Rearrangement of the Cellulose-Enriched Cell Wall in Flax Phloem Fibers over the Course of the Gravitropic Reaction
Abstract
:1. Introduction
2. Results
2.1. Total Monosaccharide Composition of the Control and Gravistimulated Flax Plants
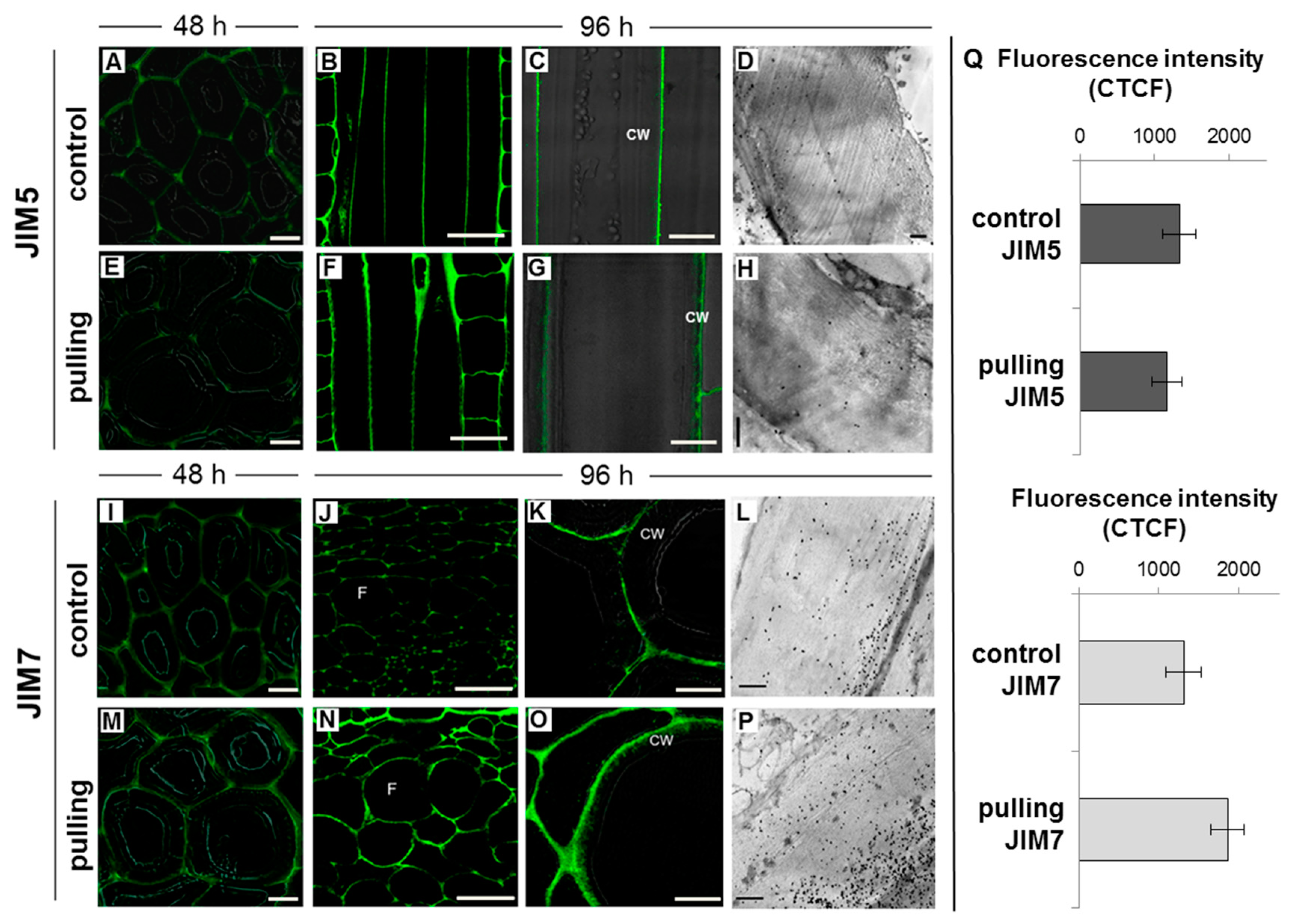
2.2. Immunolocalization of Non-Cellulosic Polysaccharides in the Cell Wall of the Control and Gravistimulated Flax Plants
2.2.1. Galactan
2.2.2. Homogalacturonan
2.2.3. Xyloglucan
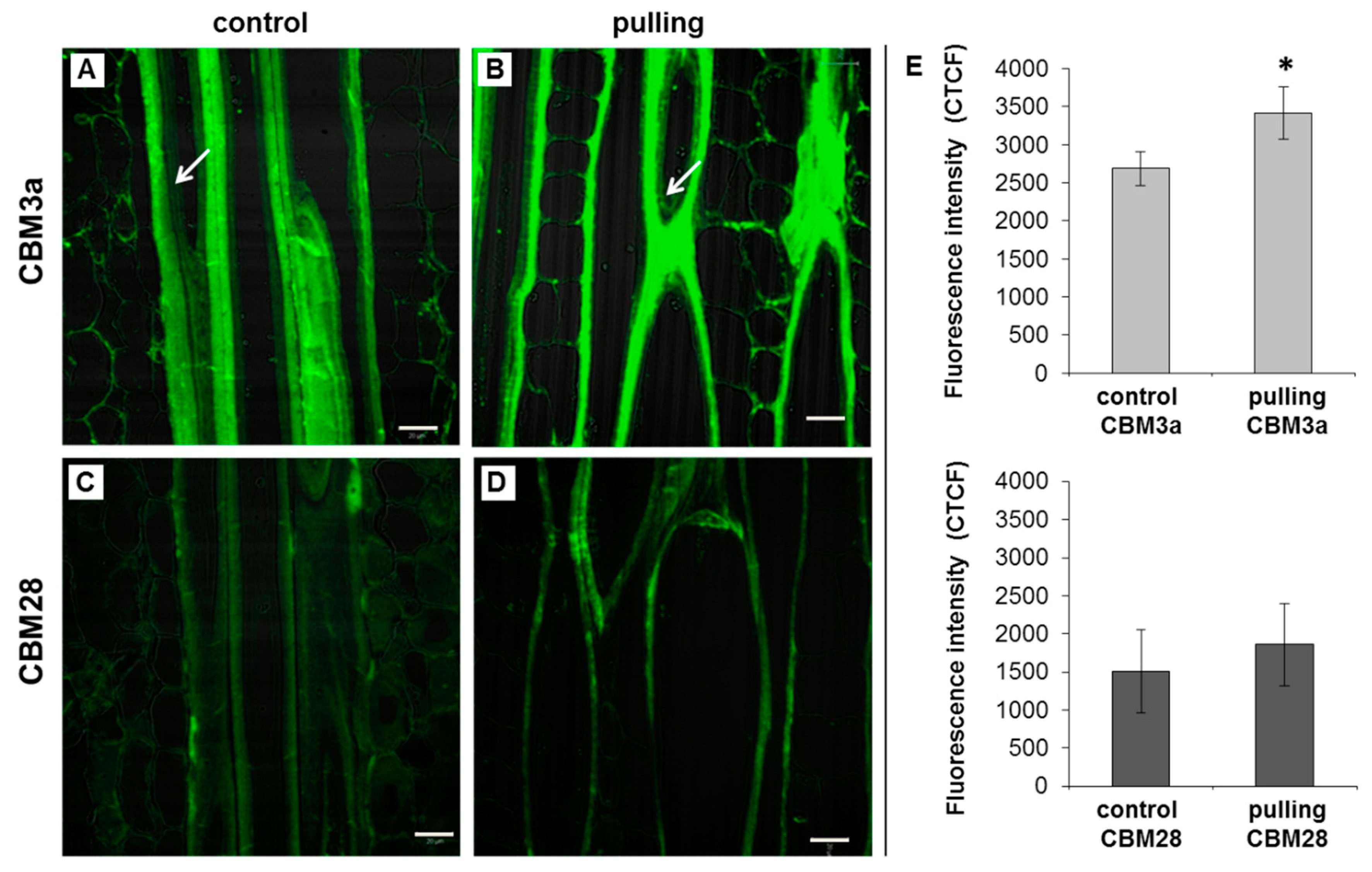
2.3. Crystalline and Amorphous Cellulose in the Fiber Cell Wall After Gravistimualtion
2.3.1. Immunocytochemistry
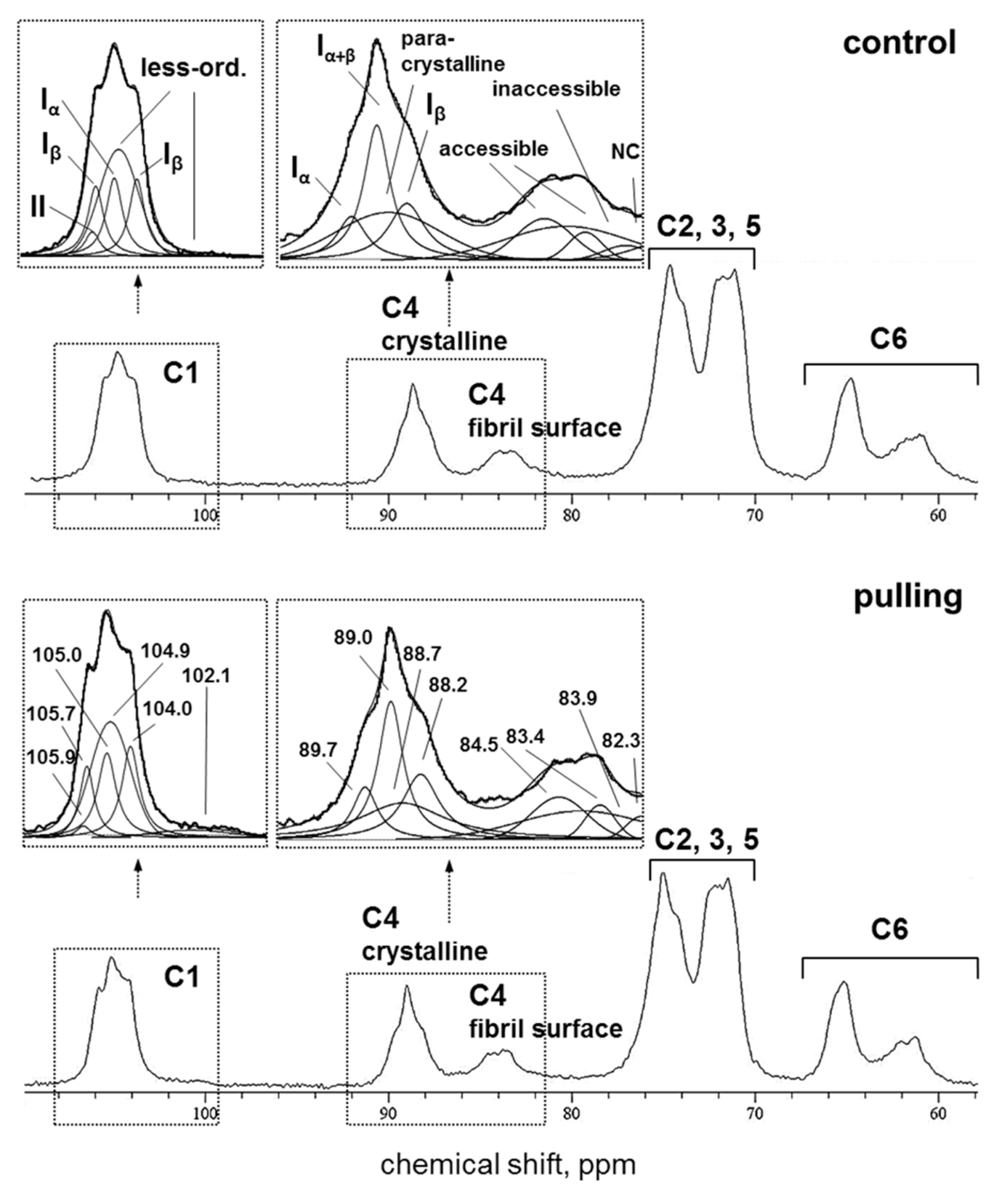
2.3.2. Solid State NMR
2.3.3. CP/MAS 13C NMR
2.3.4. SP/MAS 13C NMR
3. Discussion
- (1)
- (2)
- In fibers of the gravistimulated plants, epitopes of the LM5 antibody specific for β-(1,4)-d-galactan were localized in a wider cell wall layer compared to the control plants (Figure 3B,C), and the epitope of JIM7 specific for high methyl-esterified homogalacturonan was more abundant in the outer cell wall layer in fibers of the gravistimulated plants compared to the control plants (Figure 4N–Q). Epitopes of LM25 specific for galactosylated xyloglucan were revealed in the fiber cell walls of the gravistimulated plants without galactanase pretreatment (Figure 5B,E) while, in the control plants, epitopes of LM25 became visible only after galactanase pretreatment (Figure 5C).
- (3)
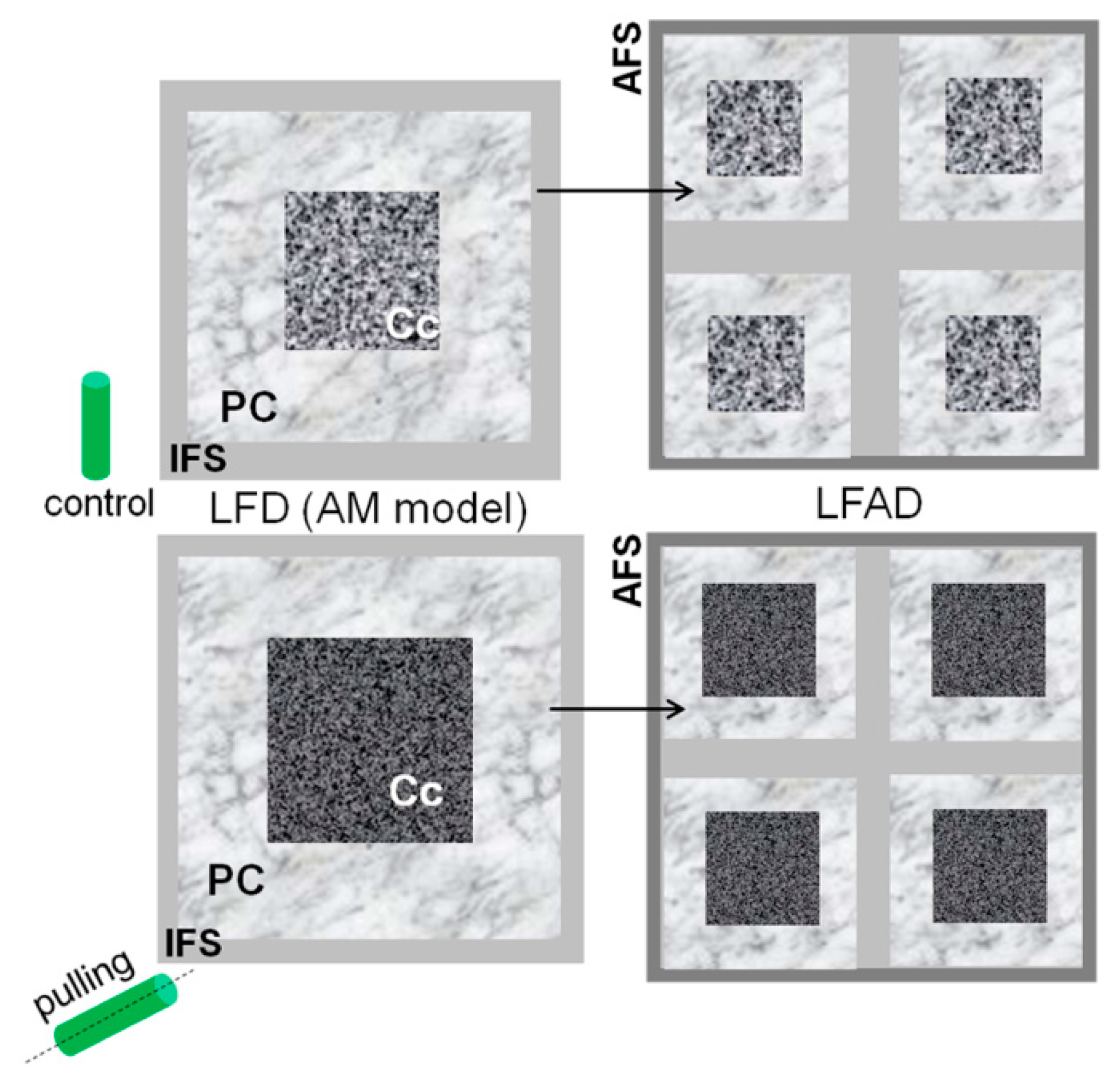
- Some changes in the cellulose structure in the flax fibers of the gravistimulated plants were revealed. The distribution of the epitopes of CBM28 and CBM3a antibodies, specific for amorphous and crystalline cellulose, respectively, as well as the signal intensity, was different in the fiber cell walls of the gravistimulated and non-gravistimulated plants. The CP MAS 13C NMR spectra revealed that the crystalline core fraction and index of crystallinity were increased for the cellulose of the gravistimulated plants. Furthermore, an increase in the proportion of β-allomorph, a slight decrease in α-allomorph, a decrease in the proportion of para-crystalline cellulose, and inaccessible fibril surface were observed in the samples of the pulling part of the stem compared to the control plants (Table 1). The proportion of signals in the C4 region relative to other signals in the SP/MAS spectrum of the cellulose from the pulling side of the stem was reduced by two times compared to the control plants (Figure 8A,C). The largest decreases in intensity were found for the signals of the fibril surface with a short relaxation time.
