Temporal and Spatial Expression Analysis of Shoot-Regeneration Regulatory Genes during the Adventitious Shoot Formation in Hypocotyl and Cotyledon Explants of Tomato (CV. Micro-Tom)
Abstract
:1. Introduction
2. Results
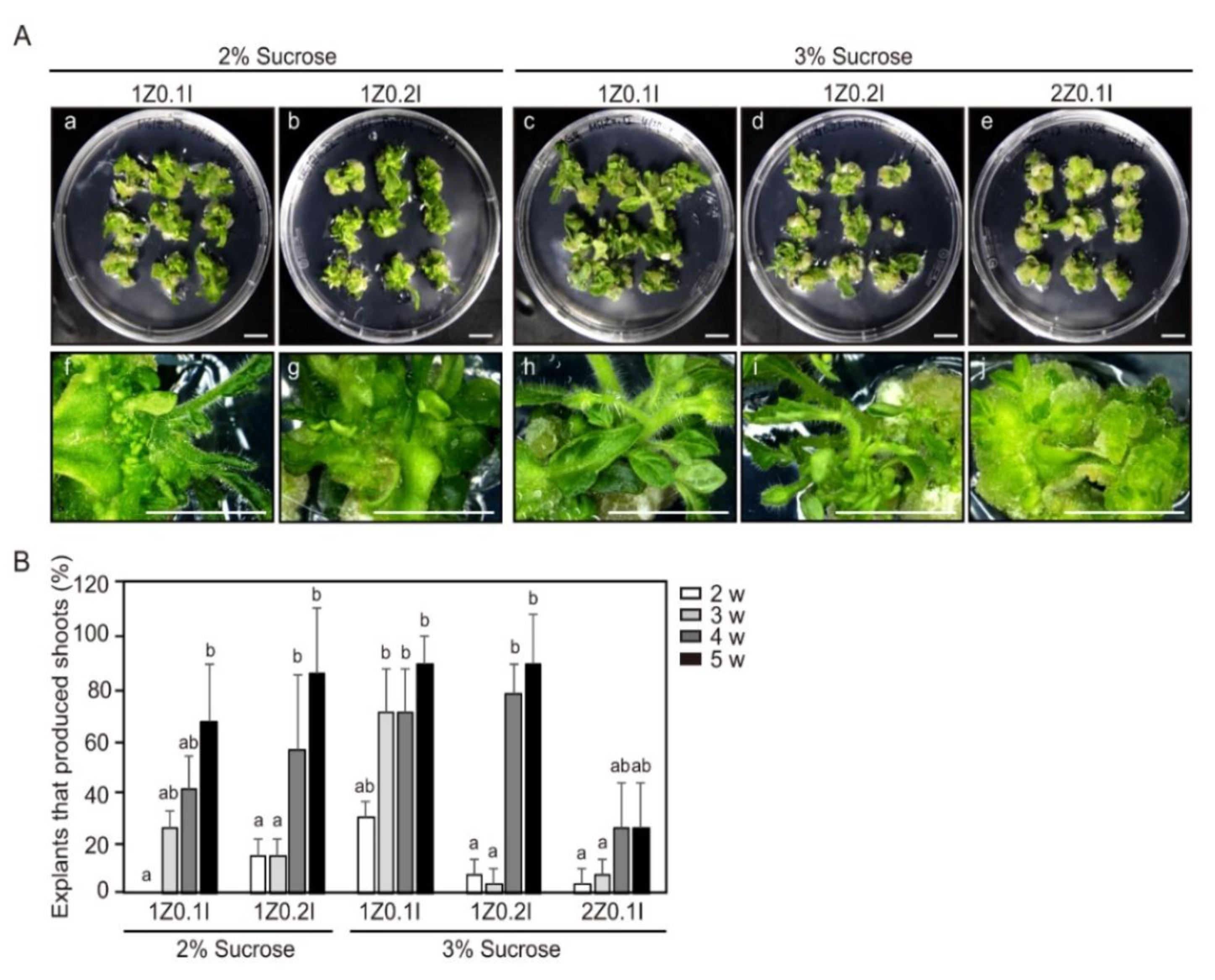
2.1. Effect of Sucrose Concentration and Plant Growth Regulators on Shoot Regeneration Efficiency
2.2. Effect of Explant Orientations on Shoot-Regeneration Efficiency
2.3. Effect of Explant Density on Shoot-Regeneration Efficiency
2.4. Effect of Explant Ages on Adventitious Shoot Formation of the Tomato
2.5. Effect of Positional Difference on Adventitious Shoot Formation Within Individual Tomato Explants
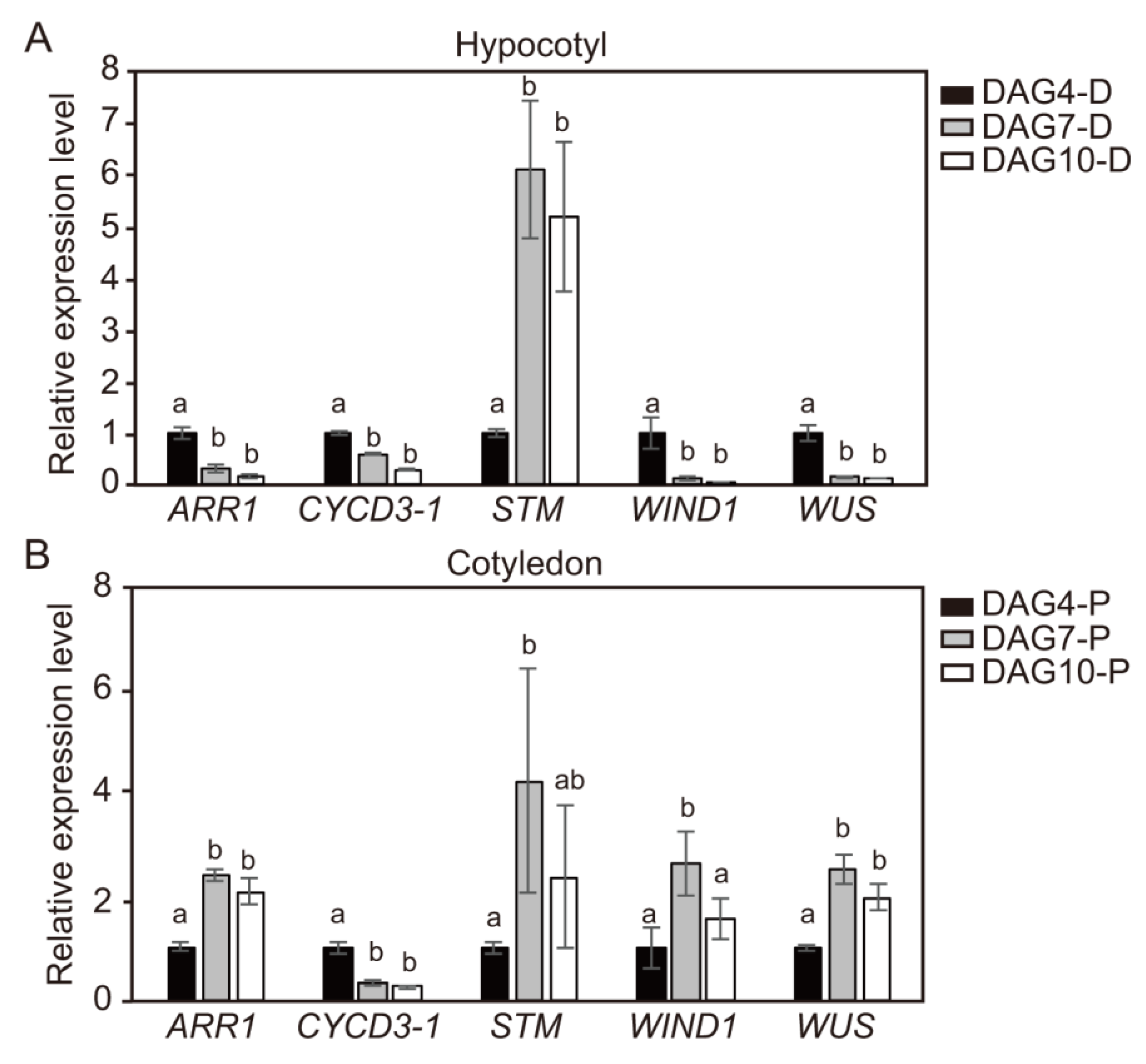
2.6. Temporal Changes in Expression Levels of CYCD3-1 and Major Shoot-Regeneration Regulatory Genes from Tomato Explants During Adventitious Shoot Formation
2.7. Spatial Changes in Expression Levels of CYCD3-1 and Major Shoot-Regeneration Regulatory Genes from Tomato Explants During Adventitious Shoot Formation
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Seeds Germination
4.2. Culture Conditions for Adventitious Shoot Formation from Tomato Explants
4.3. RNA Isolation and Quantitative Reverse Transcription PCR (qRT-PCR) Analysis
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
Accession Numbers
References
- Brooks, C.; Nekrasov, V.; Lippman, Z.B.; Van Eck, J. Efficient gene editing in tomato in the first generation using the clustered regularly interspaced short palindromic repeats/CRISPR-Associated9 system. Plant Physiol. 2014, 166, 1292–1297. [Google Scholar] [CrossRef] [Green Version]
- Ito, Y.; Nishizawa-Yokoi, A.; Endo, M.; Mikami, M.; Toki, S. CRISPR/Cas9-mediated mutagenesis of the RIN locus that regulates tomato fruit ripening. Biochem. Biophys. Res. Commun. 2015, 467, 76–82. [Google Scholar] [CrossRef]
- Ito, Y.; Nishizawa-Yokoi, A.; Endo, M.; Mikami, M.; Shima, Y.; Nakamura, N.; Kotake-Nara, E.; Kawasaki, S.; Toki, S. Re-evaluation of the rin mutation and the role of RIN in the induction of tomato ripening. Nat. Plants 2017, 3, 866–874. [Google Scholar] [CrossRef]
