miR-365 (microRNA): Potential Biomarker in Oral Squamous Cell Carcinoma Exosomes and Extracellular Vesicles
Abstract
:1. Introduction
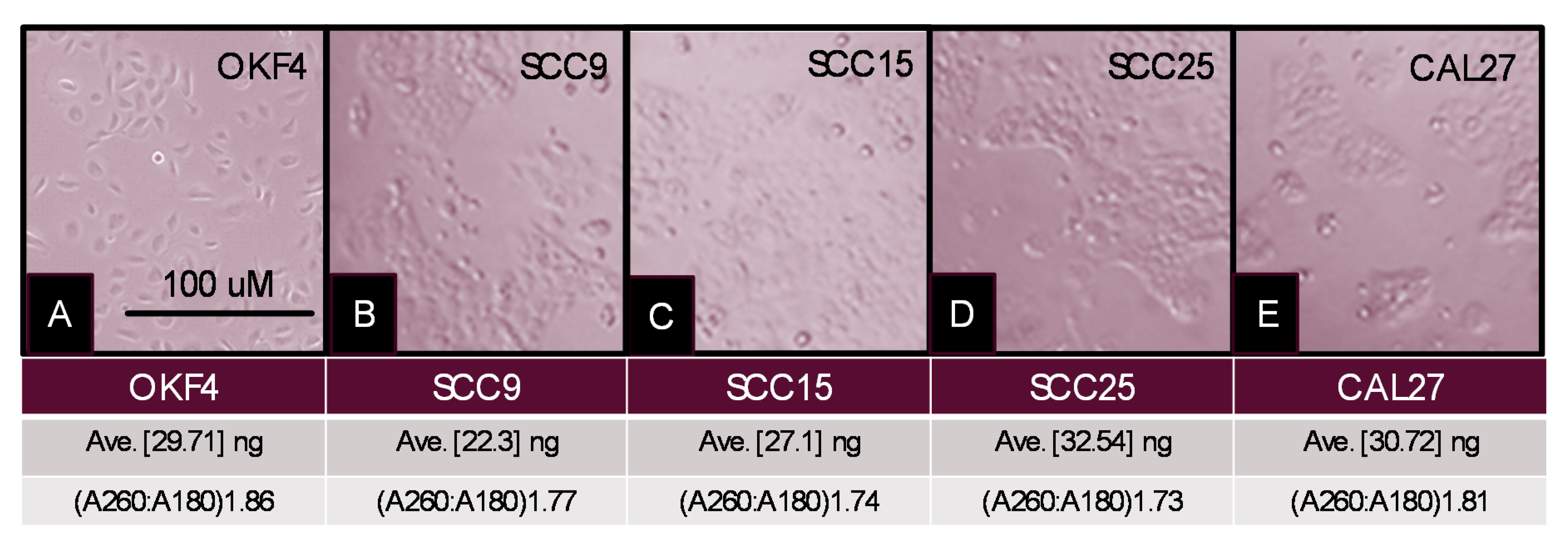
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cellular RNA Extraction
4.3. RT-PCR Screening
- Beta actin forward, 5′-GTGGGGTCCTGTGGTGTG-3′; 18 nt, 67% GC, Tm: 69 °C
- Beta actin reverse, 5′-GAAGGGGACAGGCAGTGA-3′, 18 nt, 61% GC, Tm: 67 °C
- Glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
- GAPDH forward, 5′-ATCTTCCAGGAGCGAGATCC-3′; 20 nt, 55% GC, Tm: 66 °C
- GAPDH reverse, 5′-ACCACTGACACGTTGGCAGT-3′; 20 nt, 55% GC, Tm: 70 °C
- miR-365 forward, 5′-ATAGGATCCTGAGGTCCCTTTCGTG-3′; 25 nt, 52% GC, Tm: 70 °C
- miR-365 reverse, 5′-GCGAAGCTTAAAAACAGCGGAAGAGTTTGG-3′; 30 nt, 47% GC, Tm: 72 °C
4.4. Exosome Depletion Protocol
4.5. Exosome and Extracellular Vesicle Isolation Protocol
4.6. Exosome and Extracellular Vesicle RNA Extraction
4.7. Exosome Analysis
4.8. Quantification of Exosomal RNA
4.9. cDNA Synthesis
4.10. Amplification
4.11. Real-Time PCR
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vienberg, S.; Geiger, J.; Madsen, S.; Dalgaard, L.T. MicroRNAs in metabolism. Acta Physiol. 2017, 219, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Zhan, B.; Lu, D.; Luo, P.; Wang, B. Prognostic Value of Expression of MicroRNAs in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Clin Lab. 2016, 62, 2203–2211. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.; Hosseini, M.; Ghasemi, F.; Shahidsales, S.; Maftouh, M.; Akbarzade, H.; Reza Parizadeh, S.A.; Mahdi Hassanian, S.; Avan, A. Circulating microRNAs as Potential Diagnostic, Prognostic and Therapeutic Targets in Pancreatic Cancer. Curr. Pharm. Des. 2016, 22, 6444–6450. [Google Scholar] [CrossRef]
- Liu, F.; Zhuang, L.; Wu, R.; Li, D. miR-365 inhibits cell invasion and migration of triple negative breast cancer through ADAM10. J. BUON 2019, 24, 1905–1912. [Google Scholar] [PubMed]
- Zhang, J.; Zhang, Z.; Wang, Q.; Xing, X.J.; Zhao, Y. Overexpression of microRNA-365 inhibits breast cancer cell growth and chemo-resistance through GALNT4. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4710–4718. [Google Scholar]
- Zhou, L.; Gao, R.; Wang, Y.; Zhou, M.; Ding, Z. Loss of BAX by miR-365 Promotes Cutaneous Squamous Cell Carcinoma Progression by Suppressing Apoptosis. Int. J. Mol. Sci. 2017, 18, 1157. [Google Scholar] [CrossRef]
- Zhou, M.; Zhuo, L.; Zheng, L.; Guo, L.; Wang, Y.; Liu, H.; Ou, C.; Ding, Z. miR-365 promotes cutaneous squamous cell carcinoma (CSCC) through targeting nuclear factor I/B (NFIB). PLoS ONE 2014, 9, e100620. [Google Scholar] [CrossRef]
