ding, D.-C. Exosomes and Stem Cells in Degenerative Disease Diagnosis and Therapy. Cell Transplant. 2018, 27, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 2019, 8, 467. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Gonçalves, R.M. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef]
- Phelps, J.; Sanati-Nezhad, A.; Ungrin, M.; Duncan, N.A.; Sen, A. Bioprocessing of Mesenchymal Stem Cells and Their Derivatives: Toward Cell-Free Therapeutics. Stem Cells Int. 2018, 2018, 9415367. [Google Scholar] [CrossRef]
- Samanta, S.; Rajasingh, S.; Drosos, N.; Zhou, Z.; Dawn, B.; Rajasingh, J. Exosomes: new molecular targets of diseases. Acta Pharmacol. Sin. 2017, 39, 501–513. [Google Scholar] [CrossRef]
- Ramos, T.L.; Sanchez-Abarca, L.I.; Muntion, S.; Preciado, S.; Puig, N.; López-Ruano, G.; Hernández-Hernández, A.; Redondo, A.; Ortega, R.; Rodríguez, C.; et al. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun. Signal. 2016, 14, 2. [Google Scholar] [CrossRef]
- Dabrowska, S.; Del Fattore, A.; Karnas, E.; Frontczak-Baniewicz, M.; Kozlowska, H.; Muraca, M.; Janowski, M.; Lukomska, B. Imaging of extracellular vesicles derived from human bone marrow mesenchymal stem cells using fluorescent and magnetic labels. Int. J. Nanomed. 2018, 13, 1653–1664. [Google Scholar] [CrossRef]
- Almeria, C.; Weiss, R.; Roy, M.; Tripisciano, C.; Kasper, C.; Weber, V.; Egger, D. Hypoxia Conditioned Mesenchymal Stem Cell-Derived Extracellular Vesicles Induce Increased Vascular Tube Formation in vitro. Front. Bioeng. Biotechnol. 2019, 7, 292. [Google Scholar] [CrossRef]
- Qiu, G.; Zheng, G.; Ge, M.; Wang, J.; Huang, R.; Shu, Q.; Xu, J. Functional proteins of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res. Ther. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Adamo, A.; Brandi, J.; Caligola, S.; Delfino, P.; Bazzoni, R.; Carusone, R.; Cecconi, D.; Giugno, R.; Manfredi, M.; Robotti, E.; et al. Extracellular Vesicles Mediate Mesenchymal Stromal Cell-Dependent Regulation of B Cell PI3K-AKT Signaling Pathway and Actin Cytoskeleton. Front. Immunol. 2019, 10, 446. [Google Scholar] [CrossRef] [PubMed]
- Codispoti, B.; Marrelli, M.; Paduano, F.; Tatullo, M. NANOmetric BIO-Banked MSC-Derived Exosome (NANOBIOME) as a Novel Approach to Regenerative Medicine. J. Clin. Med. 2018, 7, 357. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.M.; Shin, E.K.; Sung, J.H.; Moon, G.J.; Kim, E.H.; Cho, Y.H.; Park, H.D.; Bae, H.; Kim, J.; Bang, O.Y. Efficient scalable production of therapeutic microvesicles derived from human mesenchymal stem cells. Sci. Rep. 2018, 8, 1171. [Google Scholar] [CrossRef]
- Sagaradze, G.; Grigorieva, O.; Nimiritsky, P.; Basalova, N.; Kalinina, N.I.; Akopyan, Z.; Efimenko, A.Y. Conditioned Medium from Human Mesenchymal Stromal Cells: Towards the Clinical Translation. Int. J. Mol. Sci. 2019, 20, 1656. [Google Scholar] [CrossRef]
- Pawitan, J.A. Prospect of Stem Cell Conditioned Medium in Regenerative Medicine. BioMed Res. Int. 2014, 2014, 965849. [Google Scholar] [CrossRef]