3.1. Gravistimulation Affects the Content and Rearrangement of Non-Cellulosic Polysaccharides in the Fiber Cell Wall
3.2. Gravistimulation Affects the Fine Structure of Cellulose in the Fiber Cell Wall
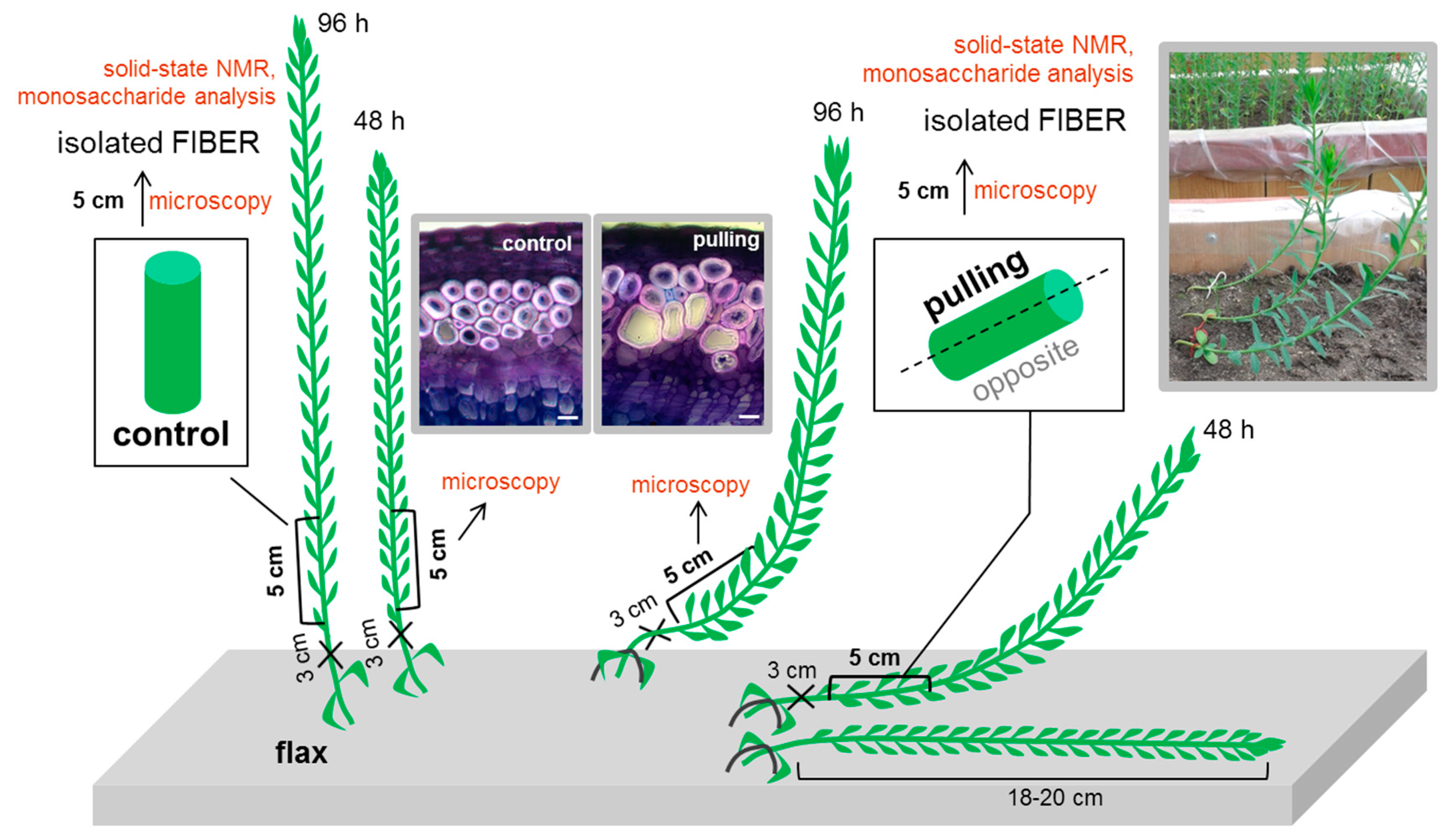
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of Fiber Cell Wall Samples for Biochemical and Nmr Analysis
4.3. Monosaccharide Analysis
4.4. Solid-State Nuclear Magnetic Resonance
4.5. Fluorescence Microscopy of the Stem Sections
4.6. Transmission Electron Microscopy of Stem Sections
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Esmon, C.A.; Pedmale, U.V.; Liscum, E. Plant tropisms: Providing the power of movement to a sessile organism. Int. J. Dev. Biol. 2005, 49, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, F.; Fortunati, A.; Tassone, P. Plant Tropisms, Physics. In Encyclopedia of Agrophysics; Gliński, J., Orabik, J., Lipiec, J., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 633–636. [Google Scholar]
- Blancaflor, E.B.; Masson, P.H. Plant gravitropism: Unraveling the ups and downs of a complex process. Plant Physiol. 2003, 133, 1677–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moulia, B.; Fournier, M. The power and control of gravitropic movements in plants: A biomechanical and systems biology view. J. Exp. Bot. 2009, 60, 461–486. [Google Scholar] [CrossRef] [Green Version]
- Toyota, M.; Gilroy, S. Gravitropism and mechanical signaling in plants. Am. J. Bot. 2013, 100, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Chauvet, H.; Moulia, B.; Legué, V.; Forterre, Y.; Pouliquen, O. Revealing the hierarchy of processes and time-scales that control the tropic response of shoots to gravi-stimulations. J. Exp. Bot. 2019, 70, 1955–1967. [Google Scholar] [CrossRef]
- Philosoph-Hadas, S.; Friedman, H.; Meir, S. Gravitropic bending and plant hormones. Vitam. Horm. 2005, 72, 31–78. [Google Scholar] [CrossRef]
- Ibragimova, N.N.; Ageeva, M.V.; Gorshkova, T.A. Development of gravitropic response: Unusual behavior of flax phloem G-fibers. Protoplasma 2017, 254, 749–762. [Google Scholar] [CrossRef]
- Okuyama, T.; Yamamoto, H.; Iguchi, M.; Yoshida, M. Generation process of growth stresses in cell walls. II. Growth stresses in tension wood. Mokuzai Gakkaishi 1990, 36, 797–803. [Google Scholar]