- Hou, M.; Liu, W.; Ma, S.; Cao, H.; Peng, X.; Guo, L.; Zhou, X.; Zheng, L.; Guo, L.; Wan, M.; et al. A novel onco-miR-365 induces cutaneous squamous cell carcinoma. Carcinogenesis 2013, 34, 1653–1659. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Yu, S.; Cheng, J.; Zhang, Y.; Huang, X. The versatile roles and clinical implications of exosomal mRNAs and microRNAs in cancer. Int. J. Biol. Markers 2020. [Google Scholar] [CrossRef]
- Groot, M.; Lee, H. Sorting Mechanisms for MicroRNAs into Extracellular Vesicles and Their Associated Diseases. Cells 2020, 9, 1044. [Google Scholar] [CrossRef]
- El-Daly, S.M.; Bayraktar, R.; Anfossi, S.; Calin, G.A. The Interplay between MicroRNAs and the Components of the Tumor Microenvironment in B-Cell Malignancies. Int. J. Mol. Sci. 2020, 21, 3387. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Meiners, S.; Lukas, C.; Stathopoulos, G.T.; Chen, J. Role of exosomal microRNAs in lung cancer biology and clinical applications. Cell Prolif. 2020, e12828. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Jang, T.H.; Tung, S.L.; Yen, T.C.; Chan, S.H.; Wang, L.H. A novel miR-365-3p/EHF/keratin 16 axis promotes oral squamous cell carcinoma metastasis, cancer stemness and drug resistance via enhancing β5-integrin/c-met signaling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Shang, W.; Zheng, F. Long non-coding RNA NEAT1 promotes migration and invasion of oral squamous cell carcinoma cells by sponging microRNA-365. Exp. Ther Med. 2018, 16, 2243–2250. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; He, X.; Wei, X.L. lncRNA NEAT1 promotes cell proliferation and invasion by regulating miR-365/RGS20 in oral squamous cell carcinoma. Oncol. Rep. 2018, 39, 1948–1956. [Google Scholar] [CrossRef]
- Coon, J.; Kingsley, K. MicroRNA (MiR)-365 Expression in Oral Squamous Cell Carcinoma. Dent. J. 2020. submitted—in review. [Google Scholar]
- Shoff, M.; Booker, T.; Leavitt, B.; Harmon, D.; Kingsley, K.; Howard, K.M. Differential exosome miRNA expression in oral cancer stem cells. ExRNA 2020, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Irani, S. Emerging insights into the biology of metastasis: A review article. Iran. J. Basic Med. Sci. 2019, 22, 833–847. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Bala, S. Emerging role of non-coding RNA in oral cancer. Cell Signal. 2018, 42, 134–143. [Google Scholar] [CrossRef]
- Zhan, C.; Yang, X.; Yin, X.; Hou, J. Exosomes and other extracellular vesicles in oral and salivary gland cancers. Oral Dis. 2019. [Google Scholar] [CrossRef]
- Shwetha, H.R.; Smitha, T. Dichotomy of exosomes in oral squamous cell carcinoma: Prey or play! J. Oral Maxillofac. Pathol. 2019, 23, 172–175. [Google Scholar] [CrossRef]