- Rani, S.; Ryan, A.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiró, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Tuazon, J.P.; Castelli, V.; Borlongan, C.V. Drug-like delivery methods of stem cells as biologics for stroke. Expert Opin. Drug Deliv. 2019, 16, 823–833. [Google Scholar] [CrossRef]
- Tuazon, J.P.; Castelli, V.; Lee, J.-Y.; Desideri, G.B.; Stuppia, L.; Cimini, A.M.; Borlongan, C.V. Neural Stem Cells. Adv. Exp. Med. Biol. 2019, 1201, 79–91. [Google Scholar] [CrossRef]
- Khanmohammadi, N.; Sameni, H.R.; Mohammadi, M.; Pakdel, A.; Mirmohammadkhani, M.; Parsaie, H.; Zarbakhsh, S. Effect of Transplantation of Bone Marrow Stromal Cell- Conditioned Medium on Ovarian Function, Morphology and Cell Death in Cyclophosphamide-Treated Rats. Cell J. 2018, 20, 10–18. [Google Scholar] [PubMed]
- Sun, D.Z.; Abelson, B.; Babbar, P.; Damaser, M.S. Harnessing the mesenchymal stem cell secretome for regenerative urology. Nat. Rev. Urol. 2019, 16, 363–375. [Google Scholar] [CrossRef]
- Castelli, V.; Benedetti, E.; Antonosante, A.; Catanesi, M.; Pitari, G.; Ippoliti, R.; Cimini, A.; D’Angelo, M. Neuronal Cells Rearrangement During Aging and Neurodegenerative Disease: Metabolism, Oxidative Stress and Organelles Dynamic. Front. Mol. Neurosci. 2019, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Calì, T.; Ottolini, D.; Brini, M. Calcium signaling in Parkinson’s disease. Cell Tissue Res. 2014, 357, 439–454. [Google Scholar] [CrossRef]
- Castelli, V.; D’Angelo, M.; Lombardi, F.; Alfonsetti, M.; Antonosante, A.; Catanesi, M.; Benedetti, E.; Palumbo, P.; Cifone, M.G.; Giordano, A.; et al. Effects of the probiotic formulation SLAB51 in in vitro and in vivo Parkinson’s disease models. Aging 2020, 12, 4641–4659. [Google Scholar] [CrossRef] [PubMed]
- Caggiu, E.; Arru, G.; Hosseini, S.; Niegowska, M.; Sechi, G.; Zarbo, I.R.; Sechi, L.A. Inflammation, Infectious Triggers, and Parkinson’s Disease. Front. Neurol. 2019, 10, 122. [Google Scholar] [CrossRef]
- Kouli, A.; Torsney, K.M.; Kuan, W.-L.; Stoker, T.B.; Greenland, J.C. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Codon Publications: Brisbane, Australia, 2018; pp. 3–26. [Google Scholar]
- Polymeropoulos, M.H. Mutation in the -Synuclein Gene Identified in Families with Parkinson’s Disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef]
- Castelli, V.; Melani, F.; Ferri, C.; D’Angelo, M.; Catanesi, M.; Grassi, D.; Benedetti, E.; Giordano, A.; Cimini, A.; Desideri, G. Neuroprotective activities of bacopa, lycopene, astaxanthin, and vitamin B12 combination on oxidative stress-dependent neuronal death. J. Cell. Biochem. 2020, 29722. [Google Scholar] [CrossRef]
- Chen, C.; Turnbull, D.M.; Reeve, A.K. Mitochondrial Dysfunction in Parkinson’s Disease—Cause or Consequence? Boilogy 2019, 8, 38. [Google Scholar] [CrossRef]
- Bandres-Ciga, S.; Cookson, M.R. Alpha-synuclein triggers T-cell response. Is Parkinson’s disease an autoimmune disorder? Mov. Disord. 2017, 32, 1327. [Google Scholar] [CrossRef]
- Chung, C.Y.; Koprich, J.B.; Siddiqi, H.; Isacson, O. Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. J. Neurosci. 2009, 29, 3365–3373. [Google Scholar] [CrossRef] [PubMed]