- Tomlinson, L.; Yang, Y.; Emenecker, R.; Smoker, M.; Taylor, J.; Perkins, S.; Smith, J.; MacLean, D.; Olszewski, N.E.; Jones, J.D.G. Using CRISPR/Cas9 genome editing in tomato to create a gibberellin-responsive dominant dwarf DELLA allele. Plant Biotechnol. J. 2019, 17, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Ueta, R.; Abe, C.; Watanabe, T.; Sugano, S.S.; Ishihara, R.; Ezura, H.; Osakabe, Y.; Osakabe, K. Rapid breeding of parthenocarpic tomato plants using CRISPR/Cas9. Sci. Rep. 2017, 7, 507. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Chen, L.; Li, R.; Zhao, R.; Yang, M.; Sheng, J.; Shen, L. Reduced drought tolerance by CRISPR/Cas9-mediated SlMAPK3 mutagenesis in tomato plants. J. Agric. Food Chem. 2017, 65, 8674–8682. [Google Scholar] [CrossRef]
- Yang, Y.F.; Zhu, G.N.; Li, R.; Yan, S.J.; Fu, D.Q.; Zhu, B.Z.; Tian, H.Q.; Luo, Y.B.; Zhu, H.L. The RNA editing factor SlORRM4 is required for normal fruit ripening in tomato. Plant Physiol. 2017, 175, 1690–1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatia, P.; Ashwath, N.; Senaratna, T.; Midmore, D. Tissue culture studies of tomato (Lycopesicun esculentum). Plant Cell Tiss. Organ Cult. 2004, 78, 1–21. [Google Scholar] [CrossRef]
- Ozcan, S.; Yildiz, M.; Sancak, C.; Ozgen, M. Adventitious shoot regeneration in sainfoin (Onobrychis viciifolia Scop.). Turk. J. Bot. 1996, 20, 497–501. [Google Scholar]
- Yıldız, M.; Saglik, C.; Telci, C.; Erkilic, E.G. The effect of in vitro competition on shoot regeneration from hypocotyl explants of Linum usitatissimum. Turk. J. Bot. 2011, 35, 211–218. [Google Scholar]
- Melanie, E.; Davis, R.; Daniel Lineberger, A.; Raymond, M. Effects of tomato cultivar, leaf age, and bacterial strain on transformation by Agrobacterium Tumefaciens. Plant Cell Tissue Organ Cult. 1991, 24, 115–121. [Google Scholar]
- Rai, G.K.; Rai, N.P.; Kumar, S.; Yadav, A.; Rathaur, S.; Singh, M. Effects of explant age, germination medium, pre-culture parameters, inoculation medium, pH, washing medium, and selection regime on Agrobacterium-mediated transformation of tomato. Vitr. Cell. Dev. Biol 2012, 48, 565–578. [Google Scholar] [CrossRef]
