Progesterone in the Brain: Hormone, Neurosteroid and Neuroprotectant
Abstract
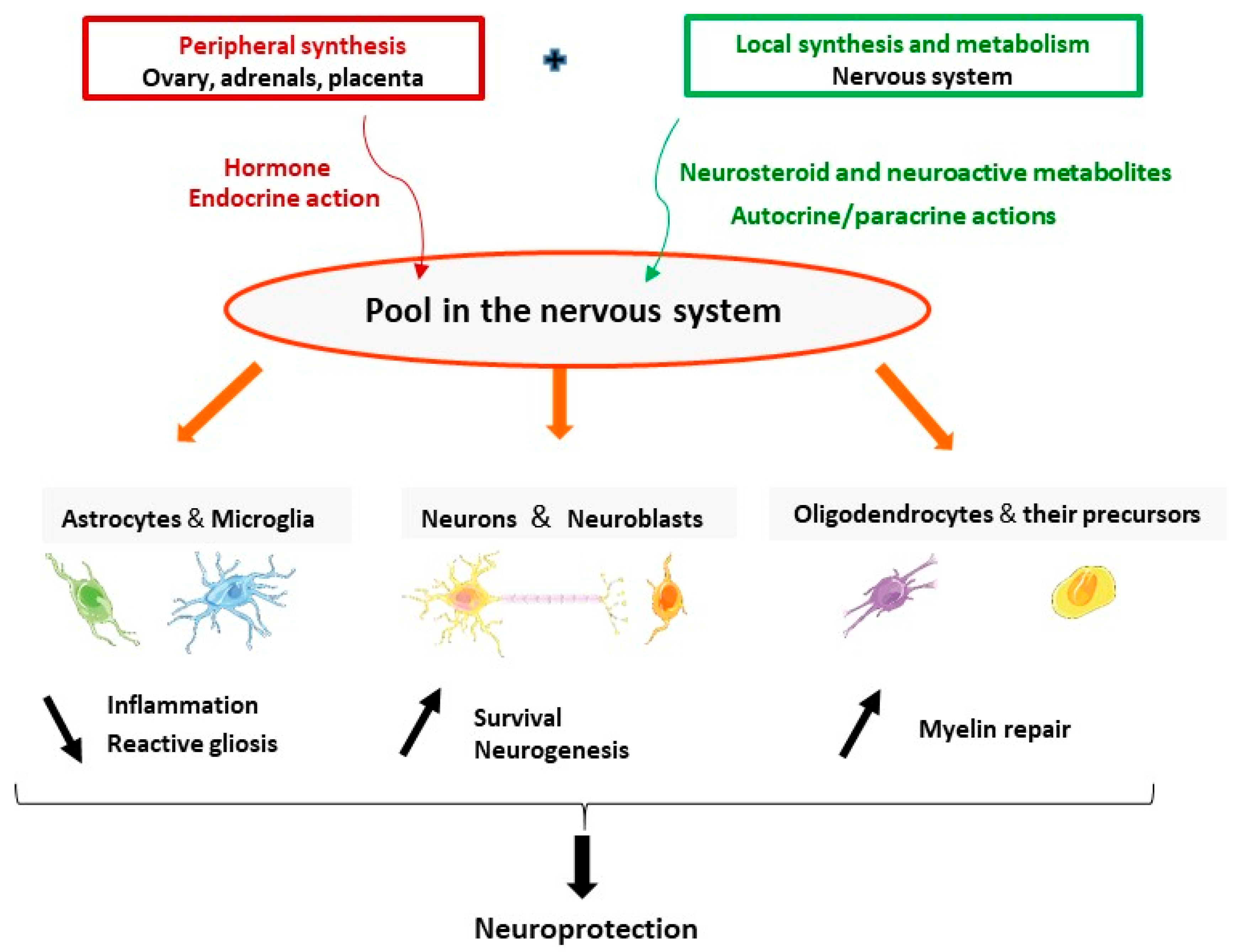
:1. Introduction
2. Progesterone Synthesis, Metabolism and Mechanism of Action in Brain
2.1. Progesterone Is an Endogenous Hormone and a Neurosteroid
2.2. Enzymes Involved in the Synthesis of Progesterone and Its Neuroactive Metabolites
2.3. Progesterone Receptors’ Expression in the Brain
3. Brain Levels of Progesterone and Its Metabolites
3.1. Sex Differences and Decline during Aging
3.2. Levels in Neurodegenerative Conditionsand in Response to Brain Injuries
4. Progesterone and Its Metabolites for Brain Neuroprotection
4.1. Neuroprotective Effects in Neurodegenerative Diseases Models
4.2. Neuroprotective Effects in TBI
4.3. Neuroprotective Effects in Stroke Models
5. Mechanism of Action of Progesterone after Stroke: A Key Role of PR
6. Summary and Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3α-HSD | 3α-hydroxysteroid dehydrogenase |
| 3β-HSD | 3β-hydroxysteroid dehydrogenase |
| 5α-DHPROG | 5α-dihydroprogesterone |
| 3α,5α-THPROG | 3α,5α-tetrahydroprogesterone (allopregnanolone) |
| 6-OHDA | 6-hydroxydopamine |
| AD | Alzheimer’s disease |
| BBB | Blood brain barrier |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| EAE | Experimental autoimmune encephalomyelitis |
| GABA | γ-aminobutyric acid |
| Il-18 | Interferon-gamma inducing factor 18 |
| MCAO/R | Middle cerebral artery occlusion/reperfusion |
| mPR | Membrane progesterone receptors |
| MS | Multiple sclerosis |
| NLRP3 | NOD-like receptor pyrin domain containing 3 |
| NP-C | Niemann–Pick type C |
| P450scc | Cholesterol side-chain cleavage |
| PD | Parkinson’s disease |
| PGRMC1 | Membrane-associated progesterone receptor membrane component 1 |
| PR | Progesterone receptors |
| PROG | Progesterone |
| TBI | Traumatic brain injury |
References
- Baulieu, E.E.; Robel, P. Neurosteroids: A new brain function? J. Steroid Biochem. Mol. Biol. 1990, 37, 395–403. [Google Scholar] [CrossRef]
- Mensah-Nyagan, A.G.; Do-Rego, J.L.; Beaujean, D.; Luu-The, V.; Pelletier, G.; Vaudry, H. Neurosteroids: Expression of steroidogenic enzymes and regulation of steroid biosynthesis in the central nervous system. Pharmacol. Rev. 1999, 51, 63–81. [Google Scholar]
- Compagnone, N.A.; Mellon, S.H. Neurosteroids: Biosynthesis and function of these novel neuromodulators. Front. Neuroendocrinol. 2000, 21, 1–56. [Google Scholar] [CrossRef] [PubMed]
- Mellon, S.H.; Griffin, L.D. Neurosteroids: Biochemistry and clinical significance. Trends Endocrinol. Metab. 2002, 13, 35–43. [Google Scholar] [CrossRef]
- Melcangi, R.C.; Garcia-Segura, L.M.; Mensah-Nyagan, A.G. Neuroactive steroids: State of the art and new perspectives. Cell Mol. Life Sci. 2008, 65, 777–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diotel, N.; Charlier, T.D.; Lefebvre d’Hellencourt, C.; Couret, D.; Trudeau, V.L.; Nicolau, J.C.; Meilhac, O.; Kah, O.; Pellegrini, E. Steroid Transport, Local Synthesis, and Signaling within the Brain: Roles in Neurogenesis, Neuroprotection, and Sexual Behaviors. Front. Neurosci. 2018, 12, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendell, A.L.; MacLusky, N.J. Neurosteroid Metabolites of Gonadal Steroid Hormones in Neuroprotection: Implications for Sex Differences in Neurodegenerative Disease. Front. Mol. Neurosci. 2018, 11, 359. [Google Scholar] [CrossRef] [Green Version]
- De Nicola, A.F.; Labombarda, F.; Gonzalez Deniselle, M.C.; Gonzalez, S.L.; Garay, L.; Meyer, M.; Gargiulo, G.; Guennoun, R.; Schumacher, M. Progesterone neuroprotection in traumatic CNS injury and motoneuron degeneration. Front. Neuroendocrinol. 2009, 30, 173–187. [Google Scholar] [CrossRef]
- Schumacher, M.; Mattern, C.; Ghoumari, A.; Oudinet, J.P.; Liere, P.; Labombarda, F.; Sitruk-Ware, R.; De Nicola, A.F.; Guennoun, R. Revisiting the roles of progesterone and allopregnanolone in the nervous system: Resurgence of the progesterone receptors. Prog. Neurobiol. 2014, 113, 6–39. [Google Scholar] [CrossRef]
- Melcangi, R.C.; Giatti, S.; Calabrese, D.; Pesaresi, M.; Cermenati, G.; Mitro, N.; Viviani, B.; Garcia-Segura, L.M.; Caruso, D. Levels and actions of progesterone and its metabolites in the nervous system during physiological and pathological conditions. Prog. Neurobiol. 2014, 113, 56–69. [Google Scholar] [CrossRef]
- Melcangi, R.C.; Panzica, G.C. Allopregnanolone: State of the art. Prog. Neurobiol. 2014, 113, 1–5. [Google Scholar] [CrossRef]
- Patte-Mensah, C.; Meyer, L.; Taleb, O.; Mensah-Nyagan, A.G. Potential role of allopregnanolone for a safe and effective therapy of neuropathic pain. Prog. Neurobiol. 2014, 113, 70–78. [Google Scholar] [CrossRef]
- Meyer, L.; Taleb, O.; Patte-Mensah, C.; Mensah-Nyagan, A.G. Neurosteroids and neuropathic pain management: Basic evidence and therapeutic perspectives. Front. Neuroendocrinol. 2019, 55, 100795. [Google Scholar] [CrossRef]
- Pardridge, W.M.; Mietus, L.J. Transport of steroid hormones through the rat blood-brain barrier. Primary role of albumin-bound hormone. J. Clin. Investig. 1979, 64, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Pardridge, W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Witt, K.A.; Sandoval, K.E. Steroids and the blood-brain barrier: Therapeutic implications. Adv. Pharmacol. 2014, 71, 361–390. [Google Scholar]
- Banks, W.A. Brain meets body: The blood-brain barrier as an endocrine interface. Endocrinology 2012, 153, 4111–4119. [Google Scholar] [CrossRef] [Green Version]
- Baulieu, E.E.; Robel, P.; Schumacher, M. Neurosteroids: Beginning of the story. Int. Rev. Neurobiol. 2001, 46, 1–32. [Google Scholar]
- Mellon, S.H.; Vaudry, H. Biosynthesis of neurosteroids and regulation of their synthesis. Int. Rev. Neurobiol. 2001, 46, 33–78. [Google Scholar]
- Guennoun, R.; Labombarda, F.; Gonzalez Deniselle, M.C.; Liere, P.; De Nicola, A.F.; Schumacher, M. Progesterone and allopregnanolone in the central nervous system: Response to injury and implication for neuroprotection. J. Steroid Biochem. Mol. Biol. 2015, 146, 48–61. [Google Scholar] [CrossRef]