- Sulzer, D.; Alcalay, R.N.; Garretti, F.; Cote, L.; Kanter, E.; Agin-Liebes, J.P.; Liong, C.; McMurtrey, C.; Hildebrand, W.H.; Mao, X.; et al. T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature 2017, 546, 656–661. [Google Scholar] [CrossRef]
- García-Sanz, P.; Orgaz, L.; Bueno-Gil, G.; Espadas, I.; Rodríguez-Traver, E.; Kulisevsky, J.; Gutierrez, A.; Dávila, J.C.; González-Polo, R.A.; Fuentes, J.M.; et al. N370S-GBA1 mutation causes lysosomal cholesterol accumulation in Parkinson’s disease. Mov. Disord. 2017, 32, 1409–1422. [Google Scholar] [CrossRef] [PubMed]
- Burbulla, L.F.; Song, P.; Mazzulli, J.R.; Zampese, E.; Wong, Y.C.; Jeon, S.; Santos, D.P.; Blanz, J.; Obermaier, C.D.; Strojny, C.; et al. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science 2017, 357, 1255–1261. [Google Scholar] [CrossRef]
- Dias, V.; Junn, E.; Mouradian, M.M. The Role of Oxidative Stress in Parkinson’s Disease. J. Park. Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [PubMed]
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxidative Med. Cell. Longev. 2019. [Google Scholar] [CrossRef]
- Zeng, X.-S.; Geng, W.-S.; Jia, J.-J. Neurotoxin-Induced Animal Models of Parkinson Disease: Pathogenic Mechanism and Assessment. ASN Neuro 2018, 10, 1–15. [Google Scholar] [CrossRef]
- Pałasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef]
- Luarte, A.; Bátiz, L.F.; Wyneken, U.; Lafourcade, C. Potential Therapies by Stem Cell-Derived Exosomes in CNS Diseases: Focusing on the Neurogenic Niche. Stem Cells Int. 2016, 2016, 5736059. [Google Scholar] [CrossRef]
- Hu, S.; Loo, J.A.; Wong, D.T. Human body fluid proteome analysis. Proteomics 2006, 6, 6326–6353. [Google Scholar] [CrossRef]
- Marrugo-Ramírez, J.; Mir, M.; Samitier, J. Blood-Based Cancer Biomarkers in Liquid Biopsy: A Promising Non-Invasive Alternative to Tissue Biopsy. Int. J. Mol. Sci. 2018, 19, 2877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Record, M.; Poirot, S.S.; Poirot, M.; Wakelam, M.J.O. Extracellular vesicles: Lipids as key components of their biogenesis and functions. J. Lipid Res. 2018, 59, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Sun, T.; An, J.; Wen, L.; Liu, F.; Bu, Z.; Cui, Y.; Feng, J. Potential Roles of Exosomes in Parkinson’s Disease: From Pathogenesis, Diagnosis, and Treatment to Prognosis. Front. Cell Dev. Boil. 2020, 8, 86. [Google Scholar] [CrossRef]
- Dorszewska, J.; Prendecki, M.; Lianeri, M.; Kozubski, W. Molecular Effects of L-dopa Therapy in Parkinson’s Disease. Curr. Genom. 2014, 15, 11–17. [Google Scholar] [CrossRef]
- Charvin, D.; Medori, R.; Hauser, R.A.; Rascol, O. Therapeutic strategies for Parkinson disease: beyond dopaminergic drugs. Nat. Rev. Drug Discov. 2018, 17, 804–822. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Nordin, J.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mäger, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef]
- Choi, H.; Lee, D.S. Illuminating the physiology of extracellular vesicles. Stem Cell Res. Ther. 2016, 7, 55. [Google Scholar] [CrossRef]