- Okuyama, T.; Yamamoto, H.; Yoshida, M.; Hattori, Y.; Archer, R.R. Growth stresses in tension wood: Role of microfibrils and lignification. Ann. Sci. For. 1994, 51, 291–300. [Google Scholar] [CrossRef]
- Yamamoto, H.; Okuyama, T.; Yoshida, M.; Sugiyama, K. Generation process of growth stresses in cell walls. III. Growth stress in compression wood. Mokuzai Gakkaishi 1991, 37, 94–100. [Google Scholar]
- Yamamoto, H.; Yoshida, M.; Okuyama, T. Growth stress controls negative gravitropism in woody plant stems. Planta 2002, 216, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Mellerowicz, E.J.; Sundberg, B. Wood cell walls: Biosynthesis, developmental dynamics and their implications for wood properties. Curr. Opin. Plant Biol. 2008, 11, 293–300. [Google Scholar] [CrossRef]
- Pilate, G.; Chabbert, B.; Cathala, B.; Yoshinaga, A.; Leplé, J.C.; Laurans, F.; Lapierre, C.; Ruel, K. Lignification and tension wood. C. R. Biol. 2004, 327, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Gorshkov, O.; Mokshina, N.; Ibragimova, N.; Ageeva, M.; Gogoleva, N.; Gorshkova, T. Phloem fibres as motors of gravitropic behaviour of flax plants: Level of transcriptome. Funct. Plant Biol. 2017, 45, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Mokshina, N.; Gorshkov, O.; Ibragimova, N.; Pozhvanov, G.; Gorshkova, T. Screenplay of flax phloem fiber behavior during gravitropic reaction. Plant Signal. Behav. 2018, 13, 1486144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morvan, C.; Andème-Onzighi, C.; Girault, R.; Himmelsbach, D.S.; Driouich, A.; Akin, D.E. Building flax fibres: More than one brick in the walls. Plant Physiol. Biochem. 2003, 41, 935–944. [Google Scholar] [CrossRef]
- Mellerowicz, E.J.; Gorshkova, T.A. Tensional stress generation in gelatinous fibres: A review and possible mechanism based on cell-wall structure and composition. J. Exp. Bot. 2012, 63, 551–565. [Google Scholar] [CrossRef] [Green Version]
- Gorshkova, T.; Chernova, T.; Mokshina, N.; Ageeva, M.; Mikshina, P. Plant ‘muscles’: Fibers with a tertiary cell wall. New Phytol. 2018, 218, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Gorshkova, T.A.; Gurjanov, O.P.; Mikshina, P.V.; Ibragimova, N.N.; Mokshina, N.E.; Salnikov, V.V.; Ageeva, M.V.; Amenitskii, S.I.; Chernova, T.E.; Chemikosova, S.B. Specific type of secondary cell wall formed by plant fibers. Russ. J. Plant Physiol. 2010, 57, 328–341. [Google Scholar] [CrossRef]