- Yildiz, M. The prerequisite of the success in plant tissue culture: High frequency shoot regeneration. In Recent Advances in Plant In Vitro Culture; Intechopen: London, UK, 2012; chapter 4; pp. 63–90. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, P.; Ashwath, N.; Midmore, D.J. Effects of genotype, explant orientation, and wounding on shoot regeneration in tomato. Vitr. Cell. Dev. Biol.-Plant 2005, 41, 457–464. [Google Scholar] [CrossRef]
- Costa, G.M.; Nogueira, F.T.S.; Otoni, W.C.; Brommorischenkel, S.H. In vitro regeneration of processing tomato (Lycopersicon esculentum Mill) ‘IPA-5’ and ‘IPA-6’. Cienc. Agroteenol. 2000, 24, 671–678. [Google Scholar]
- Abubaker, S. Effect of plant density on flowering date, yield and quality attribute of bush beans (Phaseolus vulgaris L.) under center pivot irrigation system. Am. J. Agric. Biol. Sci. 2008, 3, 666–668. [Google Scholar] [CrossRef] [Green Version]
- Stoffella, P.J.; Bryan, H.H. Plant population influences growth and yields of bell pepper. J. Am. Soc. Hortic. Sci. 1988, 113, 835–839. [Google Scholar]
- Duclercq, J.; Sangwan-Norreel, B.; Catterou, M.; Sangwan, R.S. De novo shoot organogenesis: From art to science. Trends Plant Sci. 2011, 16, 597–606. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Favero, D.S.; Sakamoto, Y.; Iwase, A.; Coleman, D.; Rymen, B.; Sugimoto, K. Molecular mechanisms of plant regeneration. Annu. Rev. Plant Biol. 2019, 70, 377–406. [Google Scholar] [CrossRef]
- Iwase, A.; Mita, K.; Nonaka, S.; Ikeuchi, M.; Koizuka, C.; Ohnuma, M.; Ezura, H.; Imamura, J.; Sugimoto, K. WIND10based acquisition of regeneration competency in Arabidopsis and rapeseed. J. Plant Res. 2015, 128, 389–397. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaew, V.; Dangle, J.L.; Mittler, R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2009, 2, ra45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwase, A.; Harashima, H.; Ikeuchi, M.; Rymen, B.; Ohnuma, M.; Komaki, S.; Morohashi, K.; Kurata, T.; Nakata, M.; Ohme-Takagi, M.; et al. WIND1 promotes shoot regeneration through transcriptional activation of ENHANCER OF SHOOT REGENERATION1 in Arabidopsis. Plant Cell 2017, 29, 54–69. [Google Scholar] [CrossRef] [Green Version]