- Schumacher, M.; Guennoun, R.; Ghoumari, A.; Massaad, C.; Robert, F.; El-Etr, M.; Akwa, Y.; Rajkowski, K.; Baulieu, E.E. Novel perspectives for progesterone in hormone replacement therapy, with special reference to the nervous system. Endocr. Rev. 2007, 28, 387–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelletier, G. Steroidogenic enzymes in the brain: Morphological aspects. Prog. Brain Res. 2010, 181, 193–207. [Google Scholar] [PubMed]
- Le Goascogne, C.; Robel, P.; Gouezou, M.; Sananes, N.; Baulieu, E.E.; Waterman, M. Neurosteroids: Cytochrome P-450scc in rat brain. Science 1987, 237, 1212–1215. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, K.; Ukena, K. Neurosteroids in the cerebellar Purkinje neuron and their actions (review). Int. J. Mol. Med. 1999, 4, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Ukena, K.; Usui, M.; Kohchi, C.; Tsutsui, K. Cytochrome P450 side-chain cleavage enzyme in the cerebellar Purkinje neuron and its neonatal change in rats. Endocrinology 1998, 139, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Kimoto, T.; Tsurugizawa, T.; Ohta, Y.; Makino, J.; Tamura, H.; Hojo, Y.; Takata, N.; Kawato, S. Neurosteroid synthesis by cytochrome p450-containing systems localized in the rat brain hippocampal neurons: N-methyl-D-aspartate and calcium-dependent synthesis. Endocrinology 2001, 142, 3578–3589. [Google Scholar] [CrossRef]
- Mellon, S.H.; Deschepper, C.F. Neurosteroid biosynthesis: Genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res. 1993, 629, 283–292. [Google Scholar] [CrossRef]
- Goascogne, C.; Gouezou, M.; Robel, P.; Defaye, G.; Chambaz, E.; Waterman, M.R.; Baulieu, E.E. The cholesterol side-chain cleavage complex in human brain white matter. J. Neuroendocrinol. 1989, 1, 153–156. [Google Scholar] [CrossRef]
- Beyenburg, S.; Stoffel-Wagner, B.; Watzka, M.; Blumcke, I.; Bauer, J.; Schramm, J.; Bidlingmaier, F.; Elger, C.E. Expression of cytochrome P450scc mRNA in the hippocampus of patients with temporal lobe epilepsy. Neuroreport 1999, 10, 3067–3070. [Google Scholar] [CrossRef]
- Watzka, M.; Bidlingmaier, F.; Schramm, J.; Klingmuller, D.; Stoffel-Wagner, B. Sex- and age-specific differences in human brain CYP11A1 mRNA expression. J. Neuroendocrinol. 1999, 11, 901–905. [Google Scholar] [CrossRef]
- Yu, L.; Romero, D.G.; Gomez-Sanchez, C.E.; Gomez-Sanchez, E.P. Steroidogenic enzyme gene expression in the human brain. Mol. Cell Endocrinol. 2002, 190, 9–17. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Bourreau, E.; Jung-Testas, I.; Robel, P.; Baulieu, E.E. Neurosteroids: Oligodendrocyte mitochondria convert cholesterol to pregnenolone. Proc. Natl. Acad. Sci. USA 1987, 84, 8215–8219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, R.C.; Cascio, C.; Papadopoulos, V. Pathways of neurosteroid biosynthesis in cell lines from human brain: Regulation of dehydroepiandrosterone formation by oxidative stress and beta-amyloid peptide. J. Neurochem. 2000, 74, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Guennoun, R.; Fiddes, R.J.; Gouezou, M.; Lombes, M.; Baulieu, E.E. A key enzyme in the biosynthesis of neurosteroids, 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4-isomerase (3 beta-HSD), is expressed in rat brain. Mol. Brain Res. 1995, 30, 287–300. [Google Scholar] [CrossRef]
- Inoue, T.; Akahira, J.; Suzuki, T.; Darnel, A.D.; Kaneko, C.; Takahashi, K.; Hatori, M.; Shirane, R.; Kumabe, T.; Kurokawa, Y.; et al. Progesterone production and actions in the human central nervous system and neurogenic tumors. J. Clin. Endocrinol. Metab. 2002, 87, 5325–5331. [Google Scholar] [CrossRef] [Green Version]
- Weidenfeld, J.; Siegel, R.A.; Chowers, I. In vitro conversion of pregnenolone to progesterone by discrete brain areas of the male rat. J. Steroid Biochem. 1980, 13, 961–963. [Google Scholar] [CrossRef]
- Bauer, H.C.; Bauer, H. Micromethod for the determination of 3-beta-HSD activity in cultured cells. J. Steroid Biochem. 1989, 33, 643–646. [Google Scholar] [CrossRef]
- Jung-Testas, I.; Hu, Z.Y.; Baulieu, E.E.; Robel, P. Neurosteroids: Biosynthesis of pregnenolone and progesterone in primary cultures of rat glial cells. Endocrinology 1989, 125, 2083–2091. [Google Scholar] [CrossRef]
- Kabbadj, K.; El-Etr, M.; Baulieu, E.E.; Robel, P. Pregnenolone metabolism in rodent embryonic neurons and astrocytes. Glia 1993, 7, 170–175. [Google Scholar] [CrossRef]
- Akwa, Y.; Sananes, N.; Gouezou, M.; Robel, P.; Baulieu, E.E.; Le Goascogne, C. Astrocytes and neurosteroids: Metabolism of pregnenolone and dehydroepiandrosterone. Regulation by cell density. J. Cell Biol. 1993, 121, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Zwain, I.H.; Yen, S.S. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinology 1999, 140, 3843–3852. [Google Scholar] [CrossRef] [PubMed]
- Gago, N.; Akwa, Y.; Sananes, N.; Guennoun, R.; Baulieu, E.E.; El-Etr, M.; Schumacher, M. Progesterone and the oligodendroglial lineage: Stage-dependent biosynthesis and metabolism. Glia 2001, 36, 295–308. [Google Scholar] [CrossRef]
- Melcangi, R.C.; Poletti, A.; Cavarretta, I.; Celotti, F.; Colciago, A.; Magnaghi, V.; Motta, M.; Negri-Cesi, P.; Martini, L. The 5alpha-reductase in the central nervous system: Expression and modes of control. J. Steroid Biochem. Mol. Biol. 1998, 65, 295–299. [Google Scholar] [CrossRef]
- Russell, D.W.; Wilson, J.D. Steroid 5 alpha-reductase: Two genes/two enzymes. Ann. Rev. Biochem. 1994, 63, 25–61. [Google Scholar] [CrossRef] [PubMed]
- Steckelbroeck, S.; Watzka, M.; Reichelt, R.; Hans, V.H.; Stoffel-Wagner, B.; Heidrich, D.D.; Schramm, J.; Bidlingmaier, F.; Klingmuller, D. Characterization of the 5alpha-reductase-3alpha-hydroxysteroid dehydrogenase complex in the human brain. J. Clin. Endocrinol. Metab. 2001, 86, 1324–1331. [Google Scholar]
- Stoffel-Wagner, B. Neurosteroid biosynthesis in the human brain and its clinical implications. Ann. N. Y. Acad. Sci. 2003, 1007, 64–78. [Google Scholar] [CrossRef]
- Thigpen, A.E.; Silver, R.I.; Guileyardo, J.M.; Casey, M.L.; McConnell, J.D.; Russell, D.W. Tissue distribution and ontogeny of steroid 5 alpha-reductase isozyme expression. J. Clin. Investig. 1993, 92, 903–910. [Google Scholar] [CrossRef]