- Clair, B.; Ruelle, J.; Beauchêne, J.; Prévost, M.-F.; Fournier Djimbi, M. Tension wood and opposite wood in 21 tropical rain forest species. 1. Occurrence and efficiency of the G-layer. IAWA J. 2006, 27, 329–338. [Google Scholar] [CrossRef] [Green Version]
- Mikshina, P.V.; Chernova, T.E.; Chemikosova, S.B.; Ibragimova, N.N.; Mokshina, N.Y.; Gorshkova, T.A. Cellulosic fibers: Role of matrix polysaccharides in structure and function. In Cellulose—Fundamental Aspects; Van de Ven, T., Godbout, L., Eds.; InTech: Rijeka, Croatia, 2013; pp. 91–112. [Google Scholar]
- Jones, L.; Seymour, G.; Knox, J.P. Localization of Pectic Galactan in Tomato Cell Wall Using a Monoclonal Antibody Specific to (1–4)-β-D-Galactan. Plant Physiol. 1997, 113, 1405–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knox, J.P.; Linstead, P.J.; King, J.; Cooper, C.; Roberts, K. Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 1990, 181, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Potapova, T.A.; Sivakumar, S.; Flynn, J.N.; Li, R.; Gorbsky, G.J. Mitotic progression becomes irreversible in prometaphase and collapses when Wee1 and Cdc25 are inhibited. Mol. Biol. Cell 2011, 22, 1191–1206. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S.E.; Verhertbruggen, Y.; Hervé, C.; Ordaz-Ortiz, J.J.; Farkas, V.; Pedersen, H.L.; Willats, W.G.T.; Knox, J.P. Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol. 2008, 8, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, H.L.; Fangel, J.U.; McCleary, B.; Ruzanski, C.; Rydahl, M.G.; Ralet, M.C.; Farkas, V.; Von Schantz, L.; Marcus, S.E.; Andersen, M.C.; et al. Versatile high resolution oligosaccharide microarrays for plant glycobiology and cell wall research. J. Biol. Chem. 2012, 287, 39429–39438. [Google Scholar] [CrossRef] [Green Version]
- Ruel, K.; Nishiyama, Y.; Joseleau, J.-P. Crystalline and amorphous cellulose in the secondary walls of Arabidopsis. Plant Sci. 2012, 193–194, 48–61. [Google Scholar] [CrossRef]
- Gorshkova, T.A.; Sal’nikov, V.V.; Chemikosova, S.B.; Ageeva, M.V.; Pavlencheva, N.V.; Van Dam, J.E.G. The snap point: A transition point in Linum usitatissimum bast fiber development. Ind. Crops Prod. 2003, 18, 213–221. [Google Scholar] [CrossRef]
- Vietor, R.J.; Newman, R.H.; Ha, M.A.; Apperley, D.C.; Jarvis, M.C. Conformational features of crystal-surface cellulose from higher plants. Plant. J. 2002, 30, 721–731. [Google Scholar] [CrossRef] [Green Version]