- Banno, H.; Ikeda, Y.; Niu, Q.W.; Chua, N.H. Overexpression of Arabidopsis ESR1 induces initiation of shoot regeneration. Plant Cell 2001, 13, 2609–2618. [Google Scholar] [CrossRef] [PubMed]
- Kirch, T.; Simon, R.; Grunewald, M.; Werr, W. The DORNROSCHEN/ENHANCER OF SHOOT REGENERATION1 gene of Arabidopsis acts in the control of meristem cell fate and lateral organ development. Plant Cell 2003, 15, 649–705. [Google Scholar] [CrossRef] [Green Version]
- Ikeuchi, M.; Iwase, A.; Rymen, B.; Lambolez, A.; Kojima, M.; Takebayashi, Y.; Heyman, J.; Watanabe, S.; Seo, M.; De Veylder, L.; et al. Wounding triggers callus formation via dynamic hormonal and transcriptional changes. Plant Physiol. 2017, 175, 1158–1174. [Google Scholar] [CrossRef]
- Iwase, A.; Mitsuda, N.; Koyama, T.; Hiratsu, K.; Kojima, M.; Arai, T.; Inoue, Y.; Seki, M.; Sakakibara, H.; Sugimoto, K.; et al. The AP2/ERF transcription factor WIND1 controls cell dedifferentiation in Arabidopsis. Curr. Biol. 2011, 21, 508–514. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.Q.; Lian, H.; Zhou, C.M.; Xu, L.; Jiao, Y.; Wang, J.W. A two-step model for de novo activation of WUSCHEL during plant shoot regeneration. Plant Cell 2017, 29, 1073–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, B.; Zhang, C.; Tian, C.; Wang, J.; Wang, Q. Two-step regulation of a meristematic cell population acting in shoot branching in Arabidopsis. PLoS Genet. 2016, 12, e1006168. [Google Scholar] [CrossRef]
- Mamidala, P.; Nanna, R.S. Effect of genotype, explant source and medium on in vitro regeneration of tomato. Int. J. Genet. Mol. Biol. 2011, 3, 45–50. [Google Scholar]
- Kaul, B.; Srivastava, S.; Sanyal, I.; Tripathi, B.; Sharma, V.; Amla, D.V. Transgenic tomato line expressing modified Bracillus thuringiensis cry1Ab gene showing complete resistance to two lepidopteran pests. Springerplus 2014, 3, 84. [Google Scholar] [CrossRef] [Green Version]