- Torres, J.M.; Ortega, E. Differential regulation of steroid 5alpha-reductase isozymes expression by androgens in the adult rat brain. FASEB J. 2003, 17, 1428–1433. [Google Scholar] [CrossRef] [Green Version]
- Luchetti, S.; Huitinga, I.; Swaab, D.F. Neurosteroid and GABA-A receptor alterations in Alzheimer’s disease, Parkinson’s disease and multiple sclerosis. Neuroscience 2011, 191, 6–21. [Google Scholar] [CrossRef]
- Giatti, S.; Diviccaro, S.; Garcia-Segura, L.M.; Melcangi, R.C. Sex differences in the brain expression of steroidogenic molecules under basal conditions and after gonadectomy. J. Neuroendocrinol. 2019, 31, e12736. [Google Scholar] [CrossRef]
- Agis-Balboa, R.C.; Pinna, G.; Zhubi, A.; Maloku, E.; Veldic, M.; Costa, E.; Guidotti, A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 14602–14607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelletier, G.; Luu-The, V.; Labrie, F. Immunocytochemical localization of 5 alpha-reductase in rat brain. Mol. Cell Neurosci. 1994, 5, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Tsuruo, Y.; Miyamoto, T.; Yokoi, H.; Kitagawa, K.; Futaki, S.; Ishimura, K. Immunohistochemical presence of 5 alpha-reductase rat type 1-containing cells in the rat brain. Brain Res. 1996, 722, 207–211. [Google Scholar] [CrossRef]
- Celotti, F.; Melcangi, R.C.; Martini, L. The 5 alpha-reductase in the brain: Molecular aspects and relation to brain function. Front. Neuroendocrinol. 1992, 13, 163–215. [Google Scholar] [PubMed]
- Saitoh, H.; Hirato, K.; Yanaihara, T.; Nakayama, T. A study of 5 alpha-reductase in human fetal brain. Endocrinol. Jpn. 1982, 29, 461–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melcangi, R.C.; Celotti, F.; Martini, L. Progesterone 5-alpha-reduction in neuronal and in different types of glial cell cultures: Type 1 and 2 astrocytes and oligodendrocytes. Brain Res. 1994, 639, 202–206. [Google Scholar] [CrossRef]
- Melcangi, R.C.; Ballabio, M.; Magnaghi, V.; Celotti, F. Metabolism of steroids in pure cultures of neurons and glial cells: Role of intracellular signalling. J. Steroid Biochem. Mol. Biol. 1995, 53, 331–336. [Google Scholar] [CrossRef]
- Khanna, M.; Qin, K.N.; Cheng, K.C. Distribution of 3 alpha-hydroxysteroid dehydrogenase in rat brain and molecular cloning of multiple cDNAs encoding structurally related proteins in humans. J. Steroid Biochem. Mol. Biol. 1995, 53, 41–46. [Google Scholar] [CrossRef]
- Meffre, D.; Delespierre, B.; Gouezou, M.; Schumacher, M.; Stein, D.G.; Guennoun, R. 3beta-Hydroxysteroid dehydrogenase/5-ene-4-ene isomerase mRNA expression in rat brain: Effect of pseudopregnancy and traumatic brain injury. J. Steroid Biochem. Mol. Biol. 2007, 104, 293–300. [Google Scholar] [CrossRef]
- Leicaj, M.L.; Pasquini, L.A.; Lima, A.; Gonzalez Deniselle, M.C.; Pasquini, J.M.; De Nicola, A.F.; Garay, L.I. Changes in neurosteroidogenesis during demyelination and remyelination in cuprizone-treated mice. J. Neuroendocrinol. 2018, 30, e12649. [Google Scholar] [CrossRef] [Green Version]
- Noorbakhsh, F.; Ellestad, K.K.; Maingat, F.; Warren, K.G.; Han, M.H.; Steinman, L.; Baker, G.B.; Power, C. Impaired neurosteroid synthesis in multiple sclerosis. Brain 2011, 134, 2703–2721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, L.D.; Gong, W.; Verot, L.; Mellon, S.H. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat. Med. 2004, 10, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Mellon, S.H.; Gong, W.; Schonemann, M.D. Endogenous and synthetic neurosteroids in treatment of Niemann-Pick Type C disease. Brain Res. Rev. 2008, 57, 410–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luchetti, S.; Bossers, K.; Frajese, G.V.; Swaab, D.F. Neurosteroid biosynthetic pathway changes in substantia nigra and caudate nucleus in Parkinson’s disease. Brain Pathol. 2010, 20, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, S.; Van Eden, C.G.; Schuurman, K.; van Strien, M.E.; Swaab, D.F.; Huitinga, I. Gender differences in multiple sclerosis: Induction of estrogen signaling in male and progesterone signaling in female lesions. J. Neuropathol. Exp. Neurol. 2014, 73, 123–135. [Google Scholar] [CrossRef] [Green Version]
- Brinton, R.D.; Thompson, R.F.; Foy, M.R.; Baudry, M.; Wang, J.; Finch, C.E.; Morgan, T.E.; Pike, C.J.; Mack, W.J.; Stanczyk, F.Z.; et al. Progesterone receptors: Form and function in brain. Front. Neuroendocrinol. 2008, 29, 313–339. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Su, C.; Ng, S. Non-genomic mechanisms of progesterone action in the brain. Front. Neurosci. 2013, 7, 159. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, S.L.; Coronel, M.F.; Raggio, M.C.; Labombarda, F. Progesterone receptor-mediated actions and the treatment of central nervous system disorders: An up-date of the known and the challenge of the unknown. Steroids 2020, 153, 108525. [Google Scholar] [CrossRef]
- Gonzalez, S.L. Progesterone for the treatment of central nervous system disorders: The many signaling roads for a single molecule. Neural Regen. Res. 2020, 15, 1846–1847. [Google Scholar] [CrossRef]
- Kastner, P.; Krust, A.; Turcotte, B.; Stropp, U.; Tora, L.; Gronemeyer, H.; Chambon, P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990, 9, 1603–1614. [Google Scholar] [CrossRef]
- Kraus, W.L.; Montano, M.M.; Katzenellenbogen, B.S. Cloning of the rat progesterone receptor gene 5′-region and identification of two functionally distinct promoters. Mol. Endocrinol. 1993, 7, 1603–1616. [Google Scholar]