- Atalla, R.H.; VanderHart, D.L. The role of solid state 13C NMR spectroscopy in studies of the structure of native celluloses. Solid State Nucl. Magn. Reson. 1999, 115, 1–19. [Google Scholar] [CrossRef]
- Larsson, P.T.; Wickholm, K.; Iversen, T. A CP/MAS 13C NMR investigation of molecular ordering in celluloses. Carbohydr. Res. 1997, 302, 19–25. [Google Scholar] [CrossRef]
- Wickholm, K.; Larsson, P.T.; Iversen, T. Assignment of non-crystalline forms in cellulose I by CP/MAS C-13 NMR spectroscopy. Carbohydr. Res. 1998, 312, 123–129. [Google Scholar] [CrossRef]
- Ibbett, R.; Domvoglou, D.; Wortmann, F.; Schuster, K.C. Carbon-13 solid state NMR investigation and modelling of the morphological reorganization in regenerated cellulose fibres induced by controlled acid hydrolysis. Cellulose 2010, 17, 231–243. [Google Scholar] [CrossRef]
- Chunilall, V.; Bush, T.; Larsson, P.T.; Iversen, T.; Kindness, A. A CP/MAS 13C NMR study of cellulose fibril aggregation in eucalyptus dissolving pulps during drying and the correlation between aggregate dimensions and chemical reactivity. Holzforschung 2010, 64, 693–698. [Google Scholar] [CrossRef]
- Newman, R.H. Estimation of the lateral dimensions of cellulose crystallites using 13C NMR signal strengths. Solid State Nucl. Magn. Reson. 1999, 15, 21–29. [Google Scholar] [CrossRef]
- Mokshina, N.; Gorshkov, O.; Ibragimova, N.; Chernova, T.; Gorshkova, T. Cellulosic fibres of flax recruit both primary and secondary cell wall cellulose synthases during deposition of thick tertiary cell walls and in the course of graviresponse. Funct. Plant Biol. 2017, 44, 820–831. [Google Scholar] [CrossRef]
- Roach, M.J.; Mokshina, N.Y.; Snegireva, A.V.; Badhan, A.; Hobson, N.; Deyholos, M.K.; Gorshkova, T.A. Development of cellulosic secondary walls in flax fibers requires β-galactosidase. Plant Physiol. 2011, 156, 1351–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bush, M.S.; Marry, M.; Huxham, I.M.; Jarvis, M.C.; McCann, M.C. Developmental regulation of pectic epitopes during potato tuberisation. Planta 2001, 213, 869–880. [Google Scholar] [CrossRef]
- Camardella, L.; Carratore, V.; Ciardiello, M.A.; Servillo, L.; Balestrieri, C.; Giovane, A. Kiwi protein inhibitor of pectin methylesterase amino-acid sequence and structural importance of two disulfide bridges. Eur. J. Biochem. 2000, 267, 4561–4565. [Google Scholar] [CrossRef]
- Micheli, F. Pectin methylesterases: Cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 2001, 6, 414–419. [Google Scholar] [CrossRef]