- Vikram, G.; Madhusudhan, K.; Srikanth, K.; Laxminarasu, M.; Swamy, N.R. Zeatin induced direct multiple shoots development and plant regeneration from cotyledon explants of cultivated tomato (Solanum lycopesicum L.). Aust. J. Crop Sci. 2012, 6, 31–35. [Google Scholar]
- Ichimura, K.; Uchiumi, T.; Tsuji, K.; Oda, M.; Nagaoka, M. Shoot regeneration of tomato (Lycopersicon esculentum Mill.) in tissue culture using several kinds of supporting materials. Plant Sci. 1995, 108, 93–100. [Google Scholar] [CrossRef]
- Kumari, A.; Ray, K.; Sadhna, S.; Pandey, A.K.; Sreelakshmi, Y.; Sharma, R. Metabolomic homeostasis shifts after callus formation and shoot regeneration in tomato. PLoS ONE 2017, 12, e0176978. [Google Scholar] [CrossRef] [PubMed]
- Yalcin-Mendi, N.Y. Effect of cotyledon age and explant location on regeneration of cucumis Sativus, Biotechnol. Biotechnol. Equip. 2003, 17, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Fagundez, S.; Katin-Grazzini, L.; Li, Y.; Li, W.; Chen, Y.; Wang, X.; Deng, Z.; Xie, S.; McAvoy, R.J.; et al. Endogenous auxin and its manipulation influence in vitro shoot organogenesis of citrus epicotyl explants. Hortic. Res. 2017, 4, 17071. [Google Scholar] [CrossRef]
- Jones, B.; Gunneras, S.A.; Petersson, S.V.; Tarkowski, P.; Graham, N.; May, S.; Dolezal, K.; Sandberg, G.; Ljung, K. Cytokinin regulation of auxin synthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal transduction. Plant Cell 2011, 22, 2956–2969 ttps://doiorg/101105/tpc110074856ccs. [Google Scholar] [CrossRef] [Green Version]
- De Veylder, L.; De Almeida Engler, J.; Burssens, S.; Manevski, A.; Lescure, B.; Van Montagu, M.; Engler, G.; Inze, D. A new D-type cyclin of Arabidopsis thaliana expressed during lateral root primordia formation. Planta 1999, 208, 453–462. [Google Scholar] [CrossRef]
- Richard, C.; Lescot, M.; Dirk Inze, D.; De Veylder, L. Effect of auxin, cytokinin, and sucrose on cell cycle gene expression in Arabidopsis thaliana cell suspension cultures. Plant Celltissue Organ Cult. 2002, 69, 167–176. [Google Scholar] [CrossRef]