- Hagan, C.R.; Daniel, A.R.; Dressing, G.E.; Lange, C.A. Role of phosphorylation in progesterone receptor signaling and specificity. Mol. Cell Endocrinol. 2012, 357, 43–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guennoun, R.; Meffre, D.; Labombarda, F.; Gonzalez, S.L.; Gonzalez Deniselle, M.C.; Stein, D.G.; De Nicola, A.F.; Schumacher, M. The membrane-associated progesterone-binding protein 25-Dx: Expression, cellular localization and up-regulation after brain and spinal cord injuries. Brain Res. Rev. 2008, 57, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Losel, R.M.; Besong, D.; Peluso, J.J.; Wehling, M. Progesterone receptor membrane component 1--many tasks for a versatile protein. Steroids 2008, 73, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Rohe, H.J.; Ahmed, I.S.; Twist, K.E.; Craven, R.J. PGRMC1 (progesterone receptor membrane component 1): A targetable protein with multiple functions in steroid signaling, P450 activation and drug binding. Pharmacol. Ther. 2009, 121, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P.; Pang, Y.; Dong, J. Enhancement of cell surface expression and receptor functions of membrane progestin receptor alpha (mPRalpha) by progesterone receptor membrane component 1 (PGRMC1): Evidence for a role of PGRMC1 as an adaptor protein for steroid receptors. Endocrinology 2014, 155, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Bond, J.; Thomas, P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc. Natl. Acad. Sci. USA 2003, 100, 2237–2242. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Rice, C.D.; Pang, Y.; Pace, M.; Thomas, P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc. Natl. Acad. Sci. USA 2003, 100, 2231–2236. [Google Scholar] [CrossRef] [Green Version]
- Pang, Y.; Dong, J.; Thomas, P. Characterization, neurosteroid binding and brain distribution of human membrane progesterone receptors delta and {epsilon} (mPRdelta and mPR{epsilon}) and mPRdelta involvement in neurosteroid inhibition of apoptosis. Endocrinology 2013, 154, 283–295. [Google Scholar] [CrossRef] [Green Version]
- Hosie, A.M.; Wilkins, M.E.; da Silva, H.M.; Smart, T.G. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature 2006, 444, 486–489. [Google Scholar] [CrossRef]
- Meffre, D.; Delespierre, B.; Gouezou, M.; Leclerc, P.; Vinson, G.P.; Schumacher, M.; Stein, D.G.; Guennoun, R. The membrane-associated progesterone-binding protein 25-Dx is expressed in brain regions involved in water homeostasis and is up-regulated after traumatic brain injury. J. Neurochem. 2005, 93, 1314–1326. [Google Scholar] [CrossRef] [PubMed]
- Meffre, D.; Labombarda, F.; Delespierre, B.; Chastre, A.; De Nicola, A.F.; Stein, D.G.; Schumacher, M.; Guennoun, R. Distribution of membrane progesterone receptor alpha in the male mouse and rat brain and its regulation after traumatic brain injury. Neuroscience 2013, 231, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Mitkari, B.; Kipp, M.; Beyer, C. Gonadal steroids prevent cell damage and stimulate behavioral recovery after transient middle cerebral artery occlusion in male and female rats. Brain Behav. Immun. 2011, 25, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.S.; Sayeed, I.; Oumarbaeva, Y.; Morrison, K.C.; Choi, P.H.; Pardue, M.T.; Stein, D.G. Progesterone treatment shows greater protection in brain vs. retina in a rat model of middle cerebral artery occlusion: Progesterone receptor levels may play an important role. Restor. Neurol. Neurosci. 2016, 34, 947–963. [Google Scholar] [CrossRef] [PubMed]
- Stanojlovic, M.; Gusevac Stojanovic, I.; Zaric, M.; Martinovic, J.; Mitrovic, N.; Grkovic, I.; Drakulic, D. Progesterone Protects Prefrontal Cortex in Rat Model of Permanent Bilateral Common Carotid Occlusion via Progesterone Receptors and Akt/Erk/eNOS. Cell Mol. Neurobiol. 2019, 40, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Su, C.; Singh, M. Let-7i inhibition enhances progesterone-induced functional recovery in a mouse model of ischemia. Proc. Natl. Acad. Sci. USA 2018, 115, E9668–E9677. [Google Scholar] [CrossRef] [Green Version]
- Gaignard, P.; Savouroux, S.; Liere, P.; Pianos, A.; Therond, P.; Schumacher, M.; Slama, A.; Guennoun, R. Effect of Sex Differences on Brain Mitochondrial Function and Its Suppression by Ovariectomy and in Aged Mice. Endocrinology 2015, 156, 2893–2904. [Google Scholar] [CrossRef]
- Lopez-Rodriguez, A.B.; Acaz-Fonseca, E.; Giatti, S.; Caruso, D.; Viveros, M.P.; Melcangi, R.C.; Garcia-Segura, L.M. Correlation of brain levels of progesterone and dehydroepiandrosterone with neurological recovery after traumatic brain injury in female mice. Psychoneuroendocrinology 2015, 56, 1–11. [Google Scholar] [CrossRef]
- Lopez-Rodriguez, A.B.; Acaz-Fonseca, E.; Spezzano, R.; Giatti, S.; Caruso, D.; Viveros, M.P.; Melcangi, R.C.; Garcia-Segura, L.M. Profiling Neuroactive Steroid Levels After Traumatic Brain Injury in Male Mice. Endocrinology 2016, 157, 3983–3993. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Frechou, M.; Liere, P.; Zhang, S.; Pianos, A.; Fernandez, N.; Denier, C.; Mattern, C.; Schumacher, M.; Guennoun, R. A Role of Endogenous Progesterone in Stroke Cerebroprotection Revealed by the Neural-Specific Deletion of Its Intracellular Receptors. J. Neurosci. 2017, 37, 10998–11020. [Google Scholar] [CrossRef] [Green Version]
- Meffre, D.; Pianos, A.; Liere, P.; Eychenne, B.; Cambourg, A.; Schumacher, M.; Stein, D.G.; Guennoun, R. Steroid profiling in brain and plasma of male and pseudopregnant female rats after traumatic brain injury: Analysis by gas chromatography/mass spectrometry. Endocrinology 2007, 148, 2505–2517. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.; Pesaresi, M.; Abbiati, F.; Calabrese, D.; Giatti, S.; Garcia-Segura, L.M.; Melcangi, R.C. Comparison of plasma and cerebrospinal fluid levels of neuroactive steroids with their brain, spinal cord and peripheral nerve levels in male and female rats. Psychoneuroendocrinology 2013, 38, 2278–2290. [Google Scholar] [CrossRef] [PubMed]
- Bixo, M.; Andersson, A.; Winblad, B.; Purdy, R.H.; Backstrom, T. Progesterone, 5alpha-pregnane-3,20-dione and 3alpha-hydroxy-5alpha-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Res. 1997, 764, 173–178. [Google Scholar] [CrossRef]
- Caruso, D.; Barron, A.M.; Brown, M.A.; Abbiati, F.; Carrero, P.; Pike, C.J.; Garcia-Segura, L.M.; Melcangi, R.C. Age-related changes in neuroactive steroid levels in 3xTg-AD mice. Neurobiol. Aging 2013, 34, 1080–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melcangi, R.C.; Caruso, D.; Levandis, G.; Abbiati, F.; Armentero, M.T.; Blandini, F. Modifications of neuroactive steroid levels in an experimental model of nigrostriatal degeneration: Potential relevance to the pathophysiology of Parkinson’s disease. J. Mol. Neurosci. 2012, 46, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Di Michele, F.; Longone, P.; Romeo, E.; Lucchetti, S.; Brusa, L.; Pierantozzi, M.; Bassi, A.; Bernardi, G.; Stanzione, P. Decreased plasma and cerebrospinal fluid content of neuroactive steroids in Parkinson’s disease. Neurol. Sci. 2003, 24, 172–173. [Google Scholar] [CrossRef]