- Willats, W.G.; Orfila, C.; Limberg, G.; Buchholt, H.C.; Van Alebeek, G.J.; Voragen, A.G.; Marcus, S.E.; Christensen, T.M.; Mikkelsen, J.D.; Murray, B.S.; et al. Modulation of the degree and pattern of methyl esterification of pectic homogalacturonan in plant cell walls. Implications for pectin methyl esterase action, matrix properties and cell adhesion. J. Biol. Chem. 2001, 276, 19404–19413. [Google Scholar] [CrossRef] [Green Version]
- Orfila, C.; Seymour, G.B.; Willats, W.G.T.; Huxham, I.M.; Jarvis, M.C.; Dover, C.J.; Thompson, A.J.; Knox, J.P. Altered middle lamella homogalacturonan and disrupted deposition of (1→5)-α-L-arabinan in the pericarp of Cnr, a ripening mutant of tomato. Plant Physiol. 2001, 126, 210–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Gall, H.; Philippe, F.; Domon, J.M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef] [PubMed]
- Mellerowicz, E.J.; Immerzeel, P.; Hayashi, T. Xyloglucan: The molecular muscle of trees. Ann. Bot. 2008, 102, 659–665. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, T.; Kaida, R.; Kaku, T.; Baba, K. Loosening xyloglucan prevents tensile stress in tree stem bending but accelerates the enzymatic degradation of cellulose. Russ. J. Plant Physiol. 2010, 57, 316–320. [Google Scholar] [CrossRef]
- Nishikubo, N.; Awano, T.; Banasiak, A.; Bourquin, V.; Ibatullin, F.; Funada, R.; Brumer, H.; Teeri, T.T.; Hayashi, T.; Sundberg, B.; et al. Xyloglucan endo-transglycosylase (XET) functions in gelatinous layers of tension wood fibers in poplar—A glimpse into the mechanism of the balancing act of trees. Plant Cell Physiol. 2007, 48, 843–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alméras, T.; Clair, B. Critical review on the mechanisms of maturation stress generation in trees. J. R. Soc. Interface 2016, 13, 20160550. [Google Scholar] [CrossRef] [Green Version]
- Guedes, F.T.P.; Laurans, F.; Quemener, B.; Assor, C.; Lainé-Prade, V.; Boizot, N.; Vigouroux, J.; Lesage-Descauses, M.C.; Leplé, J.C.; Déjardin, A.; et al. Non-cellulosic polysaccharide distribution during G-layer formation in poplar tension wood fibers: Abundance of rhamnogalacuronan I and arabinogalactan proteins but no evidence of xyloglucan. Planta 2017, 246, 857–878. [Google Scholar] [CrossRef]
- Kim, J.S.; Daniel, G. Xyloglucans in the G-layer. BioResources 2019, 14, 7675–7686. [Google Scholar]