- Soni, R.; Carmichael, J.P.; Shah, Z.H.; Murray, J.A. A family of cyclin D homologs from plants differentially controlled by growth regulators and containing the conserved retinoblastoma protein interaction motif. Plant Cell 1995, 7, 85–103. [Google Scholar] [CrossRef]
- Cheng, Z.J.; Zhu, S.S.; Gao, X.Q.; Zhang, X.S. Cytokinin and auxin regulates WUS induction and inflorescence regeneration in vitro in Arabidopsis. Plant Cell Rep. 2010, 29, 927–933. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.H.; Lee, J.; Jie, E.Y.; Choi, S.H.; Jiang, L.; Ahn, W.S.; Kim, C.Y.; Kim, S.W. Temporal and Spatial Expression Analysis of Shoot-Regeneration Regulatory Genes during the Adventitious Shoot Formation in Hypocotyl and Cotyledon Explants of Tomato (CV. Micro-Tom). Int. J. Mol. Sci. 2020, 21, 5309. https://doi.org/10.3390/ijms21155309
Lee MH, Lee J, Jie EY, Choi SH, Jiang L, Ahn WS, Kim CY, Kim SW. Temporal and Spatial Expression Analysis of Shoot-Regeneration Regulatory Genes during the Adventitious Shoot Formation in Hypocotyl and Cotyledon Explants of Tomato (CV. Micro-Tom). International Journal of Molecular Sciences. 2020; 21(15):5309. https://doi.org/10.3390/ijms21155309
Chicago/Turabian StyleLee, Myoung Hui, Jiyoung Lee, Eun Yee Jie, Seung Hee Choi, Lingmin Jiang, Woo Seok Ahn, Cha Young Kim, and Suk Weon Kim. 2020. "Temporal and Spatial Expression Analysis of Shoot-Regeneration Regulatory Genes during the Adventitious Shoot Formation in Hypocotyl and Cotyledon Explants of Tomato (CV. Micro-Tom)" International Journal of Molecular Sciences 21, no. 15: 5309. https://doi.org/10.3390/ijms21155309
APA StyleLee, M. H., Lee, J., Jie, E. Y., Choi, S. H., Jiang, L., Ahn, W. S., Kim, C. Y., & Kim, S. W. (2020). Temporal and Spatial Expression Analysis of Shoot-Regeneration Regulatory Genes during the Adventitious Shoot Formation in Hypocotyl and Cotyledon Explants of Tomato (CV. Micro-Tom). International Journal of Molecular Sciences, 21(15), 5309. https://doi.org/10.3390/ijms21155309