- Giatti, S.; D’Intino, G.; Maschi, O.; Pesaresi, M.; Garcia-Segura, L.M.; Calza, L.; Caruso, D.; Melcangi, R.C. Acute experimental autoimmune encephalomyelitis induces sex dimorphic changes in neuroactive steroid levels. Neurochem. Int. 2010, 56, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Caruso, D.; D’Intino, G.; Giatti, S.; Maschi, O.; Pesaresi, M.; Calabrese, D.; Garcia-Segura, L.M.; Calza, L.; Melcangi, R.C. Sex-dimorphic changes in neuroactive steroid levels after chronic experimental autoimmune encephalomyelitis. J. Neurochem. 2010, 114, 921–932. [Google Scholar] [CrossRef]
- Wagner, A.K.; McCullough, E.H.; Niyonkuru, C.; Ozawa, H.; Loucks, T.L.; Dobos, J.A.; Brett, C.A.; Santarsieri, M.; Dixon, C.E.; Berga, S.L.; et al. Acute serum hormone levels: Characterization and prognosis after severe traumatic brain injury. J. Neurotrauma 2011, 28, 871–888. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.; Margaill, I.; Zhang, S.; Labombarda, F.; Coqueran, B.; Delespierre, B.; Liere, P.; Marchand-Leroux, C.; O’Malley, B.W.; Lydon, J.P.; et al. Progesterone receptors: A key for neuroprotection in experimental stroke. Endocrinology 2012, 153, 3747–3757. [Google Scholar] [CrossRef] [Green Version]
- Mani, S.K.; Oyola, M.G. Progesterone signaling mechanisms in brain and behavior. Front. Endocrinol. 2012, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, J.D.; Clarke, C.L. Physiological action of progesterone in target tissues. Endocr. Rev. 1997, 18, 502–519. [Google Scholar] [PubMed] [Green Version]
- Tsutsui, K. Progesterone biosynthesis and action in the developing neuron. Endocrinology 2008, 149, 2757–2761. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Orozco, J.C.; Camacho-Arroyo, I. Progesterone Actions During Central Nervous System Development. Front. Neurosci. 2019, 13, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumacher, M.; Hussain, R.; Gago, N.; Oudinet, J.P.; Mattern, C.; Ghoumari, A.M. Progesterone synthesis in the nervous system: Implications for myelination and myelin repair. Front. Neurosci. 2012, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Carroll, J.C.; Rosario, E.R.; Chang, L.; Stanczyk, F.Z.; Oddo, S.; LaFerla, F.M.; Pike, C.J. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J. Neurosci. 2007, 27, 13357–13365. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.M.; Irwin, R.W.; Yao, J.; Liu, L.; Brinton, R.D. Allopregnanolone promotes regeneration and reduces beta-amyloid burden in a preclinical model of Alzheimer’s disease. PLoS ONE 2011, 6, e24293. [Google Scholar]
- Irwin, R.W.; Wang, J.M.; Chen, S.; Brinton, R.D. Neuroregenerative mechanisms of allopregnanolone in Alzheimer’s disease. Front. Endocrinol. 2011, 2, 117. [Google Scholar] [CrossRef] [Green Version]
- Irwin, R.W.; Brinton, R.D. Allopregnanolone as regenerative therapeutic for Alzheimer’s disease: Translational development and clinical promise. Prog. Neurobiol. 2014, 113, 40–55. [Google Scholar] [CrossRef]
- Wang, T.; Yao, J.; Chen, S.; Mao, Z.; Brinton, R.D. Allopregnanolone Reverses Bioenergetic Deficits in Female Triple Transgenic Alzheimer’s Mouse Model. Neurotherapeutics 2020, 17, 178–188. [Google Scholar] [CrossRef]
- Yu, L.; Kuo, Y.; Cherng, C.G.; Chen, H.H.; Hsu, C.H. Ovarian hormones do not attenuate methamphetamine-induced dopaminergic neurotoxicity in mice gonadectomized at 4 weeks postpartum. Neuroendocrinology 2002, 75, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.G.; Bourque, M.; Morissette, M.; Di Paolo, T. Steroids-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci. Ther. 2010, 16, e43–e71. [Google Scholar] [CrossRef] [PubMed]
- Casas, S.; Garcia, S.; Cabrera, R.; Nanfaro, F.; Escudero, C.; Yunes, R. Progesterone prevents depression-like behavior in a model of Parkinson’s disease induced by 6-hydroxydopamine in male rats. Pharmacol. Biochem. Behav. 2011, 99, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Litim, N.; Morissette, M.; Di Paolo, T. Effects of progesterone administered after MPTP on dopaminergic neurons of male mice. Neuropharmacology 2017, 117, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Bourque, M.; Dluzen, D.E.; Di Paolo, T. Neuroprotective actions of sex steroids in Parkinson’s disease. Front. Neuroendocrinol. 2009, 30, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Bourque, M.; Morissette, M.; Al Sweidi, S.; Caruso, D.; Melcangi, R.C.; Di Paolo, T. Neuroprotective Effect of Progesterone in MPTP-Treated Male Mice. Neuroendocrinology 2016, 103, 300–314. [Google Scholar] [CrossRef]
- Bourque, M.; Morissette, M.; Di Paolo, T. Repurposing sex steroids and related drugs as potential treatment for Parkinson’s disease. Neuropharmacology 2019, 147, 37–54. [Google Scholar] [CrossRef]
- Adeosun, S.O.; Hou, X.; Jiao, Y.; Zheng, B.; Henry, S.; Hill, R.; He, Z.; Pani, A.; Kyle, P.; Ou, X.; et al. Allopregnanolone reinstates tyrosine hydroxylase immunoreactive neurons and motor performance in an MPTP-lesioned mouse model of Parkinson’s disease. PLoS ONE 2012, 7, e50040. [Google Scholar] [CrossRef]
- Nezhadi, A.; Sheibani, V.; Esmaeilpour, K.; Shabani, M.; Esmaeili-Mahani, S. Neurosteroid allopregnanolone attenuates cognitive dysfunctions in 6-OHDA-induced rat model of Parkinson’s disease. Behav. Brain Res. 2016, 305, 258–264. [Google Scholar] [CrossRef]
- Ye, J.N.; Chen, X.S.; Su, L.; Liu, Y.L.; Cai, Q.Y.; Zhan, X.L.; Xu, Y.; Zhao, S.F.; Yao, Z.X. Progesterone alleviates neural behavioral deficits and demyelination with reduced degeneration of oligodendroglial cells in cuprizone-induced mice. PLoS ONE 2013, 8, e54590. [Google Scholar] [CrossRef] [Green Version]
- El-Etr, M.; Rame, M.; Boucher, C.; Ghoumari, A.M.; Kumar, N.; Liere, P.; Pianos, A.; Schumacher, M.; Sitruk-Ware, R. Progesterone and nestorone promote myelin regeneration in chronic demyelinating lesions of corpus callosum and cerebral cortex. Glia 2015, 63, 104–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aryanpour, R.; Pasbakhsh, P.; Zibara, K.; Namjoo, Z.; Beigi Boroujeni, F.; Shahbeigi, S.; Kashani, I.R.; Beyer, C.; Zendehdel, A. Progesterone therapy induces an M1 to M2 switch in microglia phenotype and suppresses NLRP3 inflammasome in a cuprizone-induced demyelination mouse model. Int. Immunopharmacol. 2017, 51, 131–139. [Google Scholar] [CrossRef]