- Hunsaker, M.; Barba, G.; Kingsley, K.; Howard, K.M. Differential MicroRNA Expression of miR-21 and miR-155 within Oral Cancer Extracellular Vesicles in Response to Melatonin. Dent. J. (Basel) 2019, 7, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Han, Y.; Zhao, Z.; Ji, X.; Wang, X.; Jin, J.; Wang, Q.; Guo, X.; Cheng, Z.; Lu, M.; et al. Oral mucosal mesenchymal stem cell-derived exosomes: A potential therapeutic target in oral premalignant lesions. Int. J. Oncol. 2019, 54, 1567–1578. [Google Scholar] [CrossRef] [PubMed]
- Galot, R.; van Marcke, C.; Helaers, R.; Mendola, A.; Goebbels, R.-M.; Caignet, X.; Ambroise, J.; Wittouck, K.; Vikkula, M.; Limaye, N.; et al. Liquid biopsy for mutational profiling of locoregional recurrent and/or metastatic head and neck squamous cell carcinoma. Oral Oncol. 2020, 104, 104631. [Google Scholar] [CrossRef]
- Rapado-González, Ó.; Martínez-Reglero, C.; Salgado-Barreira, Á.; Takkouche, B.; López-López, R.; Suárez-Cunqueiro, M.M.; Muinelo-Romay, L. Salivary biomarkers for cancer diagnosis: A meta-analysis. Ann. Med. 2020, 52, 131–144. [Google Scholar] [CrossRef]
- Rapado-González, Ó.; Martínez-Reglero, C.; Salgado-Barreira, Á.; Rodríguez-Fernández, A.; Aguín-Losada, S.; León-Mateos, L.; Muinelo-Romay, L.; López-López, R.; Suarez-Cunqueiro, M.M. Association of Salivary Human Papillomavirus Infection and Oral and Oropharyngeal Cancer: A Meta-Analysis. J. Clin. Med. 2020, 9, 1305. [Google Scholar] [CrossRef]
- Tang, K.D.; Menezes, L.; Baeten, K.; Walsh, L.J.; Whitfield, B.C.S.; Batstone, M.D.; Kenny, L.; Frazer, I.H.; Scheper, G.C.; Punyadeer, C. Oral HPV16 Prevalence in Oral Potentially Malignant Disorders and Oral Cavity Cancers. Biomolecules 2020, 10, 223. [Google Scholar] [CrossRef] [Green Version]
- Cristaldi, M.; Mauceri, R.; Di Fede, O.; Giuliana, G.; Campisi, G.; Panzarella, V. Salivary Biomarkers for Oral Squamous Cell Carcinoma Diagnosis and Follow-Up: Current Status and Perspectives. Front. Physiol. 2019, 10, 1476. [Google Scholar] [CrossRef]
- Rapado-González, Ó.; López-López, R.; López-Cedrún, J.L.; Triana-Martínez, G.; Muinelo-Romay, L.; Suárez-Cunqueiro, M.M. Cell-Free microRNAs as Potential Oral Cancer Biomarkers: From Diagnosis to Therapy. Cells 2019, 8, 1653. [Google Scholar] [CrossRef] [Green Version]
- Kodahl, A.R.; Lyng, M.B.; Binder, H.; Cold, S.; Gravgaard, K.; Knoop, A.S.; Ditzel, H.J. Novel circulating microRNA signature as a potential non-invasive multi-marker test in ER-positive early-stage breast cancer: A case control study. Mol. Oncol. 2014, 8, 874–883. [Google Scholar] [CrossRef]
- Lyng, M.B.; Kodahl, A.R.; Binder, H.; Ditzel, H.J. Prospective validation of a blood-based 9-miRNA profile for early detection of breast cancer in a cohort of women examined by clinical mammography. Mol. Oncol. 2016, 10, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Mrowczynski, O.D.; Madhankumar, A.B.; Sundstrom, J.M.; Zhao, Y.; Kawasawa, Y.I.; Slagle-Webb, B.; Mau, C.; Payne, R.A.; Rizk, E.B.; Zacharia, B.E.; et al. Exosomes impact survival to radiation exposure in cell line models of nervous system cancer. Oncotarget 2018, 9, 36083–36101. [Google Scholar] [CrossRef] [PubMed]