- Yin, K.; Wang, S.; Zhao, R.C. Exosomes from mesenchymal stem/stromal cells: A new therapeutic paradigm. Biomark. Res. 2019, 7, 8. [Google Scholar] [CrossRef]
- Busato, A.; Bonafede, R.; Bontempi, P.; Scambi, I.; Schiaffino, L.; Benati, N.; Malatesta, M.; Sbarbati, A.; Marzola, P.; Mariotti, R. Labeling and Magnetic Resonance Imaging of Exosomes Isolated from Adipose Stem Cells. Curr. Protoc. Cell Boil. 2017, 75, 3–44. [Google Scholar] [CrossRef]
- Otero-Ortega, L.; De Frutos, M.C.G.; Laso-García, F.; Rodríguez-Frutos, B.; Gutiérrez-Fernández, M.; Lopez, J.A.; Vázquez, J.; Díez-Tejedor, E.; Gutiérrez-Fernández, M. Exosomes promote restoration after an experimental animal model of intracerebral hemorrhage. Br. J. Pharmacol. 2017, 38, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Betzer, O.; Perets, N.; Angel, A.; Motiei, M.; Sadan, T.; Yadid, G.; Offen, D.; Popovtzer, R. In Vivo Neuroimaging of Exosomes Using Gold Nanoparticles. ACS Nano 2017, 11, 10883–10893. [Google Scholar] [CrossRef]
- Lai, C.P.-K.; Mardini, O.; Ericsson, M.; Prabhakar, S.; Maguire, C.A.; Chen, J.W.; Tannous, B.A.; Breakefield, X.O. Dynamic Biodistribution of Extracellular Vesicles in Vivo Using a Multimodal Imaging Reporter. ACS Nano 2014, 8, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Baek, G.; Choi, H.; Kim, Y.; Lee, H.; Choi, C. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Therapeutics and as a Drug Delivery Platform. Stem Cells Transl. Med. 2019, 8, 880–886. [Google Scholar] [CrossRef]
- Imai, T.; Takahashi, Y.; Nishikawa, M.; Kato, K.; Morishita, M.; Yamashita, T.; Matsumoto, A.; Charoenviriyakul, C.; Takakura, Y. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J. Extracell. Vesicles 2015, 4, 26238. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.L.; Medicetty, S.; Bledsoe, A.R.; Rachakatla, R.S.; Choi, M.; Merchav, S.; Luo, Y.; Rao, M.S.; Velagaleti, G.; Troyer, D. Human Umbilical Cord Matrix Stem Cells: Preliminary Characterization and Effect of Transplantation in a Rodent Model of Parkinson’s Disease. Stem Cells 2006, 24, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yasuhara, T.; Shingo, T.; Kameda, M.; Tajiri, N.; Yuan, W.J.; Kondo, A.; Kadota, T.; Baba, T.; Tayra, J.T.; et al. Intravenous administration of mesenchymal stem cells exerts therapeutic effects on parkinsonian model of rats: Focusing on neuroprotective effects of stromal cell-derived factor-1α. BMC Neurosci. 2010, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Cova, L.; Armentero, M.-T.; Zennaro, E.; Calzarossa, C.; Bossolasco, P.; Busca, G.; Deliliers, G.L.; Polli, E.; Nappi, G.; Silani, V.; et al. Multiple neurogenic and neurorescue effects of human mesenchymal stem cell after transplantation in an experimental model of Parkinson’s disease. Brain Res. 2010, 1311, 12–27. [Google Scholar] [CrossRef]
- Yalvac, M.; Yarat, A.; Mercan, D.; Rizvanov, A.; Palotás, A.; Sahin, F. Characterization of the secretome of human tooth germ stem cells (hTGSCs) reveals neuro-protection by fine-tuning micro-environment. Brain Behav. Immun. 2013, 32, 122–130. [Google Scholar] [CrossRef]