- De Nicola, A.F.; Garay, L.I.; Meyer, M.; Guennoun, R.; Sitruk-Ware, R.; Schumacher, M.; Gonzalez Deniselle, M.C. Neurosteroidogenesis and progesterone anti-inflammatory/neuroprotective effects. J. Neuroendocrinol. 2018, 30, e12502. [Google Scholar] [CrossRef] [Green Version]
- Garay, L.; Gonzalez Deniselle, M.C.; Sitruk-Ware, R.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. Efficacy of the selective progesterone receptor agonist Nestorone for chronic experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2014, 276, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Noorbakhsh, F.; Baker, G.B.; Power, C. Allopregnanolone and neuroinflammation: A focus on multiple sclerosis. Front. Cell Neurosci. 2014, 8, 134. [Google Scholar] [CrossRef] [Green Version]
- Giatti, S.; Boraso, M.; Melcangi, R.C.; Viviani, B. Neuroactive steroids, their metabolites, and neuroinflammation. J. Mol. Endocrinol. 2012, 49, R125–R134. [Google Scholar] [CrossRef] [Green Version]
- Vegeto, E.; Villa, A.; Della Torre, S.; Crippa, V.; Rusmini, P.; Cristofani, R.; Galbiati, M.; Maggi, A.; Poletti, A. The Role of Sex and Sex Hormones in Neurodegenerative Diseases. Endocr. Rev. 2020, 41, 273–319. [Google Scholar] [CrossRef]
- Yilmaz, C.; Karali, K.; Fodelianaki, G.; Gravanis, A.; Chavakis, T.; Charalampopoulos, I.; Alexaki, V.I. Neurosteroids as regulators of neuroinflammation. Front. Neuroendocrinol. 2019, 55, 100788. [Google Scholar] [CrossRef]
- Gibson, C.L.; Gray, L.J.; Bath, P.M.; Murphy, S.P. Progesterone for the treatment of experimental brain injury; a systematic review. Brain 2008, 131, 318–328. [Google Scholar] [CrossRef]
- Sayeed, I.; Stein, D.G. Progesterone as a neuroprotective factor in traumatic and ischemic brain injury. Prog. Brain Res. 2009, 175, 219–237. [Google Scholar]
- Stein, D.G. Is progesterone a worthy candidate as a novel therapy for traumatic brain injury? Dialogues Clin. Neurosci. 2011, 13, 352–359. [Google Scholar] [PubMed]
- Brotfain, E.; Gruenbaum, S.E.; Boyko, M.; Kutz, R.; Zlotnik, A.; Klein, M. Neuroprotection by Estrogen and Progesterone in Traumatic Brain Injury and Spinal Cord Injury. Curr. Neuropharmacol. 2016, 14, 641–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spani, C.B.; Braun, D.J.; Van Eldik, L.J. Sex-related responses after traumatic brain injury: Considerations for preclinical modeling. Front. Neuroendocrinol. 2018, 50, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Djebaili, M.; Hoffman, S.W.; Stein, D.G. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience 2004, 123, 349–359. [Google Scholar] [CrossRef]
- Djebaili, M.; Guo, Q.; Pettus, E.H.; Hoffman, S.W.; Stein, D.G. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J. Neurotrauma 2005, 22, 106–118. [Google Scholar] [CrossRef]
- He, J.; Hoffman, S.W.; Stein, D.G. Allopregnanolone, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restor. Neurol. Neurosci. 2004, 22, 19–31. [Google Scholar]
- Van Landingham, J.W.; Cekic, M.; Cutler, S.; Hoffman, S.W.; Stein, D.G. Neurosteroids reduce inflammation after TBI through CD55 induction. Neurosci. Lett. 2007, 425, 94–98. [Google Scholar] [CrossRef] [Green Version]
- Wright, D.W.; Kellermann, A.L.; Hertzberg, V.S.; Clark, P.L.; Frankel, M.; Goldstein, F.C.; Salomone, J.P.; Dent, L.L.; Harris, O.A.; Ander, D.S.; et al. ProTECT: A randomized clinical trial of progesterone for acute traumatic brain injury. Ann. Emerg. Med. 2007, 49, 391–402. [Google Scholar] [CrossRef]
- Xiao, G.; Wei, J.; Yan, W.; Wang, W.; Lu, Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: A randomized controlled trial. Crit. Care 2008, 12, R61. [Google Scholar] [CrossRef] [Green Version]
- Wright, D.W.; Yeatts, S.D.; Silbergleit, R.; Palesch, Y.Y.; Hertzberg, V.S.; Frankel, M.; Goldstein, F.C.; Caveney, A.F.; Howlett-Smith, H.; Bengelink, E.M.; et al. Very early administration of progesterone for acute traumatic brain injury. N. Engl. J. Med. 2014, 371, 2457–2466. [Google Scholar] [CrossRef] [Green Version]
- Skolnick, B.E.; Maas, A.I.; Narayan, R.K.; van der Hoop, R.G.; MacAllister, T.; Ward, J.D.; Nelson, N.R.; Stocchetti, N.; Investigators, S.T. A clinical trial of progesterone for severe traumatic brain injury. N. Engl. J. Med. 2014, 371, 2467–2476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, D.G. Embracing failure: What the Phase III progesterone studies can teach about TBI clinical trials. Brain Inj. 2015, 29, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Denier, C.; Oudinet, J.P.; Adams, D.; Guennoun, R. Progesterone neuroprotection: The background of clinical trial failure. J. Steroid Biochem. Mol. Biol. 2016, 160, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Guennoun, R.; Frechou, M.; Gaignard, P.; Liere, P.; Slama, A.; Schumacher, M.; Denier, C.; Mattern, C. Intranasal administration of progesterone: A potential efficient route of delivery for cerebroprotection after acute brain injuries. Neuropharmacology 2019, 145, 283–291. [Google Scholar] [CrossRef]
- Lin, C.; He, H.; Li, Z.; Liu, Y.; Chao, H.; Ji, J.; Liu, N. Efficacy of progesterone for moderate to severe traumatic brain injury: A meta-analysis of randomized clinical trials. Sci. Rep. 2015, 5, 13442. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Huang, S.; Qin, S.; You, C.; Zeng, Y. Progesterone for acute traumatic brain injury. Cochrane Database Syst. Rev. 2016, 12, CD008409. [Google Scholar] [CrossRef]
- Lu, X.Y.; Sun, H.; Li, Q.Y.; Lu, P.S. Progesterone for Traumatic Brain Injury: A Meta-Analysis Review of Randomized Controlled Trials. World Neurosurg. 2016, 90, 199–210. [Google Scholar] [CrossRef]