- Moneo-Sanchez, M.; Alonso-Chico, A.; Knox, J.P.; Dopico, B.; Labrador, E.; Martin, I. β-(1,4)-Galactan remodelling in Arabidopsis cell walls affects the xyloglucan structure during elongation. Planta 2019, 249, 351–362. [Google Scholar] [CrossRef] [Green Version]
- Norberg, P.H.; Meier, H. Physical and chemical properties of the gelatinous layer in tension wood fibre of aspen (Populus tremula L). Holzforschung 1966, 20, 174–178. [Google Scholar] [CrossRef]
- Rihouey, C.; Paynel, F.; Gorshkova, T.; Morvan, C. Flax fibers: Assessing the non-cellulosic polysaccharides and an approach to supramolecular design of the cell wall. Cellulose 2017, 24, 1985–2001. [Google Scholar] [CrossRef]
- Bourmaud, A.; Siniscalco, D.; Foucat, L.; Goudenhooft, C.; Falourd, X.; Pontoire, B.; Arnould, O.; Beaugrand, J.; Baley, C. Evolution of flax cell wall ultrastructure and mechanical properties during the retting step. Carbohydr. Polym. 2019, 206, 48–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altaner, C.; Knox, J.P.; Jarvis, M.C. In situ detection of cell wall polysaccharides in Sitka spruce (Picea sitchensis (Bong.) Carrière) wood tissue. BioResources 2007, 2, 284–295. [Google Scholar]
- Zafeiropoulos, N.E.; Baillie, C.A.; Matthews, F.L. A study of transcrystallinity and its effect on the interface in flax fibre reinforced composite materials. Compos. Part A Appl. Sci. Manuf. 2001, 32, 525–543. [Google Scholar] [CrossRef]
- Müller, M.; Burghammer, M.; Sugiyama, J. Direct investigation of the structural properties of tension wood cellulose microfibrils using microbeam x-ray fibre diffraction. Holzforschung 2006, 60, 474–479. [Google Scholar] [CrossRef]
- Villares, A.; Moreau, C.; Bennati-Granier, C.; Garajova, S.; Foucat, L.; Falourd, X.; Saake, B.; Berrin, J.G.; Cathala, B. Lytic polysaccharide monooxygenases disrupt the cellulose fibers structure. Sci. Rep. 2017, 7, 40262–40270. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal Structure and Hydrogen-Bonding System in Cellulose Ibeta From Synchrotron X-ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef]
- Martone, P.T.; Boller, M.; Burgert, I. Mechanics without muscle: Biomechanical inspiration from the plant world. Integr. Comp. Biol. 2010, 50, 888–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fry, S.C. The Growing Plant Cell Wall: Chemical Metabolic Analysis; Longman Scientific & Technical: London, UK, 1988; pp. 118–140. [Google Scholar]