- Binenbaum, Y.; Fridman, E.; Yaari, Z.; Milman, N.; Schroeder, A.; David, G.B.; Shlomi, T.; Gil, Z. Transfer of miRNA in Macrophage-Derived Exosomes Induces Drug Resistance in Pancreatic Adenocarcinoma. Cancer Res. 2018, 78, 5287–5299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, Q.-H.; Wang, X.-Z.; Zhang, J.; Chen, Q.-G.; Li, S.-Q.; Liu, X.-Q.; Li, J.; Liu, J.; Yang, W.-M.; Jiang, Y.-H.; et al. Exosomes derived from imatinib-resistant chronic myeloid leukemia cells mediate a horizontal transfer of drug-resistant trait by delivering miR-365. Exp. Cell Res. 2018, 362, 386–393. [Google Scholar] [CrossRef]
- Masterson, A.N.; Liyanage, T.; Berman, C.; Kaimakliotis, H.; Johnson, M.; Sardar, R. A novel liquid biopsy-based approach for highly specific cancer diagnostics: Mitigating false responses in assaying patient plasma-derived circulating microRNAs through combined SERS and plasmon-enhanced fluorescence analyses. Analyst 2020. [Google Scholar] [CrossRef]
- Keller, A.; Fehlmann, T.; Backes, C.; Kern, F.; Gislefoss, R.; Langseth, H.; Rounge, T.B.; Ludwig, N.; Meese, E. Competitive learning suggests circulating miRNA profiles for cancers decades prior to diagnosis. RNA Biol. 2020. [Google Scholar] [CrossRef]
- Shelke, G.V.; Lässer, C.; Gho, Y.S.; Lötvall, J. Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. J. Extracell Vesicles 2014, 3. [Google Scholar] [CrossRef] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coon, J.; Kingsley, K.; Howard, K.M. miR-365 (microRNA): Potential Biomarker in Oral Squamous Cell Carcinoma Exosomes and Extracellular Vesicles. Int. J. Mol. Sci. 2020, 21, 5317. https://doi.org/10.3390/ijms21155317
Coon J, Kingsley K, Howard KM. miR-365 (microRNA): Potential Biomarker in Oral Squamous Cell Carcinoma Exosomes and Extracellular Vesicles. International Journal of Molecular Sciences. 2020; 21(15):5317. https://doi.org/10.3390/ijms21155317
Chicago/Turabian StyleCoon, Jeffery, Karl Kingsley, and Katherine M. Howard. 2020. "miR-365 (microRNA): Potential Biomarker in Oral Squamous Cell Carcinoma Exosomes and Extracellular Vesicles" International Journal of Molecular Sciences 21, no. 15: 5317. https://doi.org/10.3390/ijms21155317
APA StyleCoon, J., Kingsley, K., & Howard, K. M. (2020). miR-365 (microRNA): Potential Biomarker in Oral Squamous Cell Carcinoma Exosomes and Extracellular Vesicles. International Journal of Molecular Sciences, 21(15), 5317. https://doi.org/10.3390/ijms21155317