- Pan, Z.Y.; Zhao, Y.H.; Huang, W.H.; Xiao, Z.Z.; Li, Z.Q. Effect of progesterone administration on the prognosis of patients with severe traumatic brain injury: A meta-analysis of randomized clinical trials. Drug Des. Dev. Ther. 2019, 13, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Wright, D.W.; Ritchie, J.C.; Mullins, R.E.; Kellermann, A.L.; Denson, D.D. Steady-state serum concentrations of progesterone following continuous intravenous infusion in patients with acute moderate to severe traumatic brain injury. J. Clin. Pharmacol. 2005, 45, 640–648. [Google Scholar] [CrossRef]
- Frechou, M.; Zhang, S.; Liere, P.; Delespierre, B.; Soyed, N.; Pianos, A.; Schumacher, M.; Mattern, C.; Guennoun, R. Intranasal delivery of progesterone after transient ischemic stroke decreases mortality and provides neuroprotection. Neuropharmacology 2015, 97, 394–403. [Google Scholar] [CrossRef]
- Stein, D.G.; Sayeed, I. Repurposing and repositioning neurosteroids in the treatment of traumatic brain injury: A report from the trenches. Neuropharmacology 2019, 147, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Schwamm, L.H. Progesterone for traumatic brain injury-resisting the sirens’ song. N. Engl. J. Med. 2014, 371, 2522–2523. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.L.; Coomber, B.; Rathbone, J. Is progesterone a candidate neuroprotective factor for treatment following ischemic stroke? Neuroscientist 2009, 15, 324–332. [Google Scholar] [CrossRef]
- Wong, R.; Renton, C.; Gibson, C.L.; Murphy, S.J.; Kendall, D.A.; Bath, P.M. Progesterone Pre-Clinical Stroke Pooling Project, C., Progesterone treatment for experimental stroke: An individual animal meta-analysis. J. Cereb. Blood Flow Metab. 2013, 33, 1362–1372. [Google Scholar] [CrossRef] [Green Version]
- Ishrat, T.; Sayeed, I.; Atif, F.; Hua, F.; Stein, D.G. Progesterone and allopregnanolone attenuate blood-brain barrier dysfunction following permanent focal ischemia by regulating the expression of matrix metalloproteinases. Exp. Neurol. 2010, 226, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Won, S.; Lee, J.H.; Wali, B.; Stein, D.G.; Sayeed, I. Progesterone attenuates hemorrhagic transformation after delayed tPA treatment in an experimental model of stroke in rats: Involvement of the VEGF-MMP pathway. J. Cereb. Blood Flow Metab. 2014, 34, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Grossman, K.J.; Goss, C.W.; Stein, D.G. Effects of progesterone on the inflammatory response to brain injury in the rat. Brain Res. 2004, 1008, 29–39. [Google Scholar] [CrossRef]
- Won, S.; Lee, J.K.; Stein, D.G. Recombinant tissue plasminogen activator promotes, and progesterone attenuates, microglia/macrophage M1 polarization and recruitment of microglia after MCAO stroke in rats. Brain Behav. Immun. 2015, 49, 267–279. [Google Scholar] [CrossRef]
- Lammerding, L.; Slowik, A.; Johann, S.; Beyer, C.; Zendedel, A. Poststroke Inflammasome Expression and Regulation in the Peri-Infarct Area by Gonadal Steroids after Transient Focal Ischemia in the Rat Brain. Neuroendocrinology 2016, 103, 460–475. [Google Scholar] [CrossRef]
- Yousuf, S.; Atif, F.; Sayeed, I.; Wang, J.; Stein, D.G. Neuroprotection by progesterone after transient cerebral ischemia in stroke-prone spontaneously hypertensive rats. Horm. Behav. 2016, 84, 29–40. [Google Scholar] [CrossRef]
- Aggarwal, R.; Medhi, B.; Pathak, A.; Dhawan, V.; Chakrabarti, A. Neuroprotective effect of progesterone on acute phase changes induced by partial global cerebral ischaemia in mice. J. Pharm. Pharmacol. 2008, 60, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Ozacmak, V.H.; Sayan, H. The effects of 17beta estradiol, 17alpha estradiol and progesterone on oxidative stress biomarkers in ovariectomized female rat brain subjected to global cerebral ischemia. Physiol. Res. 2009, 58, 909–912. [Google Scholar] [PubMed]
- Sayeed, I.; Parvez, S.; Wali, B.; Siemen, D.; Stein, D.G. Direct inhibition of the mitochondrial permeability transition pore: A possible mechanism for better neuroprotective effects of allopregnanolone over progesterone. Brain Res. 2009, 1263, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Gaignard, P.; Frechou, M.; Schumacher, M.; Therond, P.; Mattern, C.; Slama, A.; Guennoun, R. Progesterone reduces brain mitochondrial dysfunction after transient focal ischemia in male and female mice. J. Cereb. Blood Flow Metab. 2016, 36, 562–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrabi, S.S.; Parvez, S.; Tabassum, H. Progesterone induces neuroprotection following reperfusion-promoted mitochondrial dysfunction after focal cerebral ischemia in rats. Dis. Models Mech. 2017, 10, 787–796. [Google Scholar] [CrossRef] [Green Version]
- Ishrat, T.; Sayeed, I.; Atif, F.; Hua, F.; Stein, D.G. Progesterone is neuroprotective against ischemic brain injury through its effects on the phosphoinositide 3-kinase/protein kinase B signaling pathway. Neuroscience 2012, 210, 442–450. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Zuo, F.; Wang, Y.; Lu, H.; Yang, Q.; Wang, J. Progesterone Changes VEGF and BDNF Expression and Promotes Neurogenesis After Ischemic Stroke. Mol. Neurobiol. 2016, 54, 571–581. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, R.; Cai, W.; Bai, Y.; Sokabe, M.; Chen, L. Treatment with progesterone after focal cerebral ischemia suppresses proliferation of progenitor cells but enhances survival of newborn neurons in adult male mice. Neuropharmacology 2010, 58, 930–939. [Google Scholar] [CrossRef]
- Guennoun, R.; Zhu, X.; Frechou, M.; Gaignard, P.; Slama, A.; Liere, P.; Schumacher, M. Steroids in Stroke with Special Reference to Progesterone. Cell Mol. Neurobiol. 2019, 39, 551–568. [Google Scholar] [CrossRef]
- Chen, J.; Chopp, M.; Li, Y. Neuroprotective effects of progesterone after transient middle cerebral artery occlusion in rat. J. Neurol. Sci. 1999, 171, 24–30. [Google Scholar] [CrossRef]
- Wali, B.; Ishrat, T.; Won, S.; Stein, D.G.; Sayeed, I. Progesterone in experimental permanent stroke: A dose-response and therapeutic time-window study. Brain 2014, 137, 486–502. [Google Scholar] [CrossRef]
- Yousuf, S.; Atif, F.; Sayeed, I.; Tang, H.; Stein, D.G. Progesterone in transient ischemic stroke: A dose-response study. Psychopharmacology 2014, 231, 3313–3323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frechou, M.; Zhu, X.; Liere, P.; Pianos, A.; Schumacher, M.; Mattern, C.; Guennoun, R. Dose-dependent and long-term cerebroprotective effects of intranasal delivery of progesterone after ischemic stroke in male mice. Neuropharmacology 2020, 170, 108038. [Google Scholar] [CrossRef]