- Zuckerstätter, G.; Terinte, N.; Sixta, H.; Schuster, K.C. Novel insight into cellulose supramolecular structure through 13C CP-MAS NMR spectroscopy and paramagnetic relaxation enhancement. Carbohydr. Polym. 2013, 93, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, J.; Vuong, R.; Chanzy, H. Electron diffraction studies on two crystalline phases occurring in native cellulose from an algal cell wall. Macromolecules 1991, 24, 4168–4175. [Google Scholar] [CrossRef]
- Reynolds, E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Experimental Parameters Based on Signal Assignment | |||||
|---|---|---|---|---|---|
| Sample | Crystalline Cores (Si), % * | Iα/Iβ | Para-Crystalline (PC), % * | Accessible Fibril Surfaces (Sfs), % * | Inaccessible Fibril Surfaces/Non-Crystalline (Sfs), % * |
| Control | 64.0 ± 0.3 | 0.65 ± 0.00 | 23.4 ± 0.4 | 14.2 ± 0.6 | 17.6 ± 0.2 |
| Pulling | 67.2 ± 0.6 | 0.53 ± 0.02 | 20.9 ± 0.6 | 14.4 ± 0.5 | 15.5 ± 0.0 |
| Computed Parameters | |||||
| Sample | Fibril Width, nm | Lateral Fibril Aggregate Width (LFAD), 3 nm | Index of Crystallinity | ||
| Iα + Iα + β + Iβ + PC | Si/(Si + Sfs) * 100% | ||||
| Control | 6.5 ± 0.1 1 | 4.5 ± 0.2 2 | 15.5 ± 0.7 | 64.0 ± 0.3 | 66.8 ± 0.4 |
| Pulling | 7.0 ± 0.1 1 | 4.1 ± 0.1 2 | 15.3 ± 0.5 | 67.2 ± 0.6 | 69.2 ± 0.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibragimova, N.; Mokshina, N.; Ageeva, M.; Gurjanov, O.; Mikshina, P. Rearrangement of the Cellulose-Enriched Cell Wall in Flax Phloem Fibers over the Course of the Gravitropic Reaction. Int. J. Mol. Sci. 2020, 21, 5322. https://doi.org/10.3390/ijms21155322
Ibragimova N, Mokshina N, Ageeva M, Gurjanov O, Mikshina P. Rearrangement of the Cellulose-Enriched Cell Wall in Flax Phloem Fibers over the Course of the Gravitropic Reaction. International Journal of Molecular Sciences. 2020; 21(15):5322. https://doi.org/10.3390/ijms21155322
Chicago/Turabian StyleIbragimova, Nadezda, Natalia Mokshina, Marina Ageeva, Oleg Gurjanov, and Polina Mikshina. 2020. "Rearrangement of the Cellulose-Enriched Cell Wall in Flax Phloem Fibers over the Course of the Gravitropic Reaction" International Journal of Molecular Sciences 21, no. 15: 5322. https://doi.org/10.3390/ijms21155322
APA StyleIbragimova, N., Mokshina, N., Ageeva, M., Gurjanov, O., & Mikshina, P. (2020). Rearrangement of the Cellulose-Enriched Cell Wall in Flax Phloem Fibers over the Course of the Gravitropic Reaction. International Journal of Molecular Sciences, 21(15), 5322. https://doi.org/10.3390/ijms21155322