- Parga, J.A.; García-Garrote, M.; Martínez, S.; Raya, Á.; Labandeira-García, J.L.; Rodriguez-Pallares, J. Prostaglandin EP2 Receptors Mediate Mesenchymal Stromal Cell-Neuroprotective Effects on Dopaminergic Neurons. Mol. Neurobiol. 2017, 55, 4763–4776. [Google Scholar] [CrossRef]
- Nakhaeifard, M.; Kashani, M.H.G.; Goudarzi, I.; Rezaei, A. Conditioned Medium Protects Dopaminergic Neurons in Parkinsonian Rats. Cell J. 2018, 20, 348–354. [Google Scholar] [PubMed]
- Mercado, N.; Collier, T.; Sortwell, C.; Steece-Collier, K. BDNF in the Aged Brain: Translational Implications for Parkinson’s Disease. Austin Neurol. Neurosci. 2017, 2, 2. [Google Scholar]
- Oh, S.H.; Na Kim, H.; Park, H.J.; Shin, J.Y.; Kim, D.Y.; Lee, P.H. The Cleavage Effect of Mesenchymal Stem Cell and Its Derived Matrix Metalloproteinase-2 on Extracellular α-Synuclein Aggregates in Parkinsonian Models. Stem Cells Transl. Med. 2016, 6, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Pinheiro, B.; Anjo, S.I.; Manadas, B.; Da Silva, J.D.; Marote, A.; Behie, L.A.; Teixeira, F.G.; Salgado, A.J. Bone Marrow Mesenchymal Stem Cells’ Secretome Exerts Neuroprotective Effects in a Parkinson’s Disease Rat Model. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef]
- Abdelwahab, S.; Elsebay, S.A.G.; Gaber, M.F.; Hafez, S.M.N.A. Comparative study between bone marrow mesenchymal stem cell and their conditioned medium in the treatment of rat model of Parkinsonism. J. Cell. Physiol. 2020. [Google Scholar] [CrossRef]
- Chen, Y.-R.; Lai, P.-L.; Chien, Y.; Lee, P.-H.; Lai, Y.-H.; Ma, H.-I.; Shiau, C.-Y.; Wang, K.-C. Improvement of Impaired Motor Functions by Human Dental Exfoliated Deciduous Teeth Stem Cell-Derived Factors in a Rat Model of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 3807. [Google Scholar] [CrossRef]
- Li, H.; Yahaya, B.H.; Ng, W.H.; Yusoff, N.M.; Lin, J. Conditioned Medium of Human Menstrual Blood-Derived Endometrial Stem Cells Protects Against MPP+-Induced Cytotoxicity in vitro. Front. Mol. Neurosci. 2019, 12, 80. [Google Scholar] [CrossRef]
- Shintani, A.; Nakao, N.; Kakishita, K.; Itakura, T. Protection of dopamine neurons by bone marrow stromal cells. Brain Res. 2007, 1186, 48–55. [Google Scholar] [CrossRef]
- Marques, C.; Marote, A.; Mendes-Pinheiro, B.; Teixeira, F.G.; Salgado, A.J. Cell secretome based approaches in Parkinson’s disease regenerative medicine. Expert Opin. Boil. Ther. 2018, 18, 1235–1245. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, C.; Gu, P.; Wen, T. Combined MSC-Secreted Factors and Neural Stem Cell Transplantation Promote Functional Recovery of PD Rats. Cell Transplant. 2016, 25, 1101–1113. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, X.; Li, X. Exosomes Derived from Mesenchymal Stem Cells. Int. J. Mol. Sci. 2014, 15, 4142–4157. [Google Scholar] [CrossRef] [PubMed]