- Yousuf, S.; Sayeed, I.; Atif, F.; Tang, H.; Wang, J.; Stein, D.G. Delayed progesterone treatment reduces brain infarction and improves functional outcomes after ischemic stroke: A time-window study in middle-aged rats. J. Cereb. Blood Flow Metab. 2014, 34, 297–306. [Google Scholar] [CrossRef]
- Sayeed, I.; Guo, Q.; Hoffman, S.W.; Stein, D.G. Allopregnanolone, a progesterone metabolite, is more effective than progesterone in reducing cortical infarct volume after transient middle cerebral artery occlusion. Ann. Emerg. Med. 2006, 47, 381–389. [Google Scholar] [CrossRef]
- Zhu, X.; Frechou, M.; Schumacher, M.; Guennoun, R. Cerebroprotection by progesterone following ischemic stroke: Multiple effects and role of the neural progesterone receptors. J. Steroid Biochem. Mol. Biol. 2019, 185, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Kim, J.K.; Chao, D.; Kuo, L.; Mally, A.; McClean, M.E.; Pemberton, H.E.; Wilmington, A.R.; Wong, J.; Murphy, S.P. Progesterone and allopregnanolone improves stroke outcome in male mice via distinct mechanisms but neither promotes neurogenesis. J. Neurochem. 2015, 132, 32–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.; Koide, S.S.; Tsong, Y.; Sundaram, K. Nestorone: A progestin with a unique pharmacological profile. Steroids 2000, 65, 629–636. [Google Scholar] [CrossRef]
- Kumar, N.; Fagart, J.; Liere, P.; Mitchell, S.J.; Knibb, A.R.; Petit-Topin, I.; Rame, M.; El-Etr, M.; Schumacher, M.; Lambert, J.J.; et al. Nestorone(R) as a Novel Progestin for Nonoral Contraception: Structure-Activity Relationships and Brain Metabolism Studies. Endocrinology 2017, 158, 170–182. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Ogaeri, T.; Samsonov, M.; Sokabe, M. Nestorone exerts long-term neuroprotective effects against transient focal cerebral ischemia in adult male rats. Brain Res. 2019, 1719, 288–296. [Google Scholar] [CrossRef]
- Cai, W.; Zhu, Y.; Furuya, K.; Li, Z.; Sokabe, M.; Chen, L. Two different molecular mechanisms underlying progesterone neuroprotection against ischemic brain damage. Neuropharmacology 2008, 55, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, D.R.; Spoelstra, N.S.; Richer, J.K. The role of miRNAs in progesterone action. Mol. Cell Endocrinol. 2012, 357, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Theis, V.; Theiss, C. Progesterone: A universal stimulus for neuronal cells? Neural Regen. Res. 2015, 10, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Theis, V.; Theiss, C. Progesterone Effects in the Nervous System. Anat. Rec. 2019, 302, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Pieczora, L.; Stracke, L.; Vorgerd, M.; Hahn, S.; Theiss, C.; Theis, V. Unveiling of miRNA Expression Patterns in Purkinje Cells During Development. Cerebellum 2017, 16, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Herzog, R.; Zendedel, A.; Lammerding, L.; Beyer, C.; Slowik, A. Impact of 17beta-estradiol and progesterone on inflammatory and apoptotic microRNA expression after ischemia in a rat model. J. Steroid Biochem. Mol. Biol. 2017, 167, 126–134. [Google Scholar] [CrossRef] [PubMed]

| Steroid | Cycle Effect | Sex Effect | Age Effect | Species/Structures | References |
|---|---|---|---|---|---|
| PROG | D > P > E | F (D) > M | Decrease | Mice/Cerebral hemisphere | [87] |
| 5α-DHPROG | D > P > E | NS | Decrease | ||
| 3α,5αTHPROG | NS | NS | NS | ||
| PROG | NS | Rats/Hippocampus | [92] | ||
| M > F (D) | Rats/Cortex, Cerebellum | ||||
| 5α-DHPROG | F (D) > M | Rats/Hippocampus, Cortex, Cerebellum | |||
| 3α,5α-THPROG | F (D) > M | Rats/Hippocampus, Cortex | |||
| PROG | Decrease | Rats/Limbic regions | [94] | ||
| 5α-DHPROG | Increase | ||||
| 3α,5α-THPROG | Decrease | ||||
| PROG | Decrease | Human/Cortex, Amygdala, Hippocampus, Striatum, Thalamus | [93] | ||
| 5α-DHPROG | Decrease | ||||
| 3α,5α-THPROG | Decrease |
| Pathology/Experimental Model | Sex/Species | Steroids/Change | Structures | References |
|---|---|---|---|---|
| AD/3xTg-AD mice | Male mice | 5α-DHPROG ↑ | Limbic region | [94] |
| P/ 6-OHDA | Male rats | 5α-DHPROG ↓ | Cortex, Striatum | [95] |
| PD | Men | 5α-DHPROG ↓ | CSF | [96] |
| EAE acute phase | Male rats | PROG ↓ | Cerebellum | [97] |
| PROG ↓ 5α-DHPROG ↓ 3α,5α-THPROG ↑ | Cortex | |||
| Female rats | 3α,5α-THPROG ↓ | Cerebellum | ||
| EAE chronic phase | Male and female rats | PROG ↓ 5α-DHPROG ↓ 3α,5α-THPROG↓ | Cortex of males Cerebellum of females | [98] |
| MS | Men | 3α,5α-THPROG ↓ | Frontal lobe | [61] |
| TBI/Bilateral cortex contusion | Male rats | PROG ↑ 5α-DHPROG ↑ (6 h post-TBI) | Brain | [91] |
| Pseudopregnant Female rats | 5α-DHPROG ↑ (6 h post-TBI) | |||
| Male mice | PROG = 5α-DHPROG = 3α,5α-THPROG =(24 h, 72 h, 2 weeks post-TBI) | Brain | [89] | |
| TBI/weight drop model | Female mice | PROG ↓ 5α-DHPROG ↓ 3α,5α-THPROG ↓ (24 h, 72 h, 2 weeks post-TBI) | Brain | [88] |
| Severe TBI | Men | PROG ↑ (Day 0 post-TBI) | Plasma | [99] |
| Men and Women | PROG ↓ (Days 1 to 6 post-TBI) | |||
| Stroke MCAO/R | Male mice | PROG ↑ (4 h, 6 h post-MCAO) | Cerebral hemisphere | [90] |
| 5α-DHPROG ↑ 3α,5α-THPROG = (1,2,4,6, 24 h post-MCAO) | ||||
| Female mice | PROG = 5α-DHPROG = 3α,5α-THPROG = (1,2,4,6, 24 h post-MCAO) |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guennoun, R. Progesterone in the Brain: Hormone, Neurosteroid and Neuroprotectant. Int. J. Mol. Sci. 2020, 21, 5271. https://doi.org/10.3390/ijms21155271
Guennoun R. Progesterone in the Brain: Hormone, Neurosteroid and Neuroprotectant. International Journal of Molecular Sciences. 2020; 21(15):5271. https://doi.org/10.3390/ijms21155271
Chicago/Turabian StyleGuennoun, Rachida. 2020. "Progesterone in the Brain: Hormone, Neurosteroid and Neuroprotectant" International Journal of Molecular Sciences 21, no. 15: 5271. https://doi.org/10.3390/ijms21155271
APA StyleGuennoun, R. (2020). Progesterone in the Brain: Hormone, Neurosteroid and Neuroprotectant. International Journal of Molecular Sciences, 21(15), 5271. https://doi.org/10.3390/ijms21155271