- Baharlooi, H.; Azimi, M.; Salehi, Z.; Izad, M. Mesenchymal Stem Cell-Derived Exosomes: A Promising Therapeutic Ace Card to Address Autoimmune Diseases. Int. J. Stem Cells 2020, 13, 13–23. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, K.J.; Powell, K.L.; Ashton, A.W.; Morris, J.M.; McCracken, S.A. Exosomes: Mechanisms of Uptake. J. Circ. Biomark. 2015, 4, 7. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Promote Functional Recovery and Neurovascular Plasticity After Stroke in Rats. Br. J. Pharmacol. 2013, 33, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chopp, M.; Meng, Y.; Katakowski, M.; Xin, H.; Mahmood, A.; Xiong, Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J. Neurosurg. 2015, 122, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Vaccari, J.P.D.R.; Brand, F.; Adamczak, S.; Lee, S.W.; Pérez-Bárcena, J.; Wang, M.Y.; Bullock, M.R.; Dietrich, W.D.; Keane, R.W. Exosome-mediated inflammasome signaling after central nervous system injury. J. Neurochem. 2015, 136, 39–48. [Google Scholar] [CrossRef]
- Han, D.; Wu, C.; Xiong, Q.; Zhou, L.; Tian, Y. Anti-inflammatory Mechanism of Bone Marrow Mesenchymal Stem Cell Transplantation in Rat Model of Spinal Cord Injury. Cell Biophys. 2014, 71, 1341–1347. [Google Scholar] [CrossRef]
- Reza-Zaldivar, E.E.; Sapiéns, M.A.H.; Minjarez, B.; Gutiérrez-Mercado, Y.K.; Márquez-Aguirre, A.L.; Canales-Aguirre, A. Potential Effects of MSC-Derived Exosomes in Neuroplasticity in Alzheimer’s Disease. Front. Cell. Neurosci. 2018, 12, 317. [Google Scholar] [CrossRef]
- Jarmalavičiūtė, A.; Tunaitis, V.; Pivoraitė, U.; Venalis, A.; Pivoriūnas, A. Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine–induced apoptosis. Cytotherapy 2015, 17, 932–939. [Google Scholar] [CrossRef]
- Chen, H.-X.; Liang, F.-C.; Gu, P.; Xu, B.-L.; Xu, H.-J.; Wang, W.-T.; Hou, J.-Y.; Xie, D.-X.; Chai, X.-Q.; An, S.-J. Exosomes derived from mesenchymal stem cells repair a Parkinson’s disease model by inducing autophagy. Cell Death Dis. 2020, 11, 288. [Google Scholar] [CrossRef]
- Wood, H. MicroRNAs - diagnostic markers in Parkinson disease? Nat. Rev. Neurol. 2020, 16, 65. [Google Scholar] [CrossRef]
- Recasens, A.; Perier, C.; Sue, C.M. Role of microRNAs in the Regulation of α-Synuclein Expression: A Systematic Review. Front. Mol. Neurosci. 2016, 9, 128. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, Y. miR-16-1 Promotes the Aberrant α-Synuclein Accumulation in Parkinson Disease via Targeting Heat Shock Protein 70. Sci. World J. 2014, 2014, 938348. [Google Scholar] [CrossRef]
- Li, K.; Zhao, H.; Li, C.-M.; Ma, X.-X.; Chen, M.; Li, S.-H.; Wang, R.; Lou, B.-H.; Chen, H.-B.; Su, W. The Relationship between Side of Onset and Cerebral Regional Homogeneity in Parkinson’s Disease: A Resting-State fMRI Study. Park. Dis. 2020, 2020, 5146253. [Google Scholar] [CrossRef] [PubMed]
- Kabaria, S.; Choi, D.C.; Chaudhuri, A.D.; Mouradian, M.M.; Junn, E. Inhibition of miR-34b and miR-34c enhances α-synuclein expression in Parkinson’s disease. FEBS Lett. 2014, 589, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Leggio, L.; Vivarelli, S.; L’Episcopo, F.; Tirolo, C.; Caniglia, S.; Testa, N.; Marchetti, B.; Iraci, N. microRNAs in Parkinson’s Disease: From Pathogenesis to Novel Diagnostic and Therapeutic Approaches. Int. J. Mol. Sci. 2017, 18, 2698. [Google Scholar] [CrossRef] [PubMed]
- Arshad, A.R.; Sulaiman, S.A.; Saperi, A.A.; Jamal, R.; Ibrahim, N.M.; Murad, N.A.A. MicroRNAs and Target Genes As Biomarkers for the Diagnosis of Early Onset of Parkinson Disease. Front. Mol. Neurosci. 2017, 10, 352. [Google Scholar] [CrossRef]
- Konovalova, J.; Gerasymchuk, D.; Parkkinen, I.; Chmielarz, P.; Domanskyi, A. Interplay between MicroRNAs and Oxidative Stress in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 6055. [Google Scholar] [CrossRef] [PubMed]
- Vilaça-Faria, H.; Salgado, A.J.; Teixeira, F.G. Mesenchymal Stem Cells-derived Exosomes: A New Possible Therapeutic Strategy for Parkinson’s Disease? Cells 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Li, Y.; Buller, B.; Katakowski, M.; Zhang, Y.; Wang, X.; Shang, X.; Zhang, Z.G.; Chopp, M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 2012, 30, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-S.; Bandeira, E.; Shelke, G.V.; Lässer, C.; Lötvall, J. Enhancement of therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res. Ther. 2019, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wu, J.; Wang, J.; Li, Y.; Hu, X.; Luo, S.; Xiang, D.-X. Extracellular vesicles derived from different sources of mesenchymal stem cells: therapeutic effects and translational potential. Cell Biosci. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Katakowski, M.; Wang, F.; Qian, J.-Y.; Liu, X.S.; Ali, M.M.; Buller, B.; Zhang, Z.G.; Chopp, M. MicroRNA cluster miR-17-92 Cluster in Exosomes Enhance Neuroplasticity and Functional Recovery After Stroke in Rats. Stroke 2017, 48, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, P.; Gilbert, K.S.; Hu, K.; Cronin-Golomb, A. Circadian Rest-Activity Rhythms Predict Cognitive Function in Early Parkinson’s Disease Independently of Sleep. Mov. Disord. Clin. Pract. 2018, 5, 614–619. [Google Scholar] [CrossRef]
- Tatullo, M.; Marrelli, B.; Zullo, M.J.; Codispoti, B.; Paduano, F.; Benincasa, C.; Fortunato, F.; Scacco, S.; Zavan, B.; Cocco, T. Exosomes from Human Periapical Cyst-MSCs: Theranostic Application in Parkinson’s Disease. Int. J. Med Sci. 2020, 17, 657–663. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
d’Angelo, M.; Cimini, A.; Castelli, V. Insights into the Effects of Mesenchymal Stem Cell-Derived Secretome in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 5241. https://doi.org/10.3390/ijms21155241
d’Angelo M, Cimini A, Castelli V. Insights into the Effects of Mesenchymal Stem Cell-Derived Secretome in Parkinson’s Disease. International Journal of Molecular Sciences. 2020; 21(15):5241. https://doi.org/10.3390/ijms21155241
Chicago/Turabian Styled’Angelo, Michele, Annamaria Cimini, and Vanessa Castelli. 2020. "Insights into the Effects of Mesenchymal Stem Cell-Derived Secretome in Parkinson’s Disease" International Journal of Molecular Sciences 21, no. 15: 5241. https://doi.org/10.3390/ijms21155241
APA Styled’Angelo, M., Cimini, A., & Castelli, V. (2020). Insights into the Effects of Mesenchymal Stem Cell-Derived Secretome in Parkinson’s Disease. International Journal of Molecular Sciences, 21(15), 5241. https://doi.org/10.3390/ijms21155241






